Abstract
The anaphase promoting complex/cyclosome (APC/C), a large E3 ubiquitin ligase, is a key regulator of mitotic progression. Upon activation in mitosis, the APC/C targets its two essential substrates, securin and cyclin B, for proteasomal destruction. Cyclin B is the activator of cyclin-dependent kinase 1 (Cdk1), the major mitotic kinase, and both cyclin B and securin are safeguards of sister chromatid cohesion. Conversely, the degradation of securin and cyclin B promotes sister chromatid separation and mitotic exit. The negative feedback loop between Cdk1 and APC/C—Cdk1 activating the APC/C and the APC/C inactivating Cdk1—constitutes the core of the biochemical cell cycle oscillator.
Since its discovery three decades ago, the mechanisms of APC/C regulation have been intensively studied, and several in vitro assays exist to measure the activity of the APC/C in different activation states. However, most of these assays require the purification of numerous recombinant enzymes involved in the ubiquitylation process (e.g., ubiquitin, the E1 and E2 ubiquitin ligases, and the APC/C) and/or the use of radioactive isotopes. In this chapter, we describe an easy-to-implement method to continuously measure APC/C activity in Xenopus laevis egg extracts using APC/C substrates fused to fluorescent proteins and a fluorescence plate reader. Because the egg extract provides all important enzymes and proteins for the reaction, this method can be used largely without the need for recombinant protein purification. It can also easily be adapted to test the activity of APC/C mutants or investigate other mechanisms of APC/C regulation.
Keywords: Cell cycle, Anaphase promoting complex/cyclosome (APC/C), Xenopus laevis, Frog egg extracts, Plate reader assay, Enzymatic activity
1. Introduction
The anaphase promoting complex/cyclosome (APC/C) is a 1.2 MDa E3 ubiquitin ligase. In vertebrates the complex comprises 14 subunits, some in multiple copies (reviewed in [1]). APC/C-mediated ubiquitylation and subsequent proteasomal degradation of distinct substrates at different times during the cell cycle is instrumental in coordinating cell cycle events (reviewed in [2, 3]) and also plays a role in nonproliferative cells [4, 5]. The activity of the APC/C is tightly regulated by multiple mechanisms, including association with coactivators, posttranslational modifications, and inhibitory interactions. In mitosis, the APC/C in association with its coactivator Cdc20 promotes sister chromatid separation and the initiation of mitotic exit [6–9]. At the end of mitosis, APC/C in association with a second coactivator, Cdh1, facilitates cytokinesis [10]. In G1 phase, APC/CCdh1 activity opposes cell cycle progression by mediating the degradation of S-phase and mitotic cyclins [11, 12]. A third coactivator, Ama1, has a specific role in meiosis [13, 14].
APC/C activity is further regulated by phosphorylation. During mitosis, APC/C phosphorylation of an unstructured loop in the APC1 subunit promotes the recruitment of Cdc20 [15–17], whereas phosphorylation of Cdh1 prevents its association with the APC/C [18–20]. Cdh1 dephosphorylation at the end of mitosis promotes the switch from the APC/CCdc20 to the APC/CCdh1 with Cdc20 itself becoming a target of the APC/CCdh1. In somatic cells, APC/CCdc20 activity is additionally controlled by the mitotic checkpoint, a signaling network that delays APC/C activation until all chromosomes have become attached to the mitotic spindle (reviewed in [21]). However, in many organisms, during early embryogenesis this control seems to be absent or unreliable at best [22–24]. Another inhibitor, the early mitotic inhibitor 1 (Emi1), prevents APC/CCdh1 activity during S and G2 phase to allow for DNA replication and cell cycle progression [25, 26]. A close relative, Emi2, has been found to play a role in controlling the APC/CCdc20 in Xenopus laevis eggs and early embryos [26, 27].
Substrate recognition is achieved through the interaction of the coactivators and the APC/C with short linear motifs (SLIMs) within the substrate (reviewed in [28]). The most common motifs are the destruction box (D box, consensus RxxLx[D/E][Ø]xN [N/S]) and the KEN box. Both securin and cyclin B possess a D box in their unstructured N-terminus; securin furthermore has a KEN box. Abolishing these motifs or truncating the N-terminus of cyclin B and securin prevent their APC/C-mediated degradation (the so-called nondegradable versions). Other SLIMs provide additional specificity but are found less frequently; for example, Cyclin A and Nek2A have additional motifs, the ABBA ([ILVF]x[ILMVP] [FHY]x[DE]) motif and the MR-tail, respectively, that facilitate their APC/C-dependent degradation even in the presence of an active mitotic checkpoint. The combinatorial, multivalent interactions of the different motifs as well as the adherence or divergence from the core consensus motif are likely to define substrate specificity and temporal order of substrate degradation.
Two kinds of assays are commonly used to measure APC/C activity. The first one directly measures the ubiquitylation of a fluorescence-labeled or radiolabeled model substrate in vitro (e.g., [29–31]). This assay constitutes the most direct measurement of the enzymatic activity of the APC/C, but requires the purification of the APC/C, either after recombinant expression or by immunoprecipitation, as well as all other regulators and enzymes involved in the reaction (e.g., E1 and E2 ligase, ubiquitin and the model substrate).
On the other hand, APC/C activity has also been measured by proxy, following the APC/C-mediated proteasomal degradation of an APC/C substrate in a cytoplasmic extract that provides all the necessary components [32, 33]. These extracts are often derived from frog eggs. For this, a purified recombinant labeled substrate is added to the extract, samples taken every few minutes, resolved using SDS polyacrylamide gel electrophoresis and detected by immunoblotting or autoradiography. However, this assay has been difficult to scale without losing accuracy due to the necessity to analyze numerous time points for each condition as well as the somewhat limited time resolution.
Here we present an alternative way of measuring APC/C activity, which is based on measuring the degradation of an APC/C substrate fused to a fluorescent protein (substrate-FP) in Xenopus laevis egg extracts using a fluorescence plate reader. This method was presented in Yang and Ferrell [34]. By using the frog egg extract, the method preserves the advantage of not having to purify every single component of the ubiquitin ligation reaction, but streamlines the detection to enable a higher throughput of measurements.
Although we have mainly used the method to gain insight into the relationship between APC/C activity and its activating kinase Cdk1 [34], we envision that the method can be used in a variety of contexts: (1) studying substrate recognition by using different substrates or different substrate mutants, (2) studying the properties of APC/C with its different coactivators, and (3) studying APC/C regulation directly by using recombinant mutant APC/C.
The protocol can be divided into three main steps: (1) Xenopus laevis egg extract preparation and manipulation, (2) substrate preparation by in vitro transcription and translation, and (3) the actual measurement and analysis of APC/C activity.
Frog egg extracts can be manipulated in multiple ways in order to accommodate the different research interests. As a starting material we use cytostatic factor (CSF)-arrested or low-speed interphase supernatant (LSS) extracts. Excellent protocols on how to prepare either of these extracts are available [35, 36]. These extracts can then be prepared to resemble interphase or mitosis. Although frog egg extracts only contain the coactivator Cdc20, APC/C activity in the context of other coactivators can be studied by adding the purified recombinant coactivator of interest. Cdc20, the APC/C or other regulators can be immunodepleted and substituted by recombinant variants (for a protocol on how to purify the APC/C and its coactivators see [37]). Different substrate-FPs or mutant variants thereof can easily be cloned into the expression vector and tested using this method.
2. Materials
Prepare all solutions using ultrapure water and store all reagents at room temperature (unless indicated otherwise).
2.1. Xenopus laevis Egg Extract Preparation and Manipulation
If starting from CSF-arrested extracts:
-
1
40 mM CaCl2.
-
2
10 mg/ml cycloheximide (stored at −20 °C).
If mitotic extracts are desired (e.g., if APC/CCdc20 activity should be measured):
-
3
10 mg/ml cycloheximide (stored at −20 °C).
-
4
Nondegradable cyclin B (see Note 1, stored at −80 °C).
-
5
Optional: Wee1 inhibitor (e.g., PD0166285, dissolved in DMSO, stored at −20 °C).
2.2. Immunodepletion
Antibody raised against the protein of interest (for APC/C immunodepletion antibodies against the APC3/Cdc27 subunit are commonly used). An irrelevant antibody (e.g., purified IgG) as a control.
Protein A or Protein G magnetic beads (depending on the antibody).
HEPES buffered saline (HBS): 50 mM HEPES, 1.5 mM Na2HPO4, 140 mM NaCl.
The identical extract buffer used to crush the Xenopus laevis eggs (e.g., CSF-XB or ELB).
2.3. Preparation of Fluorescently Labeled Substrate by In Vitro Translation
Plasmid encoding the APC/C substrate of interest fused to a fluorescent protein (substrate-FP) usually under the control of a T7 or SP6 promoter (see Note 2, store at −20 °C).
Coupled in vitro transcription/translation system or cell-free protein expression system compatible with the plasmid promoter (see Notes 3 and 4, usually stored at −80 °C).
Temperature block (with mixing function) depending on the chosen system for protein expression.
2.4. Measuring APC/C Activity Using a Fluorescence Plate Reader
Fluorescence plate reader with fluorescence excitation and emission detection capabilities in the range of the selected fluorescent protein and compatible with 384-well plates. Temperature control around 20–25 °C is an advantage.
384-Well microwell plate, flat bottom, suitable for fluorescence measurements (see Note 5).
3. Methods
Carry out all procedures at room temperature, unless otherwise specified.
3.1. Egg Extract Preparation
Depending on the experiment, APC/C activity can be measured in interphase (e.g., APC/CCdh1) or in mitosis (e.g., APC/CCdc20). CSF-arrested or interphase-arrested frog egg extracts can be used as the starting material. These extracts can be stored at −80 °C for at least one month. Many protocols add an energy mix, though we have performed experiments successfully without it.
3.1.1. Preparing Interphase-Arrested Extract from CSF Extract
Thaw extract(s) for 5 min at 20 °C and if necessary pool the aliquots.
Add cycloheximide to a final concentration of 100 μg/ml.
Add CaCl2 to a final concentration of 0.8 mM.
Mix thoroughly by carefully pipetting up and down (at least ten times).
Incubate for 50 min at 20 °C. Stir the extract with a pipet tip every 10 min to keep the extract well mixed.
3.1.2. Preparing Mitotic Extract from Interphase Extract
If you have not added it already, add cycloheximide to 100 μg/ml.
Add nondegradable cyclin B to about 150 nM (see Note 6).
Mix thoroughly by carefully pipetting up and down (at least ten times).
-
Incubate for 1 h at 20 °C. Stir the extract with a pipet tip every 10 min to keep the extract well mixed.
Optional: Take samples from the CSF, interphase, and mitotic extract to analyse the phosphorylation status of the APC/C and other proteins later by immunoblotting.
3.2. Immunodepletion
The exact amount of antibody used for the immunodepletion needs to be established for each protein–antibody pair. Antibodies can either be covalently coupled to the beads, which allows for the possibility of eluting the protein and reusing the beads, or simply bound to the beads overnight as we describe here. In addition to the antibody coupled beads, prepare control IgG coupled beads in the same way.
Use about 10 μl magnetic beads per round of depletion for each 30 μl of extract. As two rounds of depletion are performed, prepare a total of 20 μl of magnetic beads per 30 μl of extract.
Remove supernatant and wash the magnetic beads twice with two bead volumes of HBS.
Resuspend beads in two bead volumes of HBS and add the empirically determined amount of antibody.
Rotate overnight at 4 °C.
Collect the beads using a magnet and wash five times with two bead volumes of extract buffer (e.g., CSF-XB and ELB). Remove all buffer.
Add the egg extract to one half of the antibody beads. Rotate for 40 min at 4 °C. Collect the beads using a magnet.
Transfer the extract to a fresh tube with the other half of the antibody beads. Rotate for 40 min at 4 °C. Collect the beads using a magnet.
-
Transfer immunodepleted extract to a fresh tube. Store on ice until further use (typically no longer than 1 h).
Optional: Take extract samples for immunoblotting to confirm efficient protein depletion.
3.3. Preparation of Fluorescently Labeled Substrate by In Vitro Transcription/Translation
The fluorescently labelled substrate can be prepared in parallel to the (immunodepleted) extract or ahead of time. The substrate-FB is best used fresh, but can be stored 2–3 days at 4 °C protected from light if necessary.
For best results ensure an RNase-free environment (e.g., wear gloves, clean pipettes with RNase AWAY, and use RNase-free consumables).
Add the plasmid to the coupled transcription/translation system following manufacturer’s instructions. In addition, prepare a blank control for measuring the baseline fluorescence of the extract later.
During the incubation, protect your fluorescent substrate from light by covering the temperature block or wrapping aluminium foil around the tube.
Confirm the successful transcription and translation of the substrate-FP by measuring the fluorescence relative to the blank control using a plate reader (see Subheading 3.4). The fluorescence should be at least 50-fold above the blank control for good results in the assay.
No further purification of the protein is necessary.
3.4. Measuring APC/C Activity Using a Fluorescence Plate Reader
A typical measurement is performed at least as a duplicate using about 20 μl of extract, manipulated beforehand in the desired way, per measurement. Degradation might start right after mixing the substrate-FP and the extracts; therefore, all steps are performed on ice and in proximity to the plate reader. The plate reader should be equilibrated to the desired temperature and all settings adjusted before the mixing step.
Add the substrate-FP to the extract in the ratio 1:16–1:20 (see Note 7). Additionally, set up at least one sample with the blank control to get a good background reading.
Mix vigorously by pipetting up and down (at least ten times). Do not vortex! Try to avoid air bubbles as much as possible.
Carefully pipette 20 μl of extract into each well of the micro-plate avoiding air bubbles as much as possible.
Load plate into the plate reader and start measurement (top read). We commonly measure for 120 min, with 1 min intervals and mixing before every measurement. Excitation and emission wavelengths have to be determined for the specific fluorescent protein; for CFP we use 435 nm/475 nm (see Fig. 1a for an example measurement).
Fig. 1.
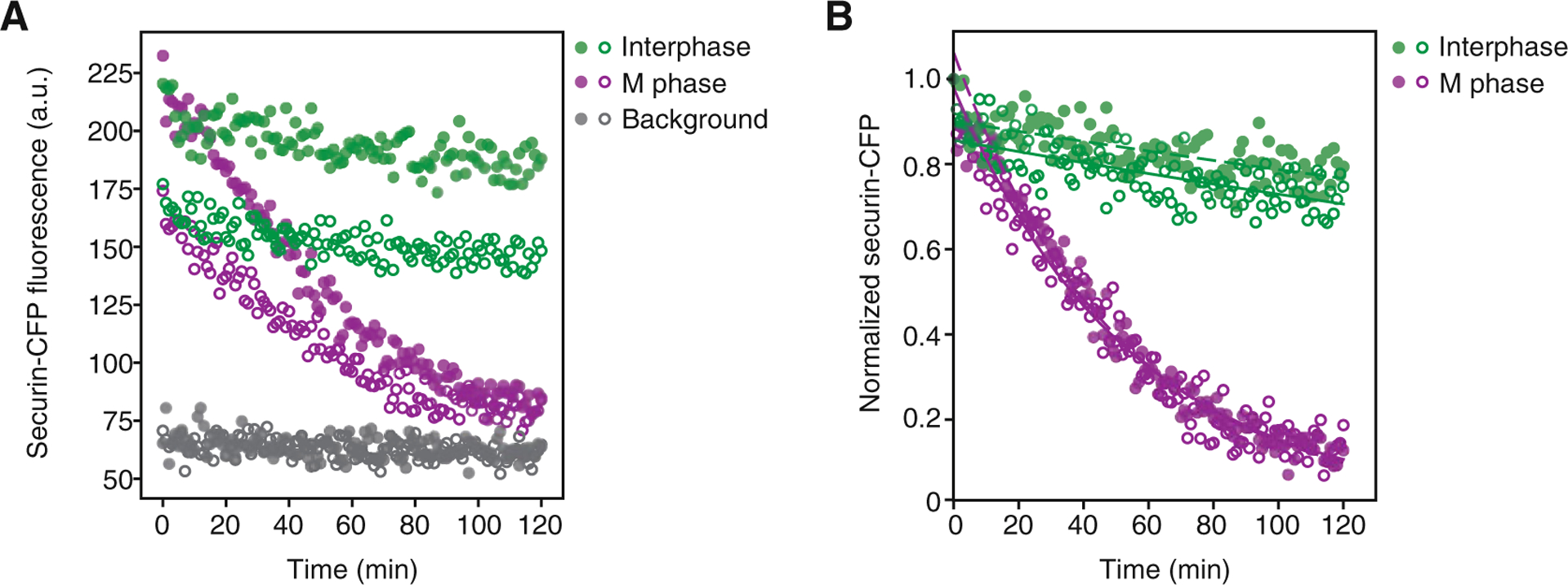
(a) Measured fluorescence of an interphase or M phase (mitotic) extract supplemented with securin-CFP as a function of time. An interphase extract supplemented with the blank control in vitro transcription/translation reaction was used as a background control. Shown are data from two technical replicates (full or empty circles respectively). (b) Data shown in (a) after background subtraction and normalization. The dashed or solid lines, respectively, show the exponential fit of the data
3.5. Data Analysis
We are using the apparent first order rate constant (k) as a measure for APC/C activity. To estimate the first order rate constant, the following steps are undertaken:
4. Notes
We commonly use recombinant nondegradable cyclin B from sea urchin purified from bacteria (for a protocol see [38]) or nondegradable cyclin B1 from Xenopus laevis purified from baculovirus-infected insect cells (for a protocol see [39]).
We commonly use SP6-securin-CFP but have also successfully used cyclin B-CFP or cyclin A-CFP as well as other fluorescent proteins (e.g., mCherry).
Do not use transcription/translation systems based on rabbit reticulocytes as their pink color interferes with the fluorescence measurements. We commonly use transcription/translation systems based on wheat germ. Depending on the system and promoter used, the plasmid might have to be linearized and purified before used for transcription/translation (though this was not applicable for our system–promoter combination).
So far, we have not been able to perform consistent measurements using purified recombinant substrate-FPs and therefore advise using the in vitro transcribed and translated protein.
Several plate suppliers exist. We use black 384-well fluotrac 200 plates from Greiner.
The exact concentration of nondegradable cyclin B necessary for promoting the mitotic state varies from batch to batch and also depends on the exact construct used (e.g., we have observed that higher concentrations of bacterially expressed sea urchin nondegradable cyclin B are necessary relative to insect cell expressed Xenopus laevis nondegradable cyclin B).
Extracts should be diluted as little as possible and more than 10% dilution should be avoided. Depending on the yield of the in vitro translation/transcription reaction 1:16–1:20 works well for us to avoid extract dilution while still having a good signal-to-noise ratio for the measurement.
To account for some variability in the starting concentration of the substrate-FP, the data is normalized.
In theory, due to the normalization and the background subtraction, A0 should be close to 1 and C should be close to 0 in the fit and we normally constraint these parameters for the fit (A0 > 0.8 and C < 0.2).
Acknowledgments
We thank Jan-Michael Peters and members of the Peters lab from the Research Institute of Molecular Pathology (IMP, Vienna, Austria) for their support and helpful discussions. The work was assisted by grants from the National Institutes of Health (R35 GM131792 to J.E.F., and R35 GM119688 to Q.Y.), the National Science Foundation (Early CAREER Grant #1553031 and MCB #1817909 to Q.Y.) and a postdoctoral fellowship from the German Research Foundation (KA 4476/1-1, J.K.).
References
- 1.Alfieri C, Zhang S, Barford D (2017) Visualizing the complex functions and mechanisms of the anaphase promoting complex/cyclosome (APC/C). Open Biol 7(11). 10.1098/rsob.170204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters JM (2006) The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7(9):644–656. 10.1038/nrm1988 [DOI] [PubMed] [Google Scholar]
- 3.Pines J (2011) Cubism and the cell cycle: the many faces of the APC/C. Nat Rev Mol Cell Biol 12(7):427–438. 10.1038/nrm3132 [DOI] [PubMed] [Google Scholar]
- 4.Delgado-Esteban M, Garcia-Higuera I, Maestre C, Moreno S, Almeida A (2013) APC/C-Cdh1 coordinates neurogenesis and cortical size during development. Nat Commun 4:2879. 10.1038/ncomms3879 [DOI] [PubMed] [Google Scholar]
- 5.Eguren M, Porlan E, Manchado E, Garcia-Higuera I, Canamero M, Farinas I, Malumbres M (2013) The APC/C cofactor Cdh1 prevents replicative stress and p53-dependent cell death in neural progenitors. Nat Commun 4:2880. 10.1038/ncomms3880 [DOI] [PubMed] [Google Scholar]
- 6.Irniger S, Piatti S, Michaelis C, Nasmyth K (1995) Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell 81(2):269–278. 10.1016/0092-8674(95)90337-2 [DOI] [PubMed] [Google Scholar]
- 7.King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW (1995) A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81(2):279–288. 10.1016/0092-8674(95)90338-0 [DOI] [PubMed] [Google Scholar]
- 8.Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca FC, Ruderman JV, Hershko A (1995) The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell 6(2):185–197. 10.1091/mbc.6.2.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tugendreich S, Tomkiel J, Earnshaw W, Hieter P (1995) CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell 81(2):261–268. 10.1016/0092-8674(95)90336-4 [DOI] [PubMed] [Google Scholar]
- 10.Floyd S, Pines J, Lindon C (2008) APC/C Cdh1 targets aurora kinase to control reorganization of the mitotic spindle at anaphase. Curr Biol 18(21):1649–1658. 10.1016/j.cub.2008.09.058 [DOI] [PubMed] [Google Scholar]
- 11.Li M, Shin YH, Hou L, Huang X, Wei Z, Klann E, Zhang P (2008) The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol 10 (9):1083–1089. 10.1038/ncb1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigrist SJ, Lehner CF (1997) Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90(4):671–681. 10.1016/s0092-8674(00)80528-0 [DOI] [PubMed] [Google Scholar]
- 13.Cooper KF, Mallory MJ, Egeland DB, Jarnik M, Strich R (2000) Ama1p is a meiosis-specific regulator of the anaphase promoting complex/cyclosome in yeast. Proc Natl Acad Sci U S A 97(26):14548–14553. 10.1073/pnas.250351297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okaz E, Arguello-Miranda O, Bogdanova A, Vinod PK, Lipp JJ, Markova Z, Zagoriy I, Novak B, Zachariae W (2012) Meiotic pro-phase requires proteolysis of M phase regulators mediated by the meiosis-specific APC/CAma1. Cell 151(3):603–618. 10.1016/j.cell.2012.08.044 [DOI] [PubMed] [Google Scholar]
- 15.Fujimitsu K, Grimaldi M, Yamano H (2016) Cyclin-dependent kinase 1-dependent activation of APC/C ubiquitin ligase. Science 352 (6289):1121–1124. 10.1126/science.aad3925 [DOI] [PubMed] [Google Scholar]
- 16.Qiao R, Weissmann F, Yamaguchi M, Brown NG, VanderLinden R, Imre R, Jarvis MA, Brunner MR, Davidson IF, Litos G, Haselbach D, Mechtler K, Stark H, Schulman BA, Peters JM (2016) Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc Natl Acad Sci U S A 113 (19):E2570–E2578. 10.1073/pnas.1604929113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Chang L, Alfieri C, Zhang Z, Yang J, Maslen S, Skehel M, Barford D (2016) Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature 533 (7602):260–264. 10.1038/nature17973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco MA, Sanchez-Diaz A, de Prada JM, Moreno S (2000) APC(ste9/srw1) promotes degradation of mitotic cyclins in G(1) and is inhibited by cdc2 phosphorylation. EMBO J 19(15):3945–3955. 10.1093/emboj/19.15.3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM (2000) Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell 11(5):1555–1569. 10.1091/mbc.11.5.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zachariae W, Schwab M, Nasmyth K, Seufert W (1998) Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282 (5394):1721–1724. 10.1126/science.282.5394.1721 [DOI] [PubMed] [Google Scholar]
- 21.Lara-Gonzalez P, Westhorpe FG, Taylor SS (2012) The spindle assembly checkpoint. Curr Biol 22(22):R966–R980. 10.1016/j.cub.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 22.Chenevert J, Roca M, Besnardeau L, Ruggiero A, Nabi D, McDougall A, Copley RR, Christians E, Castagnetti S (2020) The spindle assembly checkpoint functions during early development in non-chordate embryos. Cell 9(5). 10.3390/cells9051087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galli M, Morgan DO (2016) Cell size determines the strength of the spindle assembly checkpoint during embryonic development. Dev Cell 36(3):344–352. 10.1016/j.devcel.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minshull J, Sun H, Tonks NK, Murray AW (1994) A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79(3):475–486. 10.1016/0092-8674(94)90256-9 [DOI] [PubMed] [Google Scholar]
- 25.Dong X, Zavitz KH, Thomas BJ, Lin M, Campbell S, Zipursky SL (1997) Control of G1 in the developing Drosophila eye: rca1 regulates cyclin A. Genes Dev 11(1):94–105. 10.1101/gad.11.1.94 [DOI] [PubMed] [Google Scholar]
- 26.Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK (2001) Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105(5):645–655. 10.1016/s0092-8674(01)00361-0 [DOI] [PubMed] [Google Scholar]
- 27.Tischer T, Hormanseder E, Mayer TU (2012) The APC/C inhibitor XErp1/Emi2 is essential for Xenopus early embryonic divisions. Science 338(6106):520–524. 10.1126/science.1228394 [DOI] [PubMed] [Google Scholar]
- 28.Davey NE, Morgan DO (2016) Building a regulatory network with short linear sequence motifs: lessons from the degrons of the anaphase-promoting complex. Mol Cell 64 (1):12–23. 10.1016/j.molcel.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll CW, Morgan DO (2005) Enzymology of the anaphase-promoting complex. Methods Enzymol 398:219–230. 10.1016/S0076-6879(05)98018-X [DOI] [PubMed] [Google Scholar]
- 30.Izawa D, Pines J (2015) The mitotic checkpoint complex binds a second CDC20 to inhibit active APC/C. Nature 517(7536):631–634. 10.1038/nature13911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraft C, Gmachl M, Peters JM (2006) Methods to measure ubiquitin-dependent proteolysis mediated by the anaphase-promoting complex. Methods 38(1):39–51. 10.1016/j.ymeth.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi M, Yu S, Qiao R, Weissmann F, Miller DJ, VanderLinden R, Brown NG, Frye JJ, Peters JM, Schulman BA (2015) Structure of an APC3-APC16 complex: insights into assembly of the anaphase-promoting complex/cyclosome. J Mol Biol 427(8):1748–1764. 10.1016/j.jmb.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamano H, Trickey M, Grimaldi M, Kimata Y (2009) In vitro assays for the anaphase-promoting complex/cyclosome (APC/C) in Xenopus egg extracts. Methods Mol Biol 545:287–300. 10.1007/978-1-60327-993-2_18 [DOI] [PubMed] [Google Scholar]
- 34.Yang Q, Ferrell JE Jr (2013) The Cdk1-APC/C cell cycle oscillator circuit functions as a time-delayed, ultrasensitive switch. Nat Cell Biol 15 (5):519–525. 10.1038/ncb2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banaszynski LA, Allis CD, Shechter D (2010) Analysis of histones and chromatin in Xenopus laevis egg and oocyte extracts. Methods 51 (1):3–10. 10.1016/j.ymeth.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray AW (1991) Cell cycle extracts. Methods Cell Biol 36:581–605 [PubMed] [Google Scholar]
- 37.Jarvis MA, Brown NG, Watson ER, VanderLinden R, Schulman BA, Peters JM (2016) Measuring APC/C-dependent ubiquitylation in vitro. Methods Mol Biol 1342:287–303. 10.1007/978-1-4939-2957-3_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glotzer M, Murray AW, Kirschner MW (1991) Cyclin is degraded by the ubiquitin pathway. Nature 349(6305):132–138. 10.1038/349132a0 [DOI] [PubMed] [Google Scholar]
- 39.Ha SH, Kim SY, Ferrell JE Jr (2016) The pro-zone effect accounts for the paradoxical function of the Cdk-binding protein Suc1/Cks. Cell Rep 14(6):1408–1421. 10.1016/j.celrep.2016.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]


