Abstract
Simple Summary
In recent years, aspartate has been increasingly acknowledged as a critical player in the metabolism of cancer cells which use this metabolite for nucleotide and protein synthesis and for redox homeostasis. Most intracellular aspartate derives from the mitochondrial catabolism of glutamine. To date at least four mitochondrial transporters have been involved in this metabolic pathway. Their involvement appears to be cancer type-specific and dependent on glutamine availability. Targeting these mitochondrial transporters may represent a new attractive strategy to fight cancer. The aim of this review is to dissect the role of each of these transporters in relation to the type of cancer and the availability of nutrients in the tumoral microenvironment.
Abstract
Aspartate has a central role in cancer cell metabolism. Aspartate cytosolic availability is crucial for protein and nucleotide biosynthesis as well as for redox homeostasis. Since tumor cells display poor aspartate uptake from the external environment, most of the cellular pool of aspartate derives from mitochondrial catabolism of glutamine. At least four transporters are involved in this metabolic pathway: the glutamine (SLC1A5_var), the aspartate/glutamate (AGC), the aspartate/phosphate (uncoupling protein 2, UCP2), and the glutamate (GC) carriers, the last three belonging to the mitochondrial carrier family (MCF). The loss of one of these transporters causes a paucity of cytosolic aspartate and an arrest of cell proliferation in many different cancer types. The aim of this review is to clarify why different cancers have varying dependencies on metabolite transporters to support cytosolic glutamine-derived aspartate availability. Dissecting the precise metabolic routes that glutamine undergoes in specific tumor types is of upmost importance as it promises to unveil the best metabolic target for therapeutic intervention.
Keywords: cancer, glutamine metabolism, aspartate, mitochondrial carriers, UCP2, SLC1A5_var, aspartate/glutamate carrier, glutamate carrier
1. Introduction
For many years, following Otto Warburg’s pioneering work [1], biologists have focused their attention on glucose metabolism in cancer cells. The interest in the tight connection between tumor growth and glucose utilization further increased with the later findings that genes, usually mutated in human cancers, boosted glucose metabolism [2,3,4]. Although cancer cells convert most of the glycolytic pyruvate to lactate, even in the presence of oxygen [1,4,5], their survival and proliferation rely on mitochondrial activity to provide the building blocks for macromolecule synthesis [6]. One of the most important nutrients used by cancer cells to fuel anabolic processes is glutamine [7]. Glutamine, a source of carbon and reduced nitrogen for many biosynthetic reactions, has an anaplerotic function in cancer cells and controls redox homeostasis [6,8,9,10,11]. To support these cellular needs, glutamine enters several metabolic pathways in both the cytosol and the mitochondria. In this review, we will focus on the role of mitochondrial glutamine metabolism. For an overview of the cytosolic metabolism of glutamine the reader is refered to several excellent reviews [9,12,13].
The two main metabolites derived from mitochondrial glutamine oxidation, when the Krebs cycle (KC) is fully functioning [14,15], are malate and aspartate. Both are produced in the matrix and used in the cytosol to produce NADPH for anabolic processes and redox homeostasis. Aspartate, specifically, is crucial for protein and nucleotide biosynthesis [11,16,17]. Importantly, it was recently shown that the main purpose of the mitochondrial electron transport chain (ETC) in cancer cells’ proliferation is to enable mitochondrial aspartate synthesis [18,19,20]. Inhibitors of the ETC and hypoxic conditions negatively affect the proliferation of many cancer cells by reducing the availability of aspartate [21].
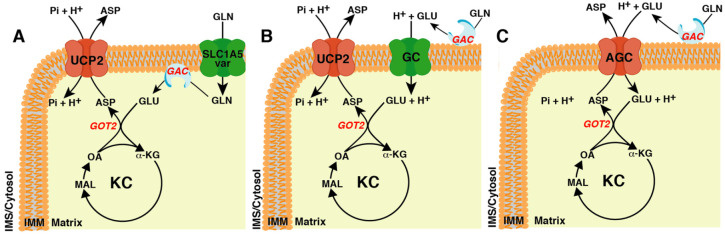
In order to produce aspartate, glutamine must enter the KC as α-ketoglutarate (α-KG). The critical enzyme in this metabolic pathway is glutaminase (GA) [22]. This enzyme converts glutamine to glutamate, which is further transformed by glutamate dehydrogenase (GDH) or glutamate-oxaloacetate transaminase (GOT) to α-KG. At least four isoforms of GA [23], with a different subcellular localization (mitochondrial, cytosolic, and nuclear) [24,25,26], are present in humans. Here, we will focus on glutaminase C (GAC or GLS C) [23], a shorter isoform of the kidney type GA (KGA or GLS) that is localized to mitochondria [26] and frequently induced in cancer cells [23]. Although it is commonly accepted that GAC is bound to the inner mitochondrial membrane, the localisation of its catalytic site, whether in the matrix or in the intermembrane space, is still a matter of debate [26,27,28,29,30]. This is a crucial point because if the catalytic site is matrix facing a glutamine transporter would be required (Figure 1A). However, if the catalytic domain faces the intermembrane space, glutamate would be released outside the mitochondria thus requiring a glutamate transporter [31,32,33].
Figure 1.
The different transporters involved in cytosolic glutamine-derived aspartate availability based on submitochondrial localization of glutaminase. (A) The localisation of glutaminase (GAC) at the matrix side of the inner mitochondrial membrane (IMM) requires the glutamine transporter and the aspartate/Pi + H+ exchanger (uncoupling protein 2, UCP2). (B) The localisation of GAC at the external side of the IMM requires the glutamate + H+ transporter (GC) and UCP2. (C) The localisation of GAC at the external side of the IMM requires only the aspartate/glutamate + H+ carrier (AGC). ASP, aspartate; GLN, glutamine; GLU, glutamate; Pi, phosphate; OA, oxaloacetate; MAL, malate; α-KG, α-ketoglutarate; KC, Krebs cycle; IMS, intermembrane space; GOT2, mitochondrial isoform of the glutamate-oxaloacetate transaminase.
The latter hypothesis poses another question: does glutamate enter into mitochondria through GC (Figure 1B) or AGC (Figure 1C)? Although the two routes would be equally efficient as far as glutamate import is concerned, they are different with respect to aspartate export. Indeed, if glutamate enters into the mitochondria through AGC, the aspartate produced in the matrix can exit through this transporter in exchange for glutamate (Figure 1C), whereas if glutamate enters through GC, an aspartate exporter is needed, e.g., UCP2 (Figure 1B).
As discussed below, data reported in the literature support all of these scenarios in different contexts suggesting the route of transport depends on the cancer type and glutamine availability in the tumor microenvironment.
2. Glutaminase: A Multifaceted Enzyme in Cancer Cell Metabolism
Before beginning with the role of the mitochondrial carriers in the aspartate metabolism of cancer cells, it is worth examining glutaminase, the first enzyme in the glutaminolysis pathway through which the majority of aspartate in cancer cells is derived. Human GA proteins are encoded by two paralogous genes named GLS and GLS2 [34], both producing several transcript variants. GLS gives rise to KGA and to a shorter isoform, GAC [23]. Splicing of GLS2 leads to a long transcript named GAB, initially identified in human breast cancer cells, and to a shorter transcript, LGA, originally identified in rat liver [34,35,36]. Although the biochemical function of GA isoenzymes is the same, they appear to have opposing roles in cancer. The c-Myc-regulated GLS correlates with tumor growth rate and malignancy, making cancer cells dependent on glutamine anaplerosis for the maintenance of mitochondrial integrity and KC functionality [37,38]. In contrast, the role of p53-regulated GLS2 is more controversial, with many reports supporting a tumor suppressive function [35,39,40,41,42,43,44].
For the purpose of this review, we will focus on GAC, which was initially cloned from a human colon carcinoma cell line [23]. GAC is the predominant glutaminase isoform expressed in cancer cells [26]. Furthermore, GAC and KGA display different subcellular localizations in a cell type-specific manner, mitochondrial (GAC) and cytosolic (KGA) [26]. Both isoforms are regulated by phosphate which increases their turnover rate and decreases their Km-app for glutamine [26]. GAC has the lowest Km-app and the highest catalytic efficiency, pointing out its central role in the increased glutaminolysis of cancer cells [26]. Glutaminase enzymatic activity in mitochondrial extracts was initially proposed in 1967 by Katunuma et al. [45], and since then, many aspects of this enzyme have been revealed: the number of encoding genes, their mechanism of splicing, expression and function regulation, kinetic constants, and inhibitors for cancer therapy. However, little has been done to shed light on the sub-mitochondrial localization of the enzyme. Early work attempted to use complementary experimental approaches to answer this question: (i) mitochondria sub-fractionation and immunoblotting with specific antisera; (ii) chemical modification with sulfhydryl group reagents of different permeability; (iii) immunological studies; (iv) enzymatic digestion at both sides of the inner mitochondrial membrane; (v) studies of intact mitochondria with [14C]glutamine and determination of the amount of produced [14C]glutamate inside and outside the mitochondria [27,28,30,46,47,48,49,50,51,52]. Unfortunately, none of these studies came to an unambiguous answer, although most studies favoured the extramitochondrial localization of the enzyme. A possible explanation for the ambiguity came from a transmission electron microscopy study of post-embedding immunogold labelling of GA in which the pig and rat renal phosphate-activated glutaminase was found partially outside and partially inside the inner mitochondrial membrane. The intermembrane space-facing GA was suggested to be the only functional enzyme, as high intramitochondrial concentrations of the inhibitor, glutamate, would keep the matrix-facing one in a dormant state [47]. The recent release of MitoCarta3.0 still reports the localization of both KGA and GAC in the IMM [53] suggesting that the true localization remains to be determined.
3. The Mitochondrial Glutamine Carrier: The Last Piece of Glutaminolysis Puzzle
Despite the central role of glutamine in the mitochondrial metabolism of normal and cancer cells, the gene encoding for the mitochondrial glutamine carrier remained unknown until recently. Yoo et al. [54] reported that the SLC1A5 gene (also known as ASCT2) contains two different transcription initiation sites, which give rise to a long transcript encoding the plasma membrane obligatory sodium-dependent transporter for neutral amino acids [55] and to a shorter transcript (SLC1A5_var) encoding the mitochondrial glutamine carrier [54], although some concern about its biochemical functional has been recently raised [56]. It should be emphasized that SLC1A5_var and the mitochondrial pyruvate carrier (MPC) [57,58] do not present a tripartite structure which characterizes all members of the MCF [59,60,61]. SLC1A5 is one of the most important transporters used by cancer cells to take up glutamine [62,63]. SLC1A5 is upregulated in many forms of cancer that are characterized by rapid progression, anti-cancer drug resistance and poor survival outcome [64,65,66,67,68]. In different types of adenocarcinoma, the expression of SLC1A5_var is higher than that of surrounding normal tissue, and its expression level is correlated with poor survival. Interestingly the only reported exception is colon cancer, where outcomes are the opposite [54].
SLC1A5_var transcription is induced in hypoxic conditions by HIF-2α [54]. Studies of gain and loss of function demonstrated that SLC1A5_var had a key role in glutaminolysis, redox homeostasis, proliferation rate, and gemcitabine resistance of pancreatic cancer cells [54]. In pancreatic ductal adenocarcinoma (PDAC), mutated KRAS induces a rewiring of glutamine metabolism vital for redox homeostasis and cell proliferation [11]. In this pathway, the mitochondrial glutamine-derived aspartate once transported into the cytosol is converted through a series of enzymatic reactions to pyruvate and NADPH that cells use for reactive oxygen species (ROS) control [11,17]. Metabolomics experiments carried out with [U-13C]-glutamine on SLC1A5_var silenced PDAC cells confirmed a reduced glutaminolysis, though unfortunately the aspartate levels were not determined, although a possible reduction should be expected since a direct link between glutaminolysis and cytosolic aspartate availability in PDAC has been already demonstrated [11,17]. In fact, the proliferation defect found in SLC1A5_var-silenced PDAC cells could be partially restored by the addition of aspartate to the growth medium [54]. The results reported by Yoo et al. demonstrated that the GAC or its catalytic site is localized in the matrix and, at least in PDAC cells, glutaminolysis requires a mitochondrial glutamine transporter and an exporter of aspartate, identified to be UCP2 [17] (Figure 1A). Importantly, silencing of SLC1A5_var or UCP2 inhibits mitochondrial glutaminolysis and abolishes gemcitabine resistance in PDAC cells [54,69,70].
4. The Mitochondrial Glutamate Carrier: The Other Side of the Coin
The discovery of the mitochondrial glutamine carrier pointed out that the catalytic site of glutaminase was located in the matrix. Interestingly, in colorectal cancer (CRC) the mitochondrial catabolism of glutamine required the presence of the SLC25A22, encoding the mitochondrial glutamate carrier isoform 1 (GC1) [71]. This suggested an external mitochondrial localization of the catalytic site of glutaminase (Figure 1B) [71].
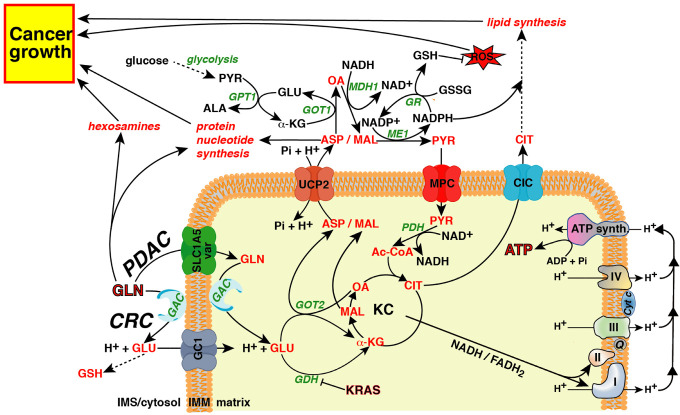
The mitochondrial glutamate carrier in humans is encoded by two different genes SLC25A22 and SLC25A18, encoding GC1 and GC2, respectively, which differ in their tissue distribution and kinetic constants [31]. Both isoforms catalyse a symport of glutamate coupled to a proton in the matrix [31]. Wong et al. demonstrated that SLC25A22 expression increased in tumor tissues compared with non-tumor colon tissues in humans. Indeed, knockdown of SLC25A22 in KRAS mutated CRC cell lines suppressed glutamine metabolism via the KC, reducing the availability of cytosolic aspartate [71,72]. SLC25A22 silencing reduced cell proliferation, migration, and invasion in vitro, as well as tumor and metastasis formation in a xenograft model. The crucial role of the mitochondrial glutamate carrier in cytosolic availability of the glutamine-derived aspartate in KRAS-mutated CRC cells was further supported by the rescue of the proliferation defect induced by SLC25A22 knockdown via the addition of aspartate in the growth medium. Most of the data reported by Wong et al. on glutamine utilization by KRAS-mutated CRC cell lines overlapped with those reported for the rewired glutamine metabolism induced by mutated KRAS in PDAC [11,17]. The only exception is that in PDAC glutamine must be transported in the matrix by the mitochondrial glutamine carrier in order to be processed by GAC, whereas in CRC this last enzymatic reaction occurs outside the mitochondria and glutamate enters into the matrix through the mitochondrial glutamate transporter (Figure 2). This difference in the glutamine utilization between PDAC and CRC may explain why although SLC1A5_var was found to be overexpressed in colon adenocarcinoma its expression was not correlated to survival outcomes [54].
Figure 2.
A bird’s eye view of glutamine utilization by cancer cells. Cancer cells can use the carbon skeleton and reduced nitrogen of glutamine to synthesize non-essential amino acids, hexosamines, reduced glutathione (GSH), nucleotides, proteins and lipids. Aspartate and malate produced in the matrix can be used in the cytosol for NADPH production, reducing power for the biosynthetic processes and redox homeostasis. The carbon skeleton of glutamine produces reducing equivalents in the KC (NADH and FADH2) which can be re-oxidized in the electron transport chain, producing chemical energy (ATP). ASP, aspartate; GLN, glutamine; GLU, glutamate, Pi, phosphate; OA, oxaloacetate; MAL, malate; α-KG, α-ketoglutarate; CIT, citrate; GSH, reduced glutathione; GSSG, oxidized glutathione; PYR, pyruvate; Ac-CoA, acetyl-CoA; ALA, alanine; Q, coenzyme Q; PDAC, pancreatic ductal adenocarcinoma; CRC, colorectal cancer; GAC, glutaminase C; SLC1A5_var, mitochondrial glutamine carrier; UCP2, uncoupling protein 2; GC1, mitochondrial glutamate carrier, isoform 1; MPC, mitochondrial pyruvate carrier; CIC, mitochondrial citrate carrier; KC, Krebs cycle; IMM, inner mitochondrial membrane; IMS, intermembrane space; GOT1/2, glutamic-oxaloacetic transaminase isoforms; GPT1, cytosolic isoform of glutamic-pyruvic transaminase; MDH1, cytosolic isoform of malic dehydrogenase; ME1, cytosolic isoform of malic enzyme; GR, glutathione reductase; PDH, pyruvic dehydrogenase; GDH, glutamic dehydrogenase.
Interestingly, the expression levels of GC2 in CRC tissues was lower than that of control tissues and its upregulation inhibited Warburg effect and cell proliferation via Wnt/β-catenin cascade [73]. Furthermore, high expression of GC2 indicated a longer disease-free survival time after surgery [73].
Similar to CRC, GC1 was also found to be upregulated in osteosarcoma and gallbladder cancer [74,75]. Studies of gain and loss of function showed that GC1 significantly increased osteosarcoma cell proliferation and promoted their invasion capability. Moreover, GC1 expression levels were associated with a poor outcome for patients [74]. In gallbladder cancer, the most common biliary tract malignancy, GC1 also promoted tumor development and metastasizing by activating the MAPK/ERK pathway [75].
5. The Mitochondrial Aspartate/Glutamate Carrier: A Key Player of Malate-Aspartate Shuttle
The first member of the MCF found to transport aspartate across the IMM was AGC [76]. In humans, there are two isoforms of AGC, AGC1, and AGC2, the former encoded by the SLC25A12 gene, also known as ARALAR1, and the latter by the SLC25A13 gene, also known as CITRIN/ARALAR2. AGC1 is mainly expressed in heart, skeletal muscle, and brain whereas AGC2 is expressed in many tissues and abundantly in liver [77,78]. In vitro, both isoforms catalyse a Ca2+-stimulated electrogenic exchange of mitochondrial aspartate for cytosolic glutamate plus a proton [76,79]. AGC, together with the mitochondrial oxoglutarate/malate carrier [80,81], the two isoforms of malate dehydrogenase and glutamic-oxaloacetic transaminase, constitute the malate-aspartate shuttle (MAS) which transfers NADH reducing equivalents from cytosol to mitochondria [82,83]. Although the key role of AGC in MAS has been demonstrated [84,85,86,87], the functionality of AGCs in certain circumstances is also crucial for cellular aspartate metabolism. In fact, AGC2 deficiency in humans causes type II citrullinemia [78,88,89], a urea cycle disease in which the low availability of cytosolic aspartate does not allow the arginine–succinate synthase to use citrulline for arginine-succinate production. Furthermore, the deficiency of AGC1 induces a global cerebral hypomyelination due to the low availability of N-acetyl-aspartate for myelin synthesis [85,90]. This means that AGC in cell metabolism may function independently of MAS, in some cases acting only to transfer aspartate out of mitochondria.
In this scenario, if GAC faces the mitochondrial intermembrane space, AGC would be enough to meet the glutamine-derived aspartate demand of cancer cells (Figure 1C). Actually, as demonstrated in a paper by Alkan et al., the knockdown of AGC1 slows down cell proliferation due to impaired aspartate synthesis [91]. Nevertheless, the total ablation of AGC1 was not sufficient to block proliferation in glutamine-replete media, suggesting the existence of at least one other pathway for the exit of aspartate from the mitochondria. Since the absence of AGC1 also sensitizes tumors to in vivo treatment with CB-839, an inhibitor of mitochondrial glutaminase, it has been proposed that this carrier sustains the growth in low glutamine conditions, and its knockdown exacerbates the growth defect observed when glutamine is limiting. To explain these observations the same authors also suggested that mitochondrial carriers that may replace AGC1 might have a higher Km for aspartate. These alternative transporters might not be sufficient to sustain aspartate export from the mitochondria when its concentration is low as seen during glutamine withdrawal [92]. This appears to be the case as the IMM contains another transporter, UCP2, able to exchange aspartate against phosphate plus a proton. The Km of UCP2 for aspartate is 6.84 mM [93], which is more than one hundred fold higher than that of AGCs (about 50 μM) [76].
A similar consideration can be made for the entry of glutamine-derived glutamate in mitochondria of CRC cells, where glutamate enters the matrix mainly through GC1 [71]; the Km of GC1 for glutamate is 5.18 mM [31] which is about thirty fold higher than that of AGCs (0.21 mM) [76]. This means that in cancer cells where glutaminolysis is very active, the high flux of metabolites across the inner mitochondrial membrane requires transporters with high Kms which are not easily saturable. Although the data reported by Alkan et al. suggest that targeting AGC might be an effective strategy to inhibit tumor growth in situations where nutrients are limited, other considerations must be made for alternative routes that might guarantee cytosolic aspartate availability.
6. Mitochondrial Uncoupling Protein 2: An Aspartate/Pi + H+ Exchanger Belonging to the Mitochondrial Carrier Family
UCP2 is one of the most commonly studied members of the MCF for its involvement in the cell redox homeostasis [94,95,96]. It was discovered in 1997 [97] and due to the level of amino acid identity with UCP1, the canonical uncoupler which regulates non-shivering thermogenesis in mammals, it was initially classified as an uncoupler, thus its moniker. Studies on animal models quickly ruled out its involvement in non-shivering thermogenesis and identified UCP2′s key role in redox homeostasis [94]. The first evidence of the crucial role of UCP2 in glutamine metabolism was published by Pecqueur et al. who demonstrated that UCP2 translation was under the positive control of glutamine [95]. In follow-up studies, the same research group demonstrated that Ucp2 knock-out (KO) macrophages presented with impaired glutaminolysis [98]. Ucp2 KO macrophages in the presence of glutamine had NADH/NAD+ ratios and ATP levels lower than that of their wild-type counterparts, the opposite that one would expect from an uncoupling protein which, by dissipating the electrochemical gradient across the IMM, should lower the ATP production [98]. Mitochondrial glutamine catabolism produces 4- and 5-carbon (C4 and C5) intermediates in the matrix that KC cannot fully oxidize, and these intermediates must be removed from the cycle via cataplerosis, otherwise some KC reactions would be inhibited [99].
The biochemical function of UCP2 in glutaminolysis was identified by Vozza et al. who showed that UCP2 plays a cataplerotic function, catalysing the exchange reaction of aspartate, malate, or oxaloacetate against phosphate plus a proton [93]. UCP2 is the only member of the MCF, with a cataplerotic function, able to catalyse a net efflux of aspartate out of mitochondria [100], thus making this transporter the only one suitable to work together with the mitochondrial glutamine or glutamate transporters to accomplish glutaminolysis in any type of cell (Figure 1A,B) [16]. It should be emphasized that, as reported above, the high Km of UCP2 for aspartate makes this protein unlikely to be saturated under physio-pathological conditions and to be able to guarantee large fluxes of substrates across the IMM preventing the overflow of KC due to the entry of glutamine-derived α-ketoglutarate (Figure 2).
UCP2 expression is tightly regulated at multiple levels, transcriptional, translational, and post-translational [70]. Its transcription is negatively controlled by the TGF-β signaling through SMAD4 [101], which is inactivated in over half of PDAC, and varying degrees in many other types of cancer [102,103]. At translational levels UCP2 is activated by glutamine [95,104,105] and inhibited by miRNAs, miR-133a [106] and miR-15a [107], both considered tumor suppressors and found downregulated in many types of cancer [108,109,110,111,112,113,114,115,116,117,118,119,120]. UCP2 is also post-translationally inhibited by glutathionylation [121,122], which may be considered a fine mechanism of control to regulate the cell redox-homeostasis since it has been demonstrated that increased ROS levels activate UCP2 by inducing its de-glutathionylation, and the active form of UCP2 decreases the ROS levels by increasing the GSH/GSSG ratio [17,123].
The role of UCP2 in cancer cell metabolism and chemoresistance has been demonstrated in many different cancer types [17,69,104,124,125,126,127,128,129,130,131]. The most commonly supported theory about the role of UCP2 in cancer cell proliferation and chemoresistance has been linked to its ability to reduce ROS levels by lowering the electrochemical gradient across the IMM thanks to its possible protonophoric activity. This theory was confuted by Bertholet et al. who, using a patch-clamp approach and KO mouse models, demonstrated that UCP2 was unable to catalyse fatty acid-mediated uncoupling activity [132]. Additionally, Raho et al. demonstrated that, at least in PDAC, UCP2 reduced ROS levels in KRAS-mutated cell lines by exporting the glutamine-derived aspartate out of mitochondria [17]. In PDAC, oncogenic KRAS induces a rewiring of the pentose phosphate pathway by decoupling the ribose 5-phosphate biogenesis from NADPH production [133]. To fulfil the NADPH needs, KRAS shifts most of the mitochondrial glutamine-derived glutamate towards GOT2 with the production of α-oxoglutarate and aspartate; the former enters in KC, and the latter, once transported to the cytosol, is converted to oxaloacetate, malate, and finally to pyruvate to produce NADPH [11] (Figure 2). In PDAC, UCP2 silencing impairs glutaminolysis, reduces the availability of cytosolic aspartate, lowers the NADPH/NADP+ and GSH/GSSG ratios, and increases ROS levels [17]. Interestingly, although the inhibitory effect of UCP2-silencing on glutaminolysis was observed both in KRAS-mutated and KRAS wild-type cell lines, in vivo and in vitro UCP2 silencing reduced the proliferation rate only of KRAS-mutated PDAC cells. These results confirmed that UCP2 is critical for glutaminolysis and the higher levels of ROS found only in UCP2-silenced KRAS-mutated PDAC cells were due to impaired glutamine oxidation, not UCP2-mediated uncoupling activity [17,134]. Of note, although UCP2 is overexpressed in many cancer types [96], its expression is not induced by KRAS mutations, since the expression of the G12V mutant in BxPC3, a PDAC cell line carrying the wild-type form of KRAS, did not alter the expression of UCP2. Interestingly, the BxPC3 cell line expressing KRASG12V showed an increased proliferation rate and clonogenic capacity which was affected by UCP2 silencing [17], suggesting that the aspartate transport catalysed by UCP2 is crucial to support the increase of cell proliferation induced by mutated KRAS. Similarly to PDAC cells lacking the mitochondrial glutamine carrier, the growth defect induced by UCP2 silencing was partially rescued by the external addition of aspartate or glutamate [17,66], suggesting that in the presence of glutamine both transporters are crucial to fulfil cytosolic aspartate needs. In contrast, when glutaminolysis is impaired or glutamine is limited, glutamate, likely through AGC [91], may enter the matrix producing aspartate which can exit with the same transporter (Figure 1C).
Most data published on the role of UCP2 in cancer comes from experiments carried out in cancer cell lines. This means that researchers have only probed the role of UCP2 in tumor maintenance and progression, whereas the role of this transporter in tumor initiation is unexplored. Recently, Aguilar et al. demonstrated that although UCP2 expression was higher in murine colorectal cancer (CRC) compared to normal tissue, its deletion enhanced colon and small intestinal tumorigenesis in carcinogen-induced and ApcMin/+ mice models, respectively. This suggested a tumor-suppressive role of UCP2 in tumorigenesis [123]. During tumor initiation, the loss of UCP2 induced a metabolic rewiring in which most of the glucose-derived pyruvate was channelled towards the biosynthesis of fatty acids/phospholipids via mitochondrial synthesis of citrate. The high amounts of NADPH spent by cells for this metabolic pathway and the parallel decrease in glucose-6-phosphate dehydrogenase activity of Ucp2 KO CRC cells reduced the GSH/GSSG ratio and increased ROS levels. The oxidative stress generated by this pathway was suggested to be the main cause of tumor initiation [123]. Although these results suggest that UCP2 has an opposite function in tumor initiation and maintenance/progression, the biochemical function of UCP2 remains the same in both situations. In fact, in CRC mice models Ucp2 deletion impacted glucose metabolism with the loss of UCP2’s cataplerotic function increasing C4 levels in the matrix, promoting the mitochondrial utilization of the glycolysis-derived pyruvate. Similar results were also found in HepG2 and human pluripotent stem cells [93,135]. On the other hand, the UCP2-dependent export of C4 out of mitochondria impairs the pyruvate utilization in the matrix which is diverted toward lactic fermentation [93,135], and a similar mechanism of control was also exerted on fatty acid oxidation [135]. Unfortunately, the effect of Ucp2 KO on glutamine utilization during tumor initiation in the CRC mice models was not investigated. Since the maintenance/progression of many tumors relies on glutamine utilization, the cataplerotic function of UCP2 is essential to cancer cells using glutamine as carbon and reduced nitrogen source for biosynthetic processes and redox homeostasis [11,16,17,93,98,99]. In other words, if the increase in ROS levels may be considered one of the main causes of tumor initiation, cancer cells, to survive and proliferate, quickly rewire their metabolism (Warburg effect/glutamine addiction) to control this oxidative stress. UCP2 may be considered a key metabolic switch in both processes.
7. Conclusions and Perspectives
A large subset of malignant tumors, in vitro and in animal models, are characterized by a glutamine addiction. As shown in Figure 2, glutamine can be used by cancer cells to synthesize most of what they need to grow and proliferate. Although in many metabolic pathways, such as nucleotide, protein, and hexosamines biosynthesis, glutamine enters directly, in others its carbon skeleton and reduced nitrogen must be reshuffled to produce other useful metabolites, such as aspartate (Figure 2, red metabolites). Aspartate can be used by cancer cells to synthesize asparagine, nucleotides, proteins and to control redox homeostasis (Figure 2).
In order to fulfil all these tasks, glutamine undergoes a series of enzymatic reactions both in mitochondria and in the cytosol, requiring continuous flux of metabolites across the IMM (Figure 2). Many mitochondrial transporters are involved in these pathways, although only those directly involved in aspartate metabolism have been covered in this review. The key role of aspartate as an endogenous metabolic limitation for tumor growth is emerging [17,21,91,92,136,137,138,139] and gain and loss of function studies have clearly demonstrated the crucial role of some of these transporters in cancer cell aspartate availability [17,54,71,72]. Many attempts aimed to target glutaminolysis of cancer cells have been focused on the design of novel and potent glutaminase inhibitors [52,140,141,142,143], some of which made it to clinical trials alone or in association with other chemotherapeutic drugs. Very little has been done to target mitochondrial transporters involved in glutaminolysis and cytosolic aspartate availability. The cause of this may be the recent discovery of their role in cancer cell metabolism [17,54,71,72] and the difficulty in carrying out inhibition assays in vitro. We believe the time has come to fill this gap. Targeting these mitochondrial transporters may provide powerful new tools to fight cancer. It should be emphasized that targeting these transporters requires a thorough understanding of the tumor-type specific metabolic signatures as the involvement of a specific transporter often depends on the type of cancer and nutrient availability (Figure 1A–C, and Figure 2). Many efforts have functionally characterized the MCF members [144] but little has been done to find specific and powerful inhibitors of the mitochondrial transporters [145]. The availability of such compounds may help fight cancer by specifically inhibiting mitochondrial metabolism as demonstrated solely for the mitochondrial citrate carrier [146,147]. An alternative strategy may be to use shRNAs or miRNAs to drastically decrease their expression levels [145]. In this context, two miRNAs, miR-15a and miR-133a known to regulate UCP2 expression, are downregulated in many kinds of cancer [70,106,107,112,113,114,115,116,117,118,148,149,150,151,152,153] and may represent an attractive therapeutic tool. CRISPR/Cas9-mediated genome editing should be considered another possible experimental approach to target these transporters by gene deletion or by knock-in of an inactivating mutation [90,100,154].
Among the four transporters considered in this review, UCP2 should be considered the most promising for three reasons:
(1) UCP2 was found to be overexpressed in many cancer types and, at least in mice, its knock-down does not produce any significant physiological alteration [97], thus it may present the advantage of obtaining drugs with minimal side effects.
(2) UCP2 would be required in both tumor types, PDAC and CRC, where glutamine and glutamate transporters are expressed (Figure 2).
(3) In tumors not dependent on KRAS mutation, glutamine-derived glutamate may enter in the KC through glutamate dehydrogenase, and in this case α-ketoglutarate should produce malate which can also exit from mitochondria through UCP2 [93]. Malate can be used in the cytosol to produce NADPH for redox homeostasis and reductive biosynthesis [16] (Figure 2), whereas pyruvate re-entering the mitochondria through the mitochondrial pyruvate carrier can fuel KC producing ATP or citrate for the lipid biosynthesis (Figure 2).
Author Contributions
Conceptualization, V.D., G.F.; Writing—original draft preparation, R.G., V.I., D.F.; Writing—review and editing, C.L.R., S.T., L.P., V.D. and G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was in part supported by a grant from the Italian Association for Cancer Research (AIRC no. IG 2014 Id.15404 to G.F.), a grant from the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (no. 2017PAB8EM_002 to G.F.), SPORE P50 CA100632, R01 CA206210, and Cancer Prevention & Research Institute of Texas (CPRIT, RP180309).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Warburg O. On the Origin of Cancer Cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Flier J.S., Mueckler M.M., Usher P., Lodish H.F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- 3.Matoba S., Kang J.-G., Patino W.D., Wragg A., Boehm M., Gavrilova O., Hurley P.J., Bunz F., Hwang P.M. p53 Regulates Mitochondrial Respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 4.Kroemer G., Pouyssegur J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 5.DeBerardinis R.J., Sayed N., Ditsworth D., Thompson C.B. Brick by brick: Metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise D.R., Thompson C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlova N.N., Thompson C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altman B.J., Stine Z.E., Dang C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer. 2016;16:619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang L., Shestov A.A., Swain P., Yang C., Parker S., Wang Q., Terada L.S., Adams N.D., McCabe M.T., Pietrak B., et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016;532:255–258. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Son J., Lyssiotis C.A., Ying H., Wang X., Hua S., Ligorio M., Perera R.M., Ferrone C.R., Mullarky E., Shyh-Chang N., et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo H.C., Yu Y.C., Sung Y., Han J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020;52:1496–1516. doi: 10.1038/s12276-020-00504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cluntun A.A., Lukey M.J., Cerione R.A., Locasale J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017;3:169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullen A.R., Wheaton W.W., Jin E.S., Chen P.-H., Sullivan L.B., Cheng T., Yang Y., Linehan W.M., Chandel N.S., DeBerardinis R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2021;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fendt S.-M., Bell E.L., Keibler M.A., Olenchock B.A., Mayers J., Wasylenko T.M., Vokes N.I., Guarente L., Heiden M.G.V., Stephanopoulos G. Reductive glutamine metabolism is a function of the α-ketoglutarate to citrate ratio in cells. Nat. Commun. 2013;4:2236. doi: 10.1038/ncomms3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raho S., Capobianco L., Malivindi R., Vozza A., Piazzolla C., de Leonardis F., Gorgoglione R., Scarcia P., Pezzuto F., Agrimi G., et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat. Metab. 2020;2:1373–1381. doi: 10.1038/s42255-020-00315-1. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan L.B., Gui D.Y., Hosios A.M., Bush L.N., Freinkman E., Vander Heiden M.G. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell. 2015;162:552–563. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gui D.Y., Sullivan L., Luengo A., Hosios A.M., Bush L.N., Gitego N., Davidson S.M., Freinkman E., Thomas C.J., Heiden M.G.V. Environment Dictates Dependence on Mitochondrial Complex I for NAD+ and Aspartate Production and Determines Cancer Cell Sensitivity to Metformin. Cell Metab. 2016;24:716–727. doi: 10.1016/j.cmet.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birsoy K., Wang T., Chen W.W., Freinkman E., Abu-Remaileh M., Sabatini D.M. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell. 2015;162:540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Bermudez J., Baudrier L., La K., Zhu X.G., Fidelin J., Sviderskiy V.O., Papagiannakopoulos T., Molina H., Snuderl M., Lewis C.A., et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat. Cell Biol. 2018;20:775–781. doi: 10.1038/s41556-018-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kvamme E., Tveit B., Svenneby G. Glutaminase from Pig Renal Cortex. J. Biol. Chem. 1970;245:1871–1877. doi: 10.1016/S0021-9258(18)63179-5. [DOI] [PubMed] [Google Scholar]
- 23.Elgadi K.M., Meguid R., Qian M., Souba W.W., Abcouwer S.F. Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol. Genom. 1999;1:51–62. doi: 10.1152/physiolgenomics.1999.1.2.51. [DOI] [PubMed] [Google Scholar]
- 24.Errera M., Greenstein J.P. Phosphate-activated glutaminase in kidney and other tissues. J. Biol. Chem. 1949;178:495–502. doi: 10.1016/S0021-9258(18)56979-9. [DOI] [PubMed] [Google Scholar]
- 25.Aoki C., Kaneko T., Starr A., Pickel V.M. Identification of mitochondrial and non-mitochondrial glutaminase within select neurons and glia of rat forebrain by electron microscopic immunocytochemistry. J. Neurosci. Res. 1991;28:531–548. doi: 10.1002/jnr.490280410. [DOI] [PubMed] [Google Scholar]
- 26.Cassago A., Ferreira A.P.S., Ferreira I.M., Fornezari C., Gomes E.R.M., Greene K.S., Pereira H.M., Garratt R.C., Dias S.M.G., Ambrosio A.L.B. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc. Natl. Acad. Sci. USA. 2012;109:1092–1097. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kvamme E., Torgner I., Roberg B. Evidence indicating that pig renal phosphate-activated glutaminase has a functionally predominant external localization in the inner mitochondrial membrane. J. Biol. Chem. 1991;266:13185–13192. doi: 10.1016/S0021-9258(18)98822-8. [DOI] [PubMed] [Google Scholar]
- 28.Roberg B. The orientation of phosphate activated glutaminase in the inner mitochondrial membrane of synaptic and non-synaptic rat brain mitochondria. Neurochem. Int. 1995;27:367–376. doi: 10.1016/0197-0186(95)00018-4. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro R.A., Haser W.G., Curthoys N.P. The orientation of phosphate-dependent glutaminase on the inner membrane of rat renal mitochondria. Arch. Biochem. Biophys. 1985;243:1–7. doi: 10.1016/0003-9861(85)90767-2. [DOI] [PubMed] [Google Scholar]
- 30.Aledo J.C., de Pedro E., Gómez-Fabre P.M., de Castro I.N., Márquez J. Submitochondrial localization and membrane topography of Ehrlich ascitic tumour cell glutaminase. Biochim. Biophys. Acta (BBA)-Biomembr. 1997;1323:173–184. doi: 10.1016/S0005-2736(96)00189-7. [DOI] [PubMed] [Google Scholar]
- 31.Fiermonte G., Palmieri L., Todisco S., Agrimi G., Palmieri F., Walker J. Identification of the Mitochondrial Glutamate Transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. J. Biol. Chem. 2002;277:19289–19294. doi: 10.1074/jbc.M201572200. [DOI] [PubMed] [Google Scholar]
- 32.Lunetti P., Cappello A.R., Marsano R.M., Pierri C.L., Carrisi C., Martello E., Caggese C., Dolce V., Capobianco L. Mitochondrial glutamate carriers from Drosophila melanogaster: Biochemical, evolutionary and modeling studies. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013;1827:1245–1255. doi: 10.1016/j.bbabio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Porcelli V., Vozza A., Calcagnile V., Gorgoglione R., Arrigoni R., Fontanesi F., Marobbio C.M., Castegna A., Palmieri F., Palmieri L. Molecular identification and functional characterization of a novel glutamate transporter in yeast and plant mitochondria. Biochim. Biophys. Acta. 2018;1859:1249–1258. doi: 10.1016/j.bbabio.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Aledo J.C., Gómez-Fabre P.M., Olalla L., Márquez J. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm. Genome. 2000;11:1107–1110. doi: 10.1007/s003350010190. [DOI] [PubMed] [Google Scholar]
- 35.Katt W.P., Lukey M.J., Cerione R.A. A tale of two glutaminases: Homologous enzymes with distinct roles in tumorigenesis. Future Med. Chem. 2017;9:223–243. doi: 10.4155/fmc-2016-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szeliga M., Obara-Michlewska M. Glutamine in neoplastic cells: Focus on the expression and roles of glutaminases. Neurochem. Int. 2009;55:71–75. doi: 10.1016/j.neuint.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Wise D.R., DeBerardinis R.J., Mancuso A., Sayed N., Zhang X.-Y., Pfeiffer H.K., Nissim I., Daikhin E., Yudkoff M., McMahon S.B., et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao P., Tchernyshyov I., Chang T.-C., Lee Y.-S., Kita K., Ochi T., Zeller K.I., de Marzo A.M., van Eyk J.E., Mendell J.T., et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki S., Tanaka T., Poyurovsky M.V., Nagano H., Mayama T., Ohkubo S., Lokshin M., Hosokawa H., Nakayama T., Suzuki Y., et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl. Acad. Sci. USA. 2010;107:7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W., Zhang C., Wu R., Sun Y., Levine A., Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl. Acad. Sci. USA. 2010;107:7455–7460. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martín-Rufián M., Nascimento-Gomes R., Higuero A., Crisma A.R., Campos-Sandoval J.A., Gomez F.J.M., Cardona C., Cheng T., Lobo C., Segura J.A., et al. Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells. J. Mol. Med. 2013;92:277–290. doi: 10.1007/s00109-013-1105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majewska E., Márquez J., Albrecht J., Szeliga M. Transfection with GLS2 Glutaminase (GAB) Sensitizes Human Glioblastoma Cell Lines to Oxidative Stress by a Common Mechanism Involving Suppression of the PI3K/AKT Pathway. Cancers. 2019;11:115. doi: 10.3390/cancers11010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukey M., Cluntun A., Katt W.P., Lin M.-C.J., Druso J.E., Ramachandran S., Erickson J.W., Le H., Wang Z.-E., Blank B., et al. Liver-Type Glutaminase GLS2 Is a Druggable Metabolic Node in Luminal-Subtype Breast Cancer. Cell Rep. 2019;29:76–88.e7. doi: 10.1016/j.celrep.2019.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C., Liu J., Zhao Y., Yue X., Zhu Y., Wang X., Wu H., Blanco F., Li S., Bhanot G., et al. Glutaminase 2 is a novel negative regulator of small GTPase Rac1 and mediates p53 function in suppressing metastasis. eLife. 2016;5:e10727. doi: 10.7554/eLife.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katunuma N., Huzino A., Tomino I. Organ specific control of glutamine metabolism. Adv. Enzym. Regul. 1967;5:55–58. doi: 10.1016/0065-2571(67)90008-8. [DOI] [PubMed] [Google Scholar]
- 46.Kovacevic Z. Properties and intracellular localization of Ehrlich ascites tumor cell glutaminase. Cancer Res. 1974;34:3403–3407. [PubMed] [Google Scholar]
- 47.Roberg B., Torgner I.A., Laake J., Takumi Y., Ottersen O.P., Kvamme E. Properties and submitochondrial localization of pig and rat renal phosphate-activated glutaminase. Am. J. Physiol. Physiol. 2000;279:C648–C657. doi: 10.1152/ajpcell.2000.279.3.C648. [DOI] [PubMed] [Google Scholar]
- 48.Crompton M., Mcgivan J.D., Chappell J.B. The intramitochondrial location of the glutaminase isoenzymes of pig kidney. Biochem. J. 1973;132:27–34. doi: 10.1042/bj1320027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laake J., Takumi Y., Eidet J., Torgner I., Roberg B., Kvamme E., Ottersen O. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience. 1999;88:1137–1151. doi: 10.1016/S0306-4522(98)00298-X. [DOI] [PubMed] [Google Scholar]
- 50.Kvamme E., Roberg B., Torgner I.A. Phosphate-activated glutaminase and mitochondrial glutamine transport in the brain. Neurochem. Res. 2000;25:1407–1419. doi: 10.1023/A:1007668801570. [DOI] [PubMed] [Google Scholar]
- 51.Bak L.K., Zieminska E., Waagepetersen H.S., Schousboe A., Albrecht J. Metabolism of [U-13C] Glutamine and [U-13C] Glutamate in Isolated Rat Brain Mitochondria Suggests Functional Phosphate-Activated Glutaminase Activity in Matrix. Neurochem. Res. 2008;33:273–278. doi: 10.1007/s11064-007-9471-1. [DOI] [PubMed] [Google Scholar]
- 52.Li B., Cao Y., Meng G., Qian L., Xu T., Yan C., Luo O., Wang S., Wei J., Ding Y., et al. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine. 2019;39:239–254. doi: 10.1016/j.ebiom.2018.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rath S., Sharma R., Gupta R., Ast T., Chan C., Durham T.J., Goodman R.P., Grabarek Z., Haas M.E., Hung W.H.W., et al. MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021;49:D1541–D1547. doi: 10.1093/nar/gkaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo H.C., Park S.J., Nam M., Kang J., Kim K., Yeo J.H., Kim J.-K., Heo Y., Lee H.S., Lee M.Y., et al. A Variant of SLC1A5 Is a Mitochondrial Glutamine Transporter for Metabolic Reprogramming in Cancer Cells. Cell Metab. 2019;31:267–283.e12. doi: 10.1016/j.cmet.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 55.Kekuda R., Prasad P.D., Fei Y.-J., Torres-Zamorano V., Sinha S., Yang-Feng T.L., Leibach F.H., Ganapathy V. Cloning of the Sodium-dependent, Broad-scope, Neutral Amino Acid Transporter Bo from a Human Placental Choriocarcinoma Cell Line. J. Biol. Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 56.Gyimesi G., Hediger M. Sequence Features of Mitochondrial Transporter Protein Families. Biomolecules. 2020;10:1611. doi: 10.3390/biom10121611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y.-C., Cox J.E., Cardon C.M., Van Vranken J.G., Dephoure N., et al. A Mitochondrial Pyruvate Carrier Required for Pyruvate Uptake in Yeast, Drosophila, and Humans. Science. 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herzig S., Raemy E., Montessuit S., Veuthey J.-L., Zamboni N., Westermann B., Kunji E.R.S., Martinou J.-C. Identification and Functional Expression of the Mitochondrial Pyruvate Carrier. Science. 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 59.Palmieri F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Asp. Med. 2013;34:465–484. doi: 10.1016/j.mam.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Curcio R., Lunetti P., Zara V., Ferramosca A., Marra F., Fiermonte G., Cappello A.R., de Leonardis F., Capobianco L., Dolce V. Drosophila melanogaster Mitochondrial Carriers: Similarities and Differences with the Human Carriers. Int. J. Mol. Sci. 2020;21:6052. doi: 10.3390/ijms21176052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vozza A., de Leonardis F., Paradies E., de Grassi A., Pierri C.L., Parisi G., Marobbio C.M.T., Lasorsa F.M., Muto L., Capobianco L., et al. Biochemical characterization of a new mitochondrial transporter of dephosphocoenzyme A in Drosophila melanogaster. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017;1858:137–146. doi: 10.1016/j.bbabio.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Cormerais Y., Massard P.A., Vucetic M., Giuliano S., Tambutté E., Durivault J., Vial V., Endou H., Wempe M.F., Parks S.K., et al. The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5) J. Biol. Chem. 2018;293:2877–2887. doi: 10.1074/jbc.RA117.001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scalise M., Pochini L., Console L., Losso M.A., Indiveri C. The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology. Front. Cell Dev. Biol. 2018;6:96. doi: 10.3389/fcell.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H., Cui K., Yao S., Yin Y., Liu D., Huang Z. Comprehensive molecular and clinical characterization of SLC1A5 in human cancers. Pathol.-Res. Pr. 2021;224:153525. doi: 10.1016/j.prp.2021.153525. [DOI] [PubMed] [Google Scholar]
- 65.Csanadi A., Oser A., Aumann K., Gumpp V., Rawluk J., Nestle U., Kayser C., Wiesemann S., Werner M., Kayser G. Overexpression of SLC1a5 in lymph node metastases outperforms assessment in the primary as a negative prognosticator in non-small cell lung cancer. Pathology. 2018;50:269–275. doi: 10.1016/j.pathol.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 66.Zhao J., Yang Z., Tu M., Meng W., Gao H., Li M.D., Li L. Correlation between Prognostic Biomarker SLC1A5 and Immune Infiltrates in Various Types of Cancers Including Hepatocellular Carcinoma. Front. Oncol. 2021;11:608641. doi: 10.3389/fonc.2021.608641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z., Liu R., Shuai Y., Huang Y., Jin R., Wang X., Luo J. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br. J. Cancer. 2020;122:82–93. doi: 10.1038/s41416-019-0637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma H., Wu Z., Peng J., Li Y., Huang H., Liao Y., Zhou M., Sun L., Huang N., Shi M., et al. Inhibition of SLC1A5 sensitizes colorectal cancer to cetuximab. Int. J. Cancer. 2018;142:2578–2588. doi: 10.1002/ijc.31274. [DOI] [PubMed] [Google Scholar]
- 69.Pozza E.D., Fiorini C., Dando I., Menegazzi M., Sgarbossa A., Costanzo C., Palmieri M., Donadelli M. Role of mitochondrial uncoupling protein 2 in cancer cell resistance to gemcitabine. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012;1823:1856–1863. doi: 10.1016/j.bbamcr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Donadelli M., Dando I., Fiorini C., Palmieri M. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 2014;71:1171–1190. doi: 10.1007/s00018-013-1407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong C.C., Qian Y., Li X., Xu J., Kang W., Tong J.H., To K.-F., Jin Y., Li W., Chen H., et al. SLC25A22 Promotes Proliferation and Survival of Colorectal Cancer Cells with KRAS Mutations and Xenograft Tumor Progression in Mice via Intracellular Synthesis of Aspartate. Gastroenterology. 2016;151:945–960.e6. doi: 10.1053/j.gastro.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 72.Li X., Chung A.C., Li S., Wu L., Xu J., Yu J., Wong C., Cai Z. LC-MS-based metabolomics revealed SLC25A22 as an essential regulator of aspartate-derived amino acids and polyamines in KRAS-mutant colorectal cancer. Oncotarget. 2017;8:101333–101344. doi: 10.18632/oncotarget.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang L., Chen Y., Yu Y., Pan W., Cui Y., Xu X., Peng K., Liu M., Rashid K., Hou Y., et al. SLC25A18 has prognostic value in colorectal cancer and represses Warburg effect and cell proliferation via Wnt signaling. Am. J. Cancer Res. 2020;10:1548–1567. [PMC free article] [PubMed] [Google Scholar]
- 74.Chen M.-W., Wu X.-J. SLC25A22 Promotes Proliferation and Metastasis of Osteosarcoma Cells via the PTEN Signaling Pathway. Technol. Cancer Res. Treat. 2018;17:1533033818811143. doi: 10.1177/1533033818811143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du P., Liang H., Fu X., Wu P., Wang C., Chen H., Zheng B., Zhang J., Hu S., Zeng R., et al. SLC25A22 promotes proliferation and metastasis by activating MAPK/ERK pathway in gallbladder cancer. Cancer Cell Int. 2019;19:33. doi: 10.1186/s12935-019-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmieri L., Pardo B., Lasorsa F., del Arco A., Kobayashi K., Iijima M., Runswick M., Walker J., Saheki T., Satrústegui J. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.del Arco A., Satrústegui J. Molecular Cloning of Aralar, a New Member of the Mitochondrial Carrier Superfamily That Binds Calcium and Is Present in Human Muscle and Brain. J. Biol. Chem. 1998;273:23327–23334. doi: 10.1074/jbc.273.36.23327. [DOI] [PubMed] [Google Scholar]
- 78.Kobayashi K., Sinasac D., Iijima M., Boright A.P., Begum L., Lee J.R., Yasuda T., Ikeda S., Hirano R., Terazono H., et al. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat. Genet. 1999;22:159–163. doi: 10.1038/9667. [DOI] [PubMed] [Google Scholar]
- 79.Lunetti P., Marsano R.M., Curcio R., Dolce V., Fiermonte G., Cappello A.R., Marra F., Moschetti R., Li Y., Aiello D., et al. The mitochondrial aspartate/glutamate carrier (AGC or Aralar1) isoforms in D. melanogaster: Biochemical characterization, gene structure, and evolutionary analysis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021;1865:129854. doi: 10.1016/j.bbagen.2021.129854. [DOI] [PubMed] [Google Scholar]
- 80.Fiermonte G., Walker J., Palmieri F. Abundant bacterial expression and reconstitution of an intrinsic membrane-transport protein from bovine mitochondria. Biochem. J. 1993;294:293–299. doi: 10.1042/bj2940293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monné M., Miniero D.V., Bisaccia F., Fiermonte G. The mitochondrial oxoglutarate carrier: From identification to mechanism. J. Bioenerg. Biomembr. 2012;45:1–13. doi: 10.1007/s10863-012-9475-7. [DOI] [PubMed] [Google Scholar]
- 82.LaNoue K.F., Tischler M.E. Electrogenic Characteristics of the Mitochondrial Glutamate-Aspartate Antiporter. J. Biol. Chem. 1974;249:7522–7528. doi: 10.1016/S0021-9258(19)81269-3. [DOI] [PubMed] [Google Scholar]
- 83.Lanoue K.F., Meijer A.J., Brouwer A. Evidence for electrogenic aspartate transport in rat liver mitochondria. Arch. Biochem. Biophys. 1974;161:544–550. doi: 10.1016/0003-9861(74)90337-3. [DOI] [PubMed] [Google Scholar]
- 84.Lasorsa F., Pinton P., Palmieri L., Fiermonte G., Rizzuto R., Palmieri F. Recombinant Expression of the Ca2+-sensitive Aspartate/Glutamate Carrier Increases Mitochondrial ATP Production in Agonist-stimulated Chinese Hamster Ovary Cells. J. Biol. Chem. 2003;278:38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- 85.Profilo E., Peña-Altamira L.E., Corricelli M., Castegna A., Danese A., Agrimi G., Petralla S., Giannuzzi G., Porcelli V., Sbano L., et al. Down-regulation of the mitochondrial aspartate-glutamate carrier isoform 1 AGC1 inhibits proliferation and N-acetylaspartate synthesis in Neuro2A cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017;1863:1422–1435. doi: 10.1016/j.bbadis.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 86.Cavero S., Vozza A., del Arco A., Palmieri L., Villa A., Blanco E., Runswick M.J., Walker J., Cerdan S., Satrústegui J. Identification and metabolic role of the mitochondrial aspartate-glutamate transporter in Saccharomyces cerevisiae. Mol. Microbiol. 2003;50:1257–1269. doi: 10.1046/j.1365-2958.2003.03742.x. [DOI] [PubMed] [Google Scholar]
- 87.Saheki T., Iijima M., Li M.X., Kobayashi K., Horiuchi M., Ushikai M., Okumura F., Meng X.J., Inoue I., Tajima A., et al. Citrin/Mitochondrial Glycerol-3-phosphate Dehydrogenase Double Knock-out Mice Recapitulate Features of Human Citrin Deficiency. J. Biol. Chem. 2007;282:25041–25052. doi: 10.1074/jbc.M702031200. [DOI] [PubMed] [Google Scholar]
- 88.Fiermonte G., Soon D., Chaudhuri A., Paradies E., Lee P.J., Krywawych S., Palmieri F., Lachmann R.H. An Adult with Type 2 Citrullinemia Presenting in Europe. N. Engl. J. Med. 2008;358:1408–1409. doi: 10.1056/NEJMc0707353. [DOI] [PubMed] [Google Scholar]
- 89.Dimmock D., Maranda B., Dionisi-Vici C., Wang J., Kleppe S., Fiermonte G., Bai R., Hainline B., Hamosh A., O’Brien W.E., et al. Citrin deficiency, a perplexing global disorder. Mol. Genet. Metab. 2009;96:44–49. doi: 10.1016/j.ymgme.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 90.Wibom R., Lasorsa F., Töhönen V., Barbaro M., Sterky F.H., Kucinski T., Naess K., Jonsson M., Pierri C.L., Palmieri F., et al. AGC1 Deficiency Associated with Global Cerebral Hypomyelination. N. Engl. J. Med. 2009;361:489–495. doi: 10.1056/NEJMoa0900591. [DOI] [PubMed] [Google Scholar]
- 91.Alkan H.F., Walter K.E., Luengo A., Madreiter-Sokolowski C.T., Stryeck S., Lau A.N., Al-Zoughbi W., Lewis C.A., Thomas C.J., Hoefler G., et al. Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting. Cell Metab. 2018;28:706–720.e6. doi: 10.1016/j.cmet.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alkan H.F., Bogner-Strauss J.G. Maintaining cytosolic aspartate levels is a major function of the TCA cycle in proliferating cells. Mol. Cell. Oncol. 2019;6:e1536843. doi: 10.1080/23723556.2018.1536843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vozza A., Parisi G., de Leonardis F., Lasorsa F.M., Castegna A., Amorese D., Marmo R., Calcagnile V.M., Palmieri L., Ricquier D., et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA. 2014;111:960–965. doi: 10.1073/pnas.1317400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arsenijevic D., Onuma H., Pecqueur C., Raimbault S., Manning B.S., Miroux B., Couplan E., Alves-Guerra M.-C., Goubern M., Surwit R., et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 95.Pecqueur C., Alves-Guerra M.-C., Gelly C., Lévi-Meyrueis C., Couplan E., Collins S., Ricquier D., Bouillaud F., Miroux B. Uncoupling Protein 2, In Vivo Distribution, Induction upon Oxidative Stress, and Evidence for Translational Regulation. J. Biol. Chem. 2001;276:8705–8712. doi: 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- 96.Baffy G. Uncoupling protein-2 and cancer. Mitochondrion. 2010;10:243–252. doi: 10.1016/j.mito.2009.12.143. [DOI] [PubMed] [Google Scholar]
- 97.Fleury C., Neverova M., Collins S., Raimbault S., Champigny O., Levi-Meyrueis C., Bouillaud F., Seldin M.F., Surwit R.S., Ricquier D., et al. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 98.Nübel T., Emre Y., Rabier D., Chadefaux B., Ricquier D., Bouillaud F. Modified glutamine catabolism in macrophages of Ucp2 knock-out mice. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008;1777:48–54. doi: 10.1016/j.bbabio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 99.Owen O.E., Kalhan S., Hanson R.W. The Key Role of Anaplerosis and Cataplerosis for Citric Acid Cycle Function. J. Biol. Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 100.Palmieri F. Mitochondrial transporters of the SLC25 family and associated diseases: A review. J. Inherit. Metab. Dis. 2014;37:565–575. doi: 10.1007/s10545-014-9708-5. [DOI] [PubMed] [Google Scholar]
- 101.Sayeed A., Meng Z., Luciani G., Chen L.-C., Bennington J.L., Dairkee S.H. Negative regulation of UCP2 by TGFβ signaling characterizes low and intermediate-grade primary breast cancer. Cell Death Dis. 2010;1:e53. doi: 10.1038/cddis.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao M., Mishra L., Deng C.X. The role of TGF-β/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018;14:111–123. doi: 10.7150/ijbs.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmed S., Bradshaw A.-D., Gera S., Dewan M.Z., Xu R. The TGF-β/Smad4 Signaling Pathway in Pancreatic Carcinogenesis and Its Clinical Significance. J. Clin. Med. 2017;6:5. doi: 10.3390/jcm6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rupprecht A., Moldzio R., Mödl B., Pohl E.E. Glutamine regulates mitochondrial uncoupling protein 2 to promote glutaminolysis in neuroblastoma cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2019;1860:391–401. doi: 10.1016/j.bbabio.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Azzu V., Affourtit C., Breen E.P., Parker N., Brand M.D. Dynamic regulation of uncoupling protein 2 content in INS-1E insulinoma cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008;1777:1378–1383. doi: 10.1016/j.bbabio.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen X., Wang K., Chen J., Guo J., Yin Y., Cai X., Guo X., Wang G., Yang R., Zhu L., et al. In Vitro Evidence Suggests That miR-133a-mediated Regulation of Uncoupling Protein 2 (UCP2) Is an Indispensable Step in Myogenic Differentiation. J. Biol. Chem. 2009;284:5362–5369. doi: 10.1074/jbc.M807523200. [DOI] [PubMed] [Google Scholar]
- 107.Sun L.-L., Jiang B.-G., Li W.-T., Zou J.-J., Shi Y.-Q., Liu Z.-M. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res. Clin. Pr. 2011;91:94–100. doi: 10.1016/j.diabres.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 108.Wang H., An H., Wang B., Liao Q., Li W., Jin X., Cui S., Zhang Y., Ding Y., Zhao L. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. Eur. J. Cancer. 2013;49:3924–3935. doi: 10.1016/j.ejca.2013.07.149. [DOI] [PubMed] [Google Scholar]
- 109.Dong Y., Zhao J., Wu C.-W., Zhang L., Liu X., Kang W., Leung W.-W., Zhang N., Chan F.K., Sung J.J.Y., et al. Tumor Suppressor Functions of miR-133a in Colorectal Cancer. Mol. Cancer Res. 2013;11:1051–1060. doi: 10.1158/1541-7786.MCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 110.Nohata N. microRNA-1/133a and microRNA-206/133b clusters: Dysregulation and functional roles in human cancers. Oncotarget. 2012;3:9. doi: 10.18632/oncotarget.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kojima S., Chiyomaru T., Kawakami K., Yoshino H., Enokida H., Nohata N., Fuse M., Ichikawa T., Naya Y., Nakagawa M., et al. Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br. J. Cancer. 2011;106:405–413. doi: 10.1038/bjc.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hua Y.-T., Xu W.-X., Li H., Xia M. Emerging roles of MiR-133a in human cancers. J. Cancer. 2021;12:198–206. doi: 10.7150/jca.48769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu W., Shi X., Wang B. microRNA-133a exerts tumor suppressive role in oral squamous cell carcinoma through the Notch signaling pathway via downregulation of CTBP2. Cancer Gene Ther. 2021:1–11. doi: 10.1038/s41417-020-0200-0. [DOI] [PubMed] [Google Scholar]
- 114.Mataki H., Enokida H., Chiyomaru T., Mizuno K., Matsushita R., Goto Y., Nishikawa R., Higashimoto I., Samukawa T., Nakagawa M., et al. Downregulation of the microRNA-1/133a cluster enhances cancer cell migration and invasion in lung-squamous cell carcinoma via regulation of Coronin1C. J. Hum. Genet. 2014;60:53–61. doi: 10.1038/jhg.2014.111. [DOI] [PubMed] [Google Scholar]
- 115.Vandewalle V., Essaghir A., Bollaert E., Lenglez S., Graux C., Schoemans H., Saussoy P., Michaux L., Valk P.J.M., Demoulin J., et al. miR-15a-5p and miR-21-5p contribute to chemoresistance in cytogenetically normal acute myeloid leukaemia by targeting PDCD4, ARL2 and BTG2. J. Cell. Mol. Med. 2020;25:575–585. doi: 10.1111/jcmm.16110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lovat F., Nigita G., Distefano R., Nakamura T., Gasparini P., Tomasello L., Fadda P., Ibrahimova N., Catricalà S., Palamarchuk A., et al. Combined loss of function of two different loci of miR-15/16 drives the pathogenesis of acute myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2020;117:12332–12340. doi: 10.1073/pnas.2003597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo S., Fesler A., Huang W., Wang Y., Yang J., Wang X., Zheng Y., Hwang G.-R., Ju J. Functional Significance and Therapeutic Potential of miR-15a Mimic in Pancreatic Ductal Adenocarcinoma. Mol. Ther.-Nucleic Acids. 2020;19:228–239. doi: 10.1016/j.omtn.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu T., Xu Z., Ou D., Liu J., Zhang J. The miR-15a/16 gene cluster in human cancer: A systematic review. J. Cell. Physiol. 2019;234:5496–5506. doi: 10.1002/jcp.27342. [DOI] [PubMed] [Google Scholar]
- 119.Guo S., Xu X., Tang Y., Zhang C., Li J., Ouyang Y., Ju J., Bie P., Wang H. miR-15a inhibits cell proliferation and epithelial to mesenchymal transition in pancreatic ductal adenocarcinoma by down-regulating Bmi-1 expression. Cancer Lett. 2014;344:40–46. doi: 10.1016/j.canlet.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 120.Aqeilan R.I., Calin G., Croce C.M. miR-15a and miR-16-1 in cancer: Discovery, function and future perspectives. Cell Death Differ. 2009;17:215–220. doi: 10.1038/cdd.2009.69. [DOI] [PubMed] [Google Scholar]
- 121.Mailloux R.J., Fu A., Robson-Doucette C., Allister E.M., Wheeler M.B., Screaton R., Harper M.-E. Glutathionylation State of Uncoupling Protein-2 and the Control of Glucose-stimulated Insulin Secretion. J. Biol. Chem. 2012;287:39673–39685. doi: 10.1074/jbc.M112.393538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mailloux R., Seifert E.L., Bouillaud F., Aguer C., Collins S., Harper M.-E. Glutathionylation Acts as a Control Switch for Uncoupling Proteins UCP2 and UCP3. J. Biol. Chem. 2011;286:21865–21875. doi: 10.1074/jbc.M111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aguilar E., Esteves P., Sancerni T., Lenoir V., Aparicio T., Bouillaud F., Dentin R., Prip-Buus C., Ricquier D., Pecqueur C., et al. UCP2 Deficiency Increases Colon Tumorigenesis by Promoting Lipid Synthesis and Depleting NADPH for Antioxidant Defenses. Cell Rep. 2019;28:2306–2316.e5. doi: 10.1016/j.celrep.2019.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Imai K., Fukuda T., Wada T., Kawanishi M., Tasaka R., Yasui T., Sumi T. UCP2 expression may represent a predictive marker of neoadjuvant chemotherapy effectiveness for locally advanced uterine cervical cancer. Oncol. Lett. 2017;14:951–957. doi: 10.3892/ol.2017.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li W., Zhang C., Jackson K., Shen X., Chunjing Z., Li G., Kevil C., Xingui S., Shi R., Zhao Y. UCP2 Knockout Suppresses Mouse Skin Carcinogenesis. Cancer Prev. Res. 2015;8:487–491. doi: 10.1158/1940-6207.CAPR-14-0297-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Donadelli M., Dando I., Pozza E.D., Palmieri M. Mitochondrial uncoupling protein 2 and pancreatic cancer: A new potential target therapy. World J. Gastroenterol. 2015;21:3232–3238. doi: 10.3748/wjg.v21.i11.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu G., Liu J., Xu K., Dong J. Uncoupling protein 2 mediates resistance to gemcitabine-induced apoptosis in hepatocellular carcinoma cell lines. Biosci. Rep. 2015;35:e00231. doi: 10.1042/BSR20150116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li W., Nichols K., Nathan C.-A., Zhao Y. Mitochondrial uncoupling protein 2 is up-regulated in human head and neck, skin, pancreatic, and prostate tumors. Cancer Biomark. 2013;13:377–383. doi: 10.3233/CBM-130369. [DOI] [PubMed] [Google Scholar]
- 129.Dando I., Fiorini C., Pozza E.D., Padroni C., Costanzo C., Palmieri M., Donadelli M. UCP2 inhibition triggers ROS-dependent nuclear translocation of GAPDH and autophagic cell death in pancreatic adenocarcinoma cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013;1833:672–679. doi: 10.1016/j.bbamcr.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 130.Pfefferle A., Mailloux R., Adjeitey C.N.-K., Harper M.-E. Glutathionylation of UCP2 sensitizes drug resistant leukemia cells to chemotherapeutics. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013;1833:80–89. doi: 10.1016/j.bbamcr.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 131.Deng S., Yang Y., Han Y., Li X., Wang X., Li X., Zhang Z., Wang Y. UCP2 Inhibits ROS-Mediated Apoptosis in A549 under Hypoxic Conditions. PLoS ONE. 2012;7:e30714. doi: 10.1371/journal.pone.0030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bertholet A.M., Chouchani E.T., Kazak L., Angelin A., Fedorenko A., Long J.Z., Vidoni S., Garrity R., Cho J., Terada N., et al. H+ transport is an integral function of the mitochondrial ADP/ATP carrier. Nature. 2019;571:515–520. doi: 10.1038/s41586-019-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ying H., Kimmelman A.C., Lyssiotis C.A., Hua S., Chu G.C., Fletcher-Sananikone E., Locasale J.W., Son J., Zhang H., Coloff J.L., et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Encarnacion-Rosado J., Kimmelman A.C. Harnessing metabolic dependencies in pancreatic cancers. Nat. Rev. Gastroenterol. Hepatol. 2021;18:482–492. doi: 10.1038/s41575-021-00431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J., Khvorostov I., Hong J.S., Oktay Y., Vergnes L., Nuebel E., Wahjudi P.N., Setoguchi K., Wang G., Do A., et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meléndez-Rodríguez F., Urrutia A.A., Lorendeau D., Rinaldi G., Roche O., Böğürcü-Seidel N., Muelas M.O., Ciller C.M., Turiel G., Bouthelier A., et al. HIF1α Suppresses Tumor Cell Proliferation through Inhibition of Aspartate Biosynthesis. Cell Rep. 2019;26:2257–2265.e4. doi: 10.1016/j.celrep.2019.01.106. [DOI] [PubMed] [Google Scholar]
- 137.Sullivan L.B., Luengo A., Danai L.V., Bush L.N., Diehl F.F., Hosios A.M., Lau A.N., Elmiligy S., Malstrom S., Lewis C.A., et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat. Cell Biol. 2018;20:782–788. doi: 10.1038/s41556-018-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng C.-T., Qi Y., Wang Y.-C., Chi K., Chung Y., Ouyang C., Chen Y.-R., Oh M.E., Sheng X., Tang Y., et al. Arginine starvation kills tumor cells through aspartate exhaustion and mitochondrial dysfunction. Commun. Biol. 2018;1:178. doi: 10.1038/s42003-018-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patel D., Menon D., Bernfeld E., Mroz V., Kalan S., Loayza D., Foster D.A. Aspartate Rescues S-phase Arrest Caused by Suppression of Glutamine Utilization in KRas-driven Cancer Cells. J. Biol. Chem. 2016;291:9322–9329. doi: 10.1074/jbc.M115.710145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matés J.M., Campos-Sandoval J.A., Santos-Jiménez J.D.L., Segura J.A., Alonso F.J., Márquez J. Metabolic Reprogramming of Cancer by Chemicals that Target Glutaminase Isoenzymes. Curr. Med. Chem. 2020;27:5317–5339. doi: 10.2174/0929867326666190416165004. [DOI] [PubMed] [Google Scholar]
- 141.Masisi B.K., El Ansari R., Alfarsi L., Rakha E.A., Green A.R., Craze M.L. The role of glutaminase in cancer. Histopathology. 2020;76:498–508. doi: 10.1111/his.14014. [DOI] [PubMed] [Google Scholar]
- 142.Wang Z., Liu F., Fan N., Zhou C., Li D., Macvicar T., Dong Q., Bruns C.J., Zhao Y. Targeting Glutaminolysis: New Perspectives to Understand Cancer Development and Novel Strategies for Potential Target Therapies. Front. Oncol. 2020;10:589508. doi: 10.3389/fonc.2020.589508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koch K., Hartmann R., Tsiampali J., Uhlmann C., Nickel A.-C., He X., Kamp M.A., Sabel M., Barker R.A., Steiger H.-J., et al. A comparative pharmaco-metabolomic study of glutaminase inhibitors in glioma stem-like cells confirms biological effectiveness but reveals differences in target-specificity. Cell Death Discov. 2020;6:20. doi: 10.1038/s41420-020-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Palmieri F., Monné M. Discoveries, metabolic roles and diseases of mitochondrial carriers: A review. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016;1863:2362–2378. doi: 10.1016/j.bbamcr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 145.Lytovchenko O., Kunji E.R. Expression and putative role of mitochondrial transport proteins in cancer. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017;1858:641–654. doi: 10.1016/j.bbabio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 146.Kolukula V.K., Sahu G., Wellstein A., Rodriguez O.C., Preet A., Iacobazzi V., D’Orazi G., Albanese C., Palmieri F., Avantaggiati M.L. SLC25A1, or CIC, is a novel transcriptional target of mutant p53 and a negative tumor prognostic marker. Oncotarget. 2014;5:1212–1225. doi: 10.18632/oncotarget.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mosaoa R., Kasprzyk-Pawelec A., Fernandez H., Avantaggiati M. The Mitochondrial Citrate Carrier SLC25A1/CIC and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond. Biomolecules. 2021;11:141. doi: 10.3390/biom11020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi W., Tang T., Li X., Deng S., Li R., Wang Y., Wang Y., Xia T., Zhang Y., Zen K., et al. Methylation-mediated silencing of miR-133a-3p promotes breast cancer cell migration and stemness via miR-133a-3p/MAML1/DNMT3A positive feedback loop. J. Exp. Clin. Cancer Res. 2018;38:429. doi: 10.1186/s13046-019-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sui Y., Zhang X., Yang H., Wei W., Wang M. MicroRNA-133a acts as a tumour suppressor in breast cancer through targeting LASP1. Oncol. Rep. 2017;39:473–482. doi: 10.3892/or.2017.6114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 150.Yuan Y., Yao Y.F., Hu S.N., Gao J., Zhang L.-L. MiR-133a Is Functionally Involved in Doxorubicin-Resistance in Breast Cancer Cells MCF-7 via Its Regulation of the Expression of Uncoupling Protein 2. PLoS ONE. 2015;10:e0129843. doi: 10.1371/journal.pone.0129843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dieter C., Assmann T.S., Costa A.R., Canani L.H., De Souza B.M., Bauer A.C., Crispim D. MiR-30e-5p and MiR-15a-5p Expressions in Plasma and Urine of Type 1 Diabetic Patients with Diabetic Kidney Disease. Front. Genet. 2019;10:563. doi: 10.3389/fgene.2019.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Groen F.L.M., Timmer L.M., Menezes R.X., Diosdado B., Hooijberg E., Meijer G.A., Steenbergen R.D.M., Carvalho B. Oncogenic Role of miR-15a-3p in 13q Amplicon-Driven Colorectal Adenoma-to-Carcinoma Progression. PLoS ONE. 2015;10:e0132495. doi: 10.1371/journal.pone.0132495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jin X., Chen N., Zheng R.-H., Zhang H., Chen Y.-P., Xiang Z. miRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J. Gastroenterol. 2017;23:76–86. doi: 10.3748/wjg.v23.i1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ferrara C.T., Boodhansingh K.E., Paradies E., Giuseppe F., Steinkrauss L.J., Topor L.S., Quintos J.B., Ganguly A., de Leon D., Palmieri F., et al. Novel Hypoglycemia Phenotype in Congenital Hyperinsulinism Due to Dominant Mutations of Uncoupling Protein 2. J. Clin. Endocrinol. Metab. 2017;102:942–949. doi: 10.1210/jc.2016-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.