Abstract
Background
Strongyloides stercoralis, a soil-transmitted helminth, occurs in humans, non-human primates, dogs, cats and wild canids. The zoonotic potential between these hosts is not well understood with data available on prevalence primarily focused on humans. To increase knowledge on prevalence, this review and meta-analysis was performed to estimate the global status of S. stercoralis infections in dogs.
Methods
Following the PRISMA guidelines, online literature published prior to November 2020 was obtained from multiple databases (Science Direct, Web of Science, PubMed, Scopus and Google Scholar). Prevalence was calculated on a global and country level, by country income and climate, and in stray/animal shelter dogs versus owned dogs. Statistical analyses were conducted using R-software (version 3.6.1).
Results
From 9428 articles, 61 met the inclusion criteria. The estimated pooled global prevalence of S. stercoralis in dogs was 6% (95% CI 3–9%). Infection was found to be the most prevalent in low-income countries with pooled prevalence of 22% (95% CI 10–36%). The highest pooled prevalence of S. stercoralis in dogs was related to regions with average temperature of 10–20 °C (6%; 95% CI 3–11%), an annual rainfall of 1001–1500 mm (9%; 95% CI 4–15%) and humidity of 40–75% (8%; 95% CI 4–13%). Prevalence was higher in stray and shelter dogs (11%; 95% CI 1–26%) than in owned dogs (3%; 95% CI 1–7%).
Conclusions
As with S. stercoralis in humans, higher prevalence in dogs is found in subtropical and tropical regions and lower-income countries, locations which also can have high dog populations. While this study presents the first estimated global prevalence of S. stercoralis in dogs, it is potentially an underestimation with 15 of 61 studies relying on diagnostic methods of lower sensitivity and a paucity of data from most locations. Standardized protocols (e.g. quantity of feces and number of samples for a Baermann) in future studies could improve reliability of results. More prevalence studies and raising veterinary awareness of S. stercoralis are needed for a One Health approach to protect humans and dogs from the impact of the infection.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-021-05135-0.
Keywords: Strongyloides stercoralis, Canine, Neglected tropical disease, Soil transmitted helminth, Systematic review
Background
A quarter of the world’s population is impacted by helminthic infections that cause substantial rates of diseases and/or disabilities. Many of these helminths are zoonotic with carnivores, particularly dogs and cats, responsible for transmission of nearly 43% of the zoonotic pathogens [1–4]. One of these zoonotic pathogens is Strongyloides stercoralis, a soil-transmitted helminth that affects 100–370 million people globally and is classified as a neglected tropical disease [5, 6]. The main manifestations of S. stercoralis infection are gastrointestinal and cutaneous signs. However, S. stercoralis infections can be asymptomatic but, in the other extreme, can cause severe pulmonary pathology with auto- and hyperinfection [7–9]. The life cycle of S. stercoralis involves homogonic and heterogonic stages. In the homogonic cycle, only females exist in the host and eggs are produced via parthenogenesis. First-stage larvae (L1) and occasionally in some hosts eggs containing L1 are excreted via the host’s feces into the environment where the heterogonic cycle occurs. Male and female larvae molt into free-living adult worms through four larval stages with female larvae also being able to molt into infectious third-stage larvae (L3) with no further development until entering a host [8]. The most common techniques to detect larvae of S. stercoralis in human feces are direct smear, Kato-Katz, flotation, sedimentation, Baermann and Koga agar plate culture with the latter three being the more sensitive for larvae. Indirect fluorescent antibody tests (IFATs), enzyme-linked immunosorbent assays (ELISAs) and molecular methods also can be used for diagnosis but are more frequently used in research versus clinical settings [10–12]. The primary treatment for S. stercoralis infection in humans is ivermectin [9].
Strongyloides stercoralis also is capable of infecting canids and a range of other vertebrate hosts such as felids and non-human primates [6, 13]. Canine S. stercoralis infection occurs most frequently in puppies and young dogs under 1 year of age and in puppies living in breeding kennels with poor sanitary conditions during hot and humid seasons [14–18]. Infection in dogs can be asymptomatic, but also can be life-threatening with clinical signs ranging from diarrhea and malabsorption to bronchopneumonia. Extraintestinal disseminations such as the nasal cavities, lungs, stomach and cranial cavity associated with severe clinical signs have been documented in immunocompromised canids as in humans (e.g. due to other pre-existing conditions or administration of immunosuppressive medicines) [19–21]. The methods for detection of S. stercoralis in dog feces are the same as those in humans with treatments including not only ivermectin but also fenbendazole, albendazole and selamectin although no products are registered for this use in dogs [22].
Dogs and humans share certain S. stercoralis genotypes. Although there are few reports of transmission from dogs to humans, experimental infections illustrate that S. stercoralis from human origin can infect dogs, suggesting dogs can be a reservoir for human infection [6, 13, 23, 24]. While there have been recent studies estimating regional and global prevalence of Strongyloides for humans and associated risk factors [5, 7, 12, 25], these data are not available for infections in dogs. In a one health context, better knowledge on the prevalence of S. stercoralis infection in dogs and the risk of zoonotic transmission to humans is needed. Therefore, this review and meta-analysis aimed to estimate the global prevalence of S. stercoralis in dogs and assess some variables that might influence prevalence.
Methods
Search strategy
This systematic review and meta-analysis followed PRISMA guidelines (http://www.prisma-statement.org/). A systematic literature search was carried out on multiple general science databases to identify all publications reporting S. stercoralis in dogs across the world published prior to November 2020. Science Direct, Web of Science, PubMed, Scopus and Google Scholar were explored using the following search terms: Strongyloides stercoralis, S. stercoralis, strongyloidiasis, dogs, puppies, gastrointestinal helminths, soil-transmitted helminths, worldwide and prevalence using AND and/or OR Boolean operators. Two independent authors involved in the search evaluated titles and abstracts and reviewed the full-text papers. After removing duplicates and irrelevant records, reference lists of full texts were examined for potential eligibility of citations not found in the database search.
Inclusion and exclusion criteria and data extracted
Literature was eligible for inclusion if it met the following priori criteria: (1) peer-reviewed articles containing original data, (2) cross-sectional studies reporting the prevalence of strongyloidiasis in dogs, (3) accessible full text and abstract and (4) numerator and denominator data available to confirm prevalence values. Literature that did not satisfy the aforementioned criteria, such as review articles with no original data, letters, editorials, articles with fecal material collected from the ground and lack of clarity about whether there were repeated samples and articles with ambiguous/undetermined conclusions, were excluded. Articles that reported S. stercoralis in humans, animals other than dogs and soil were excluded.
Using a Microsoft Excel® spreadsheet, the following information was retrieved from the included articles: first author name, year of publication, country where the study was conducted, continent, sample size and number of positive cases, diagnostic method(s) used, income level (https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups), humidity (https://www.timeanddate.com/weather/iran/tehran/climate), annual rainfall (https://en.climate-data.org/), average temperature (https://en.climate-data.org/), latitude, (https://www.geodatos.net/en/coordinates/) and climate (https://www.britannica.com/science/Koppen-climate-classification). Antibody seroprevalence studies were excluded, since they cannot confirm current infection. Experimental antigen methods also were excluded. In studies where more than one method was used to analyze a single sample (e.g. Baermann and flotation), the total number of positive samples was determined and used in the analysis.
Quality assessment
The Newcastle-Ottawa Scale was used to assess the quality of the included articles [26]. A maximum score of 9 was assigned to each article based on subject selection (0–4 points), comparability of subjects (0–2 points) and exposure (0–3 points). A total score of 0–3, 4–6 and 7–9 points was considered poor, moderate and high quality, respectively [27–29].
Data synthesis and statistical analysis
The pooled prevalence of S. stercoralis in dogs reported globally and by continent was calculated with 95% confidence intervals (CIs). In addition, prevalence for stray and shelter dogs was compared to that of owned dogs with owned dogs defined as household owned, those in pet stores and breeding dogs but excluding those with only breeding kennel data. Sub-group analysis included country income level, humidity, annual rainfall, average temperature and latitude. The probability of publication bias was surveyed using Egger’s regression test and Begg’s test. A meta-regression analysis was conducted to evaluate the impact of the year of publication on prevalence. All statistical analyses were performed using the meta-package in R (version 3.6.1). The pooled prevalence estimates were computed using the alpha method for the random-effects model, based on the inverse variance approach for measuring weight. Cochrane’s Q test and inconsistency index (I2 statistics) were used to assess the magnitude of heterogeneity among included studies, with I2 values of < 25%, 25–75% and < 75% considered as low, moderate and high heterogeneity, respectively. A P value < 0.05 was considered statistically significant.
Results
Literature search, selection and data extraction
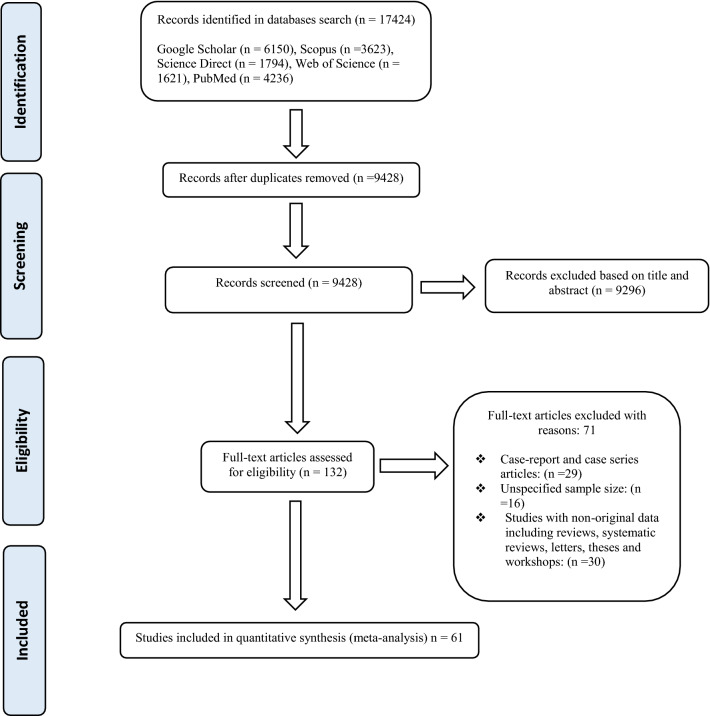
Our systematic search yielded a total of 9428 publications. One hundred thirty-two full-text articles were chosen for eligibility assessment. There were 16 studies with an unspecified sample size and 55 studies with no original data, including reviews, case reports and case series, letters, theses and workshops. Finally, 61 studies were included in the meta-analysis based on critical appraisal criteria (Fig. 1; Table 1 with full list of references in Additional file 1: References S1). The included studies utilized parasitology techniques comprising microscopic methods (flotation with and without concentration, sedimentation, Baermann, Kato-Katz and other direct smear methods, and necropsy), culture methods (Harada-Mori and agar plate culture), molecular techniques [conventional polymerase chain reactions (PCR) and real-time PCR] and serological methods (IFAT and ELISA). Of the 61 articles, 15 used only direct smears and/or flotation with most studies using sedimentation and/or Baermann often in combination with flotation or direct smears. Four studies used culture, three of which were combined with other methods. Only eight of the included articles used diagnostic methods other than microscopy and culture: three used immunological methods in addition to microscopic or culture methods; one used serology, PCR, microscopic and culture methods; one used PCR and microscopic methods; and one used only PCR methods.
Fig. 1.
Flow diagram representing the selection of studies for inclusion in the systematic review and meta-analysis of the global prevalence of Strongyloides in dogs
Table 1.
Main characteristics of the included studies reporting the prevalence of Strongyloides stercoralis in dogs
| No. | First author | Publication year | Country | Continent | Diagnostic methods used | Quality assessment based on Newcastle–Ottawa Scale |
|---|---|---|---|---|---|---|
| 1 | Rizzo and Ricciardi | 1978 | Italy | Europe | Unclear | 5 |
| 2 | Ugochukwu and Ejimadu | 1985 | Nigeria | Africa | Saturated solution flotation, formalin-ether sedimentation | 5 |
| 3 | Tarish et al. | 1986 | Iraq | Asia | Necropsy | 6 |
| 4 | Stehr-Green et al. | 1987 | USA | North America | Formalin-ethyl acetate sedimentation | 9 |
| 5 | Epe et al. | 1993 | Germany | Europe | Coproscopical examinations | 6 |
| 6 | Bugg et al. | 1999 | Australia | Oceania | Sedimentation, zinc sulfate (ZnSO4) flotation | 7 |
| 7 | Itoh et al. | 2003 | Japan | Asia | Coproscopical examination | 7 |
| 8 | Anosike et al. | 2004 | Nigeria | Africa | Direct smear, concentration methods | 5 |
| 9 | Asano et al. | 2004 | Japan | Asia | Direct smear, saturated salt (NaCl) flotation, ZnSO4 flotation, sucrose flotation | 8 |
| 10 | Ramirez-Barrios et al. | 2004 | Venezuela | South America | NaCl flotation | 8 |
| 11 | Komtangi et al. | 2005 | Cameroon | Africa | McMaster | 5 |
| 12 | Júnior et al. | 2006 | Brazil | South America | Baermann, sedimentation, ELISA, IFAT | 9 |
| 13 | Gonçalves et al. | 2007 | Brazil | South America | Baermann, sedimentation, ELISA, IFAT | 8 |
| 14 | Lorenzini et al. | 2007 | Brazil | South America | Saturated NaCl flotation, ZnSO4 flotation | 6 |
| 15 | Papazahariadou et al. | 2007 | Greece | Europe | Teleman’s sedimentation | 8 |
| 16 | Dillard et al. | 2007 | Finland | Europe | Baermann | 7 |
| 17 | Ugbomoiko et al. | 2008 | Nigeria | Africa | Kato-Katz thick smear | 7 |
| 18 | Das et al. | 2009 | India | Asia | Unclear | 5 |
| 19 | Claerebout et al. | 2009 | Belgium | Europe | Sucrose flotation | 8 |
| 20 | Gates and Nolan | 2009 | USA | North America | ZnSO4 flotation, formalin-ethyl acetate sedimentation | 8 |
| 21 | Itoh et al. | 2009 | Japan | Asia | Formalin-ethyl acetate sedimentation | 7 |
| 22 | Takano et al. | 2009 | Japan | Asia | Direct smear, agar plate culture (APC) | 7 |
| 23 | Leelayoova et al. | 2009 | Thailand | Asia | Direct smear, formalin-ethyl acetate concentration | 6 |
| 24 | Razmi | 2009 | Iran | Asia | Mini Parasep® SF (sedimentation) | 7 |
| 25 | Mariana et al. | 2010 | Bolivia | South America | Willis-Malloy flotation | 5 |
| 26 | Zewdu et al. | 2010 | Ethiopia | Africa | Necropsy | 6 |
| 27 | Awoke et al. | 2011 | Ethiopia | Africa | Direct smear, flotation | 5 |
| 28 | Jones et al. | 2011 | Ethiopia | Africa | Necropsy | 5 |
| 29 | Itoh et al. | 2011 | Japan | Asia | Formalin-ethyl acetate sedimentation | 8 |
| 30 | Itoh et al. | 2011 | Japan | Asia | Formalin-ethyl acetate sedimentation | 7 |
| 31 | Paulos et al. | 2012 | Ethiopia | Africa | Sedimentation and flotation | 5 |
| 32 | Martins et al. | 2012 | Brazil | South America | PARATEST® Diagnostek (sedimentation) | 7 |
| 33 | Getahun and Addis | 2012 | Ethiopia | Africa | Sedimentation and NaCl flotation | 6 |
| 34 | Mircean et al. | 2012 | Romania | Europe | NaCl flotation | 8 |
| 35 | Mekbib et al. | 2013 | Ethiopia | Africa | Direct smear, flotation and sedimentation | 5 |
| 36 | G/selasie et al. | 2013 | Ethiopia | Africa | McMaster, sedimentation | 5 |
| 37 | Abere et al. | 2013 | Ethiopia | Africa | Direct smear, sedimentation and NaCl flotation | 6 |
| 38 | Perera et al. | 2013 | Sri Lanka | Asia | NaCl flotation, Sheather’s sucrose flotation, direct smear | 6 |
| 39 | Riggio et al. | 2013 | Italy | Europe | Flotation, Baermann | 8 |
| 40 | Ortuno et al. | 2014 | Spain | Europe | ZnSO4 flotation | 7 |
| 41 | Alvarado-Esquivel et al. | 2015 | Mexico | North America | Sheather’s and ZnSO4 flotation | 8 |
| 42 | Puebla et al. | 2015 | Cuba | North America | Direct smear, formalin ethyl acetate sedimentation, Kato-Katz smear, Willy-Malloy flotation | 5 |
| 43 | Elom et al. | 2015 | Nigeria | Africa | NaCl and ZnSO4 floatation Formol ether sedimentation | 5 |
| 44 | Hadi and Faraj | 2016 | Iraq | Asia | Direct smear, potassium dichromate K2Cr2O4 film, NaCl flotation, Formalin-ether sedimentation | 5 |
| 45 | Wright et al. | 2016 | UK | Europe | FLOTAC technique | 7 |
| 46 | Ferreira et al. | 2016 | Brazil | South America | Sucrose and NaCl flotation, water-ether sedimentation | 7 |
| 47 | Pumidonming et al. | 2016 | Thailand | Asia | Flotation, formalin-ethyl acetate sedimentation | 7 |
| 48 | Strkolcova et al. | 2017 | Slovakia | Europe | Flotation, Baermann, APC, ELISA | 7 |
| 49 | Paradies et al. | 2017 | Italy | Europe | Direct smear, Baermann, necropsy | 8 |
| 50 | Mircean et al. | 2017 | Romania | Europe | NaCl flotation, sedimentation | 8 |
| 51 | Jaleta et al. | 2017 | Cambodia | Asia | Baermann, Kato-Katz | 9 |
| 52 | Sauda et al. | 2018 | Italy | Europe | Flotation, Baermann | 8 |
| 53 | García et al. | 2018 | Venezuela | South America | NaCl flotation | 5 |
| 54 | Hurtado and Forero | 2019 | Colombia | Asia | Formalin-gasoline concentration | 5 |
| 55 | Iatta et al. | 2019 | Italy | Europe | Direct smear, Baermann, APC, IFAT, RT-PCR | 8 |
| 56 | Sanchez-Thevenet et al. | 2019 | Spain | Europe | Modified Ritchie formalin-ether, Sheather’s sugar flotation, RT-PCR | 8 |
| 57 | Kurnosova et al. | 2019 | Russia | Europe | NaCl and ammonium nitrate flotations | 7 |
| 58 | Sanpool et al. | 2020 | Thailand | Asia | APC | 7 |
| 59 | Beknazarova et al. | 2020 | Australia | Oceania | qPCR, RT-PCR | 8 |
| 60 | Dashchenko et al. | 2020 | Ukraine | Europe | Direct smear, Baermann, modified string test | 6 |
| 61 | Nagamori et al. | 2020 | USA | North America | Direct smear, flotation, sedimentation, Baermann | 8 |
Pooled prevalence
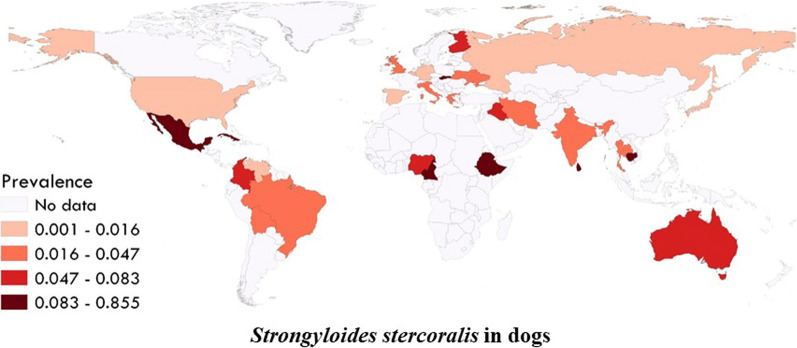
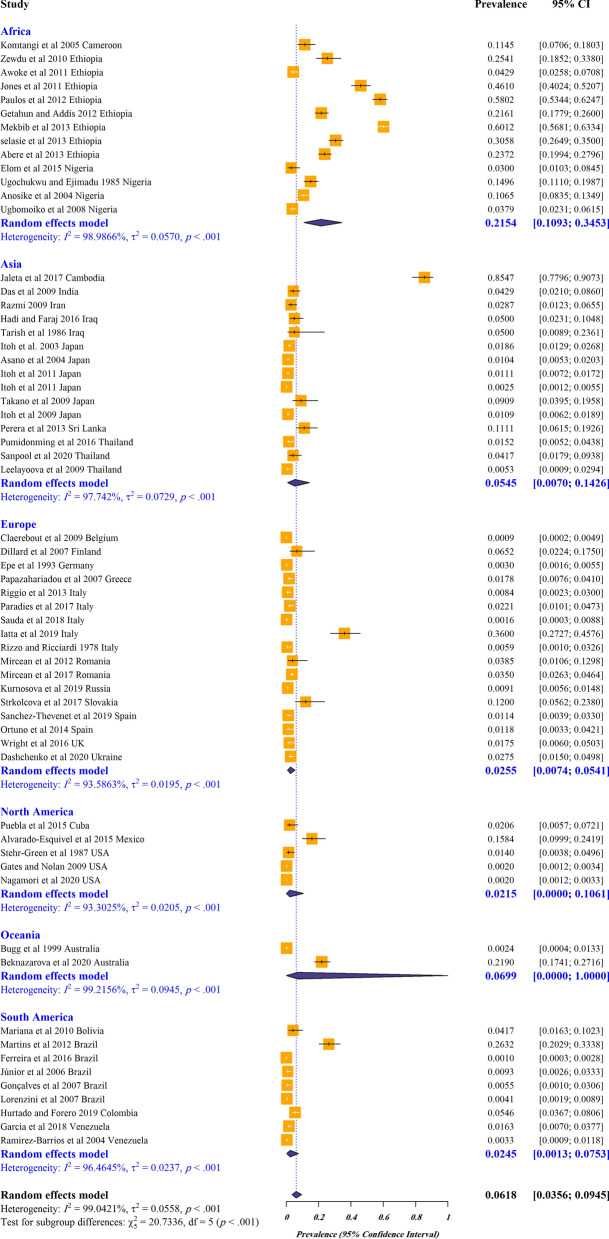
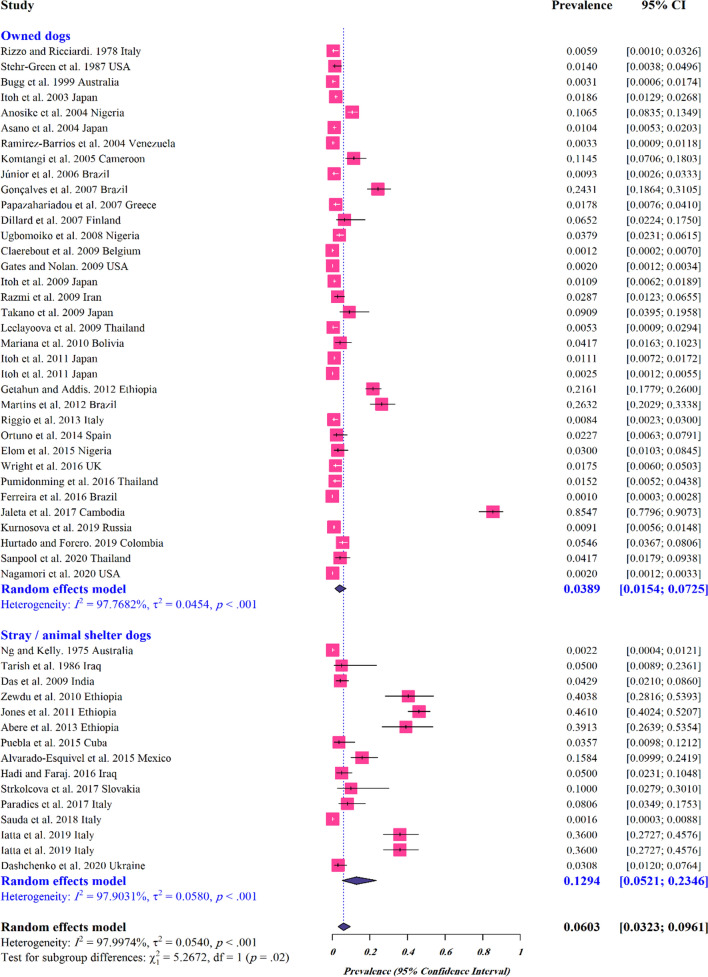
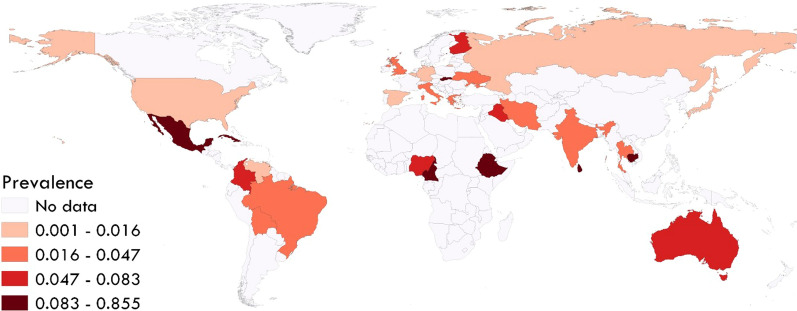
The estimated pooled global prevalence for S. stercoralis in dogs was 6% (95% CI 3–9%) with a higher estimated pooled prevalence in stray/shelter dogs (11%, 95% CI 1–26%) than in owned dogs (3%, 95% CI 1–7%) (Figs. 2; 3). Based on the manuscripts included in the analysis, S. stercoralis infection in dogs has been documented in 29 countries (Fig. 4; Additional file 2: Figure S1). The pooled prevalence on different continents ranged from 21 to 2%, with 21% (95% CI 10–34%) in Africa, 6% (95% CI 0–100%) in Oceania, 5% (95% CI 0–14%) in Asia, 2% (95% CI 0–10%) in North America, 2% (95% CI 0–7%) in South America and 2% (95% CI 0–5%) in Europe (Fig. 2; Additional file 3: Table S1).
Fig. 2.
Forest plots for random-effects meta-analysis of the global prevalence of Strongyloides stercoralis in dogs based on continent
Fig. 3.
Forest plots for random-effects meta-analysis of the global prevalence of Strongyloides stercoralis in owned and stray/shelter dogs
Fig. 4.
Global prevalence of Strongyloides stercoralis in dogs based on included studies
The largest number of studies was conducted in Ethiopia (8 studies), followed by Japan (6 studies). Analyses based on countries showed that Cambodia had the highest pooled prevalence (85%, 95% CI 78–91%) (Additional file 2: Figure S1). The estimated pooled prevalence based on country-level income groups ranged from 22 to 2%, with the highest rate in low-income countries (22%, 95% CI 10–36%) (Additional file 3: Table S1).
Our analyses revealed that regions with average temperatures of 10–20 °C had the highest prevalence of S. stercoralis (6%, 95% CI 3–11%) (Additional file 3: Table S1). Furthermore, the infection was more prevalent in regions with humidity of 40–75% (8%, 95% CI 4–13%), annual rainfall of 1001–1500 mm (9%, 95% CI 4–15%) and a tropical wet and dry climate (12%, 95% CI 5–21%). In addition, we found that the highest prevalence rate was at a latitude of 1°–25° (11%, 95% CI 5–19%).
Publication bias
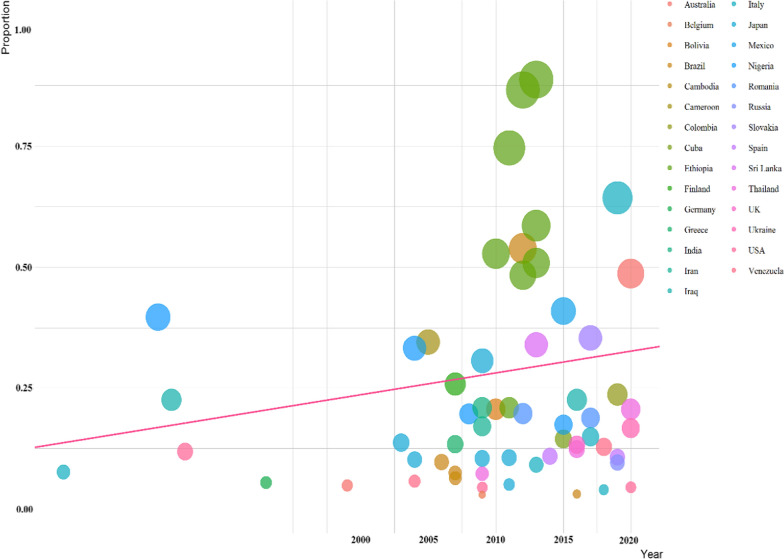
As demonstrated by funnel plot asymmetry, a highly significant publication bias was observed in our study using Egger’s test (t = 4.12, P = 0.0001) and Begg’s test (P = 0.001) (Fig. 5A, B). Meta-regression analysis demonstrated that there was a significant heterogeneity between studies regarding the year of publication (regression slope = 0.0055, P = 0.0116) (Fig. 6). Evaluation of study quality revealed that, among 61 studies, 36 had a total score of 7–9 points (high quality) and 25 had a total score of 4–6 points (moderate quality). No included studies were considered poor quality (Table 1).
Fig. 5.
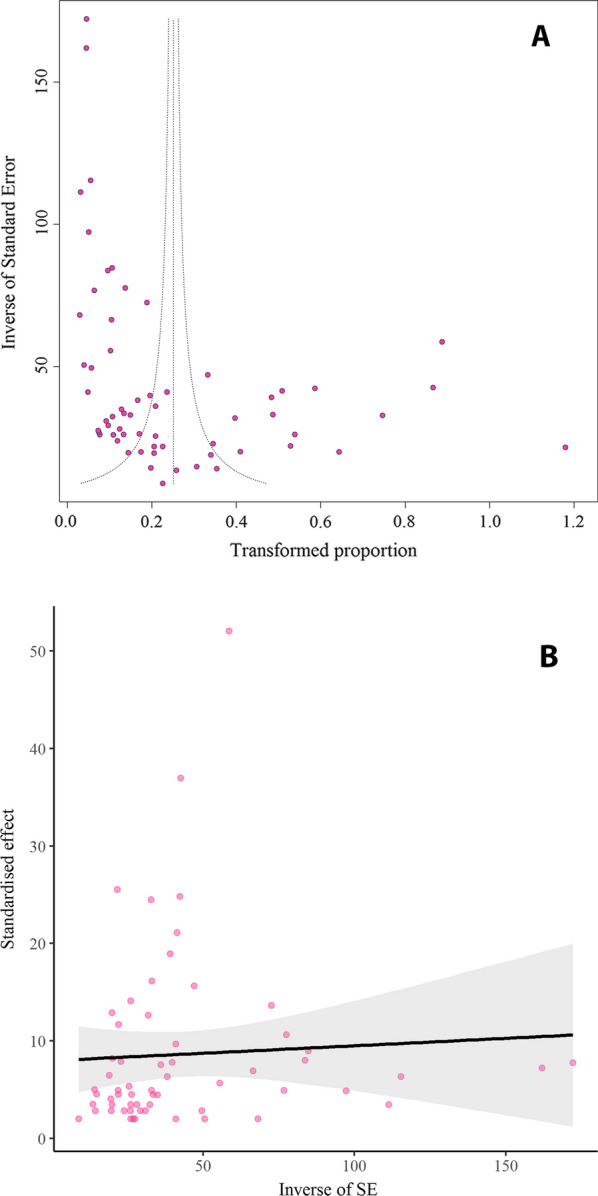
Egger’s funnel plot (A) and Begg’s funnel plot (B) to assess publication bias in included studies. (Colored circles represent each study. The middle line is the effect size and the other two lines are the corresponding confidence ranges)
Fig. 6.
A meta-regression graph for the prevalence of Strongyloides in dogs based on the year of publication. (The pink line is the regression line, which was plotted based on the intercept and the slope of the regression model. The different color bubbles represent the countries under study and their sizes indicate the effect size of each study)
Discussion
Dogs are among the most popular companion animals with a considerable positive impact on the psychological and physiological conditions of their owners [30, 31]. This close relationship with dogs, however, can pose certain risks with the potential of dogs transmitting a broad range of zoonotic pathogens, including viruses, bacteria, parasites and fungi [32–34]. Regarding S. stercoralis, some genotypes are dog specific while others can infect dogs and humans; hence, dogs could be reservoirs of zoonotic S. stercoralis [6, 13, 35]. In this review and meta-analysis, we estimated the global prevalence of S. stercoralis in dogs. Our findings show S. stercoralis in dogs being documented in 29 countries and that infections are not limited to dogs in tropical and subtropical regions, although these regions have the higher prevalence as is the general case for human infections [12].
Factors that potentially contribute to the higher prevalence in tropical regions include temperature and humidity, country income and presence of stray dogs. Similar to prior studies, the results of the meta-analysis herein presented indicate that climate conditions play a key role in the prevalence of S. stercoralis infection with the highest pooled prevalence in areas with a tropical wet and dry climate [12]. The higher humidity and temperature make favorable conditions for survival of heterogonic stages of S. stercoralis [36, 37]. While the climate plays a key role in prevalence, several studies have confirmed higher rates of helminthic infections in humans in low-income countries, attributed to sanitary conditions and limited access to health care [38–40]. This is similar to our results, with the highest prevalence of S. stercoralis in dogs in low-income countries. In these countries, access to veterinary care, specifically access to or affordability of anthelmintics, might be limited, contributing to the higher prevalence. Also, in low-income countries, the number of stray dogs can be high with the prevalence of S. stercoralis infection being greater than that in owned dogs, based on the data from the meta-analysis herein presented [41, 42].
Cambodia, where the highest prevalence was found in our analysis, serves as a potential example of the interaction of climate, income and free-roaming (individually or community owned but not contained) or stray dogs. Cambodia is a lower middle-income country with a tropical climate and limited accessibility to improved sanitation services as well as safe drinking water [43]. In the human population, S. stercoralis is a public health concern with prevalence being > 40%, one of the higher levels in human populations that have been found [44–46]. Lastly, many dogs in the country are free-roaming or stray [47]. These potential interactions support the need for a One Health approach to addressing Strongyloides infections in humans and dogs.
There is no gold standard method for detecting S. stercoralis in dogs (or people), and the current techniques have limited sensitivity due to the low burden of parasites and intermittent larval shedding [21, 48]. The most commonly used methods for the diagnosis of S. stercoralis infection in humans are direct smear and Kato-Katz, both of which have low sensitivity [12, 46]. Sedimentation, the Baermann method and agar plate culture have higher sensitivity, but they are inconvenient, time-consuming and still underestimate infections [12, 49]. While these latter methods were used in most of the studies included in the meta-analysis, technical details were inconsistent and their implementation varied. For example, with the Baermann the quantity of fecal material and the number of samples analyzed (e.g. one or three from consecutive days) were not standardized across studies, thus resulting in varied sensitivity of the method across studies. In some of the included articles, the focus was on general parasite prevalence with flotation and smears used for fecal analysis, standard screening methods for parasites in dogs but methods with low sensitivity for S. stercoralis, potentially resulting in an underestimation of prevalence. Interpretation of results from serological and molecular-based techniques, which were used in a few of the included articles, must be made with caution because of the possibility of false-positive and/or -negative results [21]. Given the high variation in how each diagnostic method was used in the studies, sensitivity ranges could not be assigned with confidence; hence, in the meta-analysis herein presented, prevalence was not adjusted based on the diagnostic method used with the resulting global prevalence likely underestimated.
In a clinical setting, S. stercoralis infections in dogs also are likely to be underestimated or overlooked because of the challenge of differentiating S. stercoralis larvae from other larvae that can occur in feces [i.e. Angiostrongylus vasorum, Crenosoma vulpis, Filaroides (Oslerus) osleri, Filaroides hirti and Filaroides milksi] [21]. Also, feces must be directly collected from the rectum or collected from the ground immediately after defecation to prevent fecal contamination with the larvae of free-living nematodes.
Most of the studies included in the meta-analysis were from tropical and subtropical regions, biasing the result towards higher prevalence in these regions. In other regions, within specific dog populations, prevalence might be higher than indicated in the meta-analysis with studies targeting S. stercoralis in susceptible dog populations (e.g. kennels, strays and shelter dogs) having prevalence similar to that seen in tropical and subtropical regions [16, 19, 50]. Hence, there is a need for more prevalence studies outside of tropical and subtropical regions to obtain a better understanding of the zoonotic risk.
Conclusion
The results of this systematic review and meta-analysis indicate the significant burden and current status of S. stercoralis infection in dogs in different parts of the world and highlight the need for studies in more geographical regions using methods with defined sensitivity. Paying attention to waste management systems, improving hygiene education and sanitary facilities in human populations as well as cleaning the environment of dog feces and establishing a preventive strategy for stray dogs could reduce the prevalence of the infection, especially in lower-income tropical and subtropical regions of the world. To decrease the burden of infective larvae in the environment contaminated with the feces of dogs, and in order to protect the canine and human population from the risk of infection, adequate deworming practices are essential. While few studies directly link infection of Strongyloides in humans to dogs, the shared genotypes and the similarity in where prevalence is higher support that the zoonotic potential of S. stercoralis infection is an important subject that should be reflected through raising awareness among dog owners and veterinarians. We recommend health authorities to organize efficient monitoring programs for protecting humans and dogs from the impact of the infection.
Supplementary Information
Additional file 1: References S1. List of articles used in the meta-analysis.
Additional file 2: Figure S1. Sub-group analysis of the prevalence of Strongyloides stercoralis in included studies based on country.
Additional file 3: Table S1. Sub-group analysis of the prevalence of Strongyloides stercoralis in included studies based on continent, income level, humidity, annual rainfall, average temperature, latitude and climate.
Acknowledgements
We sincerely thank personnel from the Metabolic Diseases Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin, Iran.
Authors’ contributions
MB, AVE and JKK designed the study. EH, EH and RM searched for primary publications, screened and appraised primary studies. AVE and MB extracted the data and, with JKK, wrote the study manuscript. MO, EH and SH contributed to data analysis and interpretation and edited the manuscript. All authors read the manuscript and participated in the preparation of the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Metabolic Diseases Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin, Iran (contract no. IR.QUMS.REC.1400.270).
Availability of data and materials
All data are included in the manuscript or as supplementary files.
Declarations
Ethics approval and consent to participate
Not applicable. No ethics approval or consent to participate were required for this work.
Consent for publication
Not applicable. No figures are included in this work that require consent for publication.
Competing interests
The authors have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aida Vafae Eslahi, Sima Hashemipour and Meysam Olfatifar contributed equally to this work
Contributor Information
Aida Vafae Eslahi, Email: vafaeeslahia@gmail.com.
Sima Hashemipour, Email: hashemipour.sima@yahoo.com.
Meysam Olfatifar, Email: ol.meysam92@gmail.com.
Elham Houshmand, Email: el_hooshmand@yahoo.com.
Elham Hajialilo, Email: e.hajialilo@gmail.com.
Razzagh Mahmoudi, Email: r.mahmodi@yahoo.com.
Milad Badri, Email: Badri22.milad@gmail.com.
Jennifer K. Ketzis, Email: JKetzis@rossu.edu
References
- 1.Zibaei M, Nosrati MRC, Shadnoosh F, Houshmand E, Karami MF, Rafsanjani MK, et al. Insights into hookworm prevalence in Asia: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2020;114(3):141–154. doi: 10.1093/trstmh/trz115. [DOI] [PubMed] [Google Scholar]
- 2.Omidinia N, Zibaei M, Hosseini H, Pourrostami K, Eslahi AV, Badri M. Human hydatidosis in Alborz province: a 5-year retrospective epidemiological analysis of hospitalized cases. Ann Parasitol. 2020;66(4):587–592. doi: 10.17420/ap6604.302. [DOI] [PubMed] [Google Scholar]
- 3.Cleaveland S, Laurenson MK, Taylor LH. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos Trans R Soc B Biol Sci. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eslahi AV, Mowlavi G, Houshmand E, Pirestani M, Majidiani H, Nahavandi KH, et al. Occurrence of Dioctophyme renale (Goeze, 1782) in road-killed canids of Iran and its public health implication. Vet Parasitol Reg Stud Rep. 2021;24:100568. doi: 10.1016/j.vprsr.2021.100568. [DOI] [PubMed] [Google Scholar]
- 5.Eslahi AV, Badri M, Nahavandi KH, Houshmand E, Dalvand S, Riahi SM, et al. Prevalence of strongyloidiasis in the general population of the world: a systematic review and meta-analysis. Pathog Glob Health. 2021;115:7–20. doi: 10.1080/20477724.2020.1851922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko PP, Suzuki K, Canales-Ramos M, Htwe MPPTH, Htike WW, Yoshida A, et al. Phylogenetic relationships of Strongyloides species in carnivore hosts. Parasitol Int. 2020;78:102151. doi: 10.1016/j.parint.2020.102151. [DOI] [PubMed] [Google Scholar]
- 7.Barroso M, Salvador F, Sánchez-Montalvá A, Bosch-Nicolau P, Molina I. Strongyloides stercoralis infection: a systematic review of endemic cases in Spain. PLoS Negl Trop Dis. 2019;13(3):e0007230. doi: 10.1371/journal.pntd.0007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viney M. Strongyloides. Parasitology. 2017;144(3):259–262. doi: 10.1017/S0031182016001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nutman T. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263–273. doi: 10.1017/S0031182016000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonfrate D, Requena-Mendez A, Angheben A, Cinquini M, Cruciani M, Fittipaldo A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. doi: 10.1371/journal.pntd.0006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levenhagen MA, Costa-Cruz JM. Update on immunologic and molecular diagnosis of human strongyloidiasis. Acta Trop. 2014;135:33–43. doi: 10.1016/j.actatropica.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barratt JLN, Lane M, Talundzic E, Richins T, Robertson G, Formenti F, et al. A global genotyping survey of Strongyloides stercoralis and Strongyloides fuelleborni using deep amplicon sequencing. PLoS Negl Trop Dis. 2019;13(9):e0007609. doi: 10.1371/journal.pntd.0007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umur Ş, Meral Y, Bölükbaş CS, Gürler AT, Acici M. First clinical Strongyloides stercoralis case in a dog in Turkey. Turk J Vet Anim Sci. 2017;41(2):312–315. [Google Scholar]
- 15.Goncalves ALR, Machado GA, Goncalves-Pires MRF, Ferreira-Junior A, Silva DAO, Costa-Cruz JM. Evaluation of strongyloidiasis in kennel dogs and keepers by parasitological and serological assays. Vet Parasitol. 2007;147(1–2):132–139. doi: 10.1016/j.vetpar.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Dillard KJ, Saari SAM, Anttila M. Strongyloides stercoralis infection in a Finnish kennel. Acta Vet Scand. 2007;49(1):1–6. doi: 10.1186/1751-0147-49-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Júnior AF, Gonçalves-Pires MRF, Silva DAO, Goncalves ALR, Costa-Cruz JM. Parasitological and serological diagnosis of Strongyloides stercoralis in domesticated dogs from southeastern Brazil. Vet Parasitol. 2006;136(2):137–145. doi: 10.1016/j.vetpar.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Eydal M, Skírnisson K. Strongyloides stercoralis found in imported dogs, household dogs and kennel dogs in Iceland. Icel Agric Sci. 2016;29:39–51. [Google Scholar]
- 19.Dashchenko S, Soroka N, Semenko O. Distribution of Strongyloides stercoralis among dogs of different housing groups in Kyiv and Kyiv region, clinical manifestations and diagnostic methods. EUREKA Health Sci. 2020;5:99–107. [Google Scholar]
- 20.Basso W, Grandt L-M, Magnenat A-L, Gottstein B, Campos M. Strongyloides stercoralis infection in imported and local dogs in Switzerland: from clinics to molecular genetics. Parasitol Res. 2019;118(1):255–266. doi: 10.1007/s00436-018-6173-3. [DOI] [PubMed] [Google Scholar]
- 21.Paradies P, Iarussi F, Sasanelli M, Capogna A, Lia RP, Zucca D, et al. Occurrence of strongyloidiasis in privately owned and sheltered dogs: clinical presentation and treatment outcome. Parasit Vectors. 2017;10(1):1–9. doi: 10.1186/s13071-017-2275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thamsborg SM, Ketzis J, Horii Y, Matthews JB. Strongyloides spp. infections of veterinary importance. Parasitology. 2017;144(3):274–84. doi: 10.1017/S0031182016001116. [DOI] [PubMed] [Google Scholar]
- 23.Faust EC, Kagy ES. Experimental studies on human and primate species of Strongyloides. I. The variability and instability of types. Am J Trop Med. 1933;13:47–65. [Google Scholar]
- 24.Lok JB, Community TCeR Strongyloides stercoralis: a model for translational research on parasitic nematode biology. WormBook. 2007 doi: 10.1895/wormbook.1.134.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9(6):468. doi: 10.3390/pathogens9060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 3 July 2021.
- 27.Badri M, Eslahi AV, Olfatifar M, Dalvand S, Houshmand E, et al. Keys to unlock the enigma of ocular toxocariasis: a systematic review and meta-analysis. Ocul Immunol Inflamm. 2021;12:1–2. doi: 10.1080/09273948.2021.1875007. [DOI] [PubMed] [Google Scholar]
- 28.Eslahi AV, Olfatifar M, Abdoli A, Houshmand E, Johkool MG, Zarabadipour M, et al. The neglected role of Trichomonastenax in oral diseases: a systematic review and meta-analysis. Acta Parasitol. 2021;66:715–732. doi: 10.1007/s11686-021-00340-4. [DOI] [PubMed] [Google Scholar]
- 29.Mirzadeh M, Olfatifar M, Eslahi AV, Abdoli A, Houshmand E, Majidiani H, et al. Global prevalence of Trichomonas vaginalis among female sex workers: a systematic review and meta-analysis. Parasitol Res. 2021;120(7):2311–2322. doi: 10.1007/s00436-021-07216-6. [DOI] [PubMed] [Google Scholar]
- 30.Kanat-Maymon Y, Antebi A, Zilcha-Mano S. Basic psychological need fulfillment in human–pet relationships and well-being. Pers Individ Dif. 2016;92:69–73. [Google Scholar]
- 31.Grajfoner D, Harte E, Potter LM, McGuigan N. The effect of dog-assisted intervention on student well-being, mood, and anxiety. Int J Environ Res Public Health. 2017;14(5):483. doi: 10.3390/ijerph14050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritossa L, Viozzi G, Flores V. The state of knowledge on intestinal helminths in free-roaming dogs in southern South America. Canine Genet Health Med. 2021 doi: 10.5772/intechopen.96125. [DOI] [Google Scholar]
- 33.Ghasemzadeh I, Namazi SH. Review of bacterial and viral zoonotic infections transmitted by dogs. J Med Life. 2015;8(Spec Iss 4):1. [PMC free article] [PubMed] [Google Scholar]
- 34.Szabová E, Juriš P, Miterpáková M, Antolová D, Papajová I, Šefčíková H. Prevalence of important zoonotic parasites in dog populations from the Slovak Republic. Helminthologia. 2007;44(4):170–176. [Google Scholar]
- 35.Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, Muth S, et al. Different but overlapping populations of Strongyloides stercoralis in dogs and humans—dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl Trop Dis. 2017;11(8):e0005752. doi: 10.1371/journal.pntd.0005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viney ME, Lok JB. The biology of Strongyloides spp. WormBook. 2015;16:1–17. doi: 10.1895/wormbook.1.141.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paula FM, Costa-Cruz JM. Epidemiological aspects of strongyloidiasis in Brazil. Parasitology. 2011;138(11):1331–1340. doi: 10.1017/S003118201100120X. [DOI] [PubMed] [Google Scholar]
- 38.Sartorius B, Cano J, Simpson H, Tusting LS, Marczak LB, Miller-Petrie MK, et al. Prevalence and intensity of soil-transmitted helminth infections of children in sub-Saharan Africa, 2000–18: a geospatial analysis. Lancet Glob Health. 2021;9(1):e52–60. doi: 10.1016/S2214-109X(20)30398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19(12):547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Beknazarova M, Whiley H, Ross K. Strongyloidiasis: a disease of socioeconomic disadvantage. Int J Environ Res Public Health. 2016;13(5):517. doi: 10.3390/ijerph13050517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abulude OA. Prevalence of intestinal helminth infections of stray dogs of public health significance in Lagos metropolis, Nigeria. Int Ann Sci. 2019;9(1):24–32. [Google Scholar]
- 42.Otranto D, Dantas-Torres F, Mihalca AD, Traub RJ, Lappin M, Baneth G. Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol. 2017;33(10):813–825. doi: 10.1016/j.pt.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Liao C-W, Chiu K-C, Chiang I-C, Cheng P-C, Chuang T-W, Kuo J-H, et al. Prevalence and risk factors for intestinal parasitic infection in schoolchildren in Battambang, Cambodia. Am J Trop Med Hyg. 2017;96(3):583. doi: 10.4269/ajtmh.16-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forrer A, Khieu V, Schindler C, Schär F, Marti H, Char MC, et al. Ivermectin treatment and sanitation effectively reduce Strongyloides stercoralis infection risk in rural communities in Cambodia. PLoS Negl Trop Dis. 2016;10(8):e0004909. doi: 10.1371/journal.pntd.0004909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khieu V, Schär F, Forrer A, Hattendorf J, Marti H, Duong S, et al. High prevalence and spatial distribution of Strongyloides stercoralis in rural Cambodia. PLoS Negl Trop Dis. 2014;8(6):e2854. doi: 10.1371/journal.pntd.0002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eslahi AV, Olfatifar M, Karim MR, AbuOdeh R, Modirian E, Houshmand E, et al. Global incidence of helminthic contamination of vegetables, cucurbits and fruits: a systematic review and meta-analysis. Food Control. 2021 doi: 10.1016/j.foodcont. [DOI] [Google Scholar]
- 47.Chevalier V, Davun H, Sorn S, Ly P, Pov V, Ly S. Large scale dog population demography, dog management and bite risk factors analysis: a crucial step towards rabies control in Cambodia. PLoS ONE. 2021;16(7):e0254192. doi: 10.1371/journal.pone.0254192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iatta R, Buonfrate D, Paradies P, Cavalera MA, Capogna A, Iarussi F, et al. Occurrence, diagnosis and follow-up of canine strongyloidiosis in naturally infected shelter dogs. Parasitology. 2019;146(2):246–252. doi: 10.1017/S0031182018001312. [DOI] [PubMed] [Google Scholar]
- 49.Vafae Eslahi A, Olfatifar M, Houshmand E, Ghanbari Johkoold M, Zibaei M, Foroutan M, Hosseinie H, Badri M, et al. Prevalence of Strongyloides stercoralis in the immunocompetent and immunocompromised individuals in Iran: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2021 doi: 10.1093/trstmh/trab104. [DOI] [PubMed] [Google Scholar]
- 50.Gates MC, Nolan TJ. Endoparasite prevalence and recurrence across different age groups of dogs and cats. Vet Parasitol. 2009;166(1–2):153–158. doi: 10.1016/j.vetpar.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: References S1. List of articles used in the meta-analysis.
Additional file 2: Figure S1. Sub-group analysis of the prevalence of Strongyloides stercoralis in included studies based on country.
Additional file 3: Table S1. Sub-group analysis of the prevalence of Strongyloides stercoralis in included studies based on continent, income level, humidity, annual rainfall, average temperature, latitude and climate.
Data Availability Statement
All data are included in the manuscript or as supplementary files.