Abstract
Background
During the COVID-19 pandemic, federal agencies relaxed buprenorphine prescribing restrictions including for incarcerated individuals. The impact of COVID-19 on the supply of MOUD in U.S. prisons and jails is not known.
Methods
We used cross-sectional national monthly data from the IQVIA National Sales Perspective (NSP) for the total volume of medicines supplied to city, county and state prisons and jails and other types of institutional facilities in the U.S. We measured the total monthly supply (or volume) as extended units (EUs) for MOUDs overall and by type. We used interrupted time series analysis to evaluate changes in monthly volume of MOUDs in prisons and jails and other types of facilities (hospitals, clinics and long-term care) before (January 2018-February 2020) and during the COVID-19 (March 2020-October 2020) pandemic.
Results
The availability of MOUD in jails and prisons increased by 471.3% between January 2018 (52,784 EU) and October 2020 (333,226 EU). This increase was largely driven by increased volume of buprenorphine/naloxone and was not observed in other institutional facilities, including hospitals, clinics and long-term care, and. Specifically, the mean monthly volume of buprenorphine/naloxone at prisons/jails increased every month before the pandemic by 1860 EU (95% CI, 1110–2360). In March 2020, the mean volume of buprenorphine/naloxone increased by 81,930 EU (95% CI, 59,040–104,820) per month, followed by a significant increase of 24,010 EU (95% CI 19,530–28,490) per month during the pandemic vs before the pandemic.
Conclusion
These findings may indicate increased availability of buprenorphine/naloxone, a safe and effective MOUD, in prisons and jails since the start of the COVID-19 pandemic in the U.S. despite previous barriers in its use.
Keywords: Addiction treatment, Justice-involved population, Buprenorphine, Pharmacy distribution, Opioid use disorder, Opioids
1. Introduction
As of 2018, over two million people are held in prisons or jails in the United States (Mauruschak and Minton, 2020). Nearly 1 in 3 of these individuals have been diagnosed with opioid use disorder (OUD) (Fazel et al., 2017), yet the vast majority of them are not treated with medications for OUD (MOUD), particularly buprenorphine.(Krawczyk et al., 2017) During the COVID-19 pandemic, federal agencies relaxed buprenorphine regulations to allow telephonic prescribing for new or existing patients, including for incarcerated individuals (Drug Enforcement Administration, 2020; Duncan et al., 2021). In response to litigation incarcerated individuals with OUD are being denied treatment at prisons in jails, many states have passed legislation requiring treatment with at least one type of MOUD in correctional settings (Weizman et al., 2021). Despite these efforts to address the under treatment of OUD in U.S. prisons and jails, information on the availability and distribution of MOUD in these justice-involved populations is not known. We examined the availability of MOUD before and during the COVID-19 pandemic in prisons and jails in the U.S. We also compare the availability of MOUD in prisons and jails with other institutional facilities, including hospitals, clinics, long-term care, and home health care facilities.
2. Methods
2.1. Data source and measures
We used IQVIA National Sales Perspective (NSP) monthly data on the total volume of medicines purchased in city, county and state prisons and jails. Other types of institutional facilities include hospitals, clinics and long-term care and home health care facilities. Hospitals and clinics are defined as non-federal private, city, county and state hospitals, psychiatric hospitals and hospice centers, outpatient centers, convenience care, HMO hospitals, clinics and pharmacies/warehouses. Long-term care includes nursing home residential care facilities, institutional providers, and nursing home pharmacies. Home health care services are organizations that provide care in patients homes.
The volume (or supply) of medicines to facilities is standardized using extended units (EUs), representing the total number of tablets, capsules, vials, grams or milliliters of a specific product. EUs are calculated by multiplying the number of units or packages (e.g., 100 bottles of methadone) by the package size (e.g., 100 tablets per bottle). MOUD include methadone, buprenorphine, buprenorphine/naloxone, naloxone, and naltrexone. We calculated the total number of monthly EUs for MOUDs overall and for specific medications between January 2018 and October 2020 in prisons and jails and other facilities.
2.2. Statistical analysis
We calculated the total number of monthly EUs for MOUDs overall and for specific medications between January 2018 and October 2020 in prisons and jails and other facilities. We also compare the mean monthly volume for MOUD overall and by type of medication during the COVID-19 period compared to the pre-COVID-19 period. We defined the COVID-19 period as March 2020 through October 2020 since the US declared COVID-19 a national emergency in March 2020. We defined MOUD as buprenorphine/naloxone, buprenorphine, naloxone, naltrexone and methadone.
We also conducted a single-group interrupted time series analysis (ITSA) to evaluate whether there was a change in level or rate of growth (slope) in monthly volume of MOUDs by medication and facility type before and after the start of the COVID-19 pandemic. Our model included three key variables: 1) time, in months; 2) an intervention indicator (before and after March 1st 2020); and 3) an interaction of time and intervention. Further, autocorrelation and seasonality were checked using the Cumby-Huizinga general test for autocorrelation in time series (Cumby and Huizinga, 1992; Newey and West, 1987). Data analysis was conducted using Stata, 16.1 (StataCorp, 2021).
3. Results
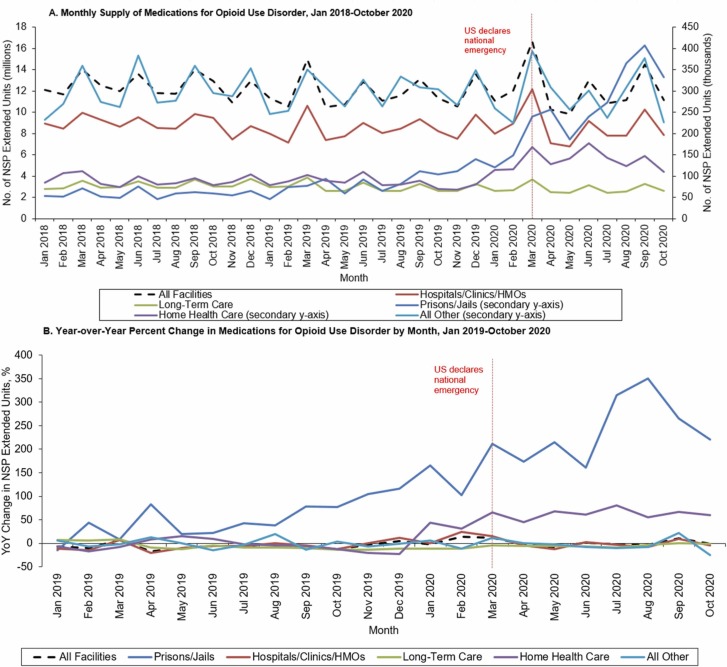
The availability of MOUD in jails and prisons increased by 471.3% between January 2018 (52,784 EU) and October 2020 (333,226 EU) ( Fig. 1) with the first peak in volume observed in March 2020 (239,910 EU). The share of MOUD distributed to prisons and jails increased from 0.4% in January 2018–3.0% of all MOUDs supplied to other types of facilities by October 2020. Although hospitals, clinics and long-term care facilities persistently account for more than 93% of MOUD volume in institutional facilities, MOUD volume has steadily declined between January 2018 and October 2020 by − 12.0% (from 8954,792 EU to 7881,793 EU) in hospitals and clinics and − 6.1% in long-term care (from 2763,076 EU to 2594,006 EU).
Fig. 1.
Trends in the Supply of Medications for Opioid Use Disorder Before and During the COVID-19 Pandemic in Prisons and Jails and Other Facilities. No. = number; NSP = National Sales Perspectives™; YoY = Year-over-Year. Monthly supply trends of medications for opioid use disorder by facility type from January 2018 through October 2020 are displayed in Panel A. Supply volume is reported in extended units, measured by the number of tablets or capsules for oral products and volume in milliliters for liquid products. The secondary y-axis amplifies low-use facility types which includes prisons and jails, home health care, and all other miscellaneous facility types (Universities and non-VA federal facilities). Panel B displays the YoY percent change in supply from January 2019 through October 2020 by month, determined by the difference in product supply in a given month and the supply in the same month the year prior. All other category includes other facilities such as federal facilities and miscellaneous universities.
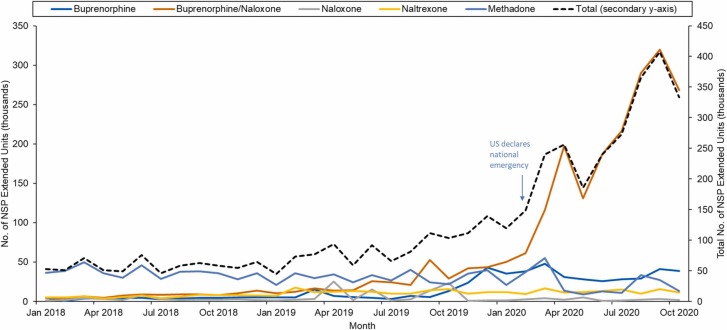
The increase in MOUD availability in prisons and jails is largely due to the substantial increase in the supply of buprenorphine/naloxone ( Fig. 2). The volume of buprenorphine/naloxone at prisons and jails increased by 2484% between January 2019 (10,380 EU) and October 2020 (268,170 EU). Prior to September 2019, when buprenorphine/naloxone exceeded methadone supply for the first time, methadone persistently accounted for the majority of MOUD distributed in prisons and jails. By October 2020, buprenorphine/naloxone accounted for 80.5% of all MOUD supplied to prisons and jails and methadone accounted for only 4%.
Fig. 2.
Trends in the Distribution of Medications for Opioid Use Disorder (MOUD) by Specific Medication in U.S. Prisons - January 2018 to October 2020. NSP = National Sales Perspectives; MOUD = medications for opioid use disorder. Data spans from January 2018 to October 2020. Monthly supply trends of MOUDs at U.S. prisons and jails from January 2018 to October 2020. Supply volume is reported in extended units, measured by the number of tablets or capsules for oral products and volume in milliliters for liquid products. The primary (left) y axis represents the number of extended units, in thousands, for specific MOUDs, while the total number of extended units for all MOUDs are charted on the secondary y-axis (right-axis). Using simple linear regression, buprenorphine, naltrexone, and overall MOUDs increased significantly annually from 2018 to 2020.
Findings from the ITSA indicate that the mean monthly supply of MOUDs to prisons and jails increased significantly in the month immediately following the COVID-19 pandemic ( Table 1). Specifically, in the March 2020, the mean monthly volume of MOUDs increased significantly (p < 0.01) by 82,690 EU (95% CI, 42,350–123,030). This increase persisted throughout the pandemic increasing by 21,870 EU (95% CI, 19,530–28,490)) per month during the pandemic when compared to before. In hospitals, clinics, long-term care facilities, and all other facilities, the monthly supply of MOUDs did not substantially change during the COVID-19 pandemic. However, the immediate effect of COVID-19 on the total monthly supply of MOUDs in home health care facilities increased significantly, specifically driven by increases in the volume of buprenorphine and naloxone (50,620 and 980, respectively).
Table 1.
Changes in Monthly Supply of Medications for Opioid Use Disorder (in thousands) in Prisons/Jails and Other Types of Facilities Before and During the COVID-19 Pandemica.
| Mean Monthly Volume, in thousands (95% CI) |
|||||
|---|---|---|---|---|---|
| Prisons and Jails | Hospitals/Clinics/HMOs | Long-Term Care | Home Health Care | All Other Facilities | |
| All MOUDs | |||||
| Prepandemic Volume | 77.62 (65.86–89.38) | 8666.29 (8308.01–9024.57) | 3014.37 (2858.03–3170.71) | 89.53 (83.89–95.16) | 295.58 (277.79–313.36) |
| Prepandemic Trend | 3.11 (1.69–4.53) | -21.99 (−62.88 to 18.89) | -15.42 (−27.72 to −3.12) | 0.15 (−0.49 to 0.78) | -0.39 (−1.24 to 0.45) |
| Pandemic Month Immediate changeb | 82.69 (42.35–123.03) | 485.60 (−1445.92–2417.12) | 70.81 (−104.40 to 246.03) | 59.54 (43.29–75.78) | 24.02 (−11.76 to 59.79) |
| Trend change during pandemicc | 21.87 (12.43–31.31) | -103.36 (−510.11 to 303.38) | -8.97 (−57.08 to 39.14) | -2.17 (−4.62 to 0.29) | -7.12 (−15.59 to 1.34) |
| Buprenorphine/Naloxone | |||||
| Prepandemc Volume | 19.74 (13.02–26.46) | 1596.87 (1483.86–1709.89) | 932.61 (870.06–995.16) | 23.38 (21.64–25.12) | 61.65 (58.06–65.24) |
| Prepandemic Trend | 1.86 (1.10–2.63) | 29.32 (25.32–33.33) | -3.86 (−12.32 to 4.60) | 0.19 (−0.06 to 0.45) | 0.72 (0.50–0.94) |
| Pandemic Month Immediate change | 81.93 (59.04–104.82) | -21.69 (−366.55 to 323.17) | 57.75 (−47.77 to 163.26) | 4.31 (−1.36 to 9.97) | 5.99 (−2.61 to 14.60) |
| Trend change during pandemic | 24.01 (19.53–28.49) | -58.17 (−126.88 to 10.54) | 2.08 (−30.11 to 34.28) | 0.23 (−1.20 to 1.67) | -3.31 (−4.97 to −1.65) |
| Buprenorphine | |||||
| Prepandemic Volume | 10.06 (5.35–14.77) | 380.12 (352.12–408.13) | 294.40 (280.05–308.75) | 10.38 (5.56–15.21) | 12.87 (12.00–13.74) |
| Prepandemic Trend | 1.03 (0.31–1.76) | 7.12 (5.90–8.35) | 0.80 (−0.28 to 1.88) | 1.07 (0.23–1.91) | 0.11 (0.02–0.21) |
| Pandemic Month Immediate change | 9.51 (−4.52 to 23.54) | -42.87 (−133.80 to 48.07) | -25.97 (−56.90 to 4.95) | 50.62 (28.36–72.87) | -0.24 (−2.02 to 1.53) |
| Trend change during pandemic | -0.06 (−2.43 to 2.31) | -8.73 (−26.13 to 8.66) | -1.29 (−6.85 to 4.26) | -3.33 (−6.45 to −0.21) | -0.43 (−0.68 to −0.18) |
| Naloxone | |||||
| Prepandemic Volume | 5.03 (2.31–7.75) | 316.76 (295.76–337.77) | 26.28 (20.32–32.23) | 0.85 (0.67–1.02) | 94.32 (83.22–105.42) |
| Prepandemic Trend | 0.27 (−0.05 to 0.60) | 1.88 (−0.59 to 4.34) | 0.17 (−0.54 to 0.88) | -0.02 (−0.03 to 0.00) | -0.44 (−1.59 to 0.72) |
| Pandemic Month Immediate change | -7.45 (−20.56 to 5.66) | 18.60 (−119.19 to 156.38) | -15.81 (−42.67 to 11.05) | 0.98 (0.56–1.41) | -2.86 (−34.88 to 29.15) |
| Trend change during pandemic | -0.55 (−3.04 to 1.94) | -9.95 (−34.16 to 14.26) | 2.43 (−1.17 to 6.03) | -0.13 (−0.19 to −0.08) | 2.85 (−3.74 to 9.44) |
| Naltrexone | |||||
| Prepandemic Volume | 9.40 (7.91–10.89) | 242.59 (227.60–257.58) | 363.17 (342.51–383.83) | 2.46 (2.20–2.71) | 41.94 (38.46–45.41) |
| Prepandemic Trend | 0.36 (0.19–0.54) | 3.79 (3.44–4.15) | -0.79 (−3.49 to 1.90) | 0.01 (−0.03 to 0.04) | 0.22 (−0.11 to 0.55) |
| Pandemic Month Immediate change | -0.68 (−5.26 to 3.90) | -15.66 (−37.53 to 6.21) | 59.25 (1.23–117.27) | -0.30 (−1.09 to 0.49) | 4.78 (−9.79 to 19.36) |
| Trend change during pandemic | -0.58 (−1.33 to 0.17) | -4.02 (−8.56 to 0.53) | -3.86 (−10.63 to 2.91) | 0.00 (−0.13 to 0.14) | -1.56 (−4.18 to 1.05) |
| Methadone | |||||
| PrepandemicVolume | 33.39 (30.41–36.37) | 6129.94 (5772.72–6487.17) | 1397.91 (1324.53–1471.29) | 52.46 (47.85–57.07) | 84.79 (74.94–94.64) |
| Prepandemic Trend | -0.42 (−0.81 to −0.04) | -64.11 (−104.93 to −23.29) | -11.73 (−13.50 to −9.96) | -1.11 (−1.56 to −0.65) | -1.01 (−2.39 to 0.37) |
| Pandemic Month Immediate change | -0.62 (−25.92 to 24.68) | 547.23 (−943.04 to 2037.49) | -4.40 (−50.21 to 41.41) | 3.93 (−8.45 to 16.31) | 16.35 (−12.29 to 44.99) |
| Trend change during pandemic | -0.96 (−5.63 to 3.70) | -22.49 (−339.55 to 294.56) | -8.33 (−18.63 to 1.98) | 1.06 (−0.97 to 3.10) | -4.67 (−9.86 to 0.52) |
MOUD = medications for opioid use disorder; COVID-19 = coronavirus disease 2019; CI = confidence interval.
Data source is IQVIA National Sales Perspective (NSP) monthly projections of the total volume of medicines purchased by facility type; All data in bold represent a statistically significant change at P < 0.05; Volume is reported in thousands.
Refers to the level change immediately after the intervention (treatment effect).
Refers to the difference between the pre/COVID-19 slopes of the outcome or the treatment effect over time (in months) (i.e., slope change).
The mean monthly volume of buprenorphine/naloxone at prisons/jails increased every month before the pandemic by 1860 EU (95% CI, 1110–2360). In March 2020, the mean volume of buprenorphine/naloxone increased by 81,930 EU (95% CI, 59,040–104,820) per month, followed by a significant increase of 24,010 EU (95% CI 19,530–28,490) per month during the pandemic vs before the pandemic. In March 2020, the mean volume of buprenorphine/naloxone increased by 81,930 EU (95% CI, 59,040–104,820) per month, followed by a significant increase of 24,010 EU (95% CI 19,530–28,490) per month during the pandemic vs before the pandemic. In contrast, naloxone volume in prisons and jails had decreased by 550 EU per month during the pandemic when compared to before.
4. Discussion
To our knowledge, this is the first study to examine the availability of MOUD overall and by type of medication in prisons and jails in the U.S. We found that supply of MOUD increased substantially in prisons and jails since January 2018; an increase not observed in hospitals, clinics or long-term care facilities. MOUD supply increased rapidly in early 2020 and then continued to increase throughout the COVID-19 pandemic. We found that this growth in prisons and jails was exclusively due to increases in the supply of buprenorphine/naloxone, a first-line treatment for OUD (Sofuoglu et al., 2019).
Importantly the decline in naloxone volume to prisons and jails, alongside an increase in buprenorphine/naloxone, may be related to increases in SAMHSA funding for state opioid response grants (Substance Abuse and Mental Health Services Administration, 2020). For example, in Los Angeles County, tens of thousands of free naloxone kits have been distributed to inmates via a vending machine and this volume is not captured in our data. These distributed units may have otherwise been purchased by traditional means which may explain at least a portion of the reduction seen (Los Angeles County Office of Diversion and Reentry, 2020).
Previous studies have shown that jails and prisons have restricted access to MOUD (Krawczyk et al., 20). The increased availability of MOUD and buprenorphine/naloxone to correctional settings may not be driven by any one catalyst. The combined effects of increased legislation requiring treatment of OUD, successful lawsuits against correctional settings for failing to provide treatment, and relaxation of regulations that allow for increased prescribing of MOUD may all contribute to these findings.
Although we did not investigate the impact of specific federal or state policy changes on the supply of MOUD, there were several policy changes that occurred over the last several years that likely contribute to an increase in MOUD supply in prisons and jails. For instance, several states have enacted legislation in 2020 that mandates treatment of inmates with buprenorphine, naltrexone, or methadone while in custody. As a result, jails and prisons would need to procure this drug supply to dispense it to patients under custody. This would directly lead to increased drug supply to jails and prisons as depicted in our data set, however to what extent this added to it (versus other potential causes) is not known.
These findings suggest that federal and state policy efforts, including the relaxing of buprenorphine prescribing regulations during the COVID-19 pandemic, may have contributed to aggregate increases in the availability of buprenorphine/naloxone in prisons and jails in the U.S. However, these increases may not be occurring in all states, as several states, including California, which has the largest correctional facilities in the U.S. still have limited pilot programs for OUD or are only available for certain populations (i.e. pregnant patients) (Leyva, 2021). Nonetheless, efforts to expand access to buprenorphine, including lifting in-person prescribing requirements, should continue after the pandemic as the effects on access to these medications is most apparent in prisons and jails.
4.1. Limitations
This study has several limitations. The data used in these analyses capture the supply of medicines and do not represent prescribing practices in jails and prisons, nor whether individuals are being treated with these medications. Our findings are reported in aggregate at the national-level and OUD treatment in prisons and jails is often regulated by state agencies and state policies vary in scope. For example, some states do not allow incarcerated individuals to be treated with any MOUD, while others require all MOUD, including buprenorphine/naloxone and methadone, to be available (Weizman et al., 2021). Last, buprenorphine, buprenorphine/naloxone and methadone may also be used in pain management, particularly in hospitals and clinics. Therefore, our findings may also reflect changes in pain management in these facilities.
5. Conclusions
Since the start of the COVID-19 pandemic, we found that MOUD supply has increased substantially in U.S. prisons and jails and was largely driven by increase in the availability of buprenorphine/naloxone. These findings were not observed in other institutional facilities, including hospitals, clinics and long-term care findings and may suggest federal efforts to increase access to buprenorphine/naloxone, a safe and effective MOUD, may differentially benefit prisons and jails in the U.S. despite previous barriers in its use.
Contributors
DD, RTS, AS, and DMQ contributed to study design, analysis and interpretation. DD and DMQ drafted the manuscript. DD, RTS, AS, and DMQ critically reviewed the intellectual content of the manuscript. All authors have contributed to, reviewed, and approved the final manuscript.
Declaration of Competing Interest
Dr. Qato serves as a paid consultant to Public Citizen’s Health Research Group.
Acknowledgements
This study was supported, in part, through the IQVIA Institute Human Data Science Research Collaborative. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IQVIA or any of its affiliated or subsidiary entities
References
- Cumby R.E., Huizinga J. Testing the autocorrelation structure of disturbances in ordinary least squares and instrumental variables regressions. Econometrica. 1992;60(1):185. doi: 10.2307/2951684. [DOI] [Google Scholar]
- Drug Enforcement Administration. (2020). COVID-19 Information Page. 〈https://www.deadiversion.usdoj.gov/coronavirus.html〉.
- Duncan A., Sanders N., Schiff M., Winkelman T.N.A. Adaptations to jail-based buprenorphine treatment during the COVID-19 pandemic. J. Subst. Abus. Treat. 2021;121(2020) doi: 10.1016/j.jsat.2020.108161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel S., Yoon I.A., Hayes A.J. Substance use disorders in prisoners: an updated systematic review and meta-regression analysis in recently incarcerated men and women. Addiction. 2017;112(10):1725–1739. doi: 10.1111/add.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N., Picher C.E., Feder K.A., Saloner B. Only one in twenty justice-referred adults in specialty treatment for opioid use receive methadone or buprenorphine. Health Aff. 2017;36(12):2046–2053. doi: 10.1377/hlthaff.2017.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva, R. (2021). Public Safety Realignment Implementation-January 2021 Update. 〈http://file.lacounty.gov/SDSInter/bos/supdocs/153155.pdf〉.
- Los Angeles County Office of Diversion and Reentry. (2020). Harm Reduction and Community-Based Diversion. 〈https://dhs.lacounty.gov/office-of-diversion-and-reentry/our-services/office-of-diversion-and-reentry/harm-reduction-and-community-based-diversion/〉.
- Mauruschak, L., & Minton, T. (2020). Correctional Populations in the United States, 2017–2018. In U.S. Department of Justice NCJ 252157 (Issue August). 〈https://bjs.ojp.gov/library/publications/correctional-populations-united-states-2017–2018〉.
- Newey W.K., West K.D. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55(3):703. doi: 10.2307/1913610. [DOI] [Google Scholar]
- Sofuoglu M., DeVito E.E., Carroll K.M. Pharmacological and behavioral treatment of opioid use disorder. Psychiatr. Res. Clin. Pract. 2019;1(1):4–15. doi: 10.1176/appi.prcp.20180006. [DOI] [Google Scholar]
- StataCorp . StataCorp LLC,; 2021. Stata Statistical Software: Release 16. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2020). 2020 Report to Congress On the State Opioid Response Grants. 〈https://www.samhsa.gov/sites/default/files/grants/pdf/other/samhsa-sor-report.pdf〉.
- Weizman, S., Perez, J., Manoff, I., Baney, M., & El-Sabawi, T. (2021). A National Snapshot: Access to Medications for Opioid Use Disorder in U.S. Jails and Prisons. 〈https://oneill.law.georgetown.edu/wp-content/uploads/2021/07/National-Snapshot-Access-to-Medications-for-Opioid-Use-Disorder-in-U.S.-Jails-and-Prisons.pdf〉.