Abstract
Cigarette smoking is associated with neurocognitive dysfunction in various populations, including those seeking treatment for an alcohol use disorder (AUD). This study compared the rate and extent of recovery on measures of processing speed, executive functions, general intelligence, visuospatial skills and working memory in treatment-seeking alcohol dependent individuals (ALC) who were never-smokers (nvsALC), former-smoker (fsALC), and active smokers (asALC), over approximately 8 months of abstinence from alcohol. Methods: ALC participants were evaluated at approximately 1 month of abstinence (AP1; n=132) and reassessed after 8 months of sobriety (AP2; n=54). Never-smoking controls (CON; n=33) completed a baseline and follow-up (n=19) assessment approximately 9 months later. Domains evaluated were executive functions, general intelligence, processing speed, visuospatial skills and working memory; a domain composite was formed from the arithmetic average of the foregoing domains. nvsALC showed greater improvement than fsALC, asALC and CON on most domains over the AP1-AP2 interval. fsALC demonstrated greater recovery than asALC on all domains except visuospatial skills; fsALC also showed greater improvements than CON on general intelligence, working memory and domain composite. asALC did not show significant improvement on any domain over the AP1-AP2 interval. At 8 months of abstinence, asALC were inferior to CON and nvsALC on multiple domains, fsALC performed worse than nvsALC on several domains, but nvsALC were not different from CON on any domain. Our results provide robust evidence that smoking status influenced the rate and extent of neurocognitive recovery between 1 and 8 months of abstinence in this ALC cohort. Chronic smoking in AUD likely contributes to the considerable heterogeneity observed in neurocognitive recovery during extended abstinence. The findings provide additional strong support for the benefits of smoking cessation and the increasing clinical movement to offer smoking cessation resources concurrent with treatment for AUD.
Keywords: alcohol dependence; cigarette smoking; cognition, recovery; longitudinal
Introduction
Approximately 55% of individuals with an alcohol use disorder (AUD) exhibit clinically significant neurocognitive dysfunction following detoxification, and demonstrate variable levels of recovery with intermediate-term (i.e., 1–12 months) and long-term (i.e., >1 year) abstinence [see (Rourke & Grant, 2009) for review]. Despite recovery with sobriety, deficiencies in several neurocognitive domains may persist with extended abstinence, principally in executive functions, processing speed, learning and memory, visuospatial skills, and postural stability (Durazzo & Meyerhoff, 2007; Durazzo, Pennington, Schmidt, & Meyerhoff, 2014; Rourke & Grant, 2009; Schmidt, Pennington, Durazzo, & Meyerhoff, 2014; Stavro, Pelletier, & Potvin, 2012). Multiple biopsychosocial factors including age, sex, alcohol consumption history, number of detoxifications, nutritional status, and comorbid psychiatric, substance and biomedical disorders are associated with the magnitude of neurocognitive dysfunction apparent following detoxification, and the rate and extent of recovery during abstinence (Durazzo & Meyerhoff, 2007; Fama et al., 2019; Loeber et al., 2010; Oscar-Berman & Marinkovic, 2007; Rourke & Grant, 2009).
Chronic cigarette smoking is the most prevalent comorbidity in AUD (Daeppen et al., 2000; Durazzo & Meyerhoff, 2007; Weinberger et al., 2019). Cigarette smoking is associated with deficits in multiple neurocognitive domains in non-clinical cohorts of adolescents and adults (Durazzo, Meyerhoff, & Nixon, 2010; Sabia et al., 2012; Sabia, Marmot, Dufouil, & Singh-Manoux, 2008; Weiser, Zarka, Werbeloff, Kravitz, & Lubin, 2010) and individuals with AUD (Durazzo et al., 2010; Durazzo et al., 2013; Glass et al., 2006; Glass et al., 2009). In those seeking treatment for AUD, we found that active cigarette smoking is also related to diminished recovery in learning and memory during short-term (Pennington et al., 2013) and extended abstinence (Durazzo et al., 2014a); in these studies, actively-smoking alcohol dependent patients (asALC) showed significantly poorer recovery than never-smoking ALC (nvsALC), and former-smoking ALC (fsALC) performed intermediate to nvsALC and asALC. However, our previous AUD studies on neurocognitive recovery during abstinence following treatment were restricted to primarily measures of learning and memory and did not thoroughly examine the effects of never-smoking, former-smoking, and active-smoking status on multiple domains of neurocognitive functioning during intermediate-term abstinence (i.e., 1–12 months). This longitudinal study significantly expands on our previous research (Durazzo et al., 2014a; Pennington et al., 2013) by comparing the rate and degree of recovery in processing speed, executive skills, general intelligence, visuospatial skills and working memory among nvsALC, fsALC and asALC between approximately 1 and 8 months of sustained abstinence from alcohol. Investigation of the effect of cigarette smoking status on recovery of multiple neurocognitive domains during abstinence may assist in better delineating the critical factors that influence neurocognitive recuperation during extended sobriety in AUD (Durazzo et al., 2014; Luhar, Sawyer, Gravitz, Ruiz, Oscar-Berman, 2013; Rourke & Grant, 2009). Based on our previous and cross-sectional (Durazzo et al., 2013) findings and longitudinal results on recovery of learning and memory over 8 months of abstinence (Durazzo et al., 2014a) we hypothesized that:
Between 1 and 8 months of abstinence, asALC and fsALC demonstrate a lower rate of recovery than nvsALC in all neurocognitive domains.
In cross-sectional comparisons at 1 and 8 months of abstinence, nvsALC perform equivalent to never-smoking controls (CON), but fsALC and asALC are inferior to nvsALC and CON on all neurocognitive domains.
For asALC, greater pack-years are related to poorer recovery on all domains between 1 and 8 months of abstinence. For fsALC, longer duration of smoking cessation is associated with greater recovery in all domains.
Material and methods
Participants
ALC were recruited from the VA Medical Center (SFVAMC) Substance Abuse Day Hospital and the Kaiser Permanente Chemical Dependence Recovery Program outpatient clinics in San Francisco. Both clinics offered abstinence-based programs that employed empirically supported cognitive-behavioral interventions for AUD, with typical treatment duration of 21 days (see Durazzo et al., 2008 for specifics of ALC outpatient treatment). CON were recruited from the local community. Written informed consent was obtained from all participants before engaging in study procedures. The protocol was approved by the University of California San Francisco and SFVAMC and conformed to the Declaration of Helsinki. One hundred thirty-two ALC (33 nvsALC, 27 fsALC, and 72 asALC) were first studied after 33 ± 9 days of abstinence (assessment point 1: AP1) and 54 (14 nvsALC, 16 fsALC, and 24 asALC) of these participants were re-assessed after 232 ± 56 days of abstinence (assessment point 2: AP2). Of the 132 ALC studied at AP1, 71 relapsed between AP1 and AP2, and seven participants were lost to follow-up (e.g., moved out-of-state or unable to contact). In this study, any alcohol consumption and or substance use between AP1 and AP2 was considered a relapse. The relapse rate of participants in this study is highly consistent with other large-scale epidemiological AUD studies (see Kirshenbaum et al., 2009; Maisto, et al., 2006; Witkiewitz, 2011). fsALC smoked for 18 ± 12 years over lifetime and had not smoked for 13 ± 10 years prior to the study. Thirty-three (33) CON completed a baseline study (AP1), and 19 were re-assessed after 269 ± 40 days (AP2); the attrition for CON was due to relocation from the San Francisco Bay Area, inability to schedule time off from work, and lack of interest in follow-up. Within the ALC subgroups studied at AP1 and AP2, no significant differences were observed on demographic, clinical, and alcohol consumption variables as well as duration of abstinence. Within CON studied at AP1 and AP2, no significant differences were apparent on demographic, clinical and alcohol consumption variables. Approximately 75% of ALC and 85% of CON in this study participated in our previous longitudinal research that reported on the effects of smoking history on intermediate-term recovery of measures of learning and memory (Durazzo et al., 2014a). Table 1 provides demographic and clinical information for the 132 ALC participants and 33 CON studied at AP1.
Table 1:
AP1 Group demographics and clinical variables
| Variable | CON (n = 33) | nvsALC (n = 33) | fsALC (n = 27) | asALC (n = 72) | Group comparison* |
|---|---|---|---|---|---|
| Age (years) | 48 (10) | 51 (10) [25–71] | 54 (12) [27–69] | 49 (9) [28–68] | fsALC > CON |
| % Male | 85 | 87 | 83 | 91 | asALC > fsALC |
| % White | 80 | 87 | 86 | 75 | NS |
| Education (years) | 15.9 (1.9) [12–20] | 14.4 (2.3) [10–18] | 14.7 (2.0) [12–18] | 13.3 (1.7) [9–20] | CON > nvsALC, fsALC, asALC nvsALC, fsALC > asALC |
| AMNART | 120 (6) [101–129] | 113 (10) [95–127] | 116 (7) [104–129] | 113 (9) [91–128] | CON > nvsALC, fsALC asALC |
| Duration of Abstinence at AP1 (days) | NA | 33 (10) [16–58] | 34 (8) [17–48] | 33 (8) [16–58] | NS |
| Beck Depression Inventory score | 3 (3) [0–8] | 10 (9) [0–28] | 7 (7) [0–31] | 11 (8) [0–37] | nvsALC, fsALC, asALC > CON |
| STAI Y-2 score | 33 (2) [21–47] | 46 (11) [24–72] | 41 (12) [22–62] | 44 (8) [21–71] | nvsALC, fsALC, asALC > CON |
| 1-yr. average drinks/month | 16 (14) [0–60] | 365 (213) [99–850] | 268 (141) [92–554] | 433 (231) [102–998] | nvsALC, fsALC, asALC > CON nvsALC, asALC > fsALC |
| Lifetime average drinks/month | 16 (14) [1–54] | 185 (118) [101–532] | 146 (83) [90–352] | 264 (138) [95–906] | nvsALC, fsALC, asALC > CON nvsALC, asALC > fsALC asALC > nvsALC |
| FTND | NA | NA | NA | 5 (2) [2–10] | NA |
| Cigarette pack-years | NA | NA | 22 (16) [3–70] | 25 (18) [2–80] | NA |
| n (%) with medical comorbidity | NA | 16 (49) | 15 (56) | 33 (46) | NS |
| n (%) taking medication for medical comorbidity | NA | 16 (48) | 15 (55) | 33 (46) | NS |
| n (%) with psychiatric comorbidity | NA | 16 (47) | 13 (48) | 22 (31) | NS |
| n (%) taking medication for psychiatric comorbidity | NA | 17 (50) | 14 (52) | 37 (52) | NS |
| n (%) with substance comorbidity | NA | 11 (34) | 9 (33) | 32 (44) | NS |
mean (standard deviation) [minimum - maximum] except where indicated;
All listed group comparisons p < .05; 1-yr. average: number of alcoholic drinks per month over 1-year prior to study; AMNART: American National Adult Reading Test; Lifetime average: number of alcoholic drinks per month over lifetime; FTND: Fagerstrom Test for Nicotine Dependence; NA: not applicable, NS: no significant group differences; STAI Y-2: State-Trait Anxiety Inventory – State
Principal inclusion criteria for ALC were a current Diagnostic and Statistical Manual of Mental Disorders-4th Edition [DSM-IV; APA (Diagnostic and statistical manual of mental disorders, 1994)] diagnosis of alcohol dependence, English fluency, consumption of greater than 150 alcohol-containing drinks/month (one drink equivalent = 13.6 g pure alcohol) for at least 8 years before enrollment for males, and intake of greater than 80 drinks per month for at least 6 years prior to enrollment for females. Primary exclusion criteria for ALC and CON are fully described elsewhere (Durazzo, Gazdzinski, Banys, & Meyerhoff, 2004). In summary, participants were free of psychiatric, neurological, physical, and medical conditions known or suspected to adversely affect brain neurobiology and/or neurocognition, except for DSM-IV anxiety disorders (post-traumatic stress disorder was exclusionary), depressive disorders, hepatitis C, hypertension, and type-2 diabetes in ALC. These comorbidities were allowed in ALC because they are highly prevalent in AUD (Mertens, Weisner, Ray, Fireman, & Walsh, 2005; Moss, Chen, & Yi, 2010). Substance dependence (other than nicotine from tobacco smoking) within the 5 years preceding study enrollment was exclusionary for ALC. All participants screened negative for alcohol and illicit substances at both APs.
Clinical Assessment
At AP1, all participants completed the Structured Clinical Interview for DSM-IV-Axis I Disorders, Patient Edition, Version 2.0 (First, Spitzer, Gibbon, & Williams, 1998), and standardized questionnaires assessing depressive [Beck Depression Inventory, BDI (Beck, 1978)] and anxiety [State-Trait Anxiety Inventory, for Y-2, STAI (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1977)] symptomatology, alcohol consumption over lifetime [Lifetime Drinking History, LDH (Sobell, Sobell, Leo, & Cancilla, 1988; Sobell & Sobell, 1992)], level of nicotine dependence [Fagerstrom Tolerance Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991)]and lifetime substance use [based on the NIDA Addiction Survey (Smith, 1991)]. Average number of drinks per month over 1 year prior to enrollment and average number of drinks per month over lifetime were calculated from the LDH. At all APs for asALC, the Fagerstrom Test for Nicotine Dependence was administered, and the total number of cigarettes smoked per day and lifetime years of smoking were obtained and cigarette pack-years [(cigarettes per day/20) x years of smoking] were calculated. For fsALC at AP1, a procedure similar to the Timeline Follow-Back Interview for alcohol was modified to assess duration of smoking over lifetime and conducted for retrospective smoking history. At AP2, the Timeline Follow-Back Interview was administered to verify self-reported abstinence from alcohol and/or other substance use for all ALC participants. Additionally, electronic medical records, and/or telephone interview of collateral sources (i.e., family or friends) were used to verify the abstinence/relapse status of ALC participants. See Durazzo and colleagues (Durazzo et al., 2008) for cohort-maintenance details.
ALC with a current or history of a non-exclusionary medical condition(s) were considered positive for the medical comorbidity factor, and the most common medical comorbidities were current hypertension and hepatitis C seropositivity. ALC were considered positive for the substance use disorder comorbidity if DSM-IV criteria were met for current or lifetime substance abuse or past dependence (>5 years prior to enrollment); most ALC with a comorbid substance use disorder diagnosis met criteria for methamphetamine or cocaine abuse/dependence. ALC were considered positive for a psychiatric comorbidity if current or lifetime criteria for a depressive or anxiety disorder were met, and the majority who were positive met criteria for current substance (alcohol)-induced mood disorder, with depressive features or recurrent major depressive disorder.
Neurocognitive Assessment
Smoking ALC were allowed to smoke ad libitum before and during neurocognitive testing to reduce the potential confound of nicotine withdrawal symptoms on test performance [for review see (Sacco, Bannon, & George, 2004)]. Approximately 40 percent of asALC took one smoke break during testing and all smoked one cigarette.
All participants completed the assessment battery in a single session. Raw scores for each measure at both AP1 and AP2 were converted to z-scores based on the performance of CON at AP1. We converted raw scores to z-scores based on CON AP1 data because the AP1 sample was larger than at AP2, and, consequently, serves as a more representative normative base. Domain scores represent the arithmetic mean of z-scores of constituent measures of the domain.
The neurocognitive domains assessed and the constituent measures were as follows: The processing speed domain incorporated all tests that were timed, or those that the time to complete the task influenced the score achieved; it was calculated by averaging the individual z-scores of the following measures: Wechsler Adult Intelligence Scale 3rd Edition (WAIS-III) Arithmetic, Block Design, Digit Symbol, Picture Completion, and Symbol Search (Wechsler, 1997), Luria-Nebraska Item 99 (Golden, Hammeke, & Purisch, 1978), Stroop word, color, and color-word tests (Golden, 1978), and Trail Making Test A and B (Reitan & Wolfson, 1985). Higher scores on these measures reflect greater speed and accuracy on principally non-verbal/visuospatial tasks. Executive functions: Short Categories Test (Wetzel & Boll, 1987), Stroop Test Color-Word subtest, Trails B, Wisconsin Card Sorting Test-64: Computer Version 2-Research Edition non-perseverative errors, perseverative errors, and perseverative responses (Kongs, Thompson, Iverson, & Heaton, 2000). General intelligence: Ward-7 Full Scale IQ, based on WAIS-III Arithmetic, Block Design, Digit Symbol, Digit Span, Information, Picture Completion, and Similarities subtests (Axelrod, Ryan, & Ward, 2001). Visuospatial skills: Luria-Nebraska Item 99 and WAIS-III Block Design. Working memory: WAIS-III Arithmetic and Digit Span. A domain composite z-score was calculated from the arithmetic mean of z-scores for all individual domains. Premorbid verbal intelligence was estimated with the American National Adult Reading Test [AMNART (Grober & Sliwinski, 1991)].
Data Analyses
Cross-sectional analyses at AP1 and AP2
Comparisons among ALC subgroups and CON on clinical and demographic variables at AP1 were conducted with multivariate analysis of variance and Fisher’s Exact Test, where appropriate. Comparisons of ALC and CON on neurocognitive measures at AP1 and AP2 were conducted with generalized linear modeling with group (nvsALC, fsALC, asALC, and CON), age, education, and AMNART as predictors. Significant main effects for group (p < .05) were followed-up with pairwise t-tests. In pairwise comparisons between nvsALC, fsALC, and asALC, in addition to age, education, and AMNART, both lifetime average number of drinks/month or 1-year-average drinks/month were also separately employed as covariates due to the significant group differences on these variables. (see Table 1). Although we specified a priori predictions, we employed a modified Bonferroni procedure (Sankoh, Huque, & Dubey, 1997) adjusted significance level (p = .05) for pairwise comparisons (all two-tailed) for each neurocognitive domain. The adjusted significance level was based on the average intercorrelation among individual neurocognitive domains (excluding the domain composite) for all groups (r =.70) and the number of pairwise comparisons (n = 6) and produced an adjusted alpha p< .029 for pairwise comparisons among nvsALC, fsALC, asALC, and CON at AP1 and AP2. Effect sizes for group pairwise comparisons at AP1 and AP2 were calculated with Cohen’s d (Cohen, 1988).
Longitudinal analyses
Comparisons of neurocognitive domains changes across the AP1-AP2 interval between nvsALC, fsALC, asALC, and CON employed linear mixed modeling; models were fit with random intercepts and time (i.e., interval between AP1 and AP2) as a random factor. Main effects and interactions for all analyses were considered statistically significant at p <.05. Group (nvsALC, fsALC, asALC, CON), age, education, AMNART, and time served as predictors in all models. For longitudinal analyses specifically comparing nvsALC, fsALC, and asALC, lifetime average drinks/month or 1-year-average drinks/month were also included as predictors. Although no significant differences were observed between nvsALC, fsALC, and asALC on frequency of medical, substance and psychiatric comorbidies, in secondary analyses, these comorbidities were added as additional predictors for domains that showed differential recovery between the ALC subgroups in primary analyses. Associations between pack-years in asALC, and duration of smoking cessation in fsALC, with changes in neurocognitive domains over AP1-AP2 employed linear mixed modeling, and models were fit with random intercepts and time as a random factor. Age, education, AMNART, lifetime average drinks/month or 1-year-average drinks/month and time served as predictors in all models. Effects for pack-years and duration of smoking cessation were considered statistically significant at p <.05. All cross-sectional and longitudinal analyses were conducted with SPSS v24.
Results
Participant demographics and clinical measures
At AP1, there were no differences between nvsALC, fsALC, asALC, and CON on the percentage of males and White race. CON were younger than fsALC, and CON had more years of education and higher AMNART than all ALC subgroups (all p < .05). asALC and nvsALC had higher lifetime and 1-year-average drinks/month than fsALC, and asALC had higher lifetime average than nvsALC (all p <. 05). No differences were observed between ALC subgroups on the AMNART, BDI, STAI, and frequency of psychiatric, medical and substance use comorbidies (see Table 1).
Group cross-sectional comparisons at AP1 and AP2
AP1: fsALC and asALC were inferior to nvsALC and CON on multiple domains (all p ≤. 029), while nvsALC and CON were not different on any domain (see Table 2). AP2: Similar to AP1, fsALC and asALC were inferior to nvsALC and CON on multiple domains (all p ≤ .029), and nvsALC and CON did not differ on any domain (see Table 3). Moderate and large effect sizes were observed for significant group differences at both APs. Higher AMNART was associated with better performance, and higher age was related to poorer performance across all neurocognitive measures at AP1 and AP2 (all p < .01). Education was not a significant predictor at either AP. In analyses comparing ALC subgroups for both APs, lifetime average and 1-year average drinks per month, and medical, substance abuse and psychiatric comorbidities not significant predictors of performance on any individual domain or the domain composite.
Table 2.
AP1 Group domain z-scores
| Measure | CON (n=33) | nvsALC (n=33) | fsALC (n=27) | asALC (n=72) | Effect Size (Cohen’s d) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON vs. nvsALC | CON vs. fsALC | CON vs. asALC | nvsALC vs. fsALC | nvsALC vs. asALC | fsALC vs. asALC | |||||
| Executive functions | −0.05 (0.92) | −0.22 (0.75) | −0.46 (0.80) | −0.78 (0.83) | 0.20 | 0.51# | 0.89# | 0.31 | 0.71# | 0.39 |
| General intelligence | −0.35 (0.86) | −0.34 (0.75) | −1.05 (0.78) | −0.93 (0.85) | 0.00 | 0.86# | 0.69# | 0.73# | 0.93# | 0.14 |
| Processing speed | −0.17 (0.75) | −0.31 (0.61) | −0.69 (0.62) | −0.75 (0.69) | 0.20 | 0.76# | 0.81# | 0.62# | 0.68# | 0.09 |
| Visuospatial skills | −0.14 (0.86) | −0.42 (0.69) | −0.72 (0.68) | −0.63 (0.76) | 0.38 | 0.79# | 0.63# | 0.44 | 0.30 | 0.12 |
| Working memory | −0.35 (0.86) | −0.48 (0.75) | −0.89 (0.78) | −0.60 (0.85) | 0.15 | 0.66# | 0.28 | 0.54 | 0.15 | 0.36 |
| Domain composite | −0.18 (0.57) | −0.36 (0.57) | −0.76 (0.49) | −0.74 (0.51) | 0.26 | 1.09# | 1.03# | 0.81# | 0.76# | 0.04 |
All values presented are estimated marginal means from cross-sectional analyses.
Indicates statistically significant group difference (p ≤ .029) for two-tailed pairwise t-tests; asALC: actively-smoking alcohol dependent participants; fsALC: former-smoking alcohol dependent participants; nvsALC: never-smoking alcohol dependent participants; CON: never-smoking controls.
Table 3.
AP2 Group domain z-scores
| Measure | CON (n=19) | nvsALC (n=14) | fsALC (n=16) | asALC (n=24) | Effect Size (Cohen’s d) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON vs. nvsALC | CON vs. fsALC | CON vs. asALC | nvsALC vs. fsALC | nvsALC vs. asALC | CON vs. nvsALC | |||||
| Executive functions | 0.34 (0.81) | 0.34 (0.67) | −0.10 (0.62) | −0.82 (0.81) | 0.00 | 0.62 | 1.37# | 0.67 | 1.49# | 0.96# |
| General intelligence | 0.16 (0.94) | 0.55 (0.86) | −0.27 (0.86) | −0.68 (1.13) | 0.44 | 0.47 | 0.81# | 0.96# | 1.23# | 0.42 |
| Processing speed | −0.05 (0.82) | 0.18 (0.61) | −0.35 (0.61) | −0.78 (0.83) | 0.32 | 0.43# | 0.88# | 0.87# | 1.32# | 0.59# |
| Visuospatial skills | 0.03 (0.66) | −0.06 (0.65) | −0.63 (0.64) | −0.62 (0.67) | 0.13 | 1.01# | 0.97# | 0.88# | 0.85# | 0.01 |
| Working memory | −0.26 (0.94) | 0.02 (0.65) | −0.34 (0.77) | −0.52 (1.03) | 0.34 | 0.09 | 0.26 | 0.47 | 0.60 | 0.20 |
| Domain composite | 0.04 (0.76) | 0.21 (0.52) | −0.34 (0.54) | −0.69 (0.78) | 0.26 | 0.59# | 0.94# | 1.03# | 1.36# | 0.52 |
All values presented are estimated marginal means from cross-sectional analyses.
Indicates statistically significant group difference (p ≤ .029) for two-tailed pairwise t-tests; asALC: actively-smoking alcohol dependent participants; fsALC: former-smoking alcohol dependent participants; nvsALC: never-smoking alcohol dependent participants; CON: never-smoking controls.
Group longitudinal neurocognitive changes over AP1 and AP2 interval
Main effects and interactions:
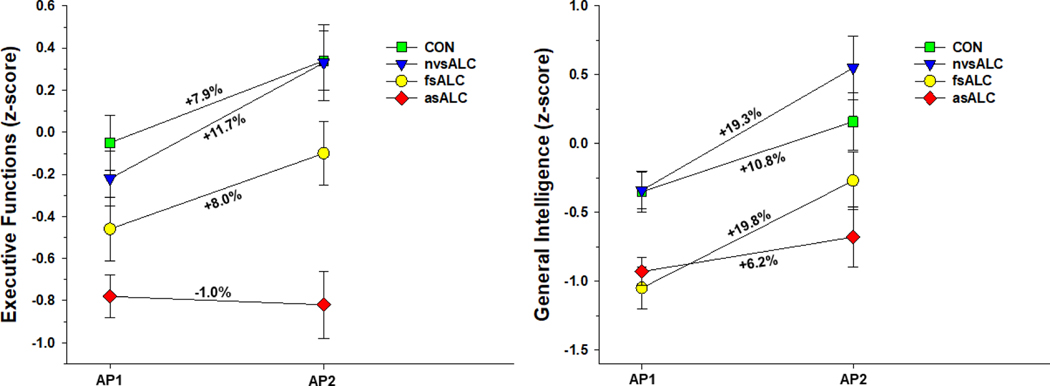
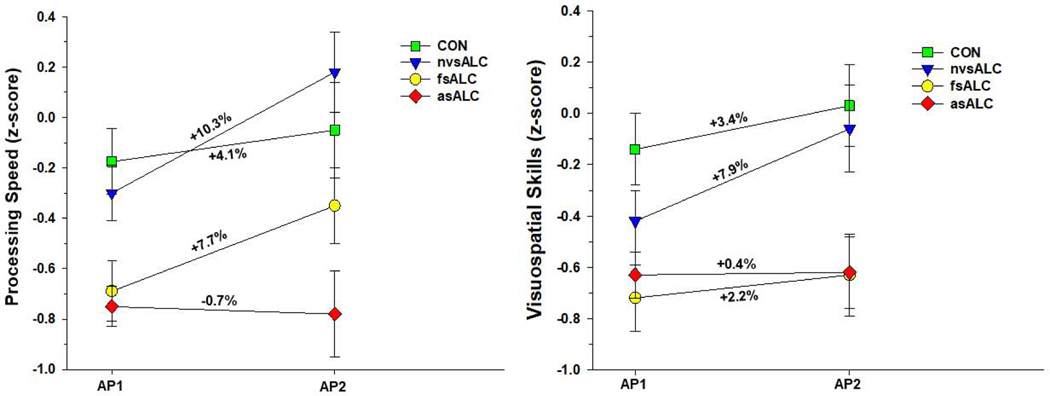
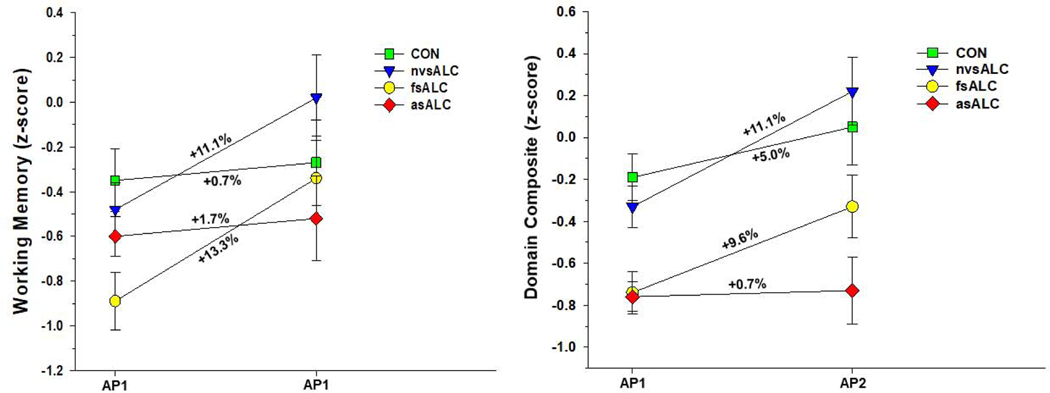
Group x time interactions were observed for processing speed [F(3, 74) = 6.18, p = .001], executive functions [F(3, 74) = 2.79, p = .047], general intelligence [F(3, 74) = 5.99, p= .001], visuospatial skills [F(3, 74) = 5.11, p = .003], working memory [F(3, 74) = 5.97, p = .001], and domain composite [F(3, 74) = 9.67, p < .001] (see Figure 1). No other significant interactions were observed. Significant main effects for AMNART and age were observed for all domains (all p < .01), with higher AMNART associated with better performance, and greater age related to poorer performance. Education did not predict any domain (all p >.30). In analyses for ALC subgroups, lifetime average, 1-year average drinks per month, and medical, substance abuse and psychiatric comorbidities were not associated with change for individual domains or the domain composite (all p > .35). nvsALC showed greater improvement than asALC and CON on all individual domains and the domain composite over the AP1-AP2 interval (all p < .05), and nvsALC exhibited greater improvement than fsALC on processing speed, executive functions, and visuospatial skills and domain composite (all p < .05). fsALC demonstrated greater recovery than asALC on processing speed, executive functions, general intelligence, working memory and domain composite, and fsALC showed greater improvement than CON on general intelligence, working memory and domain composite (all p < .05). CON showed greater improvement than asALC on processing speed, executive functions, and domain composite (p < .05).
Figure 1.
Figure 1a. Group performance on executive function and general intelligence domains over the assessment point 1 and 2 interval. Numbers listed adjacent to slopes indicate percent change between assessment points.
Figure 1b. Group performance on processing speed and visuospatial skills domains over the assessment point 1 and 2 interval. Numbers listed adjacent to slopes indicate percent change between assessment points.
Figure 1c. Group performance on working memory domain and domain composite over the assessment point 1 and 2 interval. Numbers listed adjacent to slopes indicate percent change between assessment points.
Simple effect tests:
Over the AP1-AP2 interval, nvsALC demonstrated significant improvement on all individual domains and the domain composite (p < .01). fsALC significantly improved on processing speed, executive functions, general intelligence, working memory and domain composite (p < .05). asALC did not show significant change on any individual domain or domain composite. CON demonstrated significant improvement on processing speed, executive functions, general intelligence, and domain composite (p < .05).
Relationships of smoking severity measures and neurocognitive recovery in asALC and fsALC
asALC:
Greater cigarette-pack years were associated with poorer recovery over the AP1-AP2 interval for processing speed [β = −.018, standard error (SE) = .006, p = .007], and domain composite (β = −.013, SE = .007, p = .044).
fsALC:
Longer duration of smoking cessation was associated with greater recovery over the AP1-AP2 interval for processing speed (β = .050, SE = .018, p = .011), visuospatial skills (β = .042, SE =.017, p = .023), working memory (β = .052, SE =.024, p = .047) and domain composite (β = .046, SE =.018, p = .016) .
4. Discussion
In this ALC cohort, smoking status robustly moderated recovery over approximately 8 months of abstinence on measures of processing speed, executive functions, general intelligence, visuospatial skills and working memory. The greater longitudinal improvements in nvsALC and fsALC compared to asALC and CON were not attributable to age, education, or estimated premorbid verbal intelligence. Alcohol consumption and comorbid medical, substance abuse and psychiatric conditions were not associated with change on any domain in the ALC subgroups. The improvements demonstrated by nvsALC on all domains and by fsALC on general intelligence, working memory and domain composite were significantly greater than demonstrated by CON, indicating the recovery in nvsALC and fsALC exceeded any practice effects for the constituent measures of each domain. Notably, asALC demonstrated no significant improvement on any individual domain or the domain composite and both nvsALC and fsALC showed greater recovery than asALC on processing speed, executive functions, general intelligence, working memory and domain composite. The significant improvements observed for nvsALC, fsALC and CON on general intelligence were largely driven by increases in non-verbal tasks (data not shown). nvsALC and fsALC both showed the greatest change on the processing speed, executive functions, general intelligence and working memory domains. Most measures that comprise the foregoing domains are non-verbal tasks that require efficiency (i.e., both speed and accuracy) for a strong performance. The recovery pattern for the ALC subgroups over the AP1-AP2 interval on the domains evaluated in this report was highly similar to that observed for a similar cohort on measures of auditory-verbal and visuospatial learning and memory (Durazzo et al., 2014a).
At AP1 (approximately 30 days of abstinence), nvsALC was not significantly different from CON on any neurocognitive domain, asALC performed more poorly than CON on all domains except working memory, and fsALC were inferior to CON on all domains. Despite the significant improvement of fsALC on processing speed, executive functions, general intelligence, and domain composite over the AP1-AP2 interval, fsALC remained inferior to nvsALC on these domains at AP2. Given asALC demonstrated no significant improvement on any domain over AP1-AP2, the pattern of differences between groups at AP1 was largely consistent with AP2; however, the magnitude of the inferior performance of asALC relative to the other groups was larger at AP2, because of the significant improvement of CON, nvsALC and fsALC on multiple domains. The integrity of neurocognitive function during treatment (Bates, Buckman, & Nguyen, 2013; Durazzo et al., 2008; Tapert, Ozyurt, Myers, & Brown, 2004) and subsequent changes with abstinence may inform treatment interventions that address neurocognitive dysfunction to improve treatment outcome (Nixon & Lewis, 2019).
In asALC, greater pack-years were associated with poor recovery of processing speed and domain composite, indicating an adverse dose/duration response relationship between cigarette exposure and improvement on these domains. In our previous study, we found similar associations with measures of smoking severity and recovery of auditory-verbal and visuospatial learning and memory over a comparable period of abstinence (Durazzo et al., 2014a). In fsALC, longer duration of smoking cessation was related to greater recovery on processing speed, visuospatial skills, working memory and domain composite. In addition to the significant recovery of fsALC on multiple domains over the AP1-AP2 interval, the foregoing associations support the benefits of smoking cessation on neurocognitive recovery in AUD.
In this study, smoking status and smoking severity measures, but not alcohol consumption variables, were related to neurocognitive change in this group of abstinent ALC. Despite chronic hazardous drinking and meeting criteria for alcohol dependence, nvsALC were not significantly different from CON on any domain at AP1 or AP2. Several previous cross-sectional and longitudinal AUD studies reported weak or non-significant associations of alcohol consumption level and medical, psychiatric and substance misuse comorbidities with neurocognition [see (Durazzo et al., 2014a; Durazzo et al., 2013; Pennington et al., 2013) and references therein]. The lack of neurocognitive differences between nvsALC and CON at AP1 and AP2 and the significantly greater improvement of nvsALC relative to CON on all domains indicate chronic smoking in this ALC cohort was a significant moderator of change of domains assessed. Given that chronic smoking is associated with multiple neurobiological abnormalities in “healthy” non-AUD samples (Durazzo et al., 2014b; Durazzo et al., 2016; Durazzo, Meyerhoff, & Murray, 2015; Durazzo, Meyerhoff, & Yoder, 2018; Durazzo, Meyerhoff, Yoder, & Murray, 2017; Duriez, Crivello, & Mazoyer, 2014; Franklin et al., 2014; Fritz et al., 2014; Gallinat & Schubert, 2007; Gons et al., 2011; Hanlon et al., 2016; Kuhn et al., 2012; Schubert, Seifert, Bajbouj, & Gallinat, 2006; Stoeckel, Chai, Zhang, Whitfield-Gabrieli, & Evins, 2016; Sutherland et al., 2016; Zanchi et al., 2015), nvsALC may possess greater neurobiological and neurocognitive resiliency to the adverse consequences of AUD, compared to fsALC and asALC. In addition to chronic cigarette smoking, it is possible that premorbid factors (e.g., genetic risk or resiliency factors), or comorbid factors not assessed in this study [e.g., diet/nutrition, exercise, and subclinical hepatic, pulmonary, cardiac, or cerebrovascular dysfunction (Durazzo, Hutchison, Fryer, Mon, & Meyerhoff, 2012; Fama et al., 2019; Hoefer et al., 2014; Mon et al., 2013; Tessner & Hill, 2010; Zahr, Kaufman, & Harper, 2011; Zahr et al., 2016)] influenced neurocognition in this ALC cohort. Any neurocognitive and neurobiological abnormalities observed in those with AUD following detoxification or after an extended period of abstinence are related to multiple premorbid and/or concurrent biopsychosocial factors, and not solely attributable to chronic and excessive alcohol consumption. See Durazzo and colleagues (Durazzo, Mattsson, Weiner, & Alzheimer Disease Neuroimaging Initiative, 2014) for discussion of potential smoking-related biological mechanisms contributing to the differential neurocognitive recovery demonstrated by the ALC subgroups.
This study has limitations that may influence the generalizability of the results. The ALC participants were comprised largely of US Armed Services Veterans and participant self-report was primarily used to determine post-treatment drinking status. The neurocognitive domains used in this study were based on commonly used clinical organization rather than statistical dimension reduction (e.g., principal component analysis). Therefore, the reported findings may not be congruent with similar research that employed traditional dimension reduction methods to form neurocognitive domains. The small number of female participants precluded examination of sex effects. It is imperative to include larger numbers of females, particularly Veteran females, in future research to investigate the potential influence of sex. Finally, despite all critical model assumptions being met in all analyses, the modest number of observations at AP2 increased the risk of model over-fitting and corresponding Type I error in both cross-sectional and longitudinal analyses.
Smoking status in this ALC cohort significantly moderated recovery in multiple neurocognitive domains between 1 and 8 months of abstinence; nvsALC showed significant improvement on all domains, fsALC improved on five of seven domains, and asALC demonstrated no significant recovery on any domain. Similar to our previous studies focusing on changes in learning and memory (Durazzo et al., 2014a; Pennington et al., 2013), failure to consider the influence of the common comorbidity of cigarette smoking in this ALC cohort would have yielded incomplete results and spurious conclusions regarding the rate and extent of longitudinal change on the domains assessed in this study. Overall, the results suggest that a history of chronic smoking in AUD contributes to the considerable heterogeneity in neurocognitive recovery during extended abstinence from alcohol. Our results also strongly reinforce the benefits of smoking cessation and the expanding clinical movement to offer smoking cessation resources concurrent with treatment for AUD.
AUD active-smokers showed no significant neurocognitive recovery.
AUD never-smokers showed the greatest neurocognitive recovery.
AUD former-smokers showed intermediate neurocognitive recovery.
Smoking in AUD may contribute to heterogeneity in neurocognitive recovery.
Results also strongly reinforce the benefits of smoking cessation in AUD.
Acknowledgements
This work was supported by grants from the National Institutes of Health (DA24136 to TCD and AA10788 to DJM) administered by the Northern California Institute for Research and Education, and through use of resources and facilities at the San Francisco Veterans Administration (VA) Medical Center and VA Palo Alto Health Care System. The study sponsors/supporters (National Institutes of Health and Department of Veteran Affairs) had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Timothy C. Durazzo: Conceptualization, Methodology, Formal Analyses, Writing - Original Draft, Resources, Data Curation, Supervision, Funding Acquisition. Dieter J. Meyerhoff: Conceptualization, Writing-Review and Editing, Supervision, Project Administration, Funding Acquisition.
Declarations of interest: none
The authors have no disclosures and no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association, 1994. Diagnostic and statistical manual of mental disorders. American Psychiatric Association, Washington, D.C. [Google Scholar]
- Axelrod BN, Ryan JJ, & Ward LC (2001). Evaluation of seven-subtest short forms of the Wechsler Adult Intelligence Scale-III in a referred sample. Arch Clin Neuropsychol, 16(1), 1–8. [PubMed] [Google Scholar]
- Bates ME, Buckman JF, & Nguyen TT (2013). A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol Rev, 23(1), 27–47. doi: 10.1007/s11065-013-9228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT (1978). Depression Inventory. Philadelphia: Center for Cognitive Therapy. [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Daeppen JB, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI Jr., … Schuckit MA. (2000). Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. The Collaborative Study Group on the Genetics of Alcoholism. Alcohol Alcohol, 35(2), 171–175. [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders. (1994). (4th ed.). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Durazzo T, Hutchison K, Fryer S, Mon A, & Meyerhoff D. (2012). Associations of Cigarette Smoking and Polymorphisms in Brain-Derived Neurotrophic Factor and Catechol-O-Methyltransferase with Neurocognition in Alcohol Dependent Individuals during Early Abstinence. Front Pharmacol, 3(178). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Fryer SL, Rothlind JC, Vertinski M, Gazdzinski S, Mon A, & Meyerhoff DJ (2010). Measures of Learning, Memory and Processing Speed Accurately Predict Smoking Status in Short-term Abstinent Treatment-seeking Alcohol-dependent Individuals. Alcohol Alcohol, 45(6), 507–513. doi:agq057 [pii] 10.1093/alcalc/agq057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, & Meyerhoff DJ (2004). Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res, 28(12), 1849–1860. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Yeh PH, & Meyerhoff DJ (2008). Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol and Alcoholism, 43(6), 683–691. doi:agn078 [pii] 10.1093/alcalc/agn078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW, & Alzheimer Disease Neuroimaging Initiative (2014). [Google Scholar]
- Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimer’s & Dementia, 10(3), S122–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW, Korecka M, Trojanowski JQ, & Shaw LM (2014b). History of cigarette smoking in cognitively-normal elders is associated with elevated cerebrospinal fluid biomarkers of oxidative stress. Drug Alcohol Depend, 142, 262–268. doi:S0376–8716(14)00957–0 [pii] 10.1016/j.drugalcdep.2014.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, & Meyerhoff DJ (2007). Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci, 12, 4079–4100. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Mon A, Abe C, Gazdzinski S, & Murray DE (2016). Chronic Cigarette Smoking in Healthy Middle-Aged Individuals Is Associated With Decreased Regional Brain N-acetylaspartate and Glutamate Levels. Biol Psychiatry, 79(6), 481–488. doi: 10.1016/j.biopsych.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, & Murray DE (2015). Comparison of Regional Brain Perfusion Levels in Chronically Smoking and Non-Smoking Adults. Int J Environ Res Public Health, 12(7), 8198–8213. doi: 10.3390/ijerph120708198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, & Nixon SJ (2010). Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health, 7(10), 3760–3791. doi: 10.3390/ijerph7103760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, & Yoder KK (2018). Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer’s Disease. Drug Alcohol Depend, 192, 277–284. doi: 10.1016/j.drugalcdep.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Yoder KK, & Murray DE (2017). Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug Alcohol Depend, 177, 228–236. doi: 10.1016/j.drugalcdep.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pennington DL, Schmidt TP, & Meyerhoff DJ (2014. a). Effects of cigarette smoking history on neurocognitive recovery over 8 months of abstinence in alcohol-dependent individuals. Alcohol Clin Exp Res, 38(11), 2816–2825. doi: 10.1111/acer.12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pennington DL, Schmidt TP, Mon A, Abe C, & Meyerhoff DJ (2013). Neurocognition in 1-Month-Abstinent Treatment-Seeking Alcohol-Dependent Individuals: Interactive Effects of Age and Chronic Cigarette Smoking. Alcohol Clin Exp Res. doi: 10.1111/acer.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriez Q, Crivello F, & Mazoyer B. (2014). Sex-related and tissue-specific effects of tobacco smoking on brain atrophy: assessment in a large longitudinal cohort of healthy elderly. Front Aging Neurosci, 6, 299. doi: 10.3389/fnagi.2014.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Le Berre AP, Hardcastle C, Sassoon SA, Pfefferbaum A, Sullivan EV, & Zahr NM (2019). Neurological, nutritional and alcohol consumption factors underlie cognitive and motor deficits in chronic alcoholism. Addict Biol, 24(2), 290–302. doi: 10.1111/adb.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1998). Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 revision). New York, NY: Biometrics Research Department. [Google Scholar]
- Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, … Childress AR (2014). The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One, 9(8), e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, … Lotze M. (2014). Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology, 39(11), 2594–2600. doi: 10.1038/npp.2014.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J., & Schubert F. (2007). Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry, 40(2), 64–67. [DOI] [PubMed] [Google Scholar]
- Glass JM, Adams KM, Nigg JT, Wong MM, Puttler LI, Buu A, … Zucker RA (2006). Smoking is associated with neurocognitive deficits in alcoholism. Drug Alcohol Depend, 82(2), 119–126. doi:S0376–8716(05)00261–9 [pii] 10.1016/j.drugalcdep.2005.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JM, Buu A, Adams KM, Nigg JT, Puttler LI, Jester JM, & Zucker RA (2009). Effects of alcoholism severity and smoking on executive neurocognitive function. Addiction, 104(1), 38–48. doi:ADD2415 [pii] 10.1111/j.1360-0443.2008.02415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ (1978). Stroop Color and Word Test. Chicago, IL: Stoelting Company. [Google Scholar]
- Golden CJ, Hammeke TA, & Purisch AD (1978). Diagnostic validity of a standardized neuropsychological battery derived from Luria’s neuropsychological tests. J Consult Clin Psychol, 46(6), 1258–1265. [PubMed] [Google Scholar]
- Gons RA, van Norden AG, de Laat KF, van Oudheusden LJ, van Uden IW, Zwiers MP, … de Leeuw FE (2011). Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain, 134(Pt 7), 2116–2124. doi:awr145 [pii] 10.1093/brain/awr145 [DOI] [PubMed] [Google Scholar]
- Grober E, & Sliwinski M. (1991). Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol, 13(6), 933–949. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, & Hartwell KJ (2016). Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol, 21(1), 185–195. doi: 10.1111/adb.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hoefer ME, Pennington DL, Durazzo TC, Mon A, Abe C, Truran D, … Meyerhoff DJ (2014). Genetic and behavioral determinants of hippocampal volume recovery during abstinence from alcohol. Alcohol, 48(7), 631–638. doi: 10.1016/j.alcohol.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Olsen DM, Bicke l W.K. (2009). A quantitative review of the ubiquitous relapse curve. J Subst Abuse Treat, 36, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongs S, Thompson L, Iverson G, & Heaton RK (2000). WCST-64: Wisonsin Card Sorting Test-64 Card Version, Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Kuhn S, Romanowski A, Schilling C, Mobascher A, Warbrick T, Winterer G, & Gallinat J. (2012). Brain grey matter deficits in smokers: focus on the cerebellum. Brain Struct Funct, 217(2), 517–522. doi: 10.1007/s00429-011-0346-5 [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel Marquez H, Nakovics H, Heinz A, Mann K, & Flor H. (2010). Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol, 45(6), 541–547. doi: 10.1093/alcalc/agq065 [DOI] [PubMed] [Google Scholar]
- Luhar RB, Sawyer KS, Gravitz Z, Ruiz SM, Oscar-Berman M. (2013). Brain volumes and neuropsychological performance are related to current smoking and alcoholism history. Neuropsychiatr Dis Treat, 9, 1767–84. doi: 10.2147/NDT.S52298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Clifford PR, Stout RL, Davis CM (2006) Drinking in the year after treatment as a predictor of three-year drinking outcomes. J Stud Alcohol, 67, 823–832. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, & Walsh K. (2005). Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin Exp Res, 29(6), 989–998. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Hutchison KE, Pennington D, & Meyerhoff DJ (2013). Brain-derived Neurotrophic Factor (BDNF) Genotype is Associated with Lobar Gray and White Matter Volume Recovery in Abstinent Alcohol Dependent Individuals. Genes Brain Behav, 12(1), 98–107. doi: 10.1111/j.1601-183X.2012.00854.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, & Yi HY (2010). Prospective follow-up of empirically derived Alcohol Dependence subtypes in wave 2 of the National Epidemiologic Survey on Alcohol And Related Conditions (NESARC): recovery status, alcohol use disorders and diagnostic criteria, alcohol consumption behavior, health status, and treatment seeking. Alcohol Clin Exp Res, 34(6), 1073–1083. doi:ACER1183 [pii] 10.1111/j.1530-0277.2010.01183.x [DOI] [PubMed] [Google Scholar]
- Nixon SJ, & Lewis B. (2019). Cognitive training as a component of treatment of alcohol use disorder: A review. Neuropsychology, 33(6), 822–841. doi: 10.1037/neu0000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, & Marinkovic K. (2007). Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review, 17(3), 239–257. doi: 10.1007/s11065-007-9038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt TP, Mon A, Abe C, & Meyerhoff DJ (2013). The effects of chronic cigarette smoking on cognitive recovery during early abstinence from alcohol. Alcohol Clin Exp Res, 37(7), 1220–1227. doi: 10.1111/acer.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D. (1985). The Halstead-Reitan Neuropsychological Test Battery: Theory and Interpetation. Tucson, AZ: Neuropsychological Press. [Google Scholar]
- Rourke SB, & Grant I. (2009). The Neurobehavior Correlates of Alcoholism. In Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders (Third ed., pp. 398–454). New York, NY: Oxford University Press. [Google Scholar]
- Sabia S, Elbaz A, Dugravot A, Head J, Shipley M, Hagger-Johnson G, … Singh-Manoux A. (2012). Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch Gen Psychiatry, 69(6), 627–635. doi: 10.1001/archgenpsychiatry.2011.2016archgenpsychiatry.2011.2016 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, Marmot M, Dufouil C, & Singh-Manoux A. (2008). Smoking history and cognitive function in middle age from the Whitehall II study. Arch Intern Med, 168(11), 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, & George TP (2004). Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol, 18(4), 457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, & Dubey SD (1997). Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med, 16(22), 2529–2542. doi: [pii] [DOI] [PubMed] [Google Scholar]
- Schmidt TP, Pennington DL, Durazzo TC, & Meyerhoff DJ (2014). Postural stability in cigarette smokers and during abstinence from alcohol. Alcohol Clin Exp Res, 38(6), 1753–1760. doi: 10.1111/acer.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert F, Seifert F, Bajbouj M, & Gallinat J. (2006). Proton MRS at 3 tesla reveals altered neurochemistry in smokers. Paper presented at the Proc. Intl. Soc. Mag. Reson. Med. 14. [Google Scholar]
- Smith SS (1991). Addictive Drug Survey Manual. Baltimore, MD: NIDA Addiction Research Center. [Google Scholar]
- Sobell L, Sobell M, Leo G, & Cancilla A. (1988). Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Addiction, 83(4), 393–402. [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In Litten R. & Allen J. (Eds.), Measuring Alcohol Consumption (pp. 41–72): The Humana Press Inc. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1977). Self-Evaluation Questionaire. [Google Scholar]
- Stavro K, Pelletier J, & Potvin S. (2012). Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. doi: 10.1111/j.1369-1600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Chai XJ, Zhang J, Whitfield-Gabrieli S, & Evins AE (2016). Lower gray matter density and functional connectivity in the anterior insula in smokers compared with never smokers. Addict Biol, 21(4), 972–981. doi: 10.1111/adb.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA, & Laird AR (2016). Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct, 12(1), 16. doi: 10.1186/s12993-016-0100-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Ozyurt SS, Myers MG, & Brown SA (2004). Neurocognitive ability in adults coping with alcohol and drug relapse temptations. Am J Drug Alcohol Abuse, 30(2), 445–460. [DOI] [PubMed] [Google Scholar]
- Tessner KD, & Hill SY (2010). Neural circuitry associated with risk for alcohol use disorders. Neuropsychol Rev, 20(1), 1–20. doi: 10.1007/s11065-009-9111-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1997). The Wechsler Adult Intelligence Scale. 3rd ed. The Psychological Corporation; (San Antonio, TX: ). [Google Scholar]
- Weinberger AH, Pacek LR, Giovenco D, Galea S, Zvolensky MJ, Gbedemah M, & Goodwin RD (2019). Cigarette Use Among Individuals with Alcohol Use Disorders in the United States, 2002 to 2016: Trends Overall and by Race/Ethnicity. Alcohol Clin Exp Res, 43(1), 79–90. doi: 10.1111/acer.13922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Zarka S, Werbeloff N, Kravitz E, & Lubin G. (2010). Cognitive test scores in male adolescent cigarette smokers compared to non-smokers: a population-based study. Addiction, 105(2), 358–363. doi:ADD2740 [pii] 10.1111/j.1360-0443.2009.02740.x [DOI] [PubMed] [Google Scholar]
- Wetzel L, & Boll TJ (1987). Short Category Test, Booklet Format. Los Angeles: Western Psychological Services. [Google Scholar]
- Witkiewitz K. (2011) Predictors of heavy drinking during and following treatment. Psychol Addict Behav. (25) 426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Kaufman KL, & Harper CG (2011). Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol, 7(5), 284–294. doi:nrneurol.2011.42 [pii] 10.1038/nrneurol.2011.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Sullivan EV, Rohlfing T, Mayer D, Collins AM, Luong R, & Pfefferbaum A. (2016). Concomitants of alcoholism: differential effects of thiamine deficiency, liver damage, and food deprivation on the rat brain in vivo. Psychopharmacology (Berl), 233(14), 2675–2686. doi: 10.1007/s00213-016-4313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi D, Brody AL, Montandon ML, Kopel R, Emmert K, Preti MG, … Haller S. (2015). Cigarette smoking leads to persistent and dose-dependent alterations of brain activity and connectivity in anterior insula and anterior cingulate. Addict Biol, 20(6), 1033–1041. doi: 10.1111/adb.12292 [DOI] [PubMed] [Google Scholar]