Abstract
Purpose
To determine the role tear lymphotoxin-α (LT-α) in chronic ocular graft-versus-host disease (oGVHD).
Methods
Twenty-two chronic oGVHD and 17 control tear samples were collected, and commercial test strips were used to detect LT-α concentrations. Concentration differences between patients with and without oGVHD were determined via Mann-Whitney U test. The correlation between LT-α levels and ophthalmic parameters was analyzed using Spearman’s test.
Results
The concentration of LT-α was significantly lower in oGVHD patients than in controls. LT-α levels were significantly correlated with OSDI, NIH eye score, T-BUT, and CFS among all participants. ROC analysis revealed that the area under the curve of LT-α was 0.847, and the cutoff value for chronic oGVHD diagnosis was 0.203 ng/mL.
Conclusion
Our study revealed the significant decrease of tear LT-α in oGVHD, and suggested LT-α as a promising marker for chronic oGVHD diagnosis.
Keywords: Biomarker, Lymphotoxin-α, Ocular graft-versus-host disease, Tear film
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a major therapy for many patients with hematological malignances [1]. However, chronic graft-versus-host disease (GVHD) represents a common complication of allo-HSCT, affecting 30–70% of human leukocyte antigen-matched recipients [2, 3]. As an immune disease involving tissue inflammation and fibrosis, chronic GVHD may lead to permanent dysfunction in multiple organs. Notably, up to 60–90% of chronic GVHD patients suffer from ocular GVHD (oGVHD) [4], characterized by new-onset dry eyes, keratoconjunctivitis sicca, cicatricial conjunctivitis, and confluent areas of punctate keratopathy [5]. Recently, we and others have reported the significant elevation of the levels of several proinflammatory cytokines (such as IL-1β, IL-2, IL-6, IL-8 IL-7, IL-10, IFN-γ and TNF-α) in chronic oGVHD tear films [6–9], suggesting a correlation between elevated proinflammatory cytokine levels and increased inflammation in oGVHD pathogenesis.
Lymphotoxin-alpha (LT-α, formerly named TNF-β) is a TNF superfamily cytokine that plays a specific role in the development and orchestration of immune responses [10]. The expression of LT-α is restricted to activated CD4+ Th1 and Th17 subsets, CD8+ T cells, B cells, and natural killer cells [11–13], all of which are closely implicated in GVHD. Increasing evidence supports that LT-α is vital in systemic GVHD pathogenesis. Markey KA and colleagues used multiple preclinical GVHD models and suggested that LT-α is an important contributor to GVHD [14]. Chiang EY et al. reported that LT-α expression was upregulated in activated human donor lymphocytes and that targeted depletion of these donor cells could ameliorate GVHD [15]. These studies revealed the crucial effect of LT-α in GVHD pathogenesis and suggested the potential application of LT-α antagonists for GVHD treatment; however, the role of LT-α in oGVHD remains unclear.
To reveal the role of LT-α in oGVHD, we recruited HSCT recipients with and without chronic oGVHD and determined LT-α levels in their fresh tears using colloidal gold immunochromatography strips. We then calculated the correlation of LT-α levels with ocular surface parameters and further evaluated the diagnostic value of tear LT-α for oGVHD.
Materials and methods
Ethics and clinical subjects
This study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee and performed in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all the subjects after explaining the nature and possible consequences of the study.
Chronic oGVHD patients were diagnosed in accordance with the National Institutes of Health (NIH) consensus [5], and inclusion criteria were set as we previously described [16]: (1) NIH eye score ≥ 1 point; (2) ocular surface disease index (OSDI) score > 12 points; (3) corneal fluorescein staining (CFS) ≥ 1; and (4) Schirmer’s test without topical anesthesia ≤5 mm in 5 min. Exclusion criteria included ocular surgery within the past 6 months, ocular injury, other ocular diseases (such as infection, allergy, glaucoma, retinopathy, and autoimmune disease), pregnancy, and long-term use of any topical ocular medications.
Tear collection and LT-α analysis
Tear collection was performed as we previously described [16]. First, 30 μL of a sterile normal saline solution was dropped into the conjunctival sac, and then diluted tear samples were collected with a capillary tear collector. To immediately determine the tear LT-α concentration, a commercial test strip based on a colloidal gold and immunochromatography assay (S02A, Seinda Biomedical Corporation, Guangdong, China) was utilized following the instructions. Briefly, a 2.2 μL tear sample was added to the sampling hole, three drops of the diluent were added to the diluting hole, the strip was then inserted into the analyzer (S03A, Seinda Biomedical Corporation), and the LT-α level was registered 15 min later.
Clinical evaluation
Clinical examinations were always performed by the same clinician. First, the OSDI questionnaire was administered to assess ocular symptoms over the preceding week. Then, tear samples were collected, and LT-α levels were immediately determined as we described above. Next, best corrected visual acuity and intraocular pressure were detected; fluorescein tear film break-up time (T-BUT), CFS score and NIH eye score were then evaluated in the clinic. Finally, Schirmer’s test without anesthesia was conducted using the commercial strips.
Statistical analysis
Statistical analysis and graphing were performed via GraphPad Prism 8.0 software (GraphPad Software, Inc., San Diego, CA, USA). The normality assumption was validated with the Shapiro-Wilk test. For quantitative variables, normally distributed data were analyzed with Student’s t-test and presented as the mean ± standard deviation; nonnormally distributed data were analyzed with the Mann-Whitney U test and presented as the median ± interquartile range (IQR). For qualitative data, the chi-square test was used to assess the association. Spearman’s test was used to analyze the correlation of the LT-α concentration with ocular surface parameters. The results were considered statistically significant if the P value was less than 0.05.
Results
Demographic and clinical details of the participants
Clinical and demographic characteristics are presented in Table 1. Statistical analysis confirmed that there were no significant differences in age (P = 0.173) or sex (P = 0.092) between patients with and without oGVHD.
Table 1.
Patient characteristics
| Characteristics | oGVHD (n=22) | Control (n=17) | P value |
|---|---|---|---|
| Age, years1 | 31.52 ± 9.63 | 26.86 ± 9.86 | 0.173 |
| Gender2 | 0.092 | ||
| Male, n (%) | 11 (50.0%) | 13 (76.47%) | |
| Female, n (%) | 11 (50.0%) | 4 (23.53%) | |
| Hematologic diagnosis, n (%) | 0.711 | ||
| AML (acute myelogenous leukemia) | 15 (68.2%) | 10 (58.8%) | |
| ALL (acute lymphoblastic leukemia) | 4 (18.2%) | 5 (29.4%) | |
| MDS (myelodysplastic syndromes) | 3 (13.6%) | 2 (11.8%) | |
| HLA matching | 0.291 | ||
| Haploidentical | 18 (81.8%) | 15 (88.2%) | |
| Non-identical | 0 | 1 (5.9%) | |
| Identical | 4 (18.2%) | 1 (5.9%) | |
| Other current organ involvement in cGVHD | 0.073 | ||
| Gastrointestinal tract | 2 (9.1%) | 2 (11.8%) | |
| Lung | 4 (18.2%) | 1 (6.2%) | |
| Liver | 9 (40.9%) | 0 | |
| Oral cavity | 19 (86.4%) | 1 (6.2%) | |
| Skin | 20 (90.9%) | 7 (41.2%) | |
| Current systemic immunosuppressive treatment | 0.827 | ||
| None | 6 (27.3%) | 5 (29.4%) | |
| Calcineurin inhibitor | 4 (18.2%) | 3 (17.6%) | |
| Steroid | 5 (22.7%) | 2 (11.8%) | |
| Calcineurin inhibitor + steroid | 7 (31.8%) | 7 (41.2%) | |
| Period after HSCT, months | 19 ± 19 | 15 ± 11 | 0.084 |
Mean values ± SD, median ± IQR, or percentages were shown as above. There were no significant differences in age1 (P = 0.173) or gender2 (P = 0.092) between oGVHD patients (n=22) and controls (n=17)
Ocular details were summarized in Table 2. There were significant differences in OSDI score, NIH eye score, CFS, T-BUT, and Schirmer’s test between oGVHD patients and the controls (P < 0.0001 for all), and the best corrected visual acuity of oGVHD patients was significantly poorer than that of the controls (P = 0.035).
Table 2.
Ocular parameters of patients with and without oGVHD
| Ocular parameters | oGVHD (n=22) | Control (n=17) | P value |
|---|---|---|---|
| OSDI score | 37.25 ± 20.81 | 1.60 ± 1.58 | <0.0001**** |
| NIH eye score | 2 ± 2 | 0 ± 0 | <0.0001**** |
| Corneal fluorescein score | 10 ± 7.75 | 0 ± 0 | <0.0001**** |
| Fluorescein tear film break-up time (s) | 2 ± 1.25 | 10 ± 2 | <0.0001**** |
| Schirmer’s tear secretion score (mm) | 1 ± 2.5 | 16.5 ± 8.5 | <0.0001**** |
| Best corrected visual acuity (logMAR) | 0.43 ± 0.41 | 0.19 ± 0.22 | 0.035* |
| Intraocular pressure (mmHg) | 13.0 ± 3.4 | 15.1 ± 4.2 | 0.163 |
Ocular parameters were compared between patients with (n=22) and without (n=17) oGVHD. Data were presented as mean ± SD or median ± IQR. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
Tear LT-α concentration
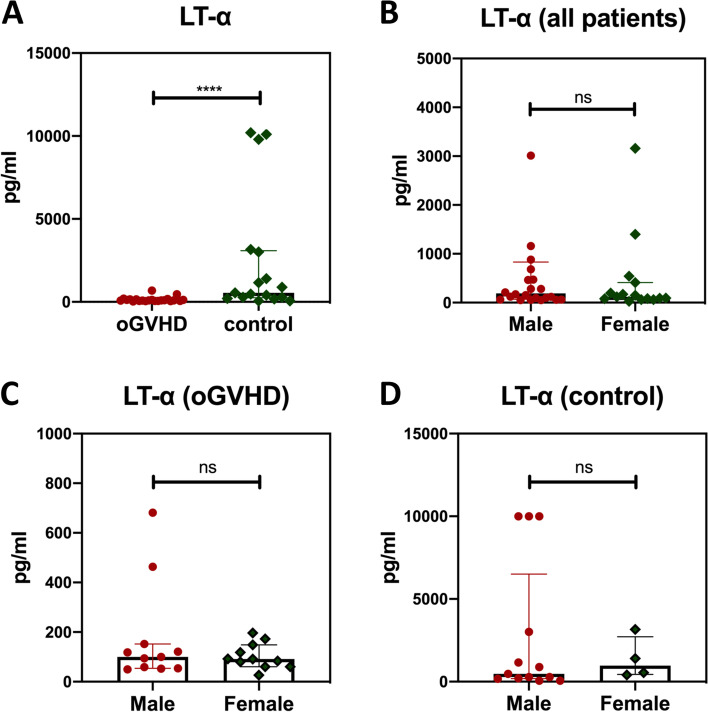
The concentration of LT-α in tears was compared between patients with (n = 22) and without chronic oGVHD (n = 17), and interestingly, we observed a significant decrease in LT-α concentration in oGVHD patients (0.093 ± 0.090 ng/mL) compared to that of controls (0.54 ± 2.84 ng/mL) (median ratio = 5.8, P < 0.0001, Fig. 1A). To determine if tear LT-α levels would be influenced by sex differences, we then compared LT-α concentration between the male and female among all patients, oGVHD subpopulations, and controls, and the analysis results revealed no significant differences among all of them (P > 0.05 for all, Fig. 1B-D).
Fig. 1.
Tear LT-α concentration. A Tear concentration of LT-α was compared between patients with (n = 22) and without (n = 17) oGVHD. B-D Tear LT-α levels were compared between the male and female among all patients (B), oGVHD subpopulations (C), and controls (D). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using Mann-Whitney U test
Correlation and ROC analysis of LT-α levels
The correlation between tear LT-α levels and ocular surface parameters was then assessed in all participants and oGVHD subpopulations (Table 3). Among all participants, tear LT-α levels correlated positively with T-BUT (r = 0.607) and negatively with OSDI score (r = − 0.565), NIH eye score (r = − 0.628), and CFS (r = − 0.608) (P < 0.001 for all), whereas in oGVHD subpopulations, LT-α level exhibited no significant correlations with all these ocular surface parameters. These results suggested tear LT-α as a potential marker for the diagnosis but not severity assessment of oGVHD.
Table 3.
Correlation analysis between tear LT-α and clinical parameters
| Ocular surface parameters | All patients (rho, P) | oGVHD patients (rho, P) |
|---|---|---|
| OSDI score | -0.565, 0.0008*** | -0.120, 0.594 |
| NIH eye score | -0.628, <0.0001**** | -0.158, 0.482 |
| Corneal fluorescein score | -0.608, <0.0001**** | -0.278, 0.211 |
| Fluorescein tear film break-up time (s) | 0.607, <0.0001**** | 0.277, 0.212 |
Correlation analysis was calculated via Spearman’s test. P value less than 0.05 was considered significant, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; rho, Spearman ranked correlation coefficient
We next performed ROC analysis to further evaluate the cutoff value of tear LT-α levels for predicting oGVHD diagnosis (Fig. 2). The area under the curve (AUC) of the LT-α concentration was 0.874 (P < 0.0001), and the cutoff value of LT-α concentration was 0.203 ng/mL (sensitivity 82.4%, specificity 90.91%).
Fig. 2.
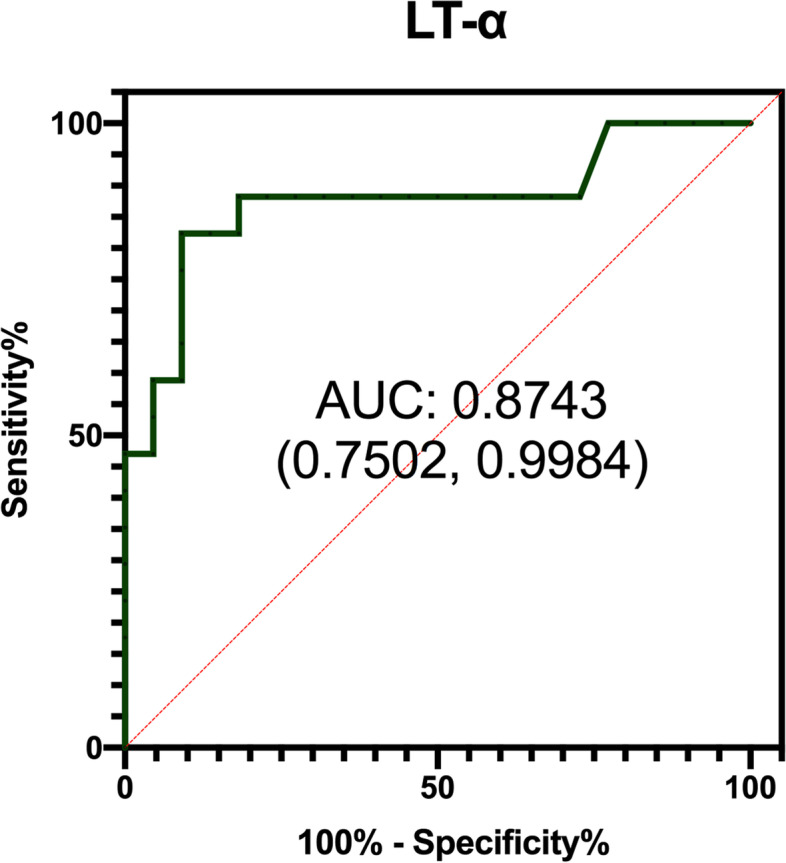
ROC curve of LT-α. Receiver operating characteristic (ROC) curve of tear LT-α to predict oGVHD. Area under the curve (AUC) = 0.8743; 95% CI (0.7502 to 0.9984); P < 0.0001
Discussion
LT-α is a cytotoxic cytokine that belongs to the TNF superfamily and is predominantly produced by lymphocytes [17]. Similar to TNF, LT-α can promote T cell function and contribute to various inflammatory responses [18]. Increasing findings support that upregulated LT-α expression plays a vital role in immune diseases and that blocking LT-α might be a potential treatment for diseases such as rheumatoid arthritis and GVHD [14, 15, 19]. However, in the present study, we observed a decreased level of tear LT-α in oGVHD patients compared with that in controls, which implied that LT-α might not simply act as a proinflammatory cytokine as previous studies have reported. In addition, we noticed that the tear LT-α level was inversely correlated with ophthalmic symptoms, suggesting that LT-α might also have a regulatory effect on chronic oGVHD. Moreover, the AUC of the LT-α concentration was 0.874, and the cutoff value of LT-α concentration was 0.203 ng/mL. These results indicated that tear LT-α levels lower than 0.203 ng/mL could be useful to predict the presence of chronic oGVHD.
Dry eye disease (DED) or keratoconjunctivitis sicca represents the most common manifestation of chronic oGVHD. Interestingly, a recent study enrolled 782 dry eye patients and 306 non-dry eye controls and reported that the tear LT-α level was significantly lower in dry eye patients than in controls [20], consistent with our results observed in oGVHD patients. Therefore, we speculated that LT-α might exert a similar influence on DED and oGVHD. Another study based on DED patients revealed that tear cytokine expression was distinct between patients with high LT-α levels and patients with low LT-α levels [21], indicating a different pathogenesis between high- and low-LT-α DED patients. In this study, we noticed a low tear LT-α level in most oGVHD patients, whereas some oGVHD patients exhibited a much higher level of tear LT-α. Based on this finding, a comparison study on oGVHD patients with different LT-α levels may reveal new insights into oGVHD mechanisms. Animal models with LT-α depletion may also provide an interesting future direction to explore the role of LT-α during the oGVHD initial and developmental stages.
Conclusions
Our study confirmed the diagnostic value of tear LT-α for chronic oGVHD. Consistent with the results in DED, we also found that the tear LT-α level was significantly decreased in chronic oGVHD, suggesting that LT-α might exhibit a regulatory effect on oGVHD-related dry eye symptoms.
Acknowledgments
We thank Dr. Jingfeng Huang for technical support.
Abbreviations
- Allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- AUC
Area under the curve
- CFS
Corneal fluorescein staining
- DED
Dry eye disease
- GVHD
Graft-versus-host disease
- IQR
Interquartile range
- LT-α
Lymphotoxin-α
- NIH
The National Institutes of Health
- oGVHD
Ocular GVHD
- OSDI
Ocular surface disease index
- ROC
Receiver operating characteristic
- T-BUT
Tear film break-up time
Authors’ contributions
JH and JM and contributed to the conception and design of this work. JM, CL,YZ, ZS, BH and RP contributed to the acquisition and analysis of data. JM drafted the work, and JH substantively revised it. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81970768 and 81800801).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee and performed in accordance with the tenets of the Declaration of Helsinki. Written consent was obtained from all the subjects after explaining the nature and possible consequences of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests in this work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kassim A, Savani B. Hematopoietic stem cell transplantation for acute myeloid leukemia: a review. Hematol Oncol Stem Cell Ther. 2017;10:245–251. doi: 10.1016/j.hemonc.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Shikari H, Antin JH, Dana R. Ocular graft-versus-host disease: a review. Surv Ophthalmol. 2013;58:233–251. doi: 10.1016/j.survophthal.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara J, Levine J, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/s0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson NG, Regillo C. Ocular manifestations of graft versus host disease. Curr Opin Ophthalmol. 2014;14:503–507. doi: 10.1097/01.icu.0000143684.22362.46. [DOI] [PubMed] [Google Scholar]
- 5.Jagasia M, Greinix H, Arora M, Williams K, Wolff D, Cowen E, Palmer J, Weisdorf D, Treister N, Cheng G, Kerr H, Stratton P, Duarte R, Mcdonald G, Inamoto Y, Vigorito A, Arai S, Datiles M, Jacobsohn D, Heller T, Kitko C, Mitchell S, Martin P, Shulman H, Wu R, Cutler C, Vogelsang G, Lee S, Pavletic S, Flowers M. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(389–401):e381. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B, Qiu Y, Hong J. Tear cytokine levels in the diagnosis and severity assessment of ocular chronic graft-versus-host disease (GVHD) Ocul Surf. 2020;18:298–304. doi: 10.1016/j.jtos.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Jung JW, Han SJ, Song MK, Kim TI, Kim EK, Min YH, Cheong JW, Seo KY. Tear cytokines as biomarkers for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:2079–2085. doi: 10.1016/j.bbmt.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Cocho L, Fernández I, Calonge M, Martínez V, González-García M, Caballero D, López-Corral L, García-Vázquez C, Vázquez L, Stern M, Enríquez-de-Salamanca A. Biomarkers in ocular chronic graft versus host disease: tear cytokine- and chemokine-based predictive model. Invest Ophthalmol Vis Sci. 2016;57:746–758. doi: 10.1167/iovs.15-18615. [DOI] [PubMed] [Google Scholar]
- 9.Riemens A, Stoyanova E, Rothova A, Kuiper J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol Vis. 2012;18:797–802. [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal B, Gupta S, Kim J. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang E, Kolumam G, Yu X, Francesco M, Ivelja S, Peng I, Gribling P, Shu J, Lee WP, Refino C, Balazs M, Paler-Martinez A, Nguyen A, Young J, Barck K, Carano R, Ferrando R, Diehl L, Chatterjea D, Grogan J. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med. 2009;15:766–773. doi: 10.1038/nm.1984. [DOI] [PubMed] [Google Scholar]
- 12.Ware C, Crowe P, Grayson M, Androlewicz M, Browning J. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol. 1992;149:3881–3888. [PubMed] [Google Scholar]
- 13.Browning J, Sizing I, Lawton P, Bourdon P, Rennert P, Majeau G, Ambrose C, Hession C, Miatkowski K, Griffiths D, Ngam-ek A, Meier W, Benjamin C, Hochman P. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J Immunol. 1997;159:3288–3298. [PubMed] [Google Scholar]
- 14.Markey K, Burman A, Banovic T, Kuns R, Raffelt N, Rowe V, Olver S, Don A, Morris E, Pettit A, Wilson Y, Robb R, Randall L, Korner H, Engwerda C, Clouston A, Macdonald K, Hill G. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood. 2010;115:122–132. doi: 10.1182/blood-2009-01-199927. [DOI] [PubMed] [Google Scholar]
- 15.Chiang E, Kolumam G, McCutcheon K, Young J, Lin Z, Balazs M, Grogan J. In vivo depletion of lymphotoxin-alpha expressing lymphocytes inhibits xenogeneic graft-versus-host-disease. PLoS One. 2012;7:e33106. doi: 10.1371/journal.pone.0033106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Shen Z, Peng R, Li C, Hu B, Hong J. Tear lipid metabolites as potential diagnostic biomarkers for ocular chronic graft-versus-host disease. Transplant cell Ther. 2021;27:232 e231–232 e236. doi: 10.1016/j.jtct.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Ruddle N. Lymphotoxin and TNF: how it all began-a tribute to the travelers. Cytokine Growth Factor Rev. 2014;25:83–89. doi: 10.1016/j.cytogfr.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaur U, Aggarwal B. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403–1408. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 19.Hirose T, Fukuma Y, Takeshita A, Nishida K. The role of lymphotoxin-alpha in rheumatoid arthritis. Inflamm Res. 2018;67:495–501. doi: 10.1007/s00011-018-1139-6. [DOI] [PubMed] [Google Scholar]
- 20.Lin X, Huang J, Liu Z. Large scale, prospective, multicenter, clinical evaluation of point-of-care Lymphotoxin alpha (LTA) test in dry eye disease. Invest Ophthalmol Vis Sci. 2020;61:116. [Google Scholar]
- 21.Chen H, Chen H, Liang L, Zhong Y, Liang Y, Yu Y, Huang S, Lu X. Evaluation of tear protein markers in dry eye disease with different Lymphotoxin-alpha expression levels. Am J Ophthalmol. 2020;217:198–211. doi: 10.1016/j.ajo.2020.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.