Abstract
Background:
Inflammatory response and oxidative stress can be found in anthrax characterized by increased level of serum Tumor Necrosis Factor Alpha (TNF-α) and Malondialdehyde (MDA). The use of antibiotics in anthrax has been known to cause some disturbing side-effects, such as allergic reaction, nausea, vomiting, and antibiotic resistance. Thus, ethanolic extract of propolis (EEP) might be the alternative regimen, due to its anti-inflammatory and antioxidant properties. This study aimed to compare the effects of ethanolic extract of propolis (EEP) on TNF-α and MDA between the inhalation and cutaneous anthrax animal model.
Materials and Methods:
This was an experimental study with a post-test-only control group design on 40 samples of Rattus norvegicus. Samples were randomized into 5 groups: control, inhalation anthrax model, inhalation anthrax model + EEP, cutaneous anthrax model, and cutaneous anthrax model + EEP. After 14 days, the level of TNF-α and MDA were measured. To compare the data, we used the ANOVA test continued by the post-hoc Turkey test.
Results:
The results obtained showed that the level of TNF-α and MDA between the inhalation and cutaneous anthrax animal models treated with EEP were statistically different (p < 0.05). The P5 group showed the lowest level of TNF-α (6.822 ± 0.383 pg/ml) and MDA (2.717 ± 0.383 nmol/ml).
Conclusion:
EEP has a better effect on reducing TNF-α and MDA in cutaneous anthrax animal models compared to the inhalation anthrax animal model.
Keywords: anthrax, ethanolic extract of propolis, TNF-α, MDA
Introduction
Anthrax is a zoonotic infectious disease caused by Bacillus anthracis. This disease is transmitted by spores via the skin, respiratory tract, or gastrointestinal tract (Martin J, et al., 2019 and Savransky V, et al., 2020). After the spore’s entrance, the vegetative form of B. anthracis develops and thereafter produces toxins. The initial responses of the infection are the production of reactive oxygen species (ROS) that can be assessed by serum malondialdehyde (MDA) level and the expression of the pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin 1β (IL-1β), IL-18, and IL-6 (Ayala et al., 2014 and Cherian DA, et al., 2019).
Cutaneous anthrax is the most common manifestation of anthrax and can be recognized early so the treatment is almost always effective. Other forms of anthrax, such as gastrointestinal and inhalational anthrax, present with very non-specific early symptoms, causing more advanced disease with relatively high mortality and morbidity (Hicks et al., 2012). The mortality rate of cutaneous anthrax without proper antibiotic treatment is 20%, while gastrointestinal anthrax is 25 - 75% and inhalation anthrax could reach 80% or higher (Kamal et al., 2011). The use of antibiotics as the treatment of anthrax has some side effects, for example, allergic reaction, nausea, vomiting, and antibiotic resistance. Thus, an alternative regimen from herbal composition might be considered.
Propolis refers to resin substances collected by the bees from various plants (Pasupuleti et al., 2017). This substance contains flavonoid that inhibits the production of some inflammatory markers, including Nitric Oxide (NO), Interleukin-1 (IL-1), IL-6, C-Reactive Proteins (CRP), and TNF-α (Zhao et al., 2016). Propolis also reduces the level of MDA by the action of Caffeic Acid Phenethyl Ester (CAPE) (Prasetyo et al., 2019). Because of these anti-inflammatory and antioxidant properties, propolis needs to be studied to compare its effect on reducing the markers of inflammation and oxidative stress on anthrax between inhalation and subcutaneous injection models.
Materials and Methods
Study Design
This study was an experimental research on 40 samples of Rattus norvegicus, using a post-test-only control group design.
Population and Sample
The population of this study was male white rats (Rattus norvegicus) obtained from the Center of Inter-University Biotechnology Laboratory, Gajah Mada University, Yogyakarta, Indonesia. Samples were chosen by purposive sampling, and the intervention was decided by randomization. The sample size was counted, and 8 samples per group were used in this study. The inclusion criteria were rats aged 3 - 4 months, weighted 175–200 grams, and healthy (have shining eyes, no dull hairs, active, and good appetite). The exclusion criteria were samples that showed some signs of sickness.
Data and Sources of Data
A total of 40 samples were randomized and placed into 5 groups: control, inhalation anthrax model, inhalation anthrax model + EEP 200 mg, cutaneous anthrax model, and cutaneous anthrax model + EEP 200 mg. The level of TNF-α and MDA were measured on the 14th day.
Statistical Analysis
Data were analyzed using SPSS 25.0 for Windows with a p-value <0.05 was considered statistically significant. To compare the effects of EEP on inhalation and cutaneous anthrax model, we used the ANOVA test continued by the post-hoc Turkey tests.
Ethical Clearance
This study was approved by Health Research Ethics Committee, Faculty of Medicine, Universitas Sebelas Maret, no. 015/UN27.06.6.1/KEPK/EC/2020 on February 5th, 2020.
Results and Discussion
Samples Characteristic
The study used 40 rats that were divided into different groups (as shown in Table 1): 8 rats were normal, 16 rats had inhaled spores at dose 4x10¹ CFU (8 rats received no treatment, 8 rats received EEP 200 mg) and 16 rats had been injected with 0.2 cc spores subcutaneously or approximately 2x1011 CFU (8 rats received no treatment, 8 rats received EEP 200 mg).
Table 1.
Samples Characteristic
| Group | n | Condition | Treatment |
|---|---|---|---|
| P1 | 8 | Normal | No treatment |
| P2 | 8 | Inhaled spores at dose 4x1010 CFU | No treatment |
| P3 | 8 | Inhaled spores at dose 4x1010 CFU | EEP 200 mg |
| P4 | 8 | Injected spores at dose 2x1011 CFU | No treatment |
| P5 | 8 | Injected spores at dose 2x1011 CFU | EEP 200 mg |
Effects of EEP in TNF-α
The level of the TNF-α on the groups were measured on the 14th day as shown in Table 2. The level of TNF-α from the highest to the lowest level were consecutively P3 group (14.282 ± 0.282 pg/ml), P4 (14.247 ± 0.311 pg/ml), P2 (14.192 ± 0.404 pg/ml), P5 (6.822 ± 0.383 pg/ml) and the lowest level found in the P1 (6.056 ± 0.394 pg/ml). All of the groups with anthrax showed a higher level of TNF-α compared to the control group. Between those anthrax groups, the group with cutaneous model receiving EEP 200 mg had the lowest level of TNF-α.
Table 2.
Results of TNF-α measurement
| Group | n | Mean | SD | p |
|---|---|---|---|---|
| P1 | 8 | 6.056 | 0.394 | 0.001 |
| P2 | 8 | 14.192 | 0.404 | |
| P3 | 8 | 14.282 | 0.282 | |
| P4 | 8 | 14.247 | 0.311 | |
| P5 | 8 | 6.822 | 0.383 |
The ANOVA test continued by post-hoc Turkey test was performed. The results were summarized in Figure 1. Every 2 groups compared showed significant difference (p < 0.05), except the P2-P3 (p = 0.983) and P2-P4 (p = 0.997). The effect of EEP in the inhalation model and cutaneous model was significantly different (P3-P5, p<0.05), with the effect of EEP in the cutaneous anthrax animal model being better.
Figure 1.
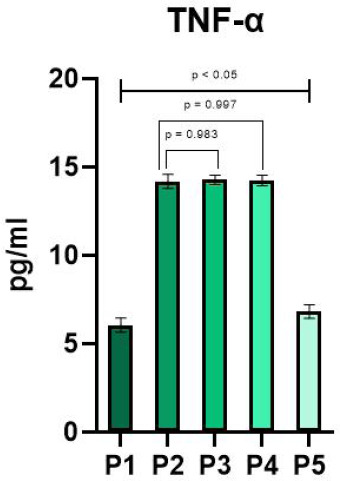
Results of the ANOVA and post-hoc Turkey tests of Serum TNF-α Level
Effects of EEP on MDA
The level of MDA was measured on the 14th day as seen in Table 3. From the highest to the lowest level of MDA between groups were consecutively P1 (11.287 ± 1.904 nmol/ml), P3 (9.866 ± 0.220 nmol/ml), P4 (9.642 ± 0.383 nmol/ml), P2 (9.533 ± 0.246 nmol/ml), and the lowest level found in P5 (2.717 ± 0.383 nmol/ml). Surprisingly, the control group showed the highest level of MDA. Between all of the anthrax groups, P5 showed the lowest level of MDA.
Table 3.
Results of MDA measurement
| Group | N | Mean | SD | p |
|---|---|---|---|---|
| P1 | 8 | 11.287 | 1.904 | 0.001 |
| P2 | 8 | 9.533 | 0.246 | |
| P3 | 8 | 9.866 | 0.220 | |
| P4 | 8 | 9.642 | 0.383 | |
| P5 | 8 | 2.717 | 0.383 |
The statistical analysis was performed using the ANOVA test continued by post-hoc Turkey test. The results can be seen in Figure 2 below. Every 2 groups compared showed significant difference (p < 0.05), except the P2-P3 (p = 0.944) and P2-P4 (p = 0.999). The effect of EEP in the inhalation model and cutaneous model was significantly different (P3-P5, p<0.05), with the effect of EEP on the level of MDA in cutaneous anthrax animal model was better.
Figure 2.
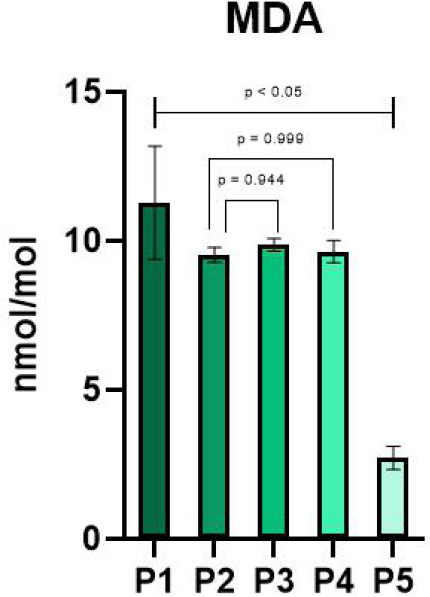
Results of the ANOVA and post-hoc Turkey tests of Serum MDA Level
Discussion
This is an experimental study aimed to compare the effect of EEP 200 mg on the level of TNF-α and MDA between the rat model of inhalation and cutaneous anthrax after 14 days. Inhaled or injected spores induce an inflammatory response, marked by TNF-α. This response might develop into oxidative stress where Reactive Oxygen Species (ROS) are produced. This oxidative stress could be assessed by measuring the serum MDA level (Ayala et al. 2014 and Cherian et al., 2019). EEP has been known to reduce the inflammatory response and oxidative stress (Hori et al., 2013 and Pahlavani et al., 2020). Thus, EEP might have some positive effects on anthrax. Kamal (2011) stated because the inhalation anthrax has higher morbidity and mortality compared to cutaneous anthrax, the effect of EEP on these 2 forms of anthrax needs to be assessed.
The findings of this study showed that all of the anthrax groups had a higher level of TNF-α and lower level of MDA compared to the control group. P5 had the lowest level of TNF-α between the anthrax groups (6.822 ± 0.383 pg/ml) and also the lowest level of MDA (2.717 ± 0.383 nmol/ml). Comparison of EEP given to the inhalation and cutaneous anthrax model also showed significant difference (p < 0.05), with the better effect shown in the cutaneous anthrax model.
The study revealed that injection of EEP also resulted in considerably decreased serum E-selectin, SGPT, and creatinine levels (Redhono et al., in press). The positive effect of EEP in this study is relevant to a previous study in rat models of cutaneous anthrax which showed no cutaneous manifestation after EEP was given (Kusumawardani et al., 2020). The absence of lesion proved that there was only minimal inflammatory reaction happened, thus oxidative stress would be minimized too. In another study with rat models of inhalation anthrax, EEP was shown to reduce the inflammatory response in the lung tissue and also showed its antioxidant effect on reducing ROS production (Redhono et al., in press).
The comparison that showed a better effect on the cutaneous model than on the inhalation model might be due to the severity of those 2 forms of anthrax. Cutaneous anthrax appears primary as a papule that progresses to a large vesicle that may develop an eschar if ruptured. Study conducted by Redhono et al. (2018), showed that clinical symptoms in the form of eschar are seen in 11.9 percent of patients with cutaneous anthrax. Patients with cutaneous anthrax may have a resolution of illness or develop signs of disseminated disease, but the risk of dissemination and death can be minimized by treating the patient with antibiotics. While inhalation anthrax is the most lethal form of anthrax with non-specific signs and symptoms, for example, malaise, fever, and nonproductive cough. It may cause the delay of diagnosis and treatment, and the worst of it is death usually results within 72 hours after symptoms start (Chambers et al., 2021).
Conclusion
Based on the results of this study, EEP has a better effect on reducing the serum level of TNF-α and MDA as markers of inflammation and oxidative stress in the cutaneous anthrax animal models.
Conflict of Interest
The authors declare that there is no actual or potential conflict of interest concerning this study.
List of Abbreviations:
- Cape:
caffeic Acid Phenethyl Ester
- CRP:
C-Reactive Protein
- EEP:
Ethanolic Extract of propolis
- IL:
Interleukin
- MDA:
Malondialdehyde
- NO:
Nitric Oxide
- ROS:
Reactive Oxygen Species
- TNF:
Tumor Necrosis Factor
Acknowledgement
The authors would like to thank the entire research team for their hard-work which ensured that this study could be resolved properly. They are also grateful to all staff and Head of Center for Veterinary Research (Balitvet) Bogor, for their dedication and commitment.
References
- 1.Ayala A, Muñoz M, Argüelles S. Lipid Peroxidation:Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers J, Yarrarapu S, Mathai J. Anthrax Infection. [online] Ncbi.nlm.nih.gov. 2021. [Accessed 12 August]. Available at: <https://www.ncbi.nlm.nih.gov/books/NBK535379/?report=classic> .
- 3.Cherian D, Peter T, Narayanan A, Madhavan S, Achammada S, Vynat G. Malondealdehyde as a marker of oxidative stress in periodontitis patients. Journal of Pharmacy and Bioallied Sciences. 2019;11(6):297. doi: 10.4103/JPBS.JPBS_17_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hicks C, Sweeney D, Cui X, Li Y, Eichacker P. An overview of anthrax infection including the recently identified form of disease in injection drug users. Intensive Care Medicine. 2012;38(7):1092–1104. doi: 10.1007/s00134-012-2541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hori J, Zamboni D, Carrão D, Goldman G, Berretta A. The Inhibition of Inflammasome by Brazilian Propolis (EPP-AF) Evidence-Based Complementary and Alternative Medicine. 2013;2013:1–11. doi: 10.1155/2013/418508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamal S, Rashid A, Bakar M, Ahad M. Anthrax:an update. Asian Pacific Journal of Tropical Biomedicine. 2011;1(6):496–501. doi: 10.1016/S2221-1691(11)60109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusumawardani A, Kariosentono H, Purwanto B, Redhono D, Arief R.C, Wasita B, Adji R.S. The Effect of Ethanolic Extract of Propolis on Skin Manifestation and Skin Tissue Necrosis in Cutaneous Anthrax Animal Model. Systematic Reviews in Pharmacy. 2020;11(11):1539–1544. [Google Scholar]
- 8.Pahlavani N, Malekahmadi M, Firouzi S, Rostami D, Sedaghat A, Moghaddam A, Ferns G, Navashenaq J, Reazvani R, Safarian M, Ghayour-Mobarhan M. Molecular and cellular mechanisms of the effects of Propolis in inflammation, oxidative stress and glycemic control in chronic diseases. Nutrition and Metabolism. 2020;17:65. doi: 10.1186/s12986-020-00485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasupuleti V, Sammugam L, Ramesh N, Gan S. Honey, Propolis and Royal Jelly:A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Medicine and Cellular Longevity. 2017;2017:1–21. doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasetyo D.H, Sarsono S, Nurwati I, Putranto P.A, Martini M, Prasetyo N.A. Hepatoprotective and antifibrotic effects of Indonesian Propolis. Majalah Kedokteran Bandung. 2019;51(3):134–140. [Google Scholar]
- 11.Redhono D, Kusumawardani A, Dirgahayu P. A comparison of the immune response between early exposed and 1 year post exposure to Bacillus anthracis in Indonesia. IOP Conference Series:Earth and Environmental Science. 2018;125:1–5. [Google Scholar]
- 12.Redhono D, Purwanto B, Wasita B, Indarto D, Adji R.S, Kusumawardani A, Cilmiaty R. The Antioxidant Effect of Indonesian Propolis In Rats Induced by Anthrax Spores. Systematic Reviews in Pharmacy. 2021;12(1):557–562. [Google Scholar]
- 13.Redhono D, Purwanto B, Wasita B, Indarto D, Setya Adji R, Kusumawardani A, Cilmiaty R. (in press). The effect of Ethanolic extract of Indonesian propolis on endothelial dysfunction and Multi Organ dysfunction syndrome in anthrax animal model. Saudi Journal of Biological Sciences. doi: 10.1016/j.sjbs.2021.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savransky V, Ionin B, Reece J. Current Status and Trends in Prophylaxis and Management of Anthrax Disease. Pathogens. 2020;9(5):370. doi: 10.3390/pathogens9050370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory J. Martin, Arthur M Friedlander. Bacillus anthracis (Anthrax). In:Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. (9th ed) 2019:2550–2569. [Google Scholar]
- 16.Zhao L, Pu L, Wei J, Li J, Wu J, Xin Z, Gao W, Guo C. Brazilian Green Propolis Improves Antioxidant Function in Patients with Type-2 Diabetes Mellitus. International Journal of Environmental Research and Public Health. 2016;13(5):498. doi: 10.3390/ijerph13050498. [DOI] [PMC free article] [PubMed] [Google Scholar]


