Abstract
Introduction:
Since the landmark publication by Smith and Robinson, approaches to the cervical spine anteriorly have undergone many modifications and even additions. Nevertheless, at its core, the anterior approach remains an elegant and efficient approach to deal with majority of cervical spine pathologies including the degenerative cervical spine.
Methodology:
For this review, we searched for all major cases series and randomized control trials of anterior cervical approaches using the PubMed databases. Articles having the details of clinical variables and outcomes were tabulated and analyzed.
Results:
A total of 9 case series for transoral, 7 case series for transmanubrial, 19 case series for anterior cervical discectomy and fusion (ACDF), 6 studies for ACDF versus posterior cervical foraminotomy, 37 case series for ACDF versus arthroplasty, and 7 studies for ACDF versus anterior cervical corpectomy and fusion have been included. The majority of the case series suggested that the anterior cervical procedures have good clinical outcomes. The upper cervical spine approached by the transoral route had good outcomes in ventral compressive pathologies, with morbidity of cerebrospinal fluid leak in 7% of patients. The midcervical spine approached by ACDF had better clinical outcomes equivalent to the majority of modifications even in multiple-level pathologies. The transsternal approach had provided greater access and stability to the cervicothoracic junction with minimal morbidity.
Conclusion:
The anterior cervical approach can address the majority of cervical pathologies. They provide adequate corridor from craniovertebral junction to T4 with minimal morbidity, thus providing a good clinical outcome.
Keywords: Anterior cervical approach, cervical spine, cervical spondylotic myelopathy, corpectomy
Point of view: The anterior approach to the cervical spine has been the bedrock of most surgical procedures of this region, and any neurosurgeon must have an overview of this technique with a short learning curve and reproducible results. We attempt to define the indications, techniques, results, complications, and tips in this short review, with an emphasis on evidence based management.
Introduction
Based on anatomical concepts, anterior approaches to the cervical spine for various cervical spine pathologies can be categorized into transoral, anterolateral cervical, and split manubrium approaches.[1,2,3,4] These anterior approaches, along with the minimally invasive techniques, provide the anterior corridor to the cervical spine pathologies. The transoral approach, although gradually going out of vogue, remains a vital cog to deal with congenital and pathological abnormalities of the craniovertebral junction (CVJ) region. More caudally, the manubrium split has been utilized with significant effect to approach the lower cervical spine up to the T4 level. While using only the manubrium split, we can access the upper thoracic and lower cervical vertebrae while avoiding the morbidity of a sternal split.[5] More recently, in expert and experienced hands, the endoscopic approach for discectomy and corpectomy of the cervical spine has expanded the surgeon's armamentarium in carefully selected cases [Table 1]. The present article provides an overview of the published literature and the technical nuances of approaching the anterior cervical spine effectively and safely.
Table 1.
Overview of the anterior approaches to the cervical spine
| Level of cervical spine | Type of anterior approach | Advantages | Disadvantages |
|---|---|---|---|
| C1–C2 | Transoral | Provides access to largely inaccessible pathologies and anatomical zones Relatively direct approach Midline approach Shorter learning curve |
Oral complications Infections Postoperative care and feeding Wound healing CSF oral fistula |
| C2–C7 | Anterolateral cervical | Reliable and reproducible Follows anatomical plane of dissection Minimal chance of injury to vital structures |
Esophageal and tracheal compression/injury Horner’s syndrome Strictly midline, difficult to deal with lateral pathologies |
| C7–T4 | Transmanubrium | Provides anterior access to upper thoracic and lower cervical spine Avoids morbidity of sternal split |
Window of surgery is limited Midline approach Vital structures injury Lung infections |
CSF – Cerebrospinal fluid
Methodology
A PubMed/Medline search was conducted to include studies completed until January 2020 and exclude systematic reviews and meta-analysis. They have been categorized into the following subgroups [Table 2 and Figures 1-6].
Table 2.
Inclusion criteria for various categories included in the study
| Category | Inclusion criteria |
|---|---|
| Transoral and trans-sternal | Case series >10 patients Clinical follow-up available Follow-up time not defined due to limited number of case series |
| Anterior cervical discectomy and fusion case series | Case series with >100 patients Case series published from January 2005 till January 2020 Clinical follow-up period of minimum 2 years |
| Case series of anterior cervical Discectomy and fusion versus anterior cervical corpectomy and fusion | Case series >10 patients comparing ACDF versus ACCF Clinical follow-up available Clinical follow-up period of minimum 2 years |
| Case series of ACDF versus posterior cervical foraminotomy | Case series >10 patients comparing ACDF versus posterior cervical foraminotomy Clinical follow-up available Clinical follow-up period of minimum 2 years |
| Case series comparing compared ACDF versus cervical arthroplasty | Case series >10 patients comparing ACDF versus arthroplasty Clinical follow-up available Clinical follow-up period of minimum 2 years |
ACDF – Anterior cervical discectomy and fusion; ACCF – Anterior cervical corpectomy and fusion
Figure 1.
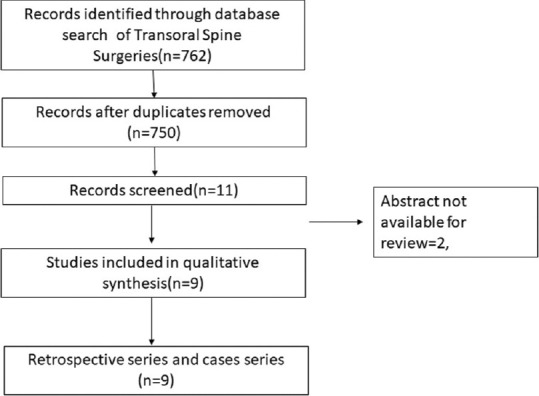
PRISMA chart depicting transoral surgeries
Figure 6.
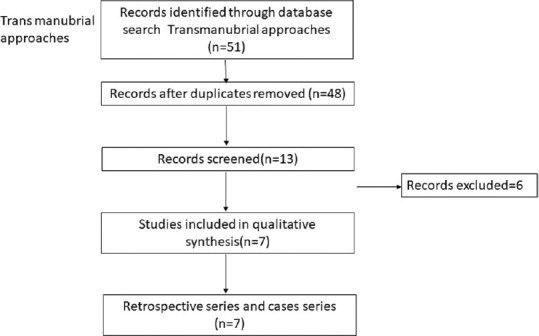
PRISMA chart comparing transternal approaches
Figure 2.
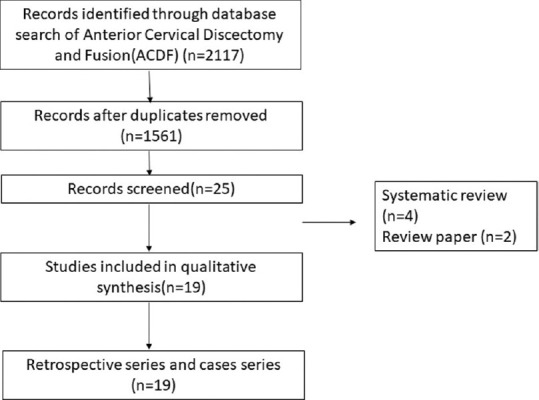
PRISMA chart for the case series of anterior cervical discectomy and fusion
Figure 3.
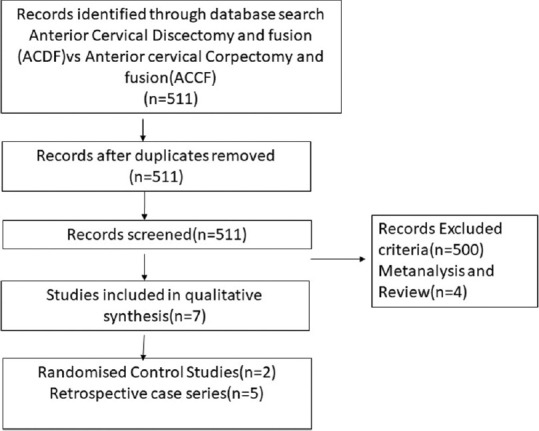
PRISMA chart depicting the case series comparing anterior AQ12 cervical discectomy and fusion and anterior cervical corpectomy and fusion
Figure 4.
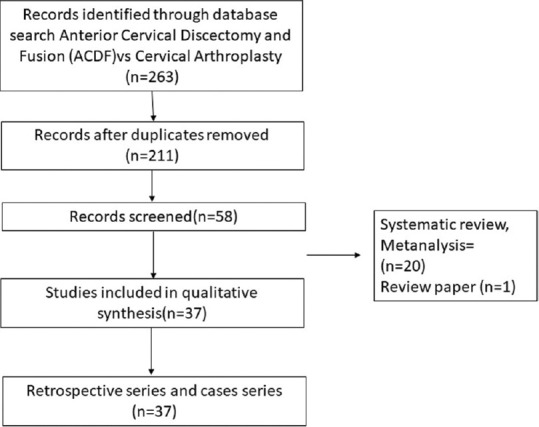
PRISMA chart depicting anterior cervical discectomy and fusion versus anterior cervical disc arthroplasty
Figure 5.
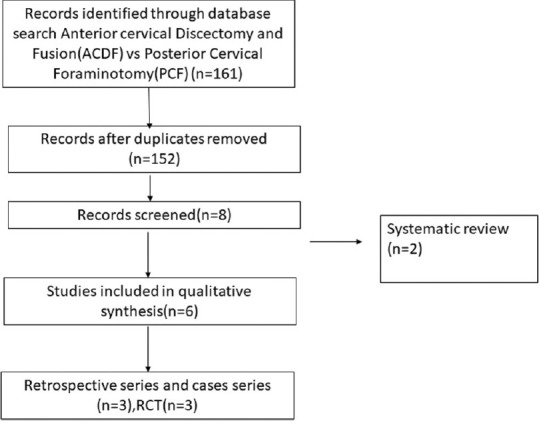
PRISMA chart comparing anterior cervical discectomy and fusion versus posterior cervical foraminotomy
MeSH keyword of “transoral,” “trans-sternal,” approaches, and case series higher than 10 patients with clinical outcomes reported included in view of limited number of the series
The remaining categories included studies that had a minimum follow-up of 2 years
MeSH keyword of “anterior cervical discectomy and fusion” was used to select all case series from 2005 till January 2020 with patients greater than 100
Studies that compared ACDF versus ACCF using MeSH word of “anterior cervical discectomy and fusion” AND and OR “anterior cervical corpectomy and fusion“
Studies that compared ACDF versus posterior cervical foraminotomy using MeSH keyword of “anterior cervical discectomy and fusion” AND and OR “posterior cervical foraminotomy“
Studies that compared ACDF versus cervical arthroplasty using MeSH keyword of “anterior cervical discectomy and fusion” AND and OR “cervical arthroplasty.“
Results
Literature search from PubMed database for the transoral cervical spine found 9 case series relevant to the study [Table 3].[6,7,8,9,10,11,12,13,14] The transoral approach has been found to be effective in treating compression up to C3 level of the cervical spine in 94% of patients; however, it is associated with complications such as cerebrospinal fluid (CSF) leak in 7%, wound dehiscence, and requirement of posterior fusion for additional stability, thus rarely being performed in recent times. Next search was done for transmanubrial approach to cervical spine and it found seven case series appropriate for the study [Table 4].[15,16,17,18,19,20,21] The transmanubrial approach is mainly indicated for the lesion of the cervicothoracic region providing an access for fusion with rare complications of transient recurrent laryngeal palsy in 0.8% of them. The next search was directed toward the more common anterolateral approaches of the cervical spine and categorized into subgroups. The first subgroup focused on case series of ACDF from 2005 till 2020 and found 19 case series eligible for the review [Table 5].[22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] The ACDF is effective up to three levels with neurological improvement in 85%–95% of the patients. The post - operative complications include transient dysphagia, hoarsness of voice in upto 3% patients, adjacent segment disease in 10%, pseudoarthosis in 3% and CSF leak and wound hematoma in less than 0.5% patients. The second subgroup comparing the ACDF versus cervical arthroplasty found 37 articles eligible [Table 6].[41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] This subgroup analysis showed that all patients have significant improvement in the neck and arm pain score in the arthroplasty group compared to ACDF with lesser rates of adjacent segment disease in the arthroplasty group. The third subgroup, which compared ACDF versus cervical foraminotomy, found 6 studies appropriate [Table 7].[78,79,80,81,82,83] The cervical foraminotomy is said to be equally effective as the ACDF in alleviating radicular pain; however, it has higher rate of recurrence up to 6% versus 4% in ACDF. The last subgroup was directed toward multiple-level cervical pathology and addressed the question if ACDF was better than anterior corpectomy and fusion. We found 7 articles among 511 articles eligible to the study [Table 8].[84,85,86,87,88,89,90] There is no difference in the clinical outcomes and complication between the groups, except radiological Cobb angle better in ACDF compared to anterior cervical corpectomy and fusion.
Table 3.
A review of some of the larger recent series of transoral approach with complications and outcomes
| Author and year | Number of cases | Complications | Outcomes | Levels fused | Remarks | Follow-up |
|---|---|---|---|---|---|---|
| Crockard et al. (1986)[6] | 68 | Vertebral artery injury-1, cord damage-1 Palatal dehiscence-2 CSF leaks-6 |
61 (90%) improved, 3 (4%) deteriorated, 1 died | Occipito-C2/C3 fusion | Transoral decompression relieve ventral compression in rheumatoid arthritis | - |
| Hadley et al. (1989)[7] | 53 | 5.6% wound dehiscence with CSF leak | 94% neurological improvement | Occipito-C3 levels | Good result for ventral pathology | 2 years |
| Dickman et al. (1992)[8] | 27 | None | 22 (81%) improved, 5 (19%) stabilized | 9 (33%) fusion of C1–C2, 10 (37%) occipitocervical fusion | Transoral decompression relieves decompression and fusion required in >70% patients and 90% of rheumatoid arthritis patients | 14 months |
| Tuite et al. (1996)[9] | 27 | Neurological deterioration-4 (15%), CSF leak-2, wound infection-3, palatal fistula-2 | 9 (33%) improved, 4 (15%) worse, 15 (52%) remained same | Occipito-C3/C5/T4 (1 patient) | Transoral surgery in congenital diseases requires less extensive surgery compared to oncological condition but associated with worse neurological outcomes | 4.6 years |
| Jain et al. (1999)[10] | 74 | Pharyngeal wound sepsis leading to dehiscence (20.3%) and hemorrhage (4%), velopharyngeal insufficiency (8.1%), CSF leak (6.7%) and inadequate decompression (6.7%) | 26 (55.3%) showed improvement from their preoperative status while 14 (29.8%) demonstrated stabilization of their neurological deficits. 7 (14.9%) of them deteriorated | C1-2-3 | TOD is logical and effective in relieving ventral compression due to craniovertebral junction anomalies; it carries the formidable risks of instability, incomplete decompression, neurological deterioration, CSF leak, infection and palatopharyngeal dysfunction | 3–24 months |
| Menezes (2008)[11] | 28 | Wound dehiscence 2, velopalatine insufficiency 5, retropharyngeal infection 1 | Neurological improvement in all patients | C1, C2, and C2–3 disc pathology | Indicated in irreducible pathology | - |
| Mouchaty et al. (2009)[12] | 53 | 2 mortality, 8 patients had morbidity – CSF leak, wound dehiscence, meningitis | 51 patients had improvement | C1, C2 | Indicated in severe BI | 4–96 months |
| Shousha et al. (2014)[13] | 139 | 3.6% wound infection early, late in 1 patient | 94% neurological improvement | - | Postoperative infections higher in rheumatic disease group | 4.5 years |
| Elbadrawi and Elkhateeb (2017)[14] | 20 | CSF leak wound dehiscence | Improvement in VAS and Nurick score | C2 | Safe and effective surgical method for the direct decompression of ventral midline extradural compressive disease of the craniovertebral junction | 29.4±3.8 months |
VAS – Visual analog scale; CSF – Cerebrospinal fluid
Table 4.
Review of the larger series reported with the transmanubrial approach along with complications and outcomes
| Author and year | Number of cases | Complications | Outcomes | Levels fused | Remarks |
|---|---|---|---|---|---|
| Xiao (2007)[15] | 28 | 11 patients had bradycardia and hypotension, 3 had recurrent laryngeal nerve paresis | Improvement in pain and neurological symptoms in all | C7–T4 | On the right side, its easier approach than left due to thoracic duct |
| Liu et al. (2009)[16] | 11 | 1 patient had recurrent laryngeal nerve palsy, 1 patient had chyle leak | Improvement in incomplete cord injury and radiculopathy | C6–T2 | Adequate access to upper cervical region |
| Falavigna et al. (2009)[17] | 14 | Hematoma - 1 Dysphonia - 1 |
Improvement in all patients | C7–T4 | C7 corpectomy and C7–T1 intervertebral disc herniation, a transcervical approach without the manubriotomy was indicated; when a T1 and/or T2 corpectomy was necessary, the transmanubrial approach usually was necessary in order to provide a good working space to perform a corpectomy and reconstruction |
| Jiang et al. (2010)[18] | 16 | 1 patient hoarseness of voice | 8 patients had neurological improvement | C7–T4 | It leads to better visulaisation |
| Zengming et al. (2010)[19] | 54 | - | Improvement in radiculopathy and myelopathy | C7–T4 | Adequate access to spine and immediate stability |
| Park et al. (2015)[20] | 13 | Chylothorax - 1 Hoarseness of voice - 2 |
Improvement in VAS and Frenkel | C7–T3 | The transmanubrial approach for CTJ lesions can achieve favorable clinical outcomes by providing direct decompression of lesion and effective reconstruction |
| Mihir et al. (2006)[21] | 28 | Left recurrent laryngeal nerve palsy 2 cases | Improvement in neurological deficits | C7–T4 | Safe approach for stabilization of anterior spine |
VAS – Visual analog scale; CTJ – Cervicothoracic junction
Table 5.
Review of the large anterior cervical discectomy series with results and complications
| Author and year | Number of cases | Complications | Outcomes | Levels fused | Follow-up (months) |
|---|---|---|---|---|---|
| Marotta et al., 2011[22] | 167 | 20% adjacent segment disease | Significant improvement in NDI, VAS score postoperative | Single level | 60 |
| Lu et al., 2013[23] | 150 | No difference in dysphagia in both groups with slight increase in pseudarthrosis in allograft group | Significant decrease in Nurick score compared to preoperatively, however, no difference with addition of r-BMP | Multiple levels up to 4 levels | 35 |
| Klingler et al., 2014[24] | 109 | 10% subsidence in follow up | VAS, NDI significant better in follow-up with no significant difference between PEEK cage and PMMA cage | 70% single-level, 30% 2-level | 29 |
| Li et al., 2017[25] | 138 | 1.5% EDH, hoarseness, dysphagia 7.4%, infection, subsidence 9.8% in cage versus 7.4% in cage+plate | Significant improvement in SF-36, VAS, NDI, JOA score in all group | Up to 4 levels performed | 26 |
| Zigler et al., 2016[26] | 186 | Adjacent segment disease high in 2-level group compared to single level | Significant improvement in NDI, VAS, SF-12 score | Single and 2 levels | 60 |
| Tasiou et al., 2017[27] | 114 | CSF leak, dysphagia, recurrent laryngeal nerve plasy, trachea-esophageal fistula, implant failure | Earlier assessment of perioperative complications – better results | Both single- and multiple-level discs | 42.5 |
| Burkhardt et al., 2018[28] | 122 | Rate of adjacent segment disease is 10%, 8% postoperative dysphagia | 89.3% had high rate of radicular pain relief | 64% single-level, 33% two-level, 3.3% 3-level | 300 |
| Grasso and Landi, 2018[29] | 100 | 2% dysphagia experienced | VAS, improved significantly immediate after surgery and continue till the last follow-up | 73% one-level, 27% 2-level | 84 |
| Tumialán et al., 2019[30] | 135 | 2.3% transient laryngeal nerve palsy | 88% had improvement with return to work | 76% single-level, 24% 2-level | 48 |
| Mullins et al., 2018[31] | 1123 | 3.6% had complication | VAS and clinical improvement seen significantly in all groups | 40% single-level, 34% 2-level, 22% 3-level, 3% 4-level | 25 |
| He et al., 2018[32] | 104 | 4% complication in zero profile device and 17% of ACDF | Clinical improvement significant in both groups with no difference between | Multiple levels | 24 |
| Muzevic et al., 2018[33] | 154 | - | 80% had clinical improvement | One to multiple levels | 24 |
| Yu et al., 2018[34] | 247 | Greater incidence of subsidence in nonfixed system | VAS and clinical outcomes improvement significant and no different between standalone cage versus fusion with cage and plate | One or two level | 24 |
| Butterman 2018[35] | 159 | 10% pseudarthrosis and 21% adjacent segment disease | 85%–95% improvement in neurological outcome | Single to 2 levels | 120 |
| Lee et al., 2018[36] | 167 | 5 cases pseudarthrosis | VAS of arm pain better in uncinate process removal compared to nonremoval | Single and 2 level | 31.4 |
| Yang et al., 2019[37] | 134 | 4% have dysphagia | VAS and NDI reduced postoperatively | 2 | 29.68 |
| Shin, 2019[38] | 165 | 20% adjacent segment disease | VAS and NDI reduced postoperatively at all levels but lesser in 3-level discectomy | Up to 3 levels | 31.9 |
| Basques et al., 2019[39] | 379 | 20% adjacent segment disease | VAS and NDI improvement after surgery, but longer duration of radiculopathy poor improvement | 45% two-level, 30% single-level, and 25% 3-level | 28.2 |
| Shousha et al., 2019[40] | 2078 | 0.9% hematoma, dysphagia, and cage subsidence seen | VAS and NDI improved significantly, however reoperation rate higher in long-segment group | 40% single-level, 40% two-level, and 20% multiple-levels | 37.8 |
NDI – Neck disability index; VAS – Visual analog scale; SF-12 – Short Form 12; JOA – Japanese Orthopaedic Scale; ACDF – Anterior cervical discectomy and fusion; PEEK – Polyether ether ketone; PMMA – Polymethyl methacrylate; EDH – Epidural hematoma; r -BMP – Recombinant human bone morphogenetic protein-2
Table 6.
Studies comparing anterior cervical discectomy and fusion versus cervical disc arthroplasty
| Study | Design | Country | n (CDA/ACDF) | Age (years) |
|---|---|---|---|---|
| Porchet and Metcalf (2004)[41] | RCT | Switzerland | 27/28 | 44 |
| Mummaneni et al. (2007), Burkus et al. (2010, 2014), Gornet et al. (2019)[42,43,44,45] | RCT | USA | 275/265 | 43.5 |
| Nabhan et al. (2007, 2011)[46,47] | RCT | Germany | 20/21 | 44 |
| Murrey et al. (2008, 2009), Delamarter et al. (2010), Kelly et al. (2011), Kesman et al. (2012), Zigler et al. (2013), Zigler et al. (2013)[48,49,50,51,52,53,54] | RCT | USA | 103/106 | 42.5 |
| Sasso et al. (2007, 2008, 2011)[55,56,57] | RCT | USA | 242/241 | 44.7 |
| Riina et al. (2008)[58] | RCT | USA | 10/9 | 39 |
| Riew et al. (2008)[59] | RCT | USA | 59/52 | 45 |
| Heller et al. (2009)[60] | RCT | USA | 106/93 | 44.5 |
| Cheng et al. (2009, 2011)[61,62] | RCT | USA | 41/42 | 47.2 |
| Mcafee et al. (2010)[63] | RCT | USA | 151/100 | 44.5 |
| Coric et al. (2011)[64] | RCT | USA | 136/139 | 44 |
| Zhang et al. (2012)[65] | RCT | China | 60/60 | 44.8/45.6 |
| Vaccaro et al. (2013)[66] | RCT | USA | 236/144 | 44 |
| Davis et al. (2013, 2015)[67,68] | RCT | USA | 225/105 | 45.7 |
| Phillips et al. (2013, 2015)[69,70] | RCT | USA | 163/130 | 45.3/43.7 |
| Rozankovic et al. (2017)[71] | RCT | Croatia | 51/50 | 49 |
| Qizhi et al. (2016) [72] | RCT | China | 14/16 | 64.2 |
| Zhang et al. (2014)[73] | RCT | USA | 55/56 | 44.8 |
| Hisey et al. (2014, 2015)[74,75] | RCT | USA | 164/81 | 43.5 |
| Skeppholm (2015)[76] | RCT | Sweden | 73/80 | 42.2 |
| Jacksont et al. (2016)[77] | RCT | USA | 179/81 (single level), 231/105 (2 level) | - |
CDA – Cervical disc arthroplasty; ACDF – Anterior cervical discectomy and fusion; RCT – Randomized controlled trials
Table 7.
Comparison of the posterior foraminotomy versus anterior cervical discectomy for a single level radiculopathy
| Study | Design | Country | Number of cases | Surgical levels | Follow up | Mean age (years) | Outcome criteria | Clinical outcome (ACDF vs PCF) |
|---|---|---|---|---|---|---|---|---|
| Ruetten et al.[78] | RCT | Germany | ACDF: 86 PCF: 89 | Single-level | 2 years each | 43 | VAS, German version NASS, Hilibrand criteria | P>0.05 |
| Herkowitz et al.[79] | RCT | USA | ACDF: 17 PCF: 16 | Single-level | 4.2 years | ACDF: 43, PCF: 39 | Relief of pain and weakness | 94% versus 75% (P>0.05) |
| Wirth et al.[80] | RCT | USA | ACDF: 25 PCF: 22 | Single-level | 60 months each | ACDF: 41.7, PCF: 43.8 | Incidence of pain relief | 96% versus 100% (P>0.05) |
| Selvanathan et al.[81] | RCoS | UK | ACDF: 150 PCF: 51 | N/A | ACDF: 24±1.4 months PCF: 25±1.2 months |
ACDF: 48, PCF: 50 | NDI VAS neck and arm | P>0.05 |
| Korinth et al.[82] | RCoS | Germany | ACDF: 124 PCF: 168 | Single-level | 72.1±25.9 months | ACDF: 45.9±8.2, PCF: 46.9±10.4 | Success rate (Odom I+II) | 93.6% versus 85.1% (P<0.05) |
| Alvin et al.[83] | RCoS | USA | ACDF: 45 PCF: 25 | Single-level | 3 years | ACDF: 49.3±9.6, PCF: 46.5±11.32 | VAS, PDQ, PHQ-9, EQ-5D | P>0.05 |
NASS – North American spine society; EQ-5D – EuroQol-5 dimensions; NDI – Neck disability index; PCF – Posterior cervical foraminotomy; PDQ – Pain disability questionnaire; PHQ – Patient health questionnaire; RCoS – Retrospective comparative study; RCT – Randomized controlled trail; VAS – Visual analog scale; EQ-5D – Euro QOF5 dimensional; ACDF – Anterior cervical discectomy and fusion; N/A – Not applicable
Table 8.
Comparison of outcomes of anterior cervical discectomy and fusion versus corpectomy and fusion in cases of cervical myelopathy
| Study (year) | Design | Sample size | Mean age (years) | Gender (male/female) | Mean follow up (months) |
|---|---|---|---|---|---|
| Oh et al. (2009)[84] | RCT | ACCF: 17 ACDF: 14 |
ACCF: 55.12 ACDF: 52.64 |
16/15 | ACCF: 27.33 ACDF: 24.9 |
| Yu et al. (2007)[85] | RCT | ACCF: 20 ACDF: 20 |
ACCF: 53.1 ACDF: 52.75 |
ACCF: 14/6 ACDF: 15/5 |
N/A |
| Liu et al. (2011)[86] | RCoS | ACCF: 23 ACDF: 23 |
ACCF: 54.4 ACDF: 56.5 |
ACCF: 18/5 ACDF: 16/7 |
ACCF: 31 ACDF: 29 |
| Park et al. (2010)[87] | RCoS | ACCF: 52 ACDF: 45 |
ACCF: 49.4 ACDF: 49.3 |
ACCF: 30/22 ACDF: 17/28 |
ACCF: 23.3 ACDF: 25.7 |
| Wang et al. (2001)[88] | RCoS | ACCF: 20 ACDF: 32 |
ACCF: 51.5 ACDF: N/A |
27/25 | 43.2 |
| Yu et al. (2012)[89] | RCoS | ACCF: 48 ACDF: 62 |
ACCF: 59.3 ACDF: N/A |
65/45 | 32 |
| Jia et al. (2012)[90] | RCoS | ACCF: 36 ACDF: 31 |
ACCF: 48.83 ACDF: 49.12 |
ACCF: 21/15 ACDF: 17/14 |
ACCF: 28.96 ACDF: 26.81 |
ACDF – Anterior cervical discectomy and fusion; ACCF – Anterior cervical corpectomy and fusion; RCoS – Retrospective comparative study; RCT – Randomized controlled trail; N/A – Not applicable
Discussion
Transoral approach
The cranial base and upper cervical spine can be approached via the transoral–transpharyngeal route. Although it provides a small corridor, it can be used for drainage of an abscess, biopsies, and small tumor surgeries. Additional maxillectomy and mandibulotomy can be added when surgery is aimed at complete resection of low-grade malignant tumors of the skull base. Thus, an anatomical classification follows: transoral approach including or excluding maxillary osteotomy, with or without palatotomy, and the combined approach with transoral and transmandibular associated with displacement of the mandible (mandibular swing transcervical and bilateral mandibular osteotomies).
Surgical anatomy and preparation for the transoral approach
The key to understanding this approach lies in understanding the anatomy of two main structures: the pharyngeal wall and the vertebral artery. The pharyngeal wall consists of mucosa, under-lying prevertebral fascia, retropharyngeal space which contains pharyngeal branches from the carotid artery and pharyngeal veins, pharyngeal veins in between them. Prevertebral fascia with prevertebral musculature containing longus capitis and longus cervicis muscles lies posterior to it. On retraction of the prevertebral muscles, the anterior longitudinal ligament is seen which continues as atlanto-occipital membrane connecting the foramen magnum to the anterior arch of the atlas.
The vertebral artery runs through the transverse foramina of the cervical spine, from C5 to C2, and then runs posterolaterally to enter C1 transverse foramen. The artery will then line the vertebral artery groove, which is located on the posterosuperior aspect of the atlas before finally entering the foramen magnum. An important aspect to be remembered while drilling the C1 arch is the anteromedial location of C2 with respect to C1.
Pathologies
The earlier case series[6,7] reported that majority of surgeries were performed for pathologies such as basilar invagination with brainstem compression and odontoid fractures. Later series[13,14] suggested its utility in rheumatoid arthritis pannus excision, surgically treatable tumors, and infections such as Koch's spine, fungal infections of the clivus, and upper cervical spine.
Clinical evaluation before transoral approach
Specific issues particular to this approach need careful evaluation [Table 9].
Table 9.
Checklist of examinations before transoral approach
| Area | Review for |
|---|---|
| Dental hygiene | Dental caries- nidus of infection |
| Loose teeth | Put dental guards or remove the loose tooth |
| Lower cranial nerves | Look for |
| Gag reflex | |
| Uvula – central or pushed to one side | |
| Swallowing impaired leading to malnutrition | |
| Consent for tracheostomy | |
| Mouth opening | Jaw excursion should be at least 2.5 cm |
| Look for temporomandibular joint stiffness | |
| Infection | Prophylactic antibiotic coverage |
| Preoperative culture swabs | |
| Airway | Review for oro-tracheal versus nasotracheal intubation |
| Especially in cases that may need prolonged ventilation |
Positioning and preparation
The patient is placed in the supine position and intubated with preferably fiber-optic scope. The neck is placed in mild extension on horseshoe clamp and traction in applied. Neuromonitoring with motor evoked potentials and somatosensory potential monitoring should be used if available. Oral wash with chlorhexidine gluconate is done, and intravenous antibiotic covering aerobic and anerobic organisms is given. A self-retaining oral retractor such as Davis–Crowe or Spetzler–Sonntag transoral retractor is placed over the teeth and expanded to keep the mouth and tongue open. This tongue retraction is released intermittently every 30 min to relieve venous compression.
Operative steps
The soft palate is divided from the hard palate in the midline preserving uvula. The posterior pharyngeal mucosa is infiltrated with 1% lidocaine in 1:100,000 epinephrine and divided by taking a midline incision from the base of the clivus to the upper border of the third Cervical vertebra. The anterior tubercle of C1 may help in identifying the midline. Pharyngeal mucosa and longus coli and longus capitis musculature are elevated together as a single myomucosal flap and retracted using Crockard retractors. The anterior longitudinal ligament is dissected subperiosteally from the clivus and bodies of C1–C3.
This approach gives a lateral exposure of roughly 15–20 mm, either side of midline extending from the inferior part of clivus to the C3 body. Further lateral exposure increases the risk to the Eustachian tube orifice, hypoglossal nerve, vidian nerve, and carotid artery.[91] The maximum amount of bone that can be drilled safely is from midline 11 mm at the foramen magnum and 14 mm at the lower border of the axis.[92] Following the drilling of the anterior arch of C1, the odontoid is drilled from above downward. This avoids leaving a free-floating fragment of the dens as it is always attached at its base. Any additional soft tissue or apical and transverse ligaments can be removed. Wound closure proceeds sequentially using monofilament 2-0 suture in an intermittent pattern. Immediate posterior stabilization is preferred in the same sitting [Figure 7].
Figure 7.
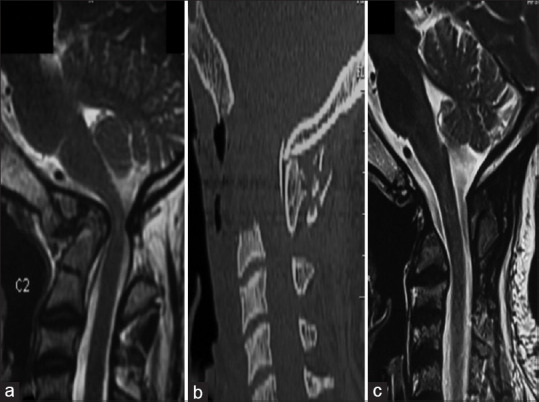
(a) Magnetic resonance imaging T2-weighted sagittal images showing atlantoaxial dislocation with retroflexed odontoid, causing compression of the cervical cord along with cord signal changes. (b and c) Postoperative sagittal computed tomography and magnetic resonance imaging T2-weighted images showing decompression of the cervical cord with transoral decompression of the odontoid
Outcomes [Table 3][6,7,8,9,10,11,12,13,14]
The analysis of our case series shows that patients who had ventral compression over the cervical spine had a good outcome in 35%–95% of patients and required stabilization in a majority of them.
Complications
Specific unique complications to this approach are present and must be kept in mind [Table 10].
Table 10.
Complications of the transoral approach
| Complication | Management |
|---|---|
| CSF leak | Avoid if possible Avoid aggressive resection of pannus Primary repair using graft Lumbar drain placement Re-exploration and repair |
| Tongue swelling | Keep endotracheal tube for 24 h to avoid respiratory distress |
| Infection | Preoperative nasogastric tube for draining gastric contents Feeding after 48–72 h Antibiotics for min 3 days |
| Wound breakdown | Tension-free closure Periodic wound inspection |
| Nutrition | High-protein diet Preoperative optimization |
CSF – Cerebrospinal fluid
Potential limitations
Instability
The approach involves resection of anterior arch of C1, C2,tectorial membrane, anterior longitudinal ligament which can lead to instability; thus, fusion was done in same setting by Crockard et al.[93] Dickman et al.[8] observed that instability in congenital Atlantoaxial-Dislocation is less frequent when compared to rheumatoid or traumatic dislocations and requires fixation in 45% of cases.
Inadequate decompression
The maximum lateral exposure is limited to 3–4 cm due to critical structures involved laterally.[10] A palatal split or open-door maxillotomy[9] facilitates rostral exposure of the clivus,[9,94] and median labiomandibular glossotomy[9] helps in caudal exposure. The dural bulge seen after excision of the tectorial membrane marks the endpoint of anterior decompression in transoral surgery.
Neurological deterioration
Tuite et al.[9] showed that neurological morbidity is proportional to the severity of preoperative neurological deficits. These can be explained by repeated trauma, which leads to gliosis of anterior horn cell, gracile, and cuneate nuclei and demyelination of the white fiber tracts.[94,95] During surgery, hypoxia secondary to venous stasis or vertebral or spinal arteries injury can worsen it.[96,97,98]
Cerebrospinal fluid leak
Dural imploding over the tip of the odontoid is responsible for most of the cases of dural breach. Pásztor[99] recommended the use of a diamond drill instead of a steel drill while approaching a deeper part of the dens to prevent injury to a posterior longitudinal ligament or the dura if the dens is breached. Drilling the dens from its base as a whole piece is facilitated due to lower bone mass strength of up to 55% compared to the rest of the axis. The posterior cortex of the dens was separated from the posterior longitudinal ligament and dura, followed by the removal of its apex. A Valsalva maneuver has to be performed to confirm the CSF leak if present.[100]
Pharyngeal sepsis
Transoral surgery is based on the belief that oral mucosa is resistant to local bacteria flora. However, the evidence is contrary to belief. Jain et al.[10] in a series of 74 patients demonstrated a high incidence of wound sepsis and dehiscence despite adequate antibiotics and layered wound closure. The various reasons are already infected oral cavity, superinfection with Candida and anaerobic organisms, retropharyngeal hematoma due to deviation from the midline, and excessive usage of cautery for longus coli dissection. Decreased oral intake in the postoperative period impairs wound healing. This can be prevented by midline approach, avoid excessive debris, obliteration of retropharyngeal cavity with fat, and opening caudal end of the suture to prevent hematoma formation. Early ambulation of the patients prevents saliva pooling at the apex of the incision, which is a relatively weaker point.[101]
Rhinolalia and regurgitation
The soft palate may need to be resected to approach the lower clivus and thus may predispose to nasal regurgitation. Rhinolalia and palatal wound dehiscence can be treated with secondary suturing of the wound. Nasal intonation and dysphagia can be due to scarred pharynx, large dead space in the posterior pharyngeal wall, leading to abnormal palatal and pharyngeal closure, or lower cranial nerve deficits. Corrective measures such as palatal prosthesis or pharyngoplasty[9] can be done.
Anterolateral approach
This is the more common approach to the anterior cervical spine, particularly C3–T1 vertebral bodies, introduced by Robinson and Smith[1] and modified later by Southwick and Robinson.[2]
Although there has been some disagreement regarding ACD versus posterior approach, it can be safely said that intervertebral disc disease remains the most common indication for it. Again, certain fractures are also best treated with the anterolateral approach. Burst fractures with or without retropulsion of the bone or disc fragments are probably best treated via the anterior route. Adequate decompression and stabilization can be achieved, leading to favorable results. However, flexion injuries with anterior displacement of one vertebra over the other with unilateral or bilateral facet locking may need posterior “unlocking” and supplementary fixation too. The most significant advantage, by far, of this approach, is the correction of kyphosis.
Various other neoplastic lesions may be amenable to anterior cervical approaches such as metastatic carcinoma or multiple myeloma, eosinophilic granuloma, and aneurysmal bone cyst. This may mainly be useful when the posterior elements have been destroyed, and anterior stabilization remains the only option.
Infections and inflammatory conditions such as rheumatoid arthritis, postlaminectomy swan neck deformity, and congenital abnormalities can also be corrected using this approach.
Technique
Positioning and side
The patient is placed in the supine position on a horseshoe-shaped headrest and with a rolled towel placed transversely in between the shoulder to allow slight extension of the neck. In general, the C2–C6 spine is approached from the right side.[102] Other considerations, such as prior surgery, local injury, or infection, guide the side of the approach. Intraoperative traction by Mayfield clamps or Garner–Wells tongs is used to allow for the interbody graft to fit in snugly and where some deformity correction is needed. The neck and head is stabilized with a head cushion. Preoperative level confirmation using a radiopaque marker is done, and the incision is taken at the middle of the level of surgery or cranial to it allowing retraction caudally.[103]
Operative steps
A transverse skin crease incision of 3–5 cm in length is enough to expose 2–3-disc levels, whereas a longitudinal incision anterior to the sternocleidomastoid muscle is taken for the extensive procedure. A subplatysmal dissection is done to increase the exposure, followed by blunt dissection to reach the vertebral body. The trachea, esophagus and recurrent laryngeal nerve are retracted medially with carotid sheath retracted laterally. The retraction must be dynamic or fixed with airway pressure variating to protect the esophagus from pressure necrosis. The exposure can be extended superiorly up to C2–C3 level by ligating the middle thyroid veins[103] and inferiorly by transecting the omohyoid muscle. The longus colli are identified and dissected off on either side of the midline.
Discectomy
After intraoperative confirmation of the level, discectomy is started following certain principles. First, the width of decompression is considered adequate only when both uncovertebral joints are seen covering a width of 15 mm. The only caveat while dissecting laterally an aberrantly medial vertebral artery might be encountered in the middle of the vertebral body, which may be 0.14 mm medial to the uncovertebral joint.[104] Second, the posterior annulus and the posterior longitudinal ligament are removed routinely to ensure all sequestrated disc fragments have been removed [Figure 8].
Figure 8.
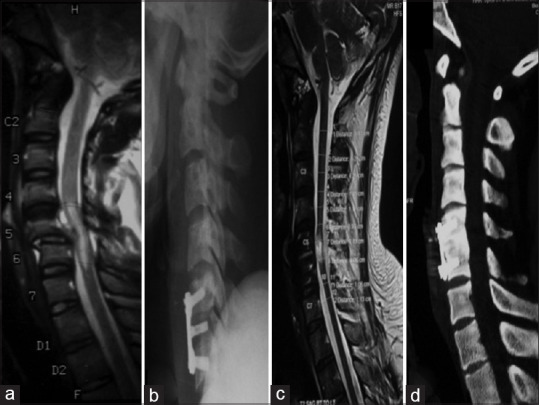
(a) Magnetic resonance imaging T2-weighted sagittal images showing subluxation of the C5–C6 vertebrae causing compression of the cervical cord along with cord signal changes. (b-d) Postoperative sagittal X-ray, computed tomography, and magnetic resonance imaging T2-weighted images showing decompression and realignment of the cervical cord with fixation using cervical plates and screws
The midcervical anterolateral approach deals with the majority of pathologies involving atraumatic dissection to the midcervical elements with minimal morbidity.
The review of the larger case series in Table 10 suggests that the symptoms improve significantly after ACDF. The additional removal of the uncinate process has a better outcome in the pain score of the arm.[36]
Complications [Table 10][22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]
The reoperation rates have been reported by up to 4% (Liu). The complications include dysphagia, hematoma, and recurrent laryngeal nerve palsy, which are generally transient, which improve from an immediate postoperative rate of 9.4%–3.4% after 3 months.[25] Long-term complications included pseudarthrosis and adjacent segment disease in 10%[94] after fusion.
Anterior cervical discectomy versus cervical disc arthroplasty [Table 5][41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]
In a systematic review by Xie, et al.,[105], cervical disc arthroplasty had better improvement in arm, neck pain score, decreased re-operation rate and adjacent segment disease compared to anterior cervical discectomy. However, the operative time was significantly higher in the arthroplasty group. The clinical improvement results were concordant with our analysis of the studies included.
Anterior cervical discectomy and fusion versus posterior foraminotomy [Table 6][78,79,80,81,82,83]
With complications of adjacent segment disease in ACDF, there has been a debate if foraminotomy could give in similar clinical results in cervical radiculopathy. In a review by Liu et al.,[106] clinical outcomes of ACDF and posterior cervical foraminotomy were similar, with no statistical difference seen. The range of motion was compared in Alvin et al.,[83] where they found that operated segment had no motion in the ACDF group, while they had 8.82° ±6.65° in the foraminotomy group. Although the complication rate in foraminotomy was lower, i.e., 4% versus 7% when compared to ACDF group, it was not significant. Despite the short-term advantages of foraminotomy, the resurgery rates are higher in the foraminotomy group, i.e., of 6% versus 4% compared to ACDF.[106] Thus, this establishes posterior cervical foraminotomy as one of the treatment modalities of radiculopathies with lower costs.
Multiple-level disease
In the review of the case series of ACDF, the surgery was performed up to two levels in 74%–93% cases; however, certain case series have extended the use up to 4 levels.[23,25,27,31,40] These studies suggest that there has been no difference in outcome score when compared either to single- or two-level disease. However, in a study by Shin et al.,[38] they found a significant decrease in range of motion with an increasing number of fusion levels and increasing adjacent segment disease of 39% versus 14% (4 vs. 1 level) fusion. This finding was further complemented by the study by Shousha et al.,[40] where they found a higher reoperation rate in the long-segment group of 7% versus 5% in short-segment group mainly due to operative site hematomas and pseudarthrosis in them.
Anterior cervical discectomy and fusion versus anterior cervical corpectomy and fusion
Anterior cervical corpectomy was found to have better outcome with decreased complication rates over multilevel ACDF in treating multi-level disc pathologies.
Our review of the cases, as tabulated in Table 7,[84,85,86,87,88,89,90] found that there was no significant difference in clinical outcomes between the two groups. However, Wang et al.[107] reported that the Cobb angle of C2–C7 was increased by higher amounts in the ACDF group compared to the corpectomy group, owing to increase points of distraction. The ACDF group had a decreased incidence of graft subsidence, but the graft dislodgment rate was similar in both groups. The fusion rate is better in the ACDF group compared to the anterior cervical corpectomy group with no difference in the complication rate of pseudarthrosis or local complication [Figure 9].
Figure 9.
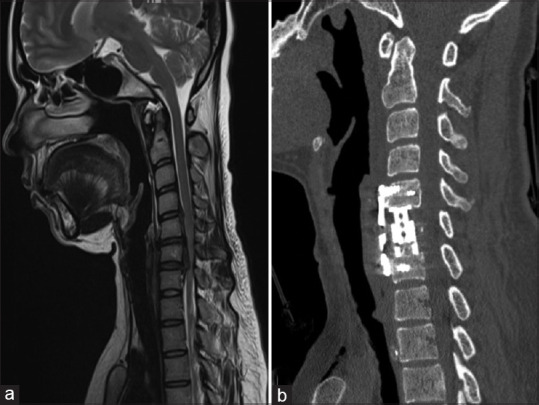
(a) Magnetic resonance imaging T2-weighted sagittal images showing C5–C6 level OPLL with kyphosis causing compression of the cervical cord pronounced at the C6 level. (b) Postoperative sagittal computed tomography images showing decompression and restoration of the cervical lordosis after C6 corpectomy and fixation using cervical plates and screws
Anterior cervical discectomy and fusion versus posterior laminoplasty
A systematic review done by Montano et al.[108] showed similar JOA score improvement and postoperative complication rate in both the groups. The cervical lordosis was better in the ACDF group up to 19.13 ± 3 grades versus 13.82 ± 0.92 grades in the laminoplasty group. Thus, ACDF is a better treatment option over laminoplasty, and further randomized control trials are required over this subject.
Transmanubrium approaches
Although the anterolateral approach allows access to the cervical column, the location of manubrial notch dictates the lower limit of this approach. A shorter stout neck with a high riding manubrium often presents a difficulty in exposing the cervicothoracic junction. The lower limit of cervical exposure, which is usually T2, can be determined by viewing the upper thoracic/lower cervical spine and then drawing a horizontal line from the upper border of the manubrial notch to the spine.
Splitting of the manubrium, with minimal morbidity, was described by Louis et al.,[109] where the anterior Smith–Robinson approach is combined with sternal splitting. It allows for exposure up to the T4 level and avoids the morbidity of a manubrial split or a clavicular osteotomy.
Indications
The cervicothoracic junction represents a transition from a lordotic mobile cervical spine to a kyphotic rigid thoracic spine,[110] thus placing it a risk of injury, owing to the transfer of weight from anterior to posterior column[111] and decrease of the vertebral index from above downward.[112]
Pathologies affecting this area include trauma, tumors, degenerative spine, and infections, which occur in the anterior segment of the vertebrae, causing instability.[113] This leads to progressive kyphosis and compression of the spinal cord, with neurological deterioration rates as high as 80%.[15] Dorsal decompression is often limited due to inadequate anterior decompression and potential destabilization of the junction with difficulty in fixation.
Preoperative considerations
Anterior approaches to the cervicothoracic spine depend on many factors: narrow corridor due to manubrium, ribs, and clavicle and vital structures nearby, such as the esophagus, trachea, great blood vessels, thoracic duct recurrent laryngeal nerve, and sympathetic ganglions. The manubrium can either be split in an L-shaped manner, which allows for an additional 4 cm width of exposure or an inverted T-shaped split, which allows for 8 cm width. Computed tomography or magnetic resonance imaging with the patient in the extended position is done. Following features are identified:
Spinal cervicothoracic curvature
A line intersecting the vertebral body from the superior border of the manubrium
The superior border of the vertebral body plateau, which is drilled until the vertebral canal is seen
Location of vessels such as aortic arch, brachiocephalic vein, and right brachiocephalic trunk.[15]
Manubriotomy is decided to depend on the surgeon's operative view. In essence, a line was drawn along the superior plateau of the vertebral body planned to be resected extending anteriorly to the sternum; if this line lies above the manubrium, manubriotomy is not required.
The patient is positioned like the Smith–Robinson method with a neck in moderate extension with a shoulder roll. Traction is applied to wrists with traction bands for better imaging.
Technique
A longitudinal incision parallel to the sternocleidomastoid extending to the midline is made starting at the manubrium and reaching caudally up to Louis angle. Blunt dissection is used to release soft tissue from manubrium posteriorly; thymus may be encountered in younger patients. The internal thoracic artery is dissected and ligated at the level of the second intercostal space, where the transverse limb of the osteotomy will be made. A unilateral or bilateral transverse cut is made with an oscillating saw to increase the width of exposure. Vertical retraction increases the exposure to the anterior mediastinum.
The structures such as the common carotid artery on the left and brachiocephalic artery and vein on the right, trachea, and esophagus on the floor are encountered. The ascending aorta can also be accessed only if dissection carried below T4, till the upper border of the heart, while the thoracic duct exposure is only possible on the left side exposure. After addressing the pathology, reconstruction is done using a mesh or interbody graft and fixed with plates. A suction drain placement is always advisable. The sternum is approximated with the number three steel wires.
Outcomes and complications [Table 8][15,16,17,18,19,20,21]
The review of our case series suggests that when performed with dissection in appropriate planes, adequate exposure up to T4 levels is obtained, allowing adequate reconstruction of the spine. Despite immediate postoperative pain, there is an improvement in the clinical outcomes of the patient.
Vocal cord paresis due to recurrent laryngeal nerve injury[15,16,17,18,19,20,21] has been reported in 4.76%–16.67% of patients. Various causes include direct injury, traction injury to nerves[114] at the point of upturn, anatomic variations, and misidentification of the nerve, leading to en masse ligation of the inferior thyroid vessels along with the nerve, rarely due to endotracheal tube compressing the nerve.[115]
Left-sided exposure has its unique complications, especially thoracic duct injury. The thoracic duct ascends behind the subclavian artery from the thoracic inlet and later arches behind carotid sheath at C7 and later enters into internal jugular vein turning forward on anterior scalene muscle. The exact location can be described as found inside a triangle, which is bounded medially by the longus coli muscles and the esophagus and posteriorly by the first rib at the level of C7–T1 vertebra.[116] Although dissection is not usually required, if the thoracic duct becomes visible, it has to be retracted laterally along with the carotid sheath. In case of injury with chyle leak, it must be double ligated both proximally and distally.[117]
Conclusion
The cervical spine is affected by several pathologies and many of which affect the anterior two-third of the construct. Consequently, anterior approaches are essential and fortunately have a shorter learning curve. Simple anatomical boundaries and meticulous soft tissue dissection are the keys to this elegant approach. From the lesser frequently used transoral approach to the turnpike of anterolateral approaches, a blood-less corridor to cervical spine is obtained. If the manubrium sterni is split, it opens a new window, which allows exposure up to even the T4 level and expands our horizon. Masterly of these is pertinent and useful.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40-A:607–24. [PubMed] [Google Scholar]
- 2.Robinson RA, Southwick WO. Surgical approaches to the cervical spine. Instr Course Lect. 1960;17:299–330. [PubMed] [Google Scholar]
- 3.Bailey RW, Badgley CE. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg Am. 1960;42-A:565–94. [PubMed] [Google Scholar]
- 4.Naderi S, Alberstone CD, Rupp FW, Benzel EC, Baldwin NG. Cervical spondylotic myelopathy treated with corpectomy: Technique and results in 44 patients. Neurosurg Focus. 1996;1:e5. doi: 10.3171/foc.1996.1.6.6. [DOI] [PubMed] [Google Scholar]
- 5.Luk KD, Cheung KM, Leong JC. Anterior approach to the cervicothoracic junction by unilateral or bilateral manubriotomy. A report of five cases. J Bone Joint Surg Am. 2002;84:1013–7. doi: 10.2106/00004623-200206000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Crockard HA, Pozo JL, Ransford AO, Stevens JM, Kendall BE, Essigman WK. Transoral decompression and posterior fusion for rheumatoid atlantoaxial subluxation. J Bone Joint Surg. 1986;68:350–6. doi: 10.1302/0301-620X.68B3.3733795. [DOI] [PubMed] [Google Scholar]
- 7.Hadley MN, Spetzler RF, Sonntag VH. The transoral approach to the superior cervical spine. J Neurosurg. 1989;71:16–23. doi: 10.3171/jns.1989.71.1.0016. [DOI] [PubMed] [Google Scholar]
- 8.Dickman CA, Locantro J, Fessler RG. The influence of transoral odontoid resection on stability of the craniovertebral junction. J Neurosurg. 1992;77:525–30. doi: 10.3171/jns.1992.77.4.0525. [DOI] [PubMed] [Google Scholar]
- 9.Tuite GF, Veres R, Crockard HA, Sell D. Pediatric transoral surgery: Indications, complications, and long-term outcome. J Neurosurg. 1996;84:573–83. doi: 10.3171/jns.1996.84.4.0573. [DOI] [PubMed] [Google Scholar]
- 10.Jain VK, Behari S, Banerji D, Bhargava V, Chhabra DK. Transoral decompression for craniovertebral osseous anomalies: Perioperative management dilemmas. Neurol India. 1999;47:188–95. [PubMed] [Google Scholar]
- 11.Menezes AH. Surgical approaches: Postoperative care and complications “transoral-transpalatopharyngeal approach to the craniocervical junction“. Childs Nerv Syst. 2008;24:1187–93. doi: 10.1007/s00381-008-0599-3. [DOI] [PubMed] [Google Scholar]
- 12.Mouchaty H, Perrini P, Conti R, Di Lorenzo N. Craniovertebral junction lesions: Our experience with the transoral surgical approach. Eur Spine J. 2009;18(Suppl 1):13–9. doi: 10.1007/s00586-009-0988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shousha M, Mosafer A, Boehm H. Infection rate after transoral approach for the upper cervical spine. Spine (Phila Pa 1976) 2014;39:1578–83. doi: 10.1097/BRS.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 14.Elbadrawi AM, Elkhateeb TM. Transoral approach for odontoidectomy efficacy and safety. HSS J. 2017;13:276–81. doi: 10.1007/s11420-016-9535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao ZM, Zhan XL, Gong DF, De Li S. Surgical management for upper thoracic spine tumors by a transmanubrium approach and a new space. Eur Spine J. 2007;16:439–44. doi: 10.1007/s00586-006-0239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YL, Hao YJ, Li T, Song YM, Wang LM. Trans-upper-sternal approach to the cervicothoracic junction. Clin Orthop Relat Res. 2009;467:2018–24. doi: 10.1007/s11999-008-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falavigna A, Righesso O, Pinto D, Teles A, Kleber FD. Anterior approach to the cervicothoracic junction: Case series and literature review. Coluna/columna. 2009:8. doi:10.1590/S1808-18512009000200010. [Google Scholar]
- 18.Jiang H, Xiao ZM, Zhan XL, He ML. Anterior transsternal approach for treatment of upper thoracic vertebral tuberculosis. Orthop Surg. 2010;2:305–9. doi: 10.1111/j.1757-7861.2010.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zengming X, He M, Xinli Z, Qianfen C. Anterior transsternal approach for a lesion in the upper thoracic vertebral body. J Neurosurg Spine. 2010;13:461–8. doi: 10.3171/2010.4.SPINE09808. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Im SB, Jeong JH, Hwang SC, Shin DS, Kim BT. The transmanubrial approach for cervicothoracic junction lesions: Feasibility, limitations, and advantages. J Korean Neurosurg Soc. 2015;58:236–41. doi: 10.3340/jkns.2015.58.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihir B, Laheri V, Umesh M, Kshitij C. Anterior instrumentation of the cervicothoracic vertebrae. Spine. 2006;31:E244–9. doi: 10.1097/01.brs.0000214883.11874.80. [DOI] [PubMed] [Google Scholar]
- 22.Marotta N, Landi A, Tarantino R, Mancarella C, Ruggeri A, Delfini R. Five-year outcome of standalone fusion using carbon cages in cervical disc arthrosis. Eur Spine J. 2011;20(Suppl 1):S8–12. doi: 10.1007/s00586-011-1747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu DC, Tumialán LM, Chou D. Multilevel anterior cervical discectomy and fusion with and without rhBMP-2: A comparison of dysphagia rates and outcomes in 150 patients. J Neurosurg Spine. 2013;18:43–9. doi: 10.3171/2012.10.SPINE10231. [DOI] [PubMed] [Google Scholar]
- 24.Klingler JH, Krüger MT, Sircar R, Kogias E, Scholz C, Volz F, et al. PEEK cages versus PMMA spacers in anterior cervical discectomy: Comparison of fusion, subsidence, sagittal alignment, and clinical outcome with a minimum 1-year follow-up. ScientificWorldJournal. 2014;2014:398396. doi: 10.1155/2014/398396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Zhao Y, Tang J, Ren D, Guo J, Wang H, et al. A comparison of a new zero-profile, stand-alone Fidji cervical cage and anterior cervical plate for single and multilevel ACDF: A minimum 2-year follow-up study. Eur Spine J. 2017;26:1129–39. doi: 10.1007/s00586-016-4739-2. [DOI] [PubMed] [Google Scholar]
- 26.Zigler JE, Rogers RW, Ohnmeiss DD. Comparison of 1-level versus 2-level anterior cervical discectomy and fusion. SPINE. 2016;41:463–9. doi: 10.1097/BRS.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 27.Tasiou A, Giannis T, Brotis AG, Siasios I, Georgiadis I, Gatos H, et al. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J Spine Surg. 2017;3:444–59. doi: 10.21037/jss.2017.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkhardt BW, Brielmaier M, Schwerdtfeger K, Oertel JM. Clinical outcome following anterior cervical discectomy and fusion with and without anterior cervical plating for the treatment of cervical disc herniation – A 25-year follow-up study. Neurosurg Rev. 2018;41:473–82. doi: 10.1007/s10143-017-0872-6. [DOI] [PubMed] [Google Scholar]
- 29.Grasso G, Landi A. Long-term clinical and radiological outcomes following anterior cervical discectomy and fusion by zero-profile anchored cage. J Craniovertebr Junction Spine. 2018;9:87–92. doi: 10.4103/jcvjs.JCVJS_36_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumialán LM, Ponton RP, Cooper AN, Gluf WM, Tomlin JM. Rate of return to military active duty after single and 2-level anterior cervical discectomy and fusion: A 4-year retrospective review. Neurosurgery. 2019;85:96–104. doi: 10.1093/neuros/nyy230. [DOI] [PubMed] [Google Scholar]
- 31.Mullins J, Pojskić M, Boop FA, Arnautović KI. Retrospective single-surgeon study of 1123 consecutive cases of anterior cervical discectomy and fusion: A comparison of clinical outcome parameters, complication rates, and costs between outpatient and inpatient surgery groups, with a literature review. J Neurosurg Spine. 2018;28:630–41. doi: 10.3171/2017.10.SPINE17938. [DOI] [PubMed] [Google Scholar]
- 32.He S, Feng H, Lan Z, Lai J, Sun Z, Wang Y, et al. A randomized trial comparing clinical outcomes between zero-profile and traditional multilevel anterior cervical discectomy and fusion surgery for cervical myelopathy. Spine (Phila Pa 1976) 2018;43:E259–66. doi: 10.1097/BRS.0000000000002323. [DOI] [PubMed] [Google Scholar]
- 33.Muzevic D, Splavski B, Boop FA, Arnautović KI. Anterior cervical discectomy with instrumented allograft fusion: Lordosis restoration and comparison of functional outcomes among patients of different age groups. World Neurosurg. 2018;109:e233–43. doi: 10.1016/j.wneu.2017.09.146. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Ha Y, Shin JJ, Oh JK, Lee CK, Kim KN, et al. Influence of plate fixation on cervical height and alignment after one- or two-level anterior cervical discectomy and fusion. Br J Neurosurg. 2018;32:188–95. doi: 10.1080/02688697.2017.1394980. [DOI] [PubMed] [Google Scholar]
- 35.Buttermann GR. Anterior cervical discectomy and fusion outcomes over 10 years: A prospective study. Spine (Phila Pa 1976) 2018;43:207–14. doi: 10.1097/BRS.0000000000002273. [DOI] [PubMed] [Google Scholar]
- 36.Lee DH, Cho JH, Baik JM, Joo YS, Park S, Min WK, et al. Does additional uncinate resection increase pseudarthrosis following anterior cervical discectomy and fusion? Spine. 2018;43:97–104. doi: 10.1097/BRS.0000000000002271. [DOI] [PubMed] [Google Scholar]
- 37.Yang S, Yu Y, Liu X, Zhang Z, Hou T, Xu J, et al. Clinical and radiological results comparison of allograft and polyetheretherketone cage for one to two-level anterior cervical discectomy and fusion: A CONSORT-compliant article. Medicine (Baltimore) 2019;98:e17935. doi: 10.1097/MD.0000000000017935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin JJ. Comparison of adjacent segment degeneration, cervical alignment, and clinical outcomes after one- and multilevel anterior cervical discectomy and fusion. Neurospine. 2019;16:589–600. doi: 10.14245/ns.1938166.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basques BA, Ahn J, Markowitz J, Harada G, Louie PK, Mormol J, et al. Does the duration of cervical radicular symptoms impact outcomes after anterior cervical discectomy and fusion? Clin Spine Surg. 2019;32:387–91. doi: 10.1097/BSD.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 40.Shousha M, Alhashash M, Allouch H, Boehm H. Reoperation rate after anterior cervical discectomy and fusion using standalone cages in degenerative disease: A study of 2,078 cases. Spine J. 2019;19:2007–12. doi: 10.1016/j.spinee.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Porchet F, Metcalf NH. Clinical outcomes with the Prestige II cervical disc: Preliminary results from a prospective randomized clinical trial. Neurosurg Focus. 2004;17:E6. doi: 10.3171/foc.2004.17.3.6. [DOI] [PubMed] [Google Scholar]
- 42.Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: A randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 43.Burkus JK, Haid RW, Traynelis VC, Mummaneni PV. Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: Results from a prospective randomized controlled clinical trial. J Neurosurg Spine. 2010;13:308–18. doi: 10.3171/2010.3.SPINE09513. [DOI] [PubMed] [Google Scholar]
- 44.Burkus JK, Traynelis VC, Haid RW, Jr, Mummaneni PV. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article. J Neurosurg Spine. 2014;21:516–28. doi: 10.3171/2014.6.SPINE13996. [DOI] [PubMed] [Google Scholar]
- 45.Gornet MF, Lanman TH, Burkus J, Dryer RF, McConnell JR, Hodges SD, et al. Two-level cervical disc arthroplasty versus anterior cervical discectomy and fusion: 10-year outcomes of a prospective, randomized investigational device exemption clinical trial. J Neurosurg Spine. 2019;31:508–18. doi: 10.3171/2019.4.SPINE19157. [DOI] [PubMed] [Google Scholar]
- 46.Nabhan A, Ishak B, Steudel WI, Ramadhan S, Steimer O. Assessment of adjacent-segment mobility after cervical disc replacement versus fusion: RCT with 1 year's results. Eur Spine J. 2011;20:934–41. doi: 10.1007/s00586-010-1588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nabhan A, Ahlhelm F, Pitzen T, Steudel WI, Jung J, Shariat K, et al. Disc replacement using Pro-Disc C versus fusion: A prospective randomized and controlled radiographic and clinical study. Eur Spine J. 2007;16:423–30. doi: 10.1007/s00586-006-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murrey DB, Janssen ME, Odum SM, Gottlieb JR, Spector LR, Darden BV. Two-year results of a randomized controlled clinical trial comparing ProDisc-C and anterior cervical discectomy and fusion. SAS J. 2008;2:76–85. doi: 10.1016/SASJ-2007-0124-RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B, Darden B. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of theProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275–86. doi: 10.1016/j.spinee.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Delamarter RB, Murrey D, Janssen ME, Goldstein JA, Zigler J, Tay BK, et al. Results at 24 months from the prospective, randomized, multicenter Investigational Device Exemption trial of ProDisc-C versus anterior cervical discectomy and fusion with 4-year follow-up and continued access patients. SAS J. 2010;4:122–8. doi: 10.1016/j.esas.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delamarter RB, Zigler J. Five-year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine. 2013;38:711–7. doi: 10.1097/BRS.0b013e3182797592. [DOI] [PubMed] [Google Scholar]
- 52.Kelly MP, Mok JM, Frisch RF, Tay BK. Adjacent segment motion after anterior cervical discectomy and fusion versus Prodisc-c cervical total disk arthroplasty: Analysis from a randomized, controlled trial. Spine. 2011;36:1171–9. doi: 10.1097/BRS.0b013e3181ec5c7d. [DOI] [PubMed] [Google Scholar]
- 53.Kesman T, Murrey D, Darden B. Single-center results at 7 years of prospective, randomized ProDisc-C total disc arthroplasty versus anterior cervical discectomy and fusion for treatment of one level symptomatic cervical disc disease. Evid Based Spine Care J. 2012;3:61–2. doi: 10.1055/s-0032-1328144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zigler JE, Delamarter R, Murrey D, Spivak J, Janssen M. ProDisc-C and anterior cervical discectomy and fusion as surgical treatment for single-level cervical symptomatic degenerative disc disease: Five-year results of a Food and Drug Administration study. Spine. 2013;38:203–9. doi: 10.1097/BRS.0b013e318278eb38. [DOI] [PubMed] [Google Scholar]
- 55.Sasso RC, Smucker JD, Hacker RJ, Heller JG. Clinical outcomes of BRYAN cervical disc arthroplasty: A prospective, randomized, controlled, multicenter trial with 24-month follow-up. J Spinal Disord Tech. 2007;20:481–91. doi: 10.1097/BSD.0b013e3180310534. [DOI] [PubMed] [Google Scholar]
- 56.Sasso RC, Best NM, Metcalf NH, Anderson PA. Motion analysis of Bryan cervical disc arthroplasty versus anterior discectomy and fusion: Results from a prospective, randomized, multicenter, clinical trial. J Spinal Disord Tech. 2008;21:393–9. doi: 10.1097/BSD.0b013e318150d121. [DOI] [PubMed] [Google Scholar]
- 57.Sasso RC, Anderson PA, Riew KD, Heller JG. Results of cervical arthroplasty compared with anterior discectomy and fusion: Four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am. 2011;93:1684–92. doi: 10.2106/JBJS.J.00476. [DOI] [PubMed] [Google Scholar]
- 58.Riina J, Patel A, Dietz JW, Hoskins JS, Trammell TR, Schwartz DD. Comparison of single-level cervical fusion and a metal-on-metal cervical disc replacement device. Am J Orthop (Belle Mead NJ) 2008;37:E71–7. [PubMed] [Google Scholar]
- 59.Riew KD, Buchowski JM, Sasso R, Zdeblick T, Metcalf NH, Anderson PA. Cervical disc arthroplasty compared with arthrodesis for the treatment of myelopathy. J Bone Joint Surg Am. 2008;90:2354–64. doi: 10.2106/JBJS.G.01608. [DOI] [PubMed] [Google Scholar]
- 60.Heller JG, Sasso RC, Papadopoulos SM, Anderson PA, Fessler RG, Hacker RJ, et al. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: Clinical and radiographic results of a randomized, controlled, clinical trial. Spine. 2009;34:101–7. doi: 10.1097/BRS.0b013e31818ee263. [DOI] [PubMed] [Google Scholar]
- 61.Cheng L, Nie L, Zhang L, Hou Y. Fusion versus Bryan Cervical Disc in two-level cervical disc disease: A prospective, randomized study. Int Orthop. 2009;33:1347–51. doi: 10.1007/s00264-008-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng L, Nie L, Li M, Huo Y, Pan X. Superiority of the Bryan((R)) disc prosthesis for cervical myelopathy: A randomized study with a 3-year follow-up. Clin Orthop Relat Res. 2011;469:3408–11. doi: 10.1007/s11999-011-2039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McAfee PC, Cappuccino A, Cunningham BW, Devine JG, Phillips FM, Regan JJ, et al. Lower incidence of dysphagia with cervical arthroplasty compared with ACDF in a prospective randomized clinical trial. J Spinal Disord Tech. 2010;23:1–8. doi: 10.1097/BSD.0b013e31819e2ab8. [DOI] [PubMed] [Google Scholar]
- 64.Coric D, Nunley PD, Guyer RD, Musante D, Carmody CN, Gordon CR, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: Clinical article. J Neurosurg Spine. 2011;15:348–58. doi: 10.3171/2011.5.SPINE10769. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Xuesong, Zhang Xuelian, Chen C, Zhang Y, Wang Z, Wang B, et al. Randomized, controlled, multicenter, clinical trial comparing BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine. 2012;37:433–8. doi: 10.1097/BRS.0b013e31822699fa. [DOI] [PubMed] [Google Scholar]
- 66.Vaccaro A, Beutler W, Peppelman W, Marzluff JM, Highsmith J, Mugglin A, et al. Clinical outcomes with selectively constrained SECURE-C cervical disc arthroplasty: Two-year results from a prospective, randomized, controlled, multicenter investigational device exemption study. Spine. 2013;38:2227–39. doi: 10.1097/BRS.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 67.Davis RJ, Kim KD, Hisey MS, Hoffman GA, Bae HW, Gaede SE, et al. Cervical total disc replacement with the Mobi-C cervical artificial disc compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: A prospective, randomized, controlled multicenter clinical trial: Clinical article. J Neurosurg Spine. 2013;19:532–45. doi: 10.3171/2013.6.SPINE12527. [DOI] [PubMed] [Google Scholar]
- 68.Davis RJ, Nunley PD, Kim KD, Hisey MS, Jackson RJ, Bae HW, et al. Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: A prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine. 2015;22:15–25. doi: 10.3171/2014.7.SPINE13953. [DOI] [PubMed] [Google Scholar]
- 69.Phillips FM, Lee JY, Geisler FH, Cappuccino A, Chaput CD, DeVine JG, et al. A prospective, randomized, controlled clinical investigation comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion.2-year results from the US FDA IDE clinical trial. Spine (Phila Pa 1976) 2013;38:E907–18. doi: 10.1097/BRS.0b013e318296232f. [DOI] [PubMed] [Google Scholar]
- 70.Phillips FM, Geisler FH, Gilder KM, Reah C, Howell KM, McAfee PC. Longterm outcomes of the US FDA IDE prospective, randomized controlled clinical trial comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. Spine. 2015;40:674–83. doi: 10.1097/BRS.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 71.Rozankovic M, Marasanov SM, Vukic M. Cervical disk replacement with discover versus fusion in a single-level cervical disk disease: A prospective single-center randomized trial with a minimum 2-year follow-up. Clin Spine Surg. 2017;30:E515–22. doi: 10.1097/BSD.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 72.Qizhi S, Lei S, Peijia L, Hanping Z, Hongwei H, Junsheng C, et al. A comparison of zero-profile devices and artificial cervical disks in patients with 2 noncontiguous levels of cervical spondylosis. Journal of Spinal Disorders and Techniques. 2016;29:E61–6. doi: 10.1097/BSD.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 73.Zhang HX, Shao YD, Chen Y, Hou Y, Cheng L, Si M, et al. A prospective, randomized, controlled multicentre study comparing cervical disc replacement with anterior cervical decompression and fusion. Int Orthop. 2014;38:2533–41. doi: 10.1007/s00264-014-2497-5. [DOI] [PubMed] [Google Scholar]
- 74.Hisey MS, Bae HW, Davis R, Gaede S, Hoffman G, Kim K, et al. Multi-center, prospective, randomized, controlled investigational device exemption clinical trial comparing Mobi-C Cervical Artificial Disc to anterior discectomy and fusion in the treatment of symptomatic degenerative disc disease in the cervical spine. Int J Spine Surg. 2014;8:7. doi: 10.14444/1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hisey MS, Bae HW, Davis RJ, Gaede S, Hoffman G, Kim KD, et al. Prospective, randomized comparison of cervical total disk replacement versus anterior cervical fusion: Results at 48 months follow-up. J Spinal Disord Tech. 2015;28:E237–43. doi: 10.1097/BSD.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 76.Skeppholm M, Lindgren L, Henriques T, Vavruch L, Lofgren H, Olerud C. The Discover artificial disc replacement versus fusion in cervical radiculopathy – A randomized controlled outcome trial with 2-year follow-up. Spine J. 2015;15:1284–94. doi: 10.1016/j.spinee.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 77.Jackson RJ, Davis RJ, Hoffman GA, Bae HW, Hisey MS, Kim KD, et al. Subsequent surgery rates after cervical total disc replacement using a Mobi-C Cervical Disc Prosthesis versus anterior cervical discectomy and fusion: A prospective randomized clinical trial with 5-year follow-up. J Neurosurg Spine. 2016;24:734–45. doi: 10.3171/2015.8.SPINE15219. [DOI] [PubMed] [Google Scholar]
- 78.Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: A prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:940–8. doi: 10.1097/BRS.0b013e31816c8b67. [DOI] [PubMed] [Google Scholar]
- 79.Herkowitz HN, Kurz LT, Overholt DP. Surgical management of cervical soft disc herniation. A comparison between the anterior and posterior approach. Spine (Phila Pa 1976) 1990;15:1026–30. doi: 10.1097/00007632-199015100-00009. [DOI] [PubMed] [Google Scholar]
- 80.Wirth FP, Dowd GC, Sanders HF, Wirth C. Cervical discectomy. A prospective analysis of three operative techniques. Surg Neurol. 2000;53:340–6. doi: 10.1016/s0090-3019(00)00201-9. [DOI] [PubMed] [Google Scholar]
- 81.Selvanathan SK, Beagrie C, Thomson S, Corns R, Deniz K, Derham C, et al. Anterior cervical discectomy and fusion versus posterior cervical foraminotomy in the treatment of brachialgia: The Leeds spinal unit experience (2008-2013) Acta Neurochir (Wien) 2015;157:1595–600. doi: 10.1007/s00701-015-2491-8. [DOI] [PubMed] [Google Scholar]
- 82.Korinth MC, Kruger A, Oertel MF, Gilsbach JM. Posterior foraminotomy or anterior discectomy with polymethyl methacrylate interbody stabilization for cervical soft disc disease: Results in 292 patients with monoradiculopathy. Spine (Phila Pa 1976) 2006;31:1207–14. doi: 10.1097/01.brs.0000217604.02663.59. [DOI] [PubMed] [Google Scholar]
- 83.Alvin MD, Lubelski D, Abdullah KG, Whitmore RG, Benzel EC, Mroz TE. Cost-utility analysis of anterior cervical discectomy and fusion with plating (ACDFP) versus posterior cervical foraminotomy (PCF) for patients with single-level cervical radiculopathy at 1-year follow-up. Clin Spine Surg. 2016;29:E67–72. doi: 10.1097/BSD.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 84.Oh MC, Zhang HY, Park JY, Kim KS. Two-level anterior cervical discectomy versus one-level corpectomy in cervical spondylotic 24.myelopathy. Spine. 2009;34:692–6. doi: 10.1097/BRS.0b013e318199690a. [DOI] [PubMed] [Google Scholar]
- 85.Yu YL, Gong WC, Xin B, et al. The comparison of therapeutic efficacy between two operative methods for the treatment of two-adjacent-level CSM. Med Coll J Qiqihaer. 2007;28:2821–3. [Google Scholar]
- 86.Liu Yong, Chen Liang, Gu Yong, Xu Yun, Yang Hui-lin, Tang Tian-si, et al. Comparison of two anterior decompression bone fusion treatments plus titanium plate implantation for two level cervical spondylotic myelopathy. J Clin Rehabil Tissue Eng Res. 2011;15:597–601. [Google Scholar]
- 87.Park Y, Maeda T, Cho W, Riew KD. Comparison of anterior cervical fusion after a two-level discectomy or single-level corpectomy: Sagittal alignment, cervical lordosis, graft collapse, and adjacent-level ossification. Spine J. 2010;10:193–9. doi: 10.1016/j.spinee.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Wang JC, McDonough PW, Endow KK, Delamarter RB. A comparison of fusion rates between single-level cervical corpectomy and two-level discectomy and fusion. J Spinal Disord. 2001;14:222–5. doi: 10.1097/00002517-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 89.Yu FB, Chen DY, Wang XW, Liu XW. Radiographic comparison of anterior cervical fusion after two-level discectomy or single-level corpectomy for two-level cervical spondylotic myelopathy. Zhonghua Yi Xue Za Zhi. 2012;92:2636–40. [PubMed] [Google Scholar]
- 90.Jia XL, Tan ZJ, Yang FB, Yang M, Wan G, et al. Comparision between single level cervical corpectomy and two level discectomy in two adjacent level cervical spondylotic myelopathy. Orthop J China. 2012;20:1931–4. [Google Scholar]
- 91.Hsu W, Wolinsky JP, Gokaslan ZL, Sciubba DM. Transoral approaches to the cervical spine. Neurosurgery. 2010;66:119–25. doi: 10.1227/01.NEU.0000365748.00721.0B. [DOI] [PubMed] [Google Scholar]
- 92.Henn JS, Lee MC, Rhoton ALJ. Transoral approach to craniocervical junction and upper cervical spine. In: Kim DH, Henn JS, Vaccaro AR, Curtis Dickman, editors. Surgical Anatomy & Techniques to the Spine. Philadelphia, PA: Saunders Elsevier; 2006. pp. 3–32. [Google Scholar]
- 93.Crockard HA, Calder 1, Ransford AO. One-stage transoral decompression and posterior fixation in rheumatoid atlantoaxial subluxation. J Bone Joint Surg. 1990;72:682–5. doi: 10.1302/0301-620X.72B4.2380227. [DOI] [PubMed] [Google Scholar]
- 94.James D, Crockard HA. Surgical access to the base of skull and upper cervical spine by extended maxillotomy. Neurosurgery. 1991;29:411–6. doi: 10.1097/00006123-199109000-00012. [DOI] [PubMed] [Google Scholar]
- 95.Wadia NH. Myelopathy complicating congenital atlantoaxial dislocation (A study of 28 cases) Brain. 1967;90:449–72. doi: 10.1093/brain/90.2.449. [DOI] [PubMed] [Google Scholar]
- 96.Greenberg AD, Scoville WB, Davey LM. Transoral decompression of atlantoaxial dislocation due to odontoid hypoplasia. J Neurosurg. 1968;28:266–9. doi: 10.3171/jns.1968.28.3.0266. [DOI] [PubMed] [Google Scholar]
- 97.Menezes AH, VanGilder JC, Clark CR, el-Khoury G. Odontoid upward migration in rheumatoid arthritis. An analysis of 45 patients with “cranial settling”. J Neurosurg. 1985;63:500–9. doi: 10.3171/jns.1985.63.4.0500. [DOI] [PubMed] [Google Scholar]
- 98.Dastur DK, Wadia NH, Desai AD, Sinh G. Medullospinal compression due to atlantoaxial dislocation and sudden haematomyelia during decompression.Pathology, pathogenesis, and clinical correlations. Brain. 1965;88:897–924. doi: 10.1093/brain/88.5.897. [DOI] [PubMed] [Google Scholar]
- 99.Pásztor E. Transoral approach to anterior brain stem compression. Acta Neurochir (Wien) 1992;118:7–19. doi: 10.1007/BF01400721. [DOI] [PubMed] [Google Scholar]
- 100.Menezes AH. Congenital and acquired abnormalities of the craniovertebral junction. In: Youmans JR, editor. Neurological Surgery. Philadelphia: WB Saunders Company; 1996. pp. 1035–89. [Google Scholar]
- 101.Menezes AH. Complications of surgery at the craniovertebral junction – Avoidance and management. Pediatr Neurosurg. 1991;17:254–66. doi: 10.1159/000120607. [DOI] [PubMed] [Google Scholar]
- 102.German J, Benzel E, Alexander J. Anatomy and surgical approaches and exposure of the vertebral column, the cervical spine. In: Benzel E, editor. Spine Surgery: Technique, Complication Avoidance, and Management. New York, NY: Churchill Livingstone; 1999. pp. 145–56. [Google Scholar]
- 103.Silber J, Albert T. Anterior and anterolateral, mid and lower cervical spine approaches: Transverse and longitudinal (C3 to C7) In: Herkowitz HN, editor. The Cervical Spine Surgery Atlas. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 91–8. [Google Scholar]
- 104.Chang U, Lee MC, Kim DH. Anterior approach to the mid cervical spine. In: Kim DH, Henn JS, Vaccaro A, Curtis Dickman, editors. Surgical Anatomy & Techniques to the Spine. Philadelphia, PA: Saunders Elsevier; 2006. pp. 45–56. [Google Scholar]
- 105.Xie L, Liu M, Ding F, Li P, Ma D. Cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) in symptomatic cervical degenerative disc diseases (CDDDs): An updated meta-analysis of prospective randomized controlled trials (RCTs) Springerplus. 2016;5:1188. doi: 10.1186/s40064-016-2851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu WJ, Hu L, Chou PH, Wang JW, Kan WS. Comparison of anterior cervical discectomy and fusion versus posterior cervical foraminotomy in the treatment of cervical radiculopathy: A systematic review. Orthop Surg. 2016;8:425–31. doi: 10.1111/os.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang T, Wang H, Liu S, An HD, Liu H, Ding WY. Anterior cervical discectomy and fusion versus anterior cervical corpectomy and fusion in multilevel cervical spondylotic myelopathy: A meta-analysis. Medicine (Baltimore) 2016;95:e5437. doi: 10.1097/MD.0000000000005437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Montano N, Ricciardi L, Olivi A. Comparison of anterior cervical decompression and fusion versus laminoplasty in the treatment of multilevel cervical spondylotic myelopathy: A meta-analysis of clinical and radiological outcomes. World Neurosurg. 2019;130:530–6.e2. doi: 10.1016/j.wneu.2019.06.144. [DOI] [PubMed] [Google Scholar]
- 109.Louis R. Chirurgische Anatomie und Operative Zugangswege. Berlin: Springer-Verlag; 1985. Die Chirurgie Der Wirbelsäule. [Google Scholar]
- 110.An HS, Wise JJ, Xu R. Anatomy of the cervicothoracic junction: A study of cadaveric dissection, cryomicrotomy, and magnetic resonance imaging. J Spinal Disord. 1999;12:519–25. [PubMed] [Google Scholar]
- 111.Pal GP, Routal RV. A study of weight transmission through the cervical and upper thoracic regions of the vertebral column in man. J Anat. 1986;148:245–61. [PMC free article] [PubMed] [Google Scholar]
- 112.Boyle JJ, Singer KP, Milne N. Morphological survey of the cervicothoracic junctional region. Spine (Phila Pa 1976) 1996;21:544–8. doi: 10.1097/00007632-199603010-00003. [DOI] [PubMed] [Google Scholar]
- 113.Le H, Balabhadra R, Park J, Kim D. Surgical treatment of tumors involving the cervicothoracic junction. Neurosurg Focus. 2003;15:E3. doi: 10.3171/foc.2003.15.5.3. [DOI] [PubMed] [Google Scholar]
- 114.Mulpuri K, LeBlanc JG, Reilly CW, Poskitt KJ, Choit RL, Sahajpal V, et al. Sternal split approach to the cervicothoracic junction in children. Spine (Phila Pa 1976) 2005;30:E305–10. doi: 10.1097/01.brs.0000164267.30422.a9. [DOI] [PubMed] [Google Scholar]
- 115.Ebraheim NA, Lu J, Yang H, Heck BE, Yeasting RA. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine (Phila Pa 1976) 2000;25:1603–6. doi: 10.1097/00007632-200007010-00002. [DOI] [PubMed] [Google Scholar]
- 116.Apfelbaum RI, Kriskovich MD, Haller JR. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine (Phila Pa 1976) 2000;25:2906–12. doi: 10.1097/00007632-200011150-00012. [DOI] [PubMed] [Google Scholar]
- 117.Boockvar JA, Philips MF, Telfeian AE, O'Rourke DM, Marcotte PJ. Results and risk factors for anterior cervicothoracic junction surgery. J Neurosurg. 2001;94:12–7. doi: 10.3171/spi.2001.94.1.0012. [DOI] [PubMed] [Google Scholar]


