Abstract
Introduction:
Salivary gland hypofunction might be associated with various local and systemic conditions and is managed with a plethora of therapeutic options with associated side effects. Transcutaneous electrical nerve stimulation (TENS) is one such option with no known systemic side effects for dealing with this crippling condition. The present study was planned with a similar intent of assessing impact of TENS on salivary flow rates in normal healthy adults according to gender and age groups.
Materials and Methods:
The present study was designed as a cross-sectional study on 130 healthy adults wherein unstimulated and stimulated saliva was collected for 5 min in graduated test tubes fitted with a funnel while mean salivary flow rates were calculated. The data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results:
In the present study, differences between mean unstimulated and stimulated salivary flow rates with TENS were found to be statistically significant for both genders (P < 0.001). Furthermore, in relation to age groups included, maximum increase in salivary flow rate was seen in 20–29 years of age group, though significant results were seen in all three age groups included namely 20–29 years, 30–39 years, and 40–49 years (P < 0.001).
Conclusions:
Based on results from the present study, it could be concluded that TENS comes out to be a safer, nonpharmacological therapeutic option for treating patients with xerostomia.
Keywords: Saliva, salivary glands, transcutaneous electric nerve stimulation, xerostomia
Introduction
Saliva is a critical fluid necessary for oral health while playing a significant role in homeostasis. At rest, this secretion ranges from 0.25 to 0.35 ml/min to constitute unstimulated saliva, while there a number of factors responsible for a decrease or, increase in its secretion, including sensory, electrical or, mechanical stimuli that can raise salivary flow rate to around 1.5 ml/min to constitute stimulated saliva, biochemically different from resting or, unstimulated saliva.[1,2] Physiologically, salivary secretion is regulated by a three-component reflex arch including afferent receptors and nerves that carry impulses created by taste and mastication activities, a central processing nucleus (salivation center in the medulla oblongata) and an efferent reflex arm which is constituted by parasympathetic and sympathetic nerves that separately but, in coordination, innervate glandular blood vessels and acini and control outflow of saliva from ducts. Given the autonomic control of salivary secretion, electrical stimulation of one of the components of salivary reflex arch can, thus, lead to potential enhancement of salivary secretion.[3,4]
Electrostimulation of neuromuscular structures is of therapeutic potential in several areas of medicine with common and well-known examples being cardiac pacemakers, phrenic stimulators and so on with the list of such applications being endless in today's era of technological advancements. Because of known autonomic control of salivary secretion, a similar approach has been tested and applied for therapeutic stimulation of saliva in the management of salivary gland hypofunction secondary to a plethora of reasons.[5,6,7,8] In clinical context, the term transcutaneous electrical nerve stimulation (TENS) is most commonly assumed to refer to the use of electrical stimulation with specific intention of providing symptomatic pain relief, though TENS does have and can be used for a whole lot of potential therapeutic advantages that can be harnessed in other areas of therapeutics. The type of stimulation delivered by TENS unit aims to excite (stimulate) sensory nerves and by so doing, activate specific natural pain relief mechanisms.[9,10,11,12]
There are two primary pain relief mechanisms which can be activated through TENS by variations in stimulation parameters, and these include the pain gate mechanism and the endogenous opioid system.[13,14,15,16,17,18] The effectiveness of TENS varies with clinical pain being treated; however, enormous research evidence suggest that when used well, TENS provides significantly greater pain relief than any kind of a placebo intervention.[9,10] The first TENS unit was developed in 1965 after publication of the well-known Gate Control Theory by Melzack and Wall.[19,20] Since 1965, TENS has become widely known throughout the world and is, also, considered to be one of the most common therapeutic resources used in clinical settings for relief of acute and chronic pain syndromes.[21,22,23,24,25,26] TENS, also, has been proposed to be effective in peripheral vascular disease, though, it is contraindicated in patients with a known history of epilepsy and/or, deep vein thrombosis or thrombophlebitis and who are at an increased risk of thromboembolic events because of the risk of initiating seizures and dislodging blood clots in patients who are more prone for thromboembolic events.[27,28,29,30,31,32,33]
Recently, many researchers have observed the therapeutic advantage of TENS in increasing salivary flow in patients with known glandular hypofunction on similar principle of stimulation of afferent nerves in salivary reflex arch. The impact of TENS has been evaluated in stimulating salivary flow in various clinical settings and has been found to be effective even in patients with xerostomia secondary to radiation therapy for head and neck cancers apart from evidence in being effective for cancer-associated pain.[34,35,36,37] The application of electrical current through oral mucosa to afferent neuronal pathways causes neuro-electrical stimulation of salivary glands, and this increases the production of saliva, eventually, reducing the symptoms of xerostomia. TENS might, also, directly stimulate the auriculotemporal nerve (efferent pathway) that supplies the secretomotor drive to parotid gland, thus, causing increased salivary flow rates.[38] There is an extensive research base for TENS in both clinical and laboratory settings and while it is worth noting that term TENS could represent the use of any electrical stimulation using skin surface electrodes which has intention of stimulating nerves, the present study employed use of TENS to stimulate salivary outflow in normal healthy adults and thus, assessing impact of TENS on salivary flow rates in normal healthy adults according to gender and age groups.
Materials and Methods
The present cross-sectional study was planned on 130 healthy adults divided into three age groups namely 20–29 years, 30–39 years, and 40–49 years to assess age-related changes in saliva production and subsequent, variation in that after stimulation with TENS. Ethical approval was obtained from Institutional Ethics Committees before start of study while subjects who were not having any positive systemic history and history of any treatment, including radiotherapy, those who did not have any habit and were not on any drugs were included in the study. On the contrary, subjects with a history of salivary gland pathology, those who were suffering from any systemic disease and were on treatment or, were with a history of radiation to head and neck region, who presented with a history of psychiatric disorders and pregnant or, possibly pregnant females were excluded from the study. The patients who were with implanted medical devices including cardiac pacemakers, cardiac defibrillator, internal pacing wires, prosthetic heart valves, cerebral, or, carotid and aortic aneurysmal clips, and those with cochlear implants were, also excluded. All the subjects were explained in detail in vernacular language about the design of the study and were asked to refrain from eating and drinking and smoking for at least 1 h prior to the appointment. The subjects were made to sit in an upright position with the head inclined slightly forward. They were asked to swallow saliva first and then instructed to stay motionless so that saliva could collect passively in anterior region of the floor of mouth. The surface electrode pads of TENS unit (Digitens) [Figure 1] were placed externally on the skin overlying parotid gland region [Figure 2] with the unit in “off” position. With low forced spitting, unstimulated saliva was collected for 5 min in a graduated test tube fitted with a funnel. The TENS unit was, then, activated while its amplitude was gradually increased to the maximum tolerable level of patient. The unit was preset at a frequency 50 Hz and then gradually increased to maximum tolerance level of patients. Stimulated saliva was collected for 5 min in a separate graduated test tube and flow rate compared with unstimulated salivary flow rate.
Figure 1.

Transcutaneous electrical nerve stimulation unit (Digitens)
Figure 2.
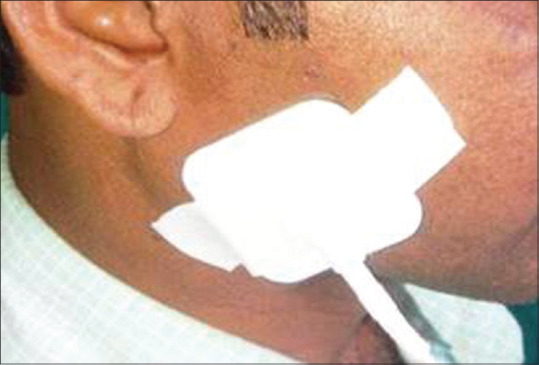
Patient positioned with surface electrode pads of the transcutaneous electrical nerve stimulation unit
Statistical analysis used
The data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Chi-square test was used to test the association between said parameters, while Student's t-test was used to compare the means. P <0.05 was considered statistically significant.
Results
In the present study, 117 subjects demonstrated increase in salivary flow rate while 10 subjects demonstrated no increase and 3 subjects showed an unexpected decrease in salivary flow rate on the application of TENS. The mean unstimulated salivary flow rate was found to be 1.395 ± 0.062 ml/min as against mean stimulated salivary flow rate of 1.498 ± 0.068 ml/min in males while in females, mean unstimulated salivary flow rate was found to be 1.264 ± 0.043 ml/min with the mean stimulated salivary flow rate of 1.377 ± 0.074 ml/min (P < 0.001) [Table 1]. In addition, five patients experienced mild twitching of facial musculature which ceased once TENS was deactivated. According to age groups, subjects were divided into three age groups namely 20–29 years (n = 42), 30–39 years (n = 48), and 40–49 years (n = 40) wherein maximum increase in salivary flow rate was seen in 20–29 years of age group with mean unstimulated and stimulated salivary flow rates being 1.313 ± 0.078 ml/min and 1.439 ± 0.089 ml/min respectively (P < 0.001), though results were found to be significant in all three age groups included (P < 0.001) [Table 2].
Table 1.
Comparison of mean unstimulated and stimulated salivary flow rates according to gender
| Gender | n | Mean unstimulated salivary flow rate (ml/min) | Mean stimulated salivary flow rate (ml/min) | t | P |
|---|---|---|---|---|---|
| Male | 65 | 1.395±0.062 | 1.498±0.068 | 9.0241 | 0.0001* |
| Female | 65 | 1.264±0.043 | 1.377±0.074 | 10.6446 | 0.0001* |
| Total | 130 | 1.330±0.054 | 1.438±0.072 | 17.4827 | 0.0001* |
*P<0.001 - highly significant
Table 2.
Comparison of mean unstimulated and stimulated salivary flow rates according to age groups
| Age group (years) | n | Mean unstimulated salivary flow rate (ml/min) | Mean stimulated salivary flow rate (ml/min) | t | P |
|---|---|---|---|---|---|
| 20-29 | 42 | 1.313±0.078 | 1.439±0.089 | 6.9001 | 0.0001* |
| 30-39 | 48 | 1.344±0.075 | 1.397±0.087 | 3.1967 | 0.0019* |
| 40-49 | 40 | 1.294±0.069 | 1.375±0.071 | 5.1744 | 0.0001* |
| Total | 130 | 1.330±0.054 | 1.438±0.072 | 17.4827 | 0.0001* |
*P<0.001 - highly significant
Discussion
In the first of its kind of studies, Steller et al.[39] reported improved salivary secretion in 3 of the 29 subjects after electrical neurostimulation in Sjogren's syndrome patients with xerostomia and suggested evaluation in further studies with larger sample sizes. In the present study, too, 117 subjects demonstrated increase in salivary flow rate while 10 subjects demonstrated no increase and 3 subjects showed an unexpected decrease in salivary flow rate on the application of TENS. These variations in results as well as an unexpected and contradictory decrease in salivary flow rate in 3 of the subjects might be explained on the basis of the settings of TENS unit used wherein there can be seen huge variations and response has to be largely modified for each individual subject to obtain desired results. In addition, five patients experienced mild twitching of facial musculature in the present study which ceased once TENS was deactivated. The results of the present study were also in close accordance with results of the study conducted by Aggarwal et al.[2] who, in their study, found 65 out of 80 subjects responding with increase in salivary flow rate on the application of TENS. Furthermore, 12 subjects showed mild reduction in salivary flow rates and 7 subjects experienced transient mild twitching of facial musculature as a side effect of TENS therapy as was observed in the present study.
Furthermore, in the present study, the subjects included were divided into three age groups, namely 20–29 years, 30–39 years, and 40–49 years to assess age-related changes in saliva production and subsequent variation in that after stimulation with TENS. Pattipati et al.,[38] also divided the study population into three groups based on their age range as group A with an age range of 21–35 years, group B with age range of 36–50 years, and group C in which the patients recruited were above 51 years of age and found subjects in group B showing statistically significant increase in the duration of stimulated parotid salivary flow following use of TENS. Similarly, Dyasnoor et al.[40] conducted a prospective study to clinically evaluate effectiveness of TENS therapy in stimulating whole salivary flow among 40 diabetic patients aged between 30 and 75 years who had subjective symptoms of xerostomia and found a statistically significant increase in stimulated whole saliva compared with unstimulated saliva after TENS application in continuous mode (P < 0.001).
On the contrary, few studies have, also, reported contradictory findings of a statistically significant decrease in salivary flow rate (P < 0.001) when TENS was used in burst mode as was observed in the present study wherein 3 subjects showed an unexpected decrease in salivary flow rate on application of TENS, attributing the decrease in salivary outflow due to variations in parameters used. Vijayalaxmi et al.,[41] also found TENS effective in stimulation of the whole saliva, though the results were found to be mixed with 39 patients on day one and 36 patients on day two out of 50 patients responding to TENS therapy with an increase in stimulated whole salivary flow rate, though there was observed a reduction in quantity of TENS stimulated saliva on day two accounting to up to 4%.
Vilas et al.,[3] also conducted a study to evaluate efficacy of TENS therapy on whole salivary flow rate in 100 healthy adult subjects with no history of any salivary gland disorder and observed 85 out of 100 subjects with increased whole salivary flow rate on application of TENS therapy. Furthermore, 11 subjects experienced no change while 4 experienced a decrease in salivary flow rate with TENS therapy similar to the observations made in the present study wherein 10 subjects demonstrated no increase while 3 subjects showed an unexpected decrease in salivary flow rate on application of TENS with the conclusion that TENS unit was effective in increasing whole salivary flow rate in 85% of the healthy adult subjects included in the said study. On similar lines, Singh et al.,[1] also conducted a study to evaluate effectiveness of TENS as a means of stimulating salivary function in normal, healthy adults with no history of salivary gland disorder and observed 43 out of 50 subjects with increased salivary flow when stimulated with TENS.
In another similar study, Bhasin et al.[42] studied 100 healthy adult subjects who were divided into five age groups and observed the mean unstimulated whole saliva flow rate to be 2.60 ml/5 min which increased to 3.60 ± 0.39 ml/5 min on application of TENS. With the said observation, there was found 38.46% increase in salivary flow rate while 96 out of 100 subjects responded positively to TENS therapy. Furthermore, salivary flow rate was observed to remain increased 30 min-as well as 24 h-poststimulation with corresponding values being 3.23 ± 0.41 ml/5 min and 2.69 ± 0.39 ml/5 min, respectively. The study concluded that not only TENS therapy was effective in stimulating whole salivary flow rate in normal, healthy controls but the effect was, also, retained for up to 24 h poststimulation, almost contradicting the findings of the study conducted by Vijayalaxmi et al.[41] who observed a reduction in the quantity of TENS stimulated saliva on day two mandating need for further studies in this regard with longer follow-up periods.
Dhillon et al.,[43] also conducted a study to assess the efficacy of extra-oral TENS as a means of stimulating salivary function in 100 healthy adult subjects divided into two age groups of 20–40 years and ≥60 years of age and observed 87 of the 100 subjects demonstrating an increased salivary flow while 10 experiencing no increase and 3 with decrease in salivary flow rate on the application of TENS. In addition, 5 of the subjects observed minimal and transient side effects similar to the findings of the present study wherein five patients experienced mild twitching of the facial musculature which ceased once TENS was deactivated. The authors, also, concluded that gender wise, no statistically significant difference could be seen among subjects in both groups while age wise, the results were found to be statistically significant (P < 0.001) with subjects in 20–40 years of age group producing more saliva similar to the observation made in the present study wherein maximum increase in salivary flow rate was seen in the 20–29 years of age group with mean unstimulated and stimulated salivary flow rates being 1.313 ± 0.078 ml/min and 1.439 ± 0.089 ml/min, respectively (P < 0.001), though the results were found to be significant in all three age groups (P < 0.001). The study concluded that TENS unit was effective in increasing parotid salivary flow in healthy controls with age-related but no gender-related variability on salivary flow rates with TENS.
Likewise, Konidena et al.[44] conducted a study to evaluate the effect of TENS therapy on whole salivary flow rate in postmenopausal females with and without oral dryness and observed a statistically significant increase in whole saliva flow rate in 90% of subjects irrespective of their oral dryness status making them conclude TENS therapy to be a suitable option for the management of xerostomia in postmenopausal females. Talal et al.,[45] also conducted a multicenter, double-blind study in patients with Sjögren's syndrome to evaluate the ability of an electro-stimulator device to increase production of saliva making 40 out of 77 Sjögren's syndrome patients assigned to active devices while 37 to placebo devices and continued the treatment for 4 weeks. The results of said study found a statistically significant increase in production of saliva in patients using active devices than those using placebo devices.
Wong et al.,[46] also, conducted a similar, single institutional Phase I-II study to assess efficacy of AL-TENS device (Codetron™) in radiation-induced xerostomia in 46 patients with residual salivary gland function. In the said study, Codetron™ treatment of acupuncture points according to traditional Chinese medicine principles was given over a period of 12 weeks with 2-week break after 6 weeks of treatment while the results of study were not only found to be encouraging in improving whole saliva production in affected individuals, but effects were found to be sustained for at least 6 months' posttreatment. In yet another study conducted by the same authors in 2012, feasibility of AL-TENS device (Codetron™) delivery in a multicenter setting and its efficacy in reducing radiation-induced xerostomia in 48 patients was evaluated while Codetron™ treatment was given for 20 min two times a week for 12 weeks and it was concluded that it was feasible to use the device in multicenter settings as authors had received 94% patients compliance while a positive treatment response was noted in 86% of patients recruited.[47]
Aparna et al.,[48] also conducted a study to assess efficacy of TENS therapy on salivary gland function in 25 subjects with a complaint of hyposalivation and found a significant increase in parotid salivary flow in 19 of 25 patients after TENS application. In addition, there was observed a statistically significant difference in salivary flow based on gender, wherein males showed a higher increase in salivary secretion when compared to female patients. The study concluded TENS to be effective in increasing salivary flow rate in patients with subjective complaints of hyposalivation and xerostomia. Similar results were obtained in yet another study conducted by Percival et al.[49] who observed a significant decrease in secretion rates of unstimulated whole saliva in relation to age in the study population (P < 0.001), however, no significant difference in flow rates of stimulated parotid saliva with increase in age. In addition, females had significantly lower mean salivary flow rates than males for both unstimulated (resting) whole saliva (P < 0.005) and stimulated parotid saliva (P < 0.05).
Mittal et al.,[6] also demonstrated increased salivation with TENS in 47 out of 50 patients in their study with similar results in studies conducted by Strietzel et al.[7] and Domingo[34] who demonstrated a significant decrease in oral dryness following TENS therapy, although they also, concluded that the effectiveness of TENS depends on functional status of glands and that TENS was not found to be effective if there was an absent residual salivary gland function and an absolute absence of salivary secretion at the baseline.
TENS, as a treatment technique, is a noninvasive technique with fewer side effects when compared with conventional drug therapy with the most common complaints being an allergic type of skin reaction reported in about 2%–3% of patients. Digital TENS machines are becoming more widely available, and extra-features such as automated frequency sweeps and more complex stimulation patterns are emerging, though the application of TENS at inadequate intensities is one of the primary factors attributed to conflicting reports of TENS efficacy. Further studies are, thus, mandated in this regard to provide a systematic approach for treatment suggesting optimal parameters that can be employed over a larger group of population for achieving desired clinical outcomes.
Conclusions
Within the limitations of the present study, the present study concluded that there was an increased salivary flow rate observed with conventional settings of TENS unit in majority of patients. TENS, thus, comes out to be a safer, nonpharmacological therapeutic option for treating patients with xerostomia wherein systemic drug therapy is contraindicated or, found to be associated with severe side effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh D, Agrawal S, Shashikanth MC, Misra N. The effects of transcutaneous electrical nerve stimulation (TENS) on salivary flow: A study. J Indian Acad Oral Med Radiol. 2015;27:16–9. [Google Scholar]
- 2.Aggarwal H, Pal-Singh M, Mathur H, Astekar S, Gulati P, Lakhani S. Evaluation of the effect of transcutaneous electrical nerve stimulation (TENS) on whole salivary flow rate. J Clin Exp Dent. 2015;7:e13–7. doi: 10.4317/jced.51828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilas SK, Shashikanth MC, Ali IM. Evaluation of the effect of transcutaneous electrical nerve stimulation on whole saliva flow: A clinical study. J Indian Acad Oral Med Radiol. 2009;21:7–11. [Google Scholar]
- 4.Sarapur S, Shilpashree HS. Salivary pacemakers: A review. Dent Res J (Isfahan) 2012;9:S20–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Lafaurie G, Fedele S, López RM, Wolff A, Strietzel F, Porter SR, et al. Biotechnological advances in neuro-electro-stimulation for the treatment of hyposalivation and xerostomia. Med Oral Patol Oral Cir Bucal. 2009;14:E76–80. [PubMed] [Google Scholar]
- 6.Mittal K, Keluskar V, Kapoor S. Evaluation of the effect of transcutaneous electrical nerve stimulation (TENS) on salivary flow in patients with Xerostomia. Annals of Dent Res. 2012;2:44–50. [Google Scholar]
- 7.Strietzel FP, Martín-Granizo R, Fedele S, Lo Russo L, Mignogna M, Reichart PA, et al. Electrostimulating device in the management of xerostomia. Oral Dis. 2007;13:206–13. doi: 10.1111/j.1601-0825.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 8.Greenspan D. Xerostomia: Diagnosis and management. Oncol (Williston Park) 1996;10:7–11. [PubMed] [Google Scholar]
- 9.Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: The state of the evidence. Pain Manag. 2014;4:197–209. doi: 10.2217/pmt.14.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson M. Transcutaneous electrical nerve stimulation: Mechanisms, clinical application and evidence. Rev Pain. 2007;1:7–11. doi: 10.1177/204946370700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh D. TENS: Clinical Applications and Related Theory. 1st ed. New York: Churchill Livingstone; 1997. [Google Scholar]
- 12.Johnson M. Transcutaneous Electrical Nerve Stimulation, in Electrotherapy: Evidence Based Practice. In: Watson T, editor. Edinburgh: Churchill Livingstone; 2008. pp. 253–96. [Google Scholar]
- 13.Garrison DW, Foreman RD. Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS) Pain. 1994;58:309–15. doi: 10.1016/0304-3959(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 14.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: Basic science mechanisms and clinical effectiveness. J Pain. 2003;4:109–21. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 15.Palmer ST, Martin DJ, Steedman WM, Ravey J. Effects of electric stimulation on C and A delta fiber-mediated thermal perception thresholds. Arch Phys Med Rehabil. 2004;85:119–28. doi: 10.1016/s0003-9993(03)00432-5. [DOI] [PubMed] [Google Scholar]
- 16.Chesterton LS, Foster NE, Wright CC, Baxter GD, Barlas P. Effects of TENS frequency, intensity and stimulation site parameter manipulation on pressure pain thresholds in healthy human subjects. Pain. 2003;106:73–80. doi: 10.1016/s0304-3959(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 17.Claydon LS, Chesterton LS, Barlas P, Sim J. Alternating-frequency TENS effects on experimental pain in healthy human participants: A randomized placebo-controlled trial. Clin J Pain. 2013;29:533–9. doi: 10.1097/AJP.0b013e318262330f. [DOI] [PubMed] [Google Scholar]
- 18.Chesterton LS, Barlas P, Foster NE, Lundeberg T, Wright CC, Baxter GD. Sensory stimulation (TENS): Effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. Pain. 2002;99:253–62. doi: 10.1016/s0304-3959(02)00118-5. [DOI] [PubMed] [Google Scholar]
- 19.Barlas P, Lundeberg T. Transcutaneous Electrical Nerve Stimulation and Acupuncture. In: McMahon S, Koltzenburg M, editors. Melzack and Wall's Textbook of Pain. Philadelphia: Elsevier Churchill Livingstone; 2006. pp. 583–90. [Google Scholar]
- 20.Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 21.Walsh DM, Baxter D. Transcutaneous electrical nerve stimulation (TENS): A review of experimental studies. Eur J Phys Med Rehabil. 1996;6:42–50. [Google Scholar]
- 22.Shanahan C, Ward AR, Robertson VJ. Comparison of the analgesic efficacy of interferential therapy and transcutaneous electrical nerve stimulation. Physiotherapy. 2006;92:247–53. [Google Scholar]
- 23.Alves-Guerreiro J, Noble JG, Lowe AS, Walsh DM. The effect of three electrotherapeutic modalities upon peripheral nerve conduction and mechanical pain threshold. Clin Physiol. 2001;21:704–11. doi: 10.1046/j.1365-2281.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson M. Transcutaneous electrical nerve stimulation (TENS) and TENS-like devices: Do they provide pain relief? Pain Rev. 2001;8:121–8. [Google Scholar]
- 25.Johnson MI. A critical review of the analgesic effects of TENS-like devices. Phys Ther Rev. 2001;6:153–73. [Google Scholar]
- 26.Johnson MI. The clinical effectiveness of TENS in pain management. Phys Rehabil Med. 2000;12:131–49. [Google Scholar]
- 27.Ainsworth L, Budelier K, Clinesmith M, Fiedler A, Landstrom R, Leeper BJ, et al. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2006;120:182–7. doi: 10.1016/j.pain.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 28.Brosseau L, Milne S, Robinson V, Marchand S, Shea B, Wells G, et al. Efficacy of the transcutaneous electrical nerve stimulation for the treatment of chronic low back pain: A meta-analysis. Spine (Phila Pa 1976) 2002;27:596–603. doi: 10.1097/00007632-200203150-00007. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Tabasam G, Johnson M. Does the pulse frequency of transcutaneous electrical nerve stimulation (TENS) influence hypoalgesia? A systematic review of studies using experimental pain and healthy human participants. Physiotherapy. 2008;94:11–20. [Google Scholar]
- 30.Jarzem PF, Harvey EJ, Arcaro N, Kaczorowski J. Transcutaneous electrical nerve stimulation (TENS) for chronic low-back pain. J Musculoskelet Pain. 2005;13:3–9. [Google Scholar]
- 31.Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: A meta-analysis of randomized controlled trials. Pain. 2007;130:157–65. doi: 10.1016/j.pain.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Cosmo P, Svensson H, Bornmyr S, Wikström SO. Effects of transcutaneous nerve stimulation on the microcirculation in chronic leg ulcers. Scand J Plast Reconstr Surg Hand Surg. 2000;34:61–4. doi: 10.1080/02844310050160187. [DOI] [PubMed] [Google Scholar]
- 33.Khadilkar A, Milne S, Brosseau L, Wells G, Tugwell P, Robinson V, et al. Transcutaneous electrical nerve stimulation for the treatment of chronic low back pain: A systematic review. Spine (Phila Pa 1976) 2005;30:2657–66. doi: 10.1097/01.brs.0000188189.21202.0f. [DOI] [PubMed] [Google Scholar]
- 34.Domingo DL. The effects of electro-stimulation on saliva production in post-radiation head and neck cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:464. [Google Scholar]
- 35.Robb K, Oxberry SG, Bennett MI, Johnson MI, Simpson KH, Searle RD. A cochrane systematic review of transcutaneous electrical nerve stimulation for cancer pain. J Pain Symptom Manage. 2009;37:746–53. doi: 10.1016/j.jpainsymman.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Robb KA, Newham DJ, Williams JE. Transcutaneous electrical nerve stimulation vs. transcutaneous spinal electroanalgesia for chronic pain associated with breast cancer treatments. J Pain Symptom Manage. 2007;33:410–9. doi: 10.1016/j.jpainsymman.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Searle RD, Bennett MI, Johnson MI, Callin S, Radford H. Transcutaneous electrical nerve stimulation (TENS) for cancer bone pain. J Pain Symptom Manage. 2009;37:424–8. doi: 10.1016/j.jpainsymman.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Pattipati S, Patil R, Kannan N, Kumar BP, Shirisharani G, Mohammed RB. Effect of transcutaneous electrical nerve stimulation induced parotid stimulation on salivary flow. Contemp Clin Dent. 2013;4:427–31. doi: 10.4103/0976-237X.123017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steller M, Chou L, Daniels TE. Electrical stimulation of salivary flow in patients with Sjögren's syndrome. J Dent Res. 1988;67:1334–7. doi: 10.1177/00220345880670101701. [DOI] [PubMed] [Google Scholar]
- 40.Dyasnoor S, Kamath S, Khader NF. Effectiveness of electrostimulation on whole salivary flow among patients with type 2 diabetes mellitus. Perm J. 2017;21:15–164. doi: 10.7812/TPP/15-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vijayalaxmi BN, Ramesh T, Sudhakara RR, Lavanya RR, Swapna LA. Effect of TENS on whole saliva in healthy adult Indians: Evaluation of influence of protocol on quantity of saliva measured. Cumhuriyet Dent J. 2012;15:235–40. [Google Scholar]
- 42.Bhasin N, Reddy S, Nagarajappa AK, Kakkad A. A study on duration of effect of transcutaneous electrical nerve stimulation therapy on whole saliva flow. J Contemp Dent Pract. 2015;16:479–85. doi: 10.5005/jp-journals-10024-1710. [DOI] [PubMed] [Google Scholar]
- 43.Dhillon M, Raju SM, Mohan RS, Tomar D. Efficacy of transcutaneous electrical nerve stimulation on parotid saliva flow rate in relation to age and gender. J Dent Shiraz Univ Med Sci. 2016;17:164–70. [PMC free article] [PubMed] [Google Scholar]
- 44.Konidena A, Sharma D, Puri G, Dixit A, Jatti D, Gupta R. Effect of TENS on stimulation of saliva in postmenopausal women with or without oral dryness – An interventional study. J Oral Biol Craniofac Res. 2016;6:S44–50. doi: 10.1016/j.jobcr.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talal N, Quinn JH, Daniels TE. The clinical effects of electro-stimulation on salivary function of Sjogren's syndrome patients. Rheumatol Int. 1992;12:43–5. doi: 10.1007/BF00300975. [DOI] [PubMed] [Google Scholar]
- 46.Wong RK, Jones GW, Sagar SM, Babjak AF, Whelan T. A Phase I-II study in the use of acupuncture-like transcutaneous nerve stimulation in the treatment of radiation-induced xerostomia in head-and-neck cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:472–80. doi: 10.1016/s0360-3016(03)00572-8. [DOI] [PubMed] [Google Scholar]
- 47.Wong RK, James JL, Sagar S, Wyatt G, Nguyen-Tân PF, Singh AK, et al. Phase 2 results from radiation therapy oncology group study 0537: A phase 2/3 study comparing acupuncture-like transcutaneous electrical nerve stimulation versus pilocarpine in treating early radiation-induced xerostomia. Cancer. 2012;118:4244–52. doi: 10.1002/cncr.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aparna PV, Sankari SL, Deivanayagi M, Priyadharshini A, Vishnupriya CK, Niveditha B. Effect of transcutaneous electrical nerve stimulation on parotid saliva flow in patients with hyposalivation. J Pharm Bioallied Sci. 2017;9:S142–6. doi: 10.4103/jpbs.JPBS_124_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Percival RS, Challacombe SJ, Marsh PD. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J Dent Res. 1994;73:1416–20. doi: 10.1177/00220345940730080401. [DOI] [PubMed] [Google Scholar]


