Abstract
Introduction:
Immunofluorescence (IF) microscopy is an essential tool for the analysis of glomerular diseases. In this study, we studied the significance of the IF technique together with light microscopy (LM) and clinical details in the diagnosis of different types of diffuse proliferative glomerulonephritis (GN). We intended to evaluate the spectrum of Diffuse Proliferative Glomerulonephritis (DPGN) in our institute.
Materials and Methods:
We evaluated a total of 95 kidney biopsies received in the past 10 years. All biopsies were scrutinized by LM and IF techniques. Clinical details were documented in a predesigned form.
Results:
The predominant clinical presentation in this study was nephrotic syndrome (49.4%) followed by systemic lupus erythromatosus with suspected renal involvement (24.2%). On microscopy, lupus nephritis (LN) was the most common DPGN in the study (35.7%), followed by immunoglobulin (Ig) A nephropathy (25.2%) and postinfectious GN (PIGN) (16.8%). The majority of patients were in the <30 years age group (72.6%), with the average age of patients being 24.4 years. The dominant deposit on IF in LN was C3 and IgG (100%). A high deposit of IgA (100%) in IgA nephropathy and of IgG and C3 (100%) in membranoproliferative GN was seen. PIGN showed dominant positive staining of IgG (92.8%).
Conclusion:
The predominant clinical presentation was of nephrotic syndrome and on LM LN was the most commonly diagnosed DPGN in this study. Direct IF is vital for classifying DPGN, followed by electron microscopy, which is an essential tool. This article describes a rational evaluation of kidney biopsies with DPGN pattern on LM in a way that guides toward the logical assessment to reach the diagnosis. Using the IF technique and comparing it with LM and clinical details, we evaluated the spectrum DPGN in our center.
Keywords: Glomerulonephritis, immunofluorescence, nephrotic
INTRODUCTION
Glomerular diseases are considered the leading cause of morbidity and mortality in renal pathology patients.[1] There are restricted approaches for the morphologic analysis of renal biopsies, as is the pathognomic morphologic changes. Deposition of immunoglobulin (Ig) and/or complement factors in glomerulus causes injury, which leads to an inflammatory response ending in proliferative glomerulonephritis (PGN).[2,3] PGN is defined by an increase in glomerular cellularity due to infiltration and proliferation of leukocytes within the glomerulus or from the proliferation of mesangial cells and parietal epithelial cells.[2,4,5] Diffuse PGN (DPGN) is PGN involving more than 50% of the glomeruli [Figure 1]. In severe forms of DPGN, the crescent is formed by the obliteration of bowman space by epithelial proliferation. Recognizing this pattern on LM and categorizing it into different disease entities with the help of direct IF (DIF) is essential for an accurate diagnosis.[6] On light microscopy (LM), the following diseases can present as DPGN.
Figure 1.
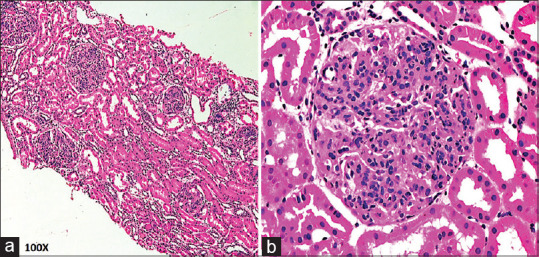
(a) Diffuse proliferative glomerulonephritis pattern-enlarged glomeruli with endocapillary proliferation (H and E, ×100). (b) Diffusely enlarged and cellular glomeruli (H and E, ×400)
Lupus nephritis (LN) Class IV
IgA nephropathy (IgAN) Class IV
Postinfectious GN (PIGN)
Membranoproliferative GN (MPGN)
C3 glomerulonephropathy (C3 GM).
Accurate diagnosis of diseases with DPGN pattern requires LM, immunofluorescence (IF) and electron microscopy (EM). It also requires correlating clinical details and biochemical parameters.[3,7] DIF is a necessary tool for categorizing cases with DPGN pattern on LM into different disease entities.[4,8,9] In this study, we studied the importance of IF technique along with LM and clinical details in the diagnosis of cases presenting as DPGN on LM.
MATERIALS AND METHODS
All cases of native renal biopsies performed from 2010 to 2019 in our institute were reviewed retrospectively in the study. A total of 95 cases with DPGN pattern on LM were selected. Clinical information was recorded in a predesigned format. Two samples of renal biopsies were taken. In each case for the first biopsy, multiple thin sections were cut for LM followed by special stains with Periodic Acid-Schiff, Gomori's methenamine silver stain and Masson's Trichome. The second biopsy core was received in Michele's fluid for DIF. The tissue is then embedded in the cryostat embedding medium, frozen and cut at 3 μ thickness at −22°C. Sections were taken on Poly L Lysine coated slide. Later, the slides were rinsed in phosphate buffer saline at pH 7.2 for 10 min. The sections were treated with fluorescein isothiocyanate labeled and optimally diluted antisera - IgG, IgA, IgM, C3, C1q and Fibrinogen. The slides were incubated in wet chamber for 1 h in dark room, washed with PBS, and mounted with glycerine jelly. The slides were observed under the green filter of the fluorescence microscope at 494 nm wavelength. DIF reporting is done based on the nature and distribution of immune deposits, glomerular localization, the intensity of staining, and the pattern of immune complex deposits. The intensity of staining was graded subjectively from 0 to3, 0 being negative and 3 maximum intensity (mild +1, moderate +2, and marked + 3).[1] After correlating clinical data with LM and IF findings, the final diagnosis was rendered.
RESULTS
The study involved biopsies from 95 patients, with 37 males (38.9.7%) and 53 (55.7%) females. The age ranged from 11 to 60 years. The majority of these patients were <30 years group (74.7%), with the mean age of patients being 24.1 years. Table 1 shows the incidence and clinical presentation of different DPGN cases in this study. The predominant clinical presentation in this study was nephrotic syndrome (49.4%), followed by systemic lupus erythematosus (SLE) with suspected renal involvement (24.2%). LN was the most common DPGN in this study (35.7%), followed by IgA nephropathy IgAN (25.2%) and PIGN (16.8%) [Table 1].
Table 1.
Clinical presentation of diffuse proliferative glomerulonephritis in this study
| Mode of presentation | Lupus nephritis | IgA nephropathy | PIGN | MPGN | C3 glomerulopathy | Total (%) |
|---|---|---|---|---|---|---|
| Total cases (%) | 34 (35.7) | 24 (25.2) | 16 (16.8) | 9 (9.4) | 7 (7.3) | 95 |
| Nephrotic syndrome | 8 | 12 | 13 | 8 | 6 | 47 (49.4) |
| Nephritic syndrome | 1 | 6 | 3 | 0 | 1 | 11 (11.5) |
| SLE | 23 | 0 | 0 | 0 | 0 | 23 (24.2) |
| CKD | 0 | 2 | 0 | 0 | 0 | 2 (2.1) |
| AKI | 2 | 2 | 0 | 0 | 0 | 4 (4.2) |
| Others | 1 | 2 | 1 | 1 | 0 | 5 (5.2) |
PIGN: Postinfectious glomerulonephritis, MPGN: Membranoproliferative glomerulonephritis, SLE: Systemic lupus erythromatosus, CKD: Chronic kidney disease, AKI: Acute kidney injury, Ig: Immunoglobulin
Table 2 shows the clinical profile of different diseases with DPGN pattern. The mean age of presentation was almost similar in different diseases. However, the gender ratio was significantly different. In LN, there was a female preponderance with a male to female ratio of 1:11. Similarly, C3 GN also had female predominance with a ratio of 2:7. Rest all diseases had male predominance. Maximum number of pediatric patients was seen in PIGN (25%). The mean duration of symptoms was maximum in case of LN which was 13.1 months while it was more or less similar in other diseases. The most common mode of presentation was SLE in LN, while in others, it was nephrotic syndrome.
Table 2.
Clinical profile of different diseases with diffuse proliferative glomerulonephritis pattern
| Lupus nephritis | IgA nephropathy | PIGN | MPGN | C3 glomerulopathy | |
|---|---|---|---|---|---|
| Age (years, mean±SD) | 24.8 | 26.3 | 21.8 | 24.3 | 19.8 |
| Gender ratio (male: female) | 1:11 | 1:1 | 2.2:1 | 8:1 | 2:5 |
| Number of pediatric patients (%) | 1 (2.8) | Nil | 4 (25) | 2 (22.2) | 1 (14.2) |
| Duration of illness (months) | 13.1 | 2.94 | 1.4 | 0.5 | 0.5 |
| Most common mode of presentaion | SLE | Nephrotic syndrome | Nephrotic syndrome | Nephrotic syndrome | Nephrotic syndrome |
SD: Standard deviation, PIGN: Postinfectious glomerulonephritis, MPGN: Membranoproliferative glomerulonephritis, SLE: Systemic lupus erythromatosus, Ig: Immunoglobulin
On LM, LN showed endocapillary proliferation (100%), leukocytic infiltration (85.2%), interstitial inflammation (88.2%), subendothelial deposits (85.2%), fibrinoid necrosis (17.6%), and cellular crescents (55.8%). The presence of wire loop and hyaline thrombi formation seen in 91.1% of cases indicates the subendothelial form of immune deposits, which can be seen on H and E, but much better appreciated by PAS stain.[10,11] However, there were three cases (8.8%) that could not be diagnosed as LN on LM in view of absence of above features which were finally labeled as LN based on IF findings. We found that 31 cases (100%) of LN which were available for IF presented with full house positivity for IgG, IgA, IgM, C3 and C1q in the glomerular basement membrane and mesangium [Figure 2]. It was noted that the intensity of IF varied. IgG, C3 and C1q were more intensely stained as compared to IgA and IgM. 46.8% of cases had high intensity of staining (3+), 38.2% of cases had 2 + and 9.3% had intensity 1+ with IgG. Similar findings were also noted with the intensity of C3 and C1q staining.
Figure 2.
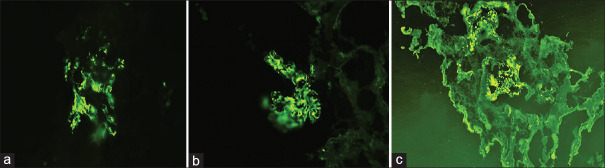
Lupus nephritis direct immunofluorescence (a) immunoglobulin A - 2+ mesangial and capillary wall (b) IgG - 2+ mesangial and capillary wall (c) C1q - 2+ mesangial and capillary wall (d) F - 2+ in crescents
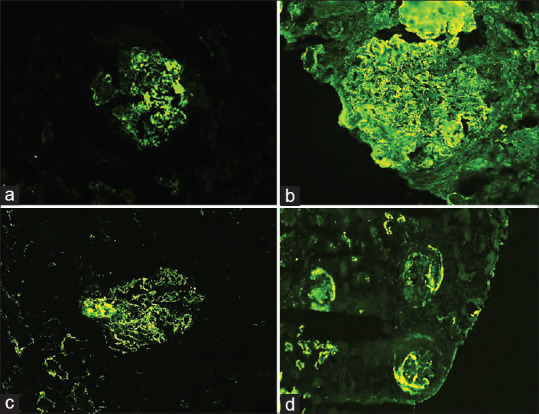
In the present study, fifteen cases were diagnosed as PIGN, which on LM showed slightly enlarged glomeruli with diffuse endocapillary proliferation, neutrophilic infiltration, and localized thickening of the glomerular capillary walls. Four cases (25%) also showed the presence of crescents. All cases in the study showed C3 only staining or atleast 2+ more C3 staining than Ig. One case (6.25%) had mesangial and capillary wall staining for C3 only without IgG. 42.8% showed 1+ and 50% cases showed >1+ staining intensity for IgG. Two cases had garland patterns, one had starry sky pattern and one case showed lumpy, bumpy deposits of IgG [Figure 3].
Figure 3.
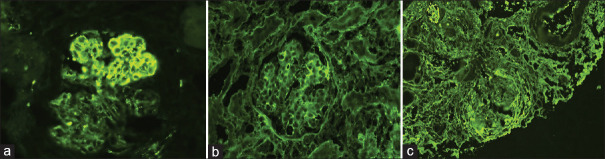
Immunoglobulin A direct immunofluorescence (a) immunoglobulin A - 2+ mesangial and capillary wall (b) immunoglobulin G - 2+ capillary wall (c) C3-1+ mesangial
A single case with DPGN pattern on LM had codominant staining of IgG, IgA and C3 on IF, which was diagnosed as IgA predominant PIGN after clinicopathological correlation.
The incidence of IgA nephropathy was 25.2%. LM showed mesangial expansion with hypercellularity. Ten cases (41.6%) showed crescents involving <50% glomeruli. On IF, there was a prominent deposition of IgA (100%), which had strong intensity in the mesangium. In 80.9% of the cases, there was codeposition of IgA and C3 [Figure 4]. IgG was prevalent in 71.4.1% and IgM in 73.6% of cases, along with IgA.
Figure 4.
Postinfectious glomerulonephritis direct immunofluorescence (a) C3 – 3+ starry sky pattern and garland pattern (b) immunoglobulin G – 1+ capillary wall (c) F – 2+ in crescents
There were 9 cases (9.4%) of MPGN, which on LM showed glomerular hypercellularity and lobulation (100%) and double contouring of basement membrane (66.7%). Crescents were not seen in any cases. Glomerular capillary wall and mesangial staining of IgG and C3 was seen in all the cases. IgM staining was seen in 71.4% of cases.
There were 7 cases (7.36%) of C3 GM, which showed membranoproliferative pattern on LM with the thickened basement membrane. IF showed mesangial and capillary wall thickening for C3 (3+) in all cases. Three cases showed mild segmental staining for IgM. IgA, C1q, and F were negative in all cases.
Of the total 95 cases, IF findings were available in 84 (88.4%) cases. Eleven cases were considered inadequate for DIF in view of the absence of even single glomerulus. Table 3 shows the concordance and discordance of LM and IF results. The provisional diagnosis of LM was confirmed by IF in 34 cases (35.7%), whereas in 50 cases (59.5%), the diagnosis was finalized only after correlating the IF findings with morphology on LM. Out of these 50 cases, IF helped in diagnosing three cases of LN, all cases of IgA nephropathy, all cases of PIGN, three cases of MPGN, and all seven cases of C3 GM.
Table 3.
The concordance and discordance of light microscopy and immunofluorescence results
| Number of cases (n=95), n (%) | LM | IF diagnosis | Final diagnosis after correlation with IF |
|---|---|---|---|
| 28 (29.2) | Consistent with LN | LN | LN |
| 3 (3.1) | DPGN | LN | LN |
| 3 (3.1) | Consistent with LN | Inadequate for IF | Consistent with LN |
| 22 (23.1) | Chronic mesangioproliferative GN | IgAN | IgAN |
| 2 (2.1) | Chronic mesangioproliferativeGN | Inadequate for IF | Possibility of IgAN cannot be ruled out |
| 14 (14.7) | DPGN | PIGN | PIGN |
| 1 (1.05) | DPGN | IgA predominant PIGN | IgA predominant PIGN |
| 1 (1.05) | DPGN | Inadequate for IF | DPGN possibility of PIGN cannot be ruled out |
| 4 (4.21) | MPGN | MPGN | MPGN |
| 3 (3.1) | DPGN | MPGN | MPGN |
| 2 (2.1) | MPGN | Inadequate for IF | DPGN possibility of MPGN cannot be ruled out |
| 7 (7.3) | MPGN | C3 GM | C3 GM |
| 2 (2.1) | Mesangioproliferative GN | Positive-IgG, IgM and C3 negative-IgA | Inconclusive |
| 3 (3.1) | DPGN | Inadequate for IF | DPGN |
DPGN: Diffuse proliferative glomerulonephritis, MPGN: Membranoproliferative glomerulonephritis, IF: Immunofluorescence, PIGN: Postinfectious glomerulonephritis, LN: Lupus nephritis, Ig: Immunoglobulin, IgAN: IgA nephropathy, C3 GM: C3 glomerulonephropathy, LM: Light microscopy
Table 4 also shows the distribution of immune deposits in studied cases. The dominant deposit on IF in LN was C3 and IgG (100%). A high deposit of IgA (100%) in IgA nephropathy and of IgG (100%) and C3 (100%) in MPGN was seen. PIGN showed dominant positive staining of granular C3 (100%) and IgG (93.3%). C3 glomerulopathy was diagnosed on the basis of C3 staining (100%) with the absence of other immunodeposits.
Table 4.
Immunofluorescence findings in the studied 95 biopsy specimens
| Disease | IgG (%) | IgA (%) | IgM (%) | C3 (%) | C1q (%) | Fibrinogen (%) | Cases inadequate for IF | Total cases |
|---|---|---|---|---|---|---|---|---|
| LN | 31/31 (100) | 28/30 (94.1) | 31/32 (97) | 31/31 (100) | 28/30 (94.11) | 9/26 (37.5) | 3 | 34 |
| IgAN | 15/21 (71.4) | 22/22 (100) | 14/19 (73.6) | 17/21 (80.9) | 2/16 (12.5) | 3/15 (20) | 2 | 24 |
| PIGN | 14/15 (93.3) | 5/15 (33.3) | 10/15 (66.7) | 15/15 (100) | 2/8 (25) | 3/10 (30) | 1 | 16 |
| MPGN | 7/7 (100) | 2/7 (28.5) | 5/7 (71.4) | 7/7 (100) | 3/5 (60) | 1/5 (20) | 2 | 9 |
| C3 glomerulopathy | 0/7 (0) | 1/7 (14.2) | 2/7 (28.5) | 7/7 (100) | 1/7 (14.2) | 2/7 (28.5) | 0 | 7 |
| MPGN | 2/2 (100) | 0/2 (0) | 2/2 (100) | 2/2 (100) | 0/0 | 0/0 | 0 | 2 |
| DPGN | - | - | - | - | - | - | 3 | 3 |
DPGN: Diffuse proliferative glomerulonephritis, MPGN: Membranoproliferative glomerulonephritis, IF: Immunofluorescence, PIGN: Postinfectious glomerulonephritis, LN: Lupus nephritis, Ig: Immunoglobulin, IgAN: IgA nephropathy
Others category included five cases. Three cases were reported as DPGN based on LM findings as the tissue sent for DIF was inadequate. There were two other cases which were reported as suggestive of mesangioproliferative GN with positive IgG, IgM, and C3 and negative IgA.
DISCUSSION
Glomerular diseases are the pathologic processes identified in renal biopsy specimens. The complexity and variety of these glomerular diseases pose a considerable challenge for the pathologist. The pattern of injury in a renal biopsy is just a glimpse of the dynamic process of glomerular injury, which may have a different pattern over time. Thus, a patient may have a mild mesangial PGN early in the course of the disease that may evolve into focal PGN and still later to DPGN. The identification of these patterns on LM is essential as there are many different causes with different prognosis with each pattern.[10] All forms of these DPGN can lead to either focal or diffuse global glomerulosclerosis and end-stage kidney disease.[2]
DPGN is a form of PGN involving >50% of the glomeruli with an increase in glomerular cellularity due to infiltration and proliferation of leukocytes within the glomerulus or from proliferation of endogenous cells like mesangial cells and parietal epithelial cells. Diseases which can present with DPGN pattern during their dynamic process of glomerular injury include LN Class IV, IgA nephropathy Class IV, PIGN, MPGN, and C3 glomerulonephropathy. These glomerular diseases in spite of being common histologic phenotype, sometimes do not have adequate features required for their diagnosis on LM and special stains and are reported as DPGN. Table 5 shows the different patterns of DPGN on DIF.
Table 5.
Immunofluorescence microscopy patterns in diffuse proliferative glomerulonephritis
| Pattern | Associated disorders |
|---|---|
| All classes of Igs with C3 and C1q | LN (31 cases) |
| Predominently mesangial IgA deposites (±IgM, C3) | IgAN (22 cases) |
| Granular deposits of IgG and C3 along capillary loops | PIGN (15 cases) |
| Mesangial deposits of IgA with bright C3 | IgA predominant PIGN (1 case) |
| IgG and C3 deposits along capillary loops | MPGN (7 cases) |
| C3 deposits with abscent/scanty Igs | C3 GN (7 cases) |
>MPGN: Membranoproliferative glomerulonephritis, PIGN: Postinfectious glomerulonephritis, LN: Lupus nephritis, IgAN: IgA nephropathy, C3 GN: C3 glomerulonephritis, Ig: Immunoglobulin
Many a times, in LN, the pathognomic subendothelial deposits are not large enough to be seen on LM and special stains. In such a situation, full house pattern of IF findings avoid a misdiagnosis. Similarly, PIGN and MPGN have precisely the same morphology on LM. It is the double contour appearance seen in MPGN that differentiates the two on LM and SS. In early stages of MPGN, sometimes, this feature is not seen on LM, which then requires a granular pattern of C3 and IgG on IF to render a correct diagnosis. Due to a varied histological presentation, the diagnosis of IgA glomeulopathy depends on the demonstration of glomerular staining of IgA dominant or IgA codominant involving the mesangium on IF.
Similarly, C3 GM is an IF dependent diagnosis with varied morphological patterns.
Many studies have been done in the past to study the different patterns of GN. However, the present study emphasizes on a single pattern of DPGN seen as a part of the dynamic process of glomerular injury in different diseases. In such situation, IF it becomes an essential tool in categorizing this pattern into different diseases. IF is used to determine the distribution, pattern, and composition of glomerular immune deposits.[10]
In our study, maximum cases were of LN (35.7%) The mean age of presentation was 24.4 years which was in concordance with the study done by Gomaa W et al.[11] and buch et al.[12] There was with female preponderance which was similar to the study of buch et al.[12] Most common mode of presentation was also comparable to other studies. In LN, IF studies reveal mesangial and subendothelial capillary wall staining of immune reactants, which is mostly full house.[12,13,14] In the present study, 34 cases (35.7%) showing DPGN patterns were diagnosed as LN. Of these 34 cases, 3 cases were inadequate for IF. Of rest 31 cases, 28 cases (91.1%) on LM showed the pathognomic subendothelial deposits in the form of hyaline thrombi deposits and wire loop lesions. However, 3 cases (8.8%) lacked these features on LM and were diagnosed as DPGN on LM. Thus, in 28 cases, IF confirmed the LM findings, but in 3 cases the final diagnosis was given based on IF findings. These findings were similar to the data from different cases series of Appel et al.[13] and Cameron.[14]
On IF, diffuse mesangial IgA deposits is the defining hallmark of the IgAN.[15] IgG, IgM, and C3 deposition may accompany IgA.[16,17] All 24 cases (25.2%) diagnosed with IgAN showed mesangioproliferative pattern on LM. These cases could have been misdiagnosed if only LM was taken into account. This is in concordance with the study of Kumar et al. where all 9 cases with the varied morphological presentations were diagnosed based on IF findings.[6]
Granular polyclonal IgG deposit in the capillary walls with bright C3 is typical of PIGN.[18,19] This granular pattern (lumpy bumpy deposits) is usually more coarse than in membranous GN.[5] Sorger et al. has described different IF microscopy patterns called the garland pattern, mesangial pattern and starry sky pattern.[18] Sixteen cases diagnosed as PIGN showed DPGN pattern on LM. Clinical details and IF confirmed the diagnoses on the basis of granular C3 and IgG staining.
IgA dominant PIGN is a distinct disease of adults, frequently seen in diabetics.[20] On IF dominant or codominant glomerular mesangial staining of IgA and C3 is seen in all cases. IgG is variably present. A single case with DPGN pattern on LM and codominant staining of IgG, IgA, and C3 on IF was diagnosed with IgA predominant PIGN.
MPGN was previously classified as type I, II, and III. However, recent classification is based on DIF results includes two categories-immune complex-mediated MPGN which involves over activation of complement and another category of complement-mediated MPGN.[21,22] In the present study, the most common mode of presentation was nephrotic syndrome, similar to the study of Nakagawa et al.[21] Mean age of presentation and gender ratio was dissimilar to the study of Nakagawa et al.[21] On LM, the double contoured BM was seen in 66.7% of cases, which was in concordance to the study of Himmani.[22] However, 3 cases were diagnosed on the basis of IF findings.
C3 glomerulopathy is a new entity characterized by C3 accumulation with absent or scanty Ig deposition.[23] There were 7 cases of C3 GM, which showed MPGN pattern on LM. This is an IF dependent diagnosis which cannot be based on LM findings. In our study all cases had MPGN pattern on LM. Six of these cases had isolated C3 staining, out of which five cases had 3+ intensity and a single case with 2+ intensity on IF. One case showed a dominant C3 with >2 order of intensity than of IgA. This was similar to the study done by Mathur et al. which studied 6 cases of C3 GM with a predominant MPGN pattern on LM and C3 deposits of 3+ intensity on IF in the majority of cases.[23]
CONCLUSION
DIF is vital for classifying DPGN, followed by EM, which is an essential tool. This article describes a rational evaluation of kidney biopsies with DPGN pattern on LM in a way that guides toward the logical assessment to reach the diagnosis. It also tries to standardize the diagnosis of DPGN with the aid of IF and represents an attempt to amalgamate the current knowledge on its bases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hossain MT, Begum M, Rahman AJ, Kamal M. Immune deposits in glomerular diseases and their clinical, histopathological and immunopathological correlation. Bangladesh Renl J. 2008;27:22–7. [Google Scholar]
- 2.Sethi S. Etiology-based diagnostic approach to proliferative glomerulonephritis. Am J Kidney Dis. 2014;63:561–6. doi: 10.1053/j.ajkd.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med. 2012;366:1119–31. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: Pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31:341–8. doi: 10.1016/j.semnephrol.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Sethi S, Rajkumar SV. Monoclonal gammopathy-associated proliferative glomerulonephritis. Mayo Clin Proc. 2013;88:1284–93. doi: 10.1016/j.mayocp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Kumar H, Bamanikar S, Swapnil K, Buch A, Sood S, Chandanwale S. Role of direct immunofluorescence in the diagnosis of glomerulonephritis. Med J Dr DY Patil Univ. 2015;8:452. [Google Scholar]
- 7.Schneider W. Value of immunofluorescence in the diagnosis of kidney diseases. Z Gesamte Inn Med. 1983;38:115–20. [PubMed] [Google Scholar]
- 8.Nasir H, Chaudhry S, Raza W, Moatasim A, Mamoon N, Akhtar N. Role of immunoflourescence in the diagnosis of glomerulonephritis. J Pak Med Assoc. 2012;62:240–3. [PubMed] [Google Scholar]
- 9.Mubarak M, Kazi JI. Role of immunofluorescence and electron microscopy in the evaluation of renal biopsies in nephrotic syndrome in a developing country. Ultrastruct Pathol. 2009;33:260–4. doi: 10.3109/01913120903296952. [DOI] [PubMed] [Google Scholar]
- 10.Jenette JC, Silva FG. Primer on the pathologic classification and diagnosis of kidney disease. In: Jennete JC, Oslon JL, editors. Heptinstall Pathology of the Kidney. 7th ed. Philadelphia: Lippincott Williams&Wilkins; 2007. pp. 194–9. [Google Scholar]
- 11.Bahlas S, Al-Maghrabi J, Al-Ghamdi S, Habhab W, Gomaa W, Mushtaq M. Clinicopathological characteristics of lupus nephritis in Western region of Saudi Arabia: An experience from two tertiary medical centres. J Microsc Ultrastruct. 2014;2:12. [Google Scholar]
- 12.Ansari J, Karnik S, Bavikar R, Patel SR, Buch A. Role of renal biopsy in evaluation of morphological spectrum and pathogenesis of lupus nephritis. Ann Pathol Lab Med. 2018;4:A732–41. [Google Scholar]
- 13.Appel GB, Silva FG, Pirani CL, Meltzer JI, Estes D. Renal involvement in systemic lupud erythematosus (SLE): A study of 56 patients emphasizing histologic classification. Medicine (Baltimore) 1978;57:371–410. doi: 10.1097/00005792-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Cameron JS. Lupus nephritis in childhood and adolescence. Pediatr Nephrol. 1994;8:230–49. doi: 10.1007/BF00865490. [DOI] [PubMed] [Google Scholar]
- 15.NEVES, Precil Diego Miranda de Menezes et al. IgA nephropathy: histological analysis and clinicomorfological correlation in patients from Minas Gerais State. J. Bras. Nefrol. 2012;34:101–8. doi: 10.1590/s0101-28002012000200001. [DOI] [PubMed] [Google Scholar]
- 16.Rumana J, Iyengar A, Vasudevan A. Clinical spectrum and outcome of childhood iga nephropathy-A single centre experience. North Int Med Coll J. 2015;7:97–100. [Google Scholar]
- 17.Barros Silva GE, Costa RS, Viana Lobato ET, Salgado JV, Vieira-Neto OM, Moyses-Neto M, et al. A case series of diffuse crescentic IgA nephropathy: An omitted entity in the Oxford classification. Int J Clin Exp Pathol. 2016;9:1909–16. [Google Scholar]
- 18.Sorger K, Gessler U, Hübner FK, Köhler H, Schulz W, Stühlinger W, et al. Subtypes of acute postinfectious glomerulonephritis. Synopsis of clinical and pathological features. Clin Nephrol. 1982;17:114–28. [PubMed] [Google Scholar]
- 19.Siddappa S, Kowsalya R, Mythri KM. A review of postinfectius glomerulonephritis cases from a tertiary care renal referral center in South India. Int J Health Allied Sci. 2013;2:264–9. [Google Scholar]
- 20.Gaut JP, Mueller S, Liapis H, Gaut JP, Mueller S, Liapis H, et al. IgA dominant post-infectious glomerulonephritis update: Pathology spectrum and disease mechanisms. Diagn Histopathol. 2017;23:126–32. [Google Scholar]
- 21.Nakagawa N, Hasebe N, Hattori M, Nagata M, Yokoyama H, Sato H, et al. Clinical features and pathogenesis of membranoproliferative glomerulonephritis: A nationwide analysis of the Japan renal biopsy registry from 2007 to 2015. Clin Exp Nephrol. 2018;22:797–807. doi: 10.1007/s10157-017-1513-7. [DOI] [PubMed] [Google Scholar]
- 22.Himamani S. Membranoproliferative Glomerulo Nephritis Common Glomerular Disease - Changing Pattern of Biopsy Proven Renal Disease in a Tertiary Care Hospital. Int J Sci Stud. 2016;3:193–6. [Google Scholar]
- 23.Mathur M, Sharma S, Prasad D, Garsa R, Singh AP, Kumar R, et al. Incidence and profile of C3 Glomerulopathy: A single center study. Indian J Nephrol. 2015;25:8–11. doi: 10.4103/0971-4065.136889. [DOI] [PMC free article] [PubMed] [Google Scholar]