ABSTRACT
A 55-year-old man with a history of ulcerative colitis status post ileal pouch-anal anastomosis underwent evaluation for pouchitis. He was found to have signet ring cell cancer within the pouch. Primary cancers of the ileal pouch are rare. Signet ring cell carcinoma is a rare form of adenocarcinoma that can involve the gastrointestinal tract and other organs. We present a case of a signet ring cell carcinoma arising from an ileal pouch.
INTRODUCTION
Cancers related to ileal pouch-anal anastomosis (IPAA) are rare. Signet ring cell carcinoma (SRCC) is an especially rare adenocarcinoma that most commonly arises in the stomach but can rarely involve other organs, such as the small intestine and colon. It is poorly differentiated and has a poor prognosis.1 We present a case of SRCC within an ileal pouch.
CASE REPORT
A 55-year-old man with long-standing ulcerative colitis (UC) diagnosed at age 14 years, status post 2-stage proctocolectomy with S-pouch for medically refractory disease in 1989, presented for pouchoscopy to evaluate fecal urgency. The patient had been lost to follow-up after his restorative surgery. He had no history of primary sclerosing cholangitis or colon dysplasia. A pouchoscopy 2 years before presentation showed inflammation of the pouch consistent with pouchitis, without evidence of dysplasia. He responded well to ciprofloxacin and metronidazole and was later maintained on a probiotic (Visbiome, ExeGi Pharma).
Follow-up pouchoscopy revealed severe diffuse inflammation with edema, erosions, friability, granularity, mucus, pseudopolyps, scarring, and shallow ulcerations in the ileoanal pouch. There were a benign-appearing mild stricture in the distal body of the ileoanal pouch and a nodular lesion at the surgical raphe (Figure 1). The pouch inlet and prepouch ileum appeared normal. Biopsies from the pouch body, surgical raphe, and rectal cuff showed invasive signet ring/goblet cell carcinoma with mucinous features and lymphovascular invasion (Figure 2). Ileal biopsies were normal. Thoracic, abdominal, and pelvic computed tomography with contrast did not show evidence of distant metastases. Magnetic resonance imaging of the pelvis revealed pouch wall thickening and multiple enlarged lymph nodes. His serum carcinoembryonic antigen was 3.7 ng/mL, and his cancer antigen 19-9 was 9 units/mL.
Figure 1.
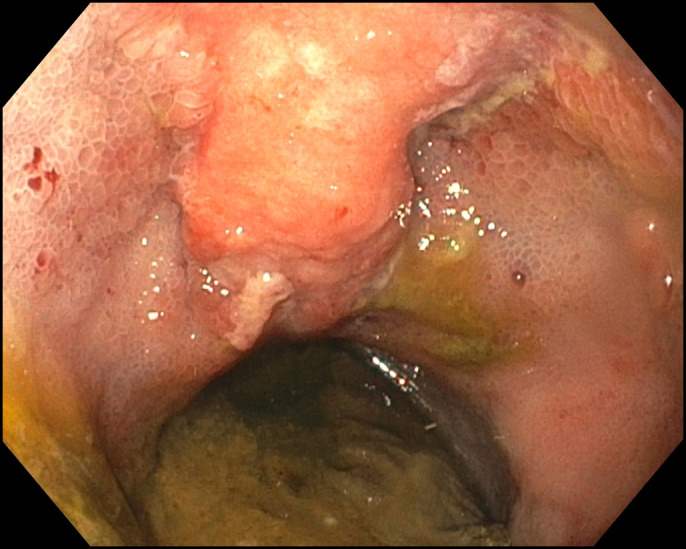
Pouchoscopy showed a nodular lesion at the surgical raphe and diffuse inflammation with edema, erosions, friability, and shallow ulcerations in the ileoanal pouch.
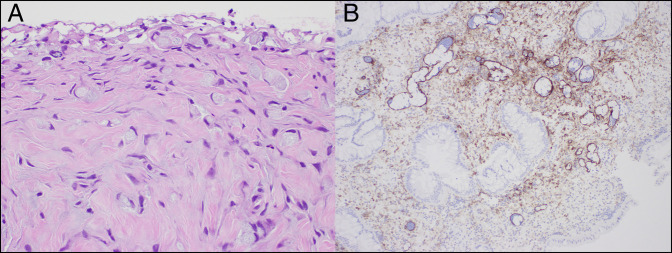
Figure 2.
Biopsies of the pouch body showing (A) invasive signet ring/goblet cell carcinoma with mucinous features on hematoxylin and eosin stain (40× magnification) and (B) with lymphovascular invasion on D2-40 immunostain (20× magnification).
Laparotomy was performed with the intent of pouch excision and permanent ileostomy; however, multiple intra-abdominal adhesions were identified, as well as several areas of exophytic nodularity throughout the abdomen. Frozen section analysis revealed evidence of signet cell carcinoma. Given the evidence of carcinomatosis, the decision was made to forego the planned excision procedure and instead use fecal diversion along with the creation of a loop ileostomy. Subsequently, leucovorin, fluorouracil, oxaliplatin and bevacizumab therapy were initiated. Unfortunately, the patient developed frequent small bowel obstructions upstream from the ileostomy requiring total parenteral nutrition, limiting his ability to proceed with chemotherapy.
DISCUSSION
IPAA is the standard of care for medically refractory or complicated UC.2 Pouch-related cancers are rare, with a systematic review reporting a pooled cumulative incidence of 0.33% 20 years after IPAA.2 Chronic pouchitis and severe villous atrophy have not emerged as well-defined risk factors for neoplasia in pelvic pouch mucosa of patients with UC.3–5 A history of colorectal neoplasia has been the only established risk factor for pouch neoplasia.6 In a systematic review of 5,136 patients receiving IPAA, there were 49 cases of “de novo” adenocarcinoma of the cuff or ileal pouch, excluding patients with local recurrences or disease persistence. Of these, 28.6% arose from the pouch and 67.3% arose from the anorectal mucosa. The subtypes of adenocarcinomas were not specified. The authors mentioned other pouch-related cancers that were excluded from their analysis, namely, 7 cases of squamous cell carcinoma, 4 cases of lymphoma, and 1 case of pouch well-differentiated neuroendocrine tumor (carcinoid).2
Although there are no universally accepted pouch surveillance guidelines after IPAA for UC, the American Society of Gastrointestinal Endoscopy suggests annual pouchoscopy with biopsies for patients at highest risk of pouch neoplasia, that is, those with a history of dysplasia or cancer.7,8 Yearly surveillance may be considered in patients at high risk, namely, those with a history of primary sclerosing cholangitis, refractory pouchitis, or atrophic pouch mucosa with severe inflammation. The American Society of Gastrointestinal Endoscopy makes no recommendations on patients without risk factors.8
Signet ring cell carcinomas are mucin-secreting tumors that originate from undifferentiated stem cells. The abundance of intracellular mucin peripherally displaces the nucleus, giving it the characteristic appearance of a signet ring.9 Within hollow viscera, SRCC typically produces minimal mucosal alterations. The “linitis plastica” appearance, referring to the rigid and contracted structure that the organ takes, is a result of the tumor invading the wall of the stomach. However, this feature is not unique to SRCC.10
SRCC has a poor prognosis, with higher T, N, and M categories, lymphatic invasion, and worse grading than adenocarcinoma. It is often diagnosed at more advanced stages and frequently with peritoneal metastases.11,12 SRCC of the ileum is rare because most cases involve the stomach. Cases of small bowel SRCC have been reported in patients with Crohn's disease.13,14 A study of 24,171 patients in the Surveillance, Epidemiology, and End Results database from 1988 to 2012 showed a prevalence of 63.4% for gastric SRCC, 18.2% for colon, 5% for esophageal, 3.1% each for rectal and lung, 1.8% for pancreatic, 1.5% for breast, 1.3% for bladder, 1.1% for small intestine, and 1% for gallbladder. Multivariate analyses showed that the primary tumor location was an independent prognostic factor of survival. Gastric cancer had a 5-year cause-specific survival of 22.1%. Patients with lung, small intestine, and bladder SRCC had comparable survival while breast and colorectal SRCC had better survival than gastric SRCC. Esophageal, gallbladder, and pancreatic SRCC were significantly associated with poor survival compared with gastric SRCC.9 To the best of our knowledge, this is the first reported case of SRCC within an ileal pouch. This rare entity carries a poor prognosis, is often diagnosed at an advanced stage, and has a survival rate similar to that of gastric SRCC.
DISCLOSURES
Author contributions: E.P. Tetangco wrote the article and is the article guarantor. K. Krogh and G. Yang edited the article. E. Bellaguarda revised the article for intellectual content and approved the final article.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Eula Plana Tetangco, Email: eptetangco@gmail.com.
Katrina Krogh, Email: Katrina.Krogh@northwestern.edu.
Guang-Yu Yang, Email: g-yang@northwestern.edu.
Emanuelle Bellaguarda, Email: emanuelle.bellaguarda@northwestern.edu.
REFERENCES
- 1.Tajiri K, Sudou T, Fujita F, Hisaka T, Kinugasa T, Akagi Y. Clinicopathological and corresponding genetic features of colorectal signet ring cell carcinoma. Anticancer Res. 2017;37(7):3817–23. [DOI] [PubMed] [Google Scholar]
- 2.Selvaggi F, Pellino G, Canonico S, Sciaudone G. Systematic review of cuff and pouch cancer in patients with ileal pelvic pouch for ulcerative colitis. Inflamm Bowel Dis. 2014;20(7):1296–308. [DOI] [PubMed] [Google Scholar]
- 3.Gullberg K, Ståhlberg D, Liljeqvist L, et al. Neoplastic transformation of the pelvic pouch mucosa in patients with ulcerative colitis. Gastroenterology. 1997;112(5):1487–92. [DOI] [PubMed] [Google Scholar]
- 4.Thompson-Fawcett MW, Marcus V, Redston M, Cohen Z, McLeod RS. Risk of dysplasia in long-term ileal pouches and pouches with chronic pouchitis. Gastroenterology. 2001;121(2):275–81. [DOI] [PubMed] [Google Scholar]
- 5.Vento P, Lepistö A, Kärkkäinen P, Ristimäki A, Haglund C, Järvinen HJ. Risk of cancer in patients with chronic pouchitis after restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2011;13(1):58–66. [DOI] [PubMed] [Google Scholar]
- 6.Derikx LA, Kievit W, Drenth JP, et al. Prior colorectal neoplasia is associated with increased risk of ileoanal pouch neoplasia in patients with inflammatory bowel disease. Gastroenterology. 2014;146(1):119–28.e1. [DOI] [PubMed] [Google Scholar]
- 7.Derikx LA, Nissen LH, Oldenburg B, Hoentjen F. Controversies in pouch surveillance for patients with inflammatory bowel disease. J Crohns Colitis. 2016;10(6):747–51. [DOI] [PubMed] [Google Scholar]
- 8.American Society for Gastrointestinal Endoscopy Standards of Practice C, Shergill AK, Shergill AK, Lightdale JR, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81(5):1101–21.e1–13. [DOI] [PubMed] [Google Scholar]
- 9.Wu SG, Chen XT, Zhang WW, et al. Survival in signet ring cell carcinoma varies based on primary tumor location: A surveillance, Epidemiology, and End results database analysis. Expert Rev Gastroenterol Hepatol. 2018;12(2):209–14. [DOI] [PubMed] [Google Scholar]
- 10.Almagro UA. Primary signet-ring carcinoma of the colon. Cancer. 1983;52(8):1453–7. [DOI] [PubMed] [Google Scholar]
- 11.Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25(3):651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitsche U, Friess H, Agha A, et al. Prognosis of mucinous and signet-ring cell colorectal cancer in a population-based cohort. J Cancer Res Clin Oncol. 2016;142(11):2357–66. [DOI] [PubMed] [Google Scholar]
- 13.Hammami MB, Aboushaar R, Musmar A, Azhar M. Ileal signet ring cell carcinoma masked by Crohn disease. Ochsner J. 2020;20(3):323–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho JR, Tavares J, Goulart I, et al. Signet ring cell carcinoma, ileal Crohn disease or both?—A case of diagnostic challenge. GE Port J Gastroenterol. 2018;25(1):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]