Abstract
Background:
Lower capsular contracture rates have been observed with peri-prosthetic fat grafting. We investigated the effect of fat grafting on capsular characteristics and peri-prosthetic collagen density, content, and fiber alignment.
Methods:
Forty miniature tissue expanders were placed on the backs of 20 rats. After four weeks, both inguinal fat pads were harvested, homogenized, and injected into peri-prosthetic tissue of the right tissue expander (fat graft) while the left served as control. The animals were killed at three (10 rats) and 12 weeks (10 rats), and full thickness peri-prosthetic samples were histologically processed for morphology (H&E) and collagen type and content (picrosirius red).
Results:
An 8.1% increase in adipose peri-prosthetic thickness was associated with a 10% decrease in collagen content at any time point (P = 0.004). Fat-grafted capsules displayed a 59% reduction in % total collagen when compared with controls (P < 0.001). There were no differences in capsular thickness. Fat-grafted samples were 54 times more likely to have a higher inflammation score and 69 times more likely to have a lower capsular density score than their nongrafted counterparts (P < 0.001 and P = 0.001, respectively). The extent of inflammation decreased over time in all samples (P = 0.002). Additionally, fat-grafted samples were 67 times more likely to have a lower fiber alignment score than the controls (P < 0.001).
Conclusions:
Enhancement of peri-prosthetic tissue with fat grafting decreases collagen content, density, and fiber alignment of implant capsules. These findings support clinical application of fat grafting in prosthetic breast surgery to potentially decrease capsular contracture.
INTRODUCTION
Peri-prosthetic capsular formation is a vexing phenomenon in breast surgery. The unfavorable tightening of the fibrous collagen layer around an implant, capsular contracture, has been the most common reason for reoperation after cosmetic or reconstructive breast procedures.1–3 Capsular contracture has been reported to complicate over 50% of breast implant procedures.3,4 Various therapeutic strategies have been employed to alleviate capsular contracture, including noninvasive treatments and operative interventions.2 Recently, the traditional surgical approach involving release or removal of the fibrous implant envelope has been successfully combined with the application of acellular dermal matrix (ADM) and fat grafting.5 The observed beneficial effect of ADM and fat transfer on resolution of capsular contracture could be attributed to the disruption of constricting forces by ADM as well as to the enhancement of the peri-prosthetic soft tissue by fat grafting. In fact, preconditioning of the breast envelope with preemptive fat transfer has been noted to yield lower contracture rates after implant procedure switch from subpectoral to prepectoral position.6,7 Repetitive fat transfer to the peri-prosthetic envelope in patients with Baker IV capsular contracture has resulted in resolution of breast pain and downstaging of capsular contracture, especially if the implant was placed in subglandular position.8 The shared feature of the cited studies consists of fat deposition in proximity of the implant capsule leading to obvious soft tissue augmentation but also possibly causing disruption of the peri-prosthetic scar anatomy and behavior. To date, the structural basis of this clinically observed positive capsular alteration after fat grafting is not known, as the histological changes accompanying peri-prosthetic fat transfer have not been well described. Therefore, we designed a study to investigate the changes of peri-prosthetic structure after fat grafting in the rat model. We hypothesized that deposition of fat graft around an implant enhances soft tissue envelope and reduces the content and alignment of collagen fibers in peri-prosthetic capsules.
MATERIALS AND METHODS
Surgical Protocol
Forty miniature tissue expanders were implanted in the back of 20 Sprague-Dawley male rats weighing 250–300 g following the surgical protocol outlined below. The study was approved by the Institutional Animal Care and Use Committee at West Michigan Regional Laboratory, Grand Rapids, Mich.
Tissue Expander Placement
The animals were anesthetized using isoflurane and placed in prone position. A prophylactic dose of cefazolin (120 mg/kg/dose) was administered intraperitoneally. In each rat, two noncommunicating pockets measuring 3 cm by 4 cm were dissected under the panniculus carnosus muscle under sterile conditions. The pockets were irrigated with a bacitracin solution consisting of 50,000 units of bacitracin per 1 L of sterile saline solution. A miniature tissue expander with an adjacent filling port (SHR-EL-010S, Sientra, Inc, Santa Barbara, Calif.) was placed in each dorsal pocket. The incisions were closed with 5-0 absorbable monofilament suture (Monocryl, Johnson&Johnson, New Brunswick, N.J.) in dermal and subcuticular fashion. Each tissue expander was then filled with 5.5 ml of sterile saline through the adjacent port palpable through the skin.
Fat Grafting
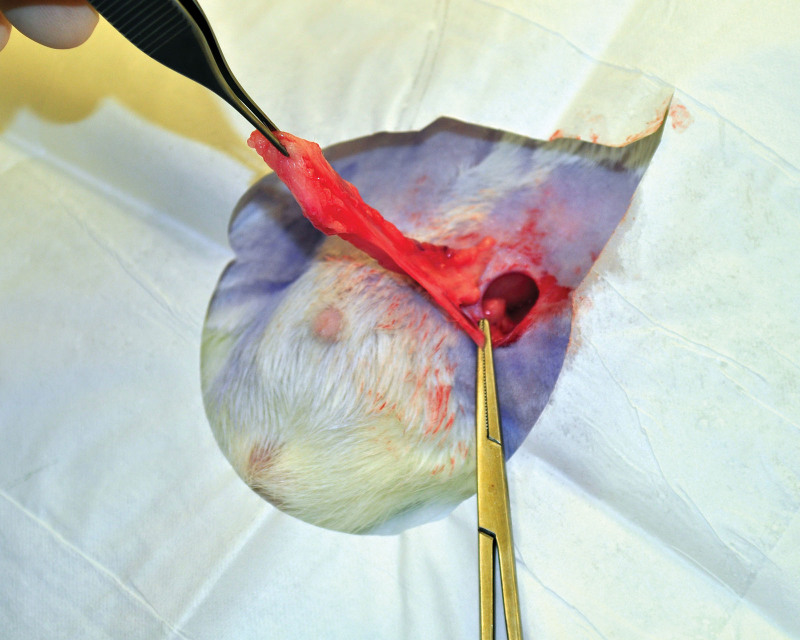
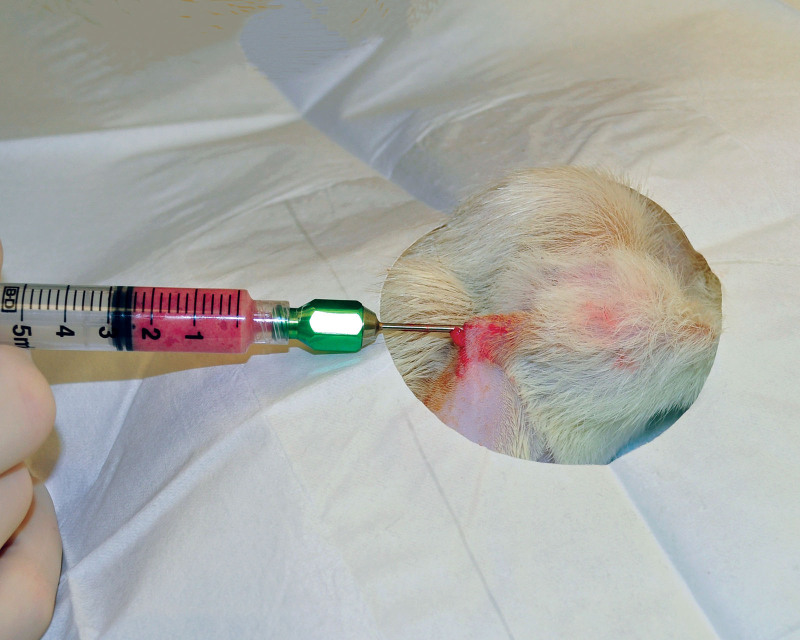
The animals were reanesthetized after four weeks. In each animal, a 1-cm incision was performed in each groin under sterile conditions. Subsequently, the bilateral inguinal fat pads were harvested through groin incisions and finely minced with a scalpel and subsequently by multiple passes through a 1.4-mm diameter Luer to Luer connector (ATLLLL1.4mm Tulip Medical, San Diego, Calif.) until the fat graft structure was uniform (Fig. 1). (See Video online], which displays homogenization of the harvested fat graft using a 1.4-mm Luer to Luer connector.) The amount of the obtained morselized fat graft was recorded. Both groin incisions were closed with 5-0 monofilament suture (Monocryl, Johnson&Johnson, New Brunswick, N.J.). Next, the animal was turned in the prone position and the implant domes were shaved with a surgical clipper and sterilely prepared. A small 2-mm stab incision was performed in the midline to allow for passage of a 2-mm blunt cannula (Col-17, Mentor Worldwide LLC, Irvine, Calif.). The prepared fat graft was deposited under the skin overlaying the right implant capsule using a fanning technique (Fig. 2). Attention was paid to placement of each fat graft pass in a separate channel to avoid placement of clamps on graft. The contralateral side underwent a 2 mL saline injection with the blunt cannula using a similar fanning technique through the same incision. The back incision was closed with tissue glue (Exofin, Chemence Medical, Inc. Alpharetta, Ga.).
Fig. 1.
Harvest of the left inguinal fat pad with ligation of the inferior epigastric vessels.
Fig. 2.
Injection of morselized fat graft under the skin overlaying the right tissue expander using the fanning technique.
Video. Video 1 from “Peri-prosthetic Fat Grafting Decreases Collagen Content, Density, and Fiber Alignment of Implant Capsules.” See Video 1 [online] which displays homogenization of the harvested fat graft using a 1.4 mm Luer to Luer connector.
Tissue Harvesting
After three (10 animals) and 12 weeks (10 animals) from fat grafting, the rats were euthanized with an isoflurane overdose and their backs were shaved. The surgical sites were inspected for signs of infection, such as erythema and edema, quality of hair regrowth, presence of wounds or excoriations. Next, incisions were made in the caudal portion of the back overlaying the port and the dissection was continued toward each implant dome, paying attention to harvesting the entire thickness of tissues overlaying each prosthetic en bloc and not leaving any residual tissue on the implant. Specimens of fat-grafted tissues overlaying the right implant (fat graft group) and nongrafted tissues overlaying the left implant (control group) containing skin, subcutaneous tissue, and capsule were placed in cassettes and fixated in 10% buffered formaldehyde solution.
Tissue Processing
Hematoxylin and Eosin Staining
After adequate fixation in formaldehyde solution, tissues underwent routine tissue processing using a Sakrua VIP5 within 24 hours of tissue harvest.
Picrosirius Red Staining
Picrosirius red staining was performed manually on the bench top. Coverslip only protocol on the Ventana Symphony was used. The samples were treated in phosphomolybdic acid 0.2% aqueous solution and stained in sirius red, 0.1% in saturated picric acid following the described protocol.9
Histology and Digital Slide Analysis
Routinely prepared hematoxylin and eosin slides were examined by a board-certified veterinary pathologist using an Olympus BX51 light microscope, and images were captured with an Olympus DP70 digital camera using CellSens Entry 1.7 imaging software (Olympus, Waltham, Mass.). For quantification of picrosirius red staining, slides were digitized at 20× objective magnification (0.5 µm/pixel resolution) on a digital slide scanner (Leica Aperio AT2, Leica Biosystems, Buffalo Grove, Ill.). Annotated areas for quantitation were digitally drawn, and digital quantification of staining was performed on picrosirius red-stained sections using commercial algorithms for positive pixel detection (Leica Aperio Positive Pixel analysis, v 9.1). Digital false color overlay images reflecting the digitally determined intensity thresholds (negative, weak positive, moderate positive, strong positive) were generated for each image by the software and visually checked to verify accuracy as follows: dense organized collagen (strongly positive pixels) was correlated with strong pixel count and loose haphazard collagen (weakly positive pixels) was correlated with weak pixel count. Total number of pixels (N total) was expressed as a sum of total positive pixels (N positive) and total negative pixels (N negative). Collagen positivity defined the total collagen content specific to each sample. Additionally, the surface area of each section (area) was measured (mm2). The contents of dense collagen, loose collagen, collagen positivity, and %total collagen in each sample were calculated as follows:
Dense collagen = strongly positive pixels/area
Loose collagen = weakly positive pixels/area
Collagen positivity = n positive/ n total
%total collagen = collagen positivity/area
Quantification of Histology Endpoints
Quantitative measurements: measurements of capsular thickness and adipose layer thickness were made using measurement functions in CellSens Entry 1.7 imaging software, taking the average of three separate measurements at the thickest areas. The adipose thickness reflected the thickness of the fat graft or any of the preexisting fat in the sample outside the tissue expander up to the muscular layer of the panniculus carnosus measured in microns. A grading scale was developed, and qualitative (semiquantitative) measurements taken for fat degeneration, extent of inflammation, and neovascularization as follows: 0 = no alteration (histology within normal limits); 1 = minimal alteration; 2 = mild alteration; 3 = moderate alteration; 4 = marked alteration. Capsular cellularity was graded as low, moderate, or high, and capsular density was graded as loose, moderate, or dense. Average fiber alignment was characterized as poor (haphazard, disorganized), moderate, or high (parallel, organized). Synovial metaplasia described as a lining of columnar cells that resembles synovium was marked as present or not present.
Statistical Analysis
Summary statistics were calculated. Data are expressed as the median, along with the minimum and maximum. Continuous, nonordinal data were log transformed before regression analysis, due to the nonnormal distribution of the variables. Synovial metaplasia was analyzed using Fisher’s exact test. Percent collagen was analyzed as a dependent variable in a multiple regression analysis, with time (three versus 12 weeks) and fat engrafting as the independent variables. Capsular contracture and adipose thickness were analyzed as dependent variables in multiple regression analyses, whereas inflammation extent, epidermal thickness, capsule cellularity, fiber alignment, and capsular density were analyzed as dependent variables in ordered logistic regression analyses. For these analyses, the independent variables were percent collagen, time (three versus 12 weeks), and fat engrafting.
All regression analyses, including the analysis of percent collagen, were first tested with the inclusion of an interaction term, time versus fat engrafting. With two exceptions, the analyses indicated a nonsignificant interaction. The exceptions were for capsule cellularity and capsular density, where in each case one of the subgroups failed to show any variability (all values were given the same score); so the analysis could not run properly. For these reasons, the final regression analyses performed for this study did not include any interaction terms. For the ordinal logistic regression analyses, the area under the curve (AUC) was calculated with Somers’ D (AUC = (1 + Somer’s D)/2), using the somersd routine described by Newson.10 Assessment of the AUC was determined using the criteria of Hosmer et al.11 Significance was assessed at P value less than 0.05. All analyses were performed using Stata v.15.1 (StataCorp, College Station, Tex.).
RESULTS
Complications
Three rats that died in recovery after the initial tissue expander placement (two rats) and after fat grafting (one rat) were replaced in the study. Similarly, one rat with a left peri-prosthetic seroma and one animal with left implant exposure were euthanized and replaced. The remaining rats did not display systemic or prosthesis-related complications. Four animals presented with unilateral or bilateral groin wound dehiscence requiring washout and reclosure on postoperative days 1–4. The technique of groin closure using tucking stitches implemented after the initial groin dehiscences eliminated this complication. The amount of fat graft derived in each animal averaged 2.1 ± 0.2 mL (1.5–3.0 mL).
Histological Analysis
Epidermal Thickness
There were no significant effects of fat grafting, time, or % total collagen content with regard to epidermal thickness amongst the groups (Table 1). The AUC for this analysis was 0.60, considered to be poor discrimination.11
Table 1.
Histological Characteristics of Peri-prosthetic Capsules
| Control Week 3 |
Fat Graft Week 3 |
Control Week 12 |
Fat Graft Week 12 |
Significance | AUC | |
|---|---|---|---|---|---|---|
| Epidermal thickness | 0 (0, 0) | 1 (0.25, 1.25) | 0 (0, 0) | 0 (0, 0) | NS | 0.60 |
| Inflammation | 1 (0, 1) | 3 (2.75, 3.5) | 0 (0, 0.5) | 1 (1, 2) | S* | 0.83 |
| Capsular cellularity | 2 (1, 2) | 2.5 (2, 3) | 1 (1, 1) | 1 (1, 2) | S† | 0.60 |
AUC: Area under the curve; NS: No significant findings (P ≥ 0.05); S: Significant finding (P < 0.05).
Ordinal data (0 = Histology within normal limits; 1 = Minimal alteration; 2 = Mild alteration; 3 = Moderate alteration; 4 = Marked alteration) are expressed as the median, with the 25th percentile and the 75th percentile in parentheses; independent variables for each analysis were fat graft, time, and % collagen.
Control, week 3, n = 12; Fat Graft group, week 3, n = 16; Control, week 12, n = 10; Fat Graft group, week 12, n = 10.
*No significant effect of % collagen; those with a fat graft were 54 times more likely to have a higher inflammation extent score than those without a fat engraft (P < 0.001); those at 12 weeks were 10 times more likely to have a lower inflammation extent score than those at 3 weeks (P = 0.012).
†No significant effect of % collagen or fat engraft; those at 12 weeks were 10 times more likely to have a lower capsule cellularity score than those at 3 weeks (P = 0.014).
Adipose Thickness and Fat Degeneration
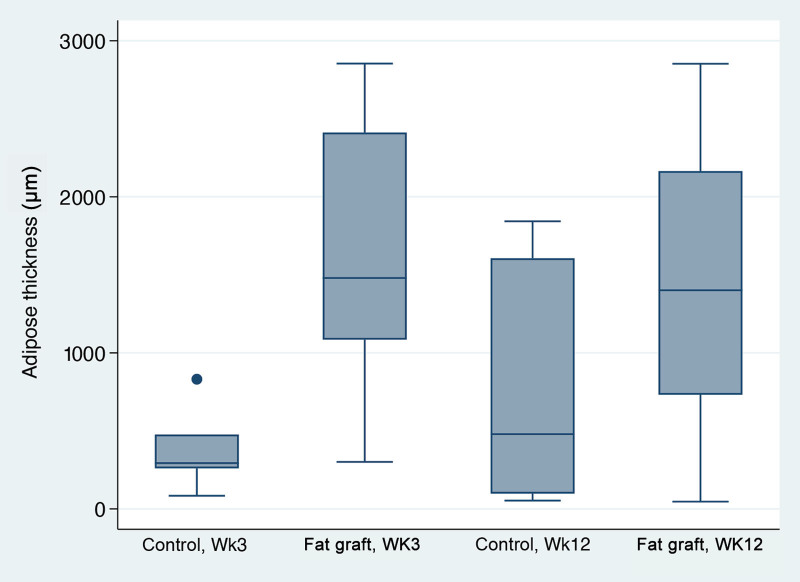
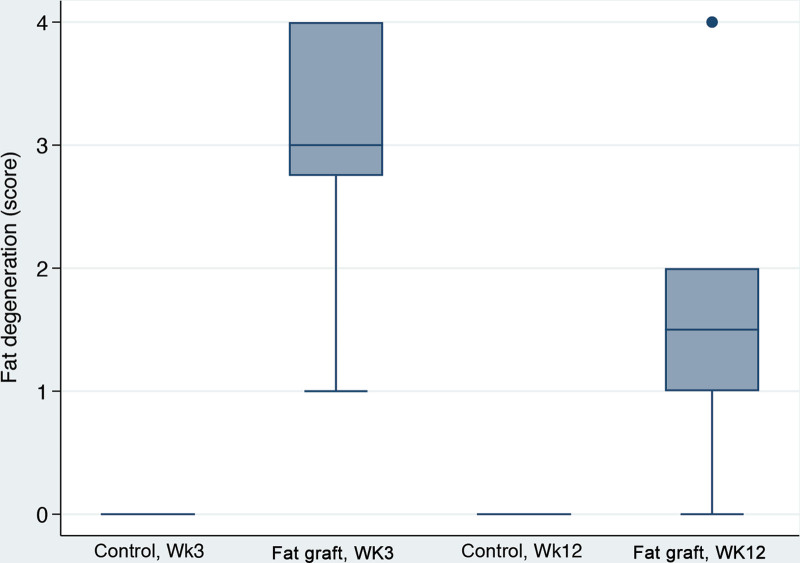
Adipose thickness was substantially accentuated in fat-grafted samples at three weeks with median values over five times higher than for the control group (Fig. 3). This ratio was not quite as great at 12 weeks, where the fat-grafted values showed less than twice as much adipose thickness as the control group (Figs. 4, 5). Despite these numerical effects, there were no statistically significant effect of fat grafting on adipose thickness (P = 0.325). Interestingly, an 8.1% increase in adipose thickness was associated with 10% decrease in collagen content at any time point (P = 0.004). Fat grafting was also associated with fat degeneration, which was noted only in fat-grafted samples (Fig. 6). Fat degeneration and necrosis were more prevalent at the earlier time point and significantly decreased with time.
Fig. 3.
Adipose thickness box plots. No significant effect of fat grafting or time (P > 0.05); a 10% increase in collagen content was associated with an 8.1% decrease in adipose thickness (P = 0.004).
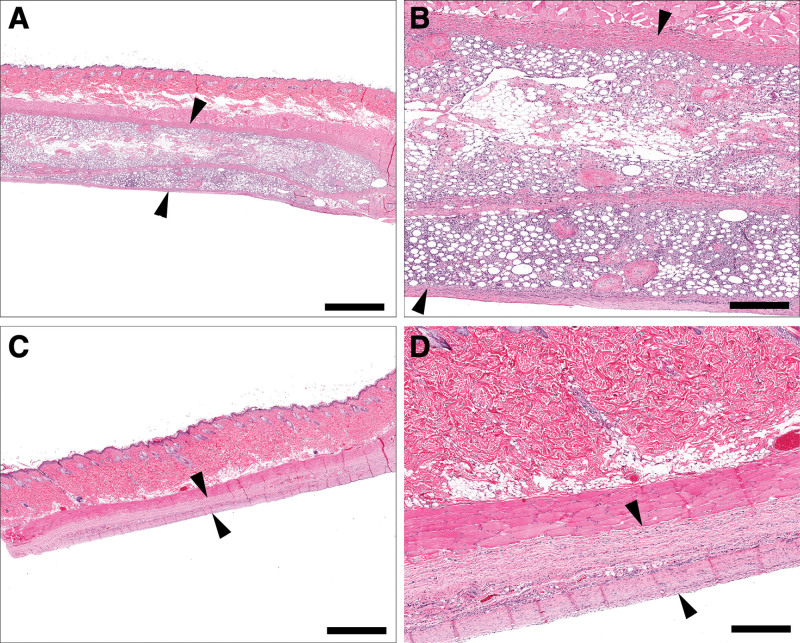
Fig. 4.
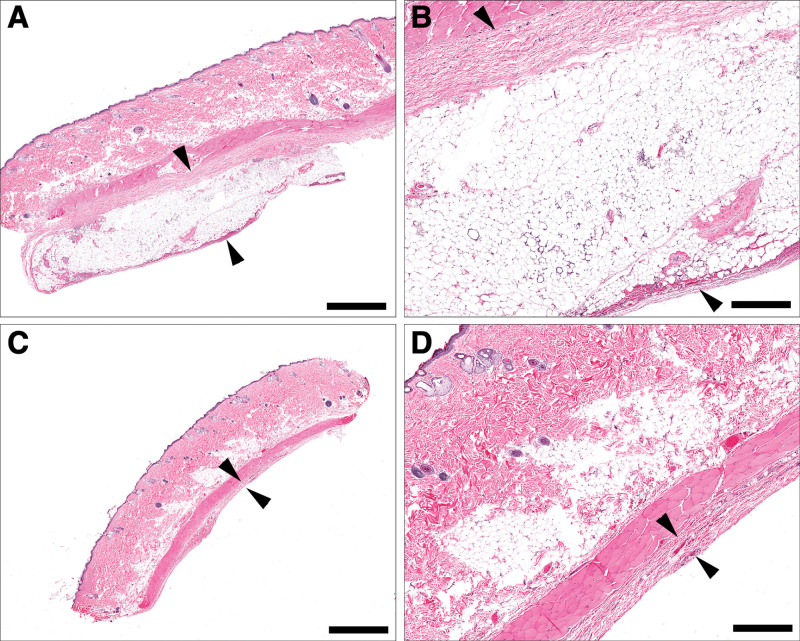
Comparative thickness of peri-prosthetic tissue sections between fat-grafted (A, B) and control (C, D) samples at 3 weeks. Fat-grafted samples are markedly thicker than nongrafted samples due to the presence of the graft (A, B, arrowheads), as well as a mixture of haphazard collagen bundles and inflammatory cell infiltrates, compared with control samples, which have variably dense capsule (D, arrowhead). (A, C, bar = 2 mm; B, D, bar = 500 μm).
Fig. 5.
Comparative thickness of peri-prosthetic tissue sections between fat graft (A, B) and control (C, D) samples at 12 weeks. Grafted samples are markedly thicker than nonengrafted samples at 12 weeks due to persistence of the engrafted fat layer and associated collagen (A, B, arrowheads) when compared with nonengrafted samples, which are associated with collagen and a variably dense capsule (C, D, arrowheads). (A, C, bar = 2 mm; B, D, bar = 500 μm).
Fig. 6.
Fat degeneration box plots. Score: 0, Histology within normal limits; 1, Minimal alteration; 2, Mild alteration; 3, Moderate alteration; 4, Marked alteration. Comparison was only between the fat graft group at 3 weeks versus the fat graft group at 12 weeks, P = 0.021.
Inflammation and Capsular Cellularity
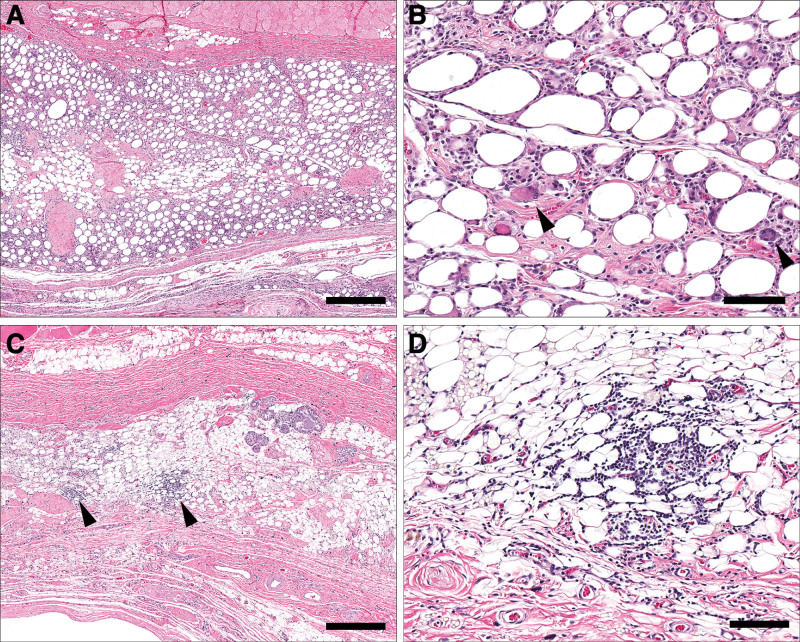
Fat degeneration appeared to elicit a lymphohistocytic or foreign body type of inflammatory response. Samples with fat graft displayed more active inflammation with obvious remodeling processes reflected by the presence of granulation tissue, edema, and mixed inflammatory cells. In contrast to fat-grafted samples, the control group displayed mononuclear inflammation with lymphocytes and plasma cells being the predominant cellular components. Fat grafting caused significantly greater inflammatory response than a sham operation in controls. Moreover, fat-grafted samples were 54 times more likely to have higher inflammation score then their nongrafted counterparts (P < 0.001). The extent of inflammation decreased overtime in all samples (Fig. 7). The observed subsiding inflammation was paralleled by changes in capsular cellularity which significantly decreased with time. In fact, 12-week samples were 10 times more likely to have lower capsular cellularity score than their 3-week counterparts irrespective of fat grafting status (Table 1). The AUC for the inflammation analysis was 0.83, considered to be excellent discrimination, while the AUC for capsular cellularity was considered to be poor discrimination.11
Fig. 7.
Resolution of inflammation in fat-grafted samples over time. At three weeks (A, B), fat-grafted samples are associated with generally diffuse and dense lymphohistiocytic inflammation including the presence of multinucleated macrophages (B, arrowheads). By 12 weeks (C, D), this inflammation significantly decreases in severity or resolves, with residual foci of mononuclear inflammatory cells (C, arrowheads). (A, C, bar = 500 μm; B, D, bar = 100 μm).
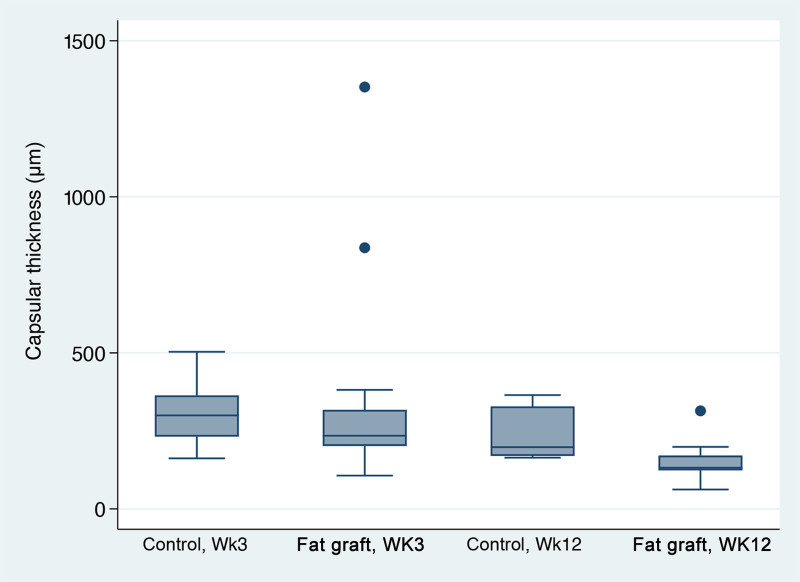
Capsular Thickness
Median capsular thickness in fat-grafted samples measured 234 µm versus 300 µm in the control group at 3 weeks and 132 µm in the fat graft group versus 198 µm in the control group at 12 weeks. Though the capsular thickness was not shown to be statistically different amongst the groups by multiple linear regression analysis, all samples displayed significantly reduced capsular thickness from week 3 to week 12 (Fig. 8).
Fig. 8.
Capsular thickness box plots. No significant effect of % collagen or fat engraft (P > 0.05); those at 12 weeks had a capsular thickness of 45.9% lower than those at 3 weeks (P = 0.004).
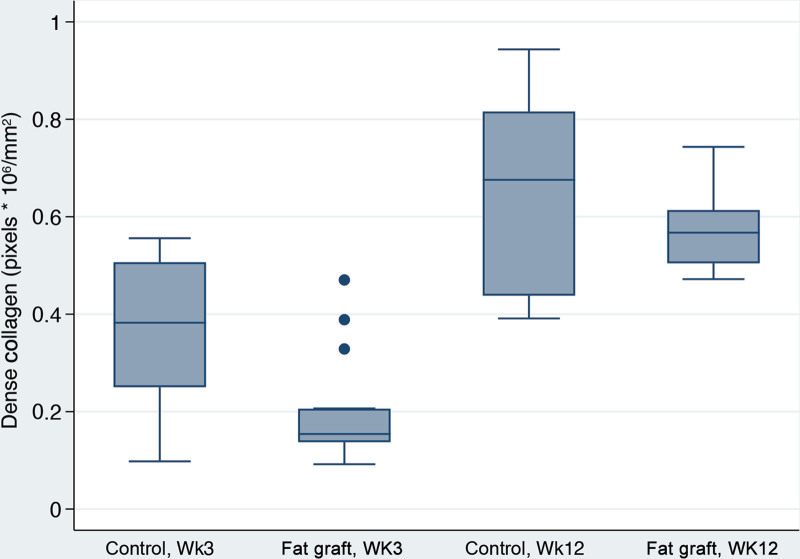
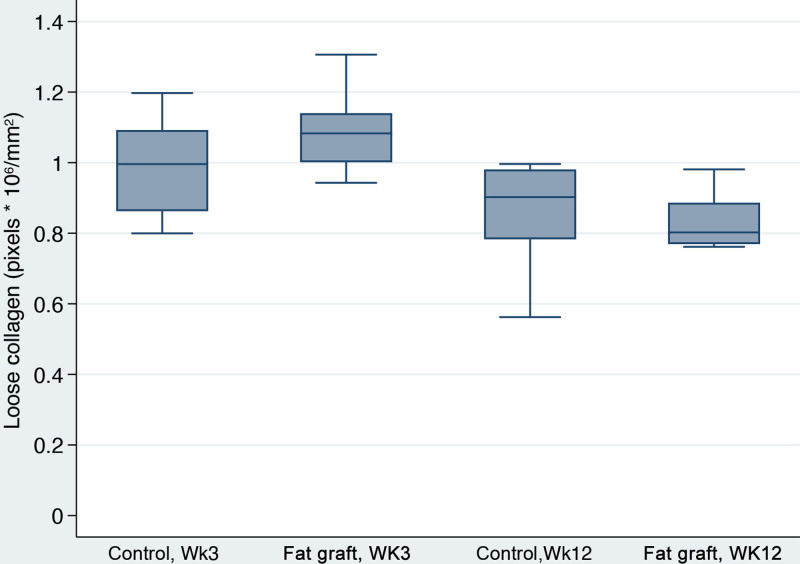
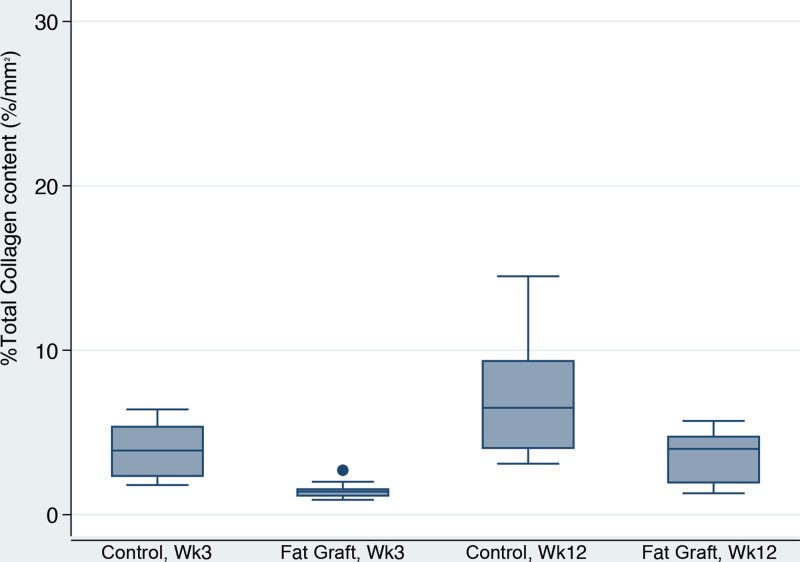
Capsular Density, Dense and Loose Collagen, %Total Collagen and Collagen Fiber Alignment
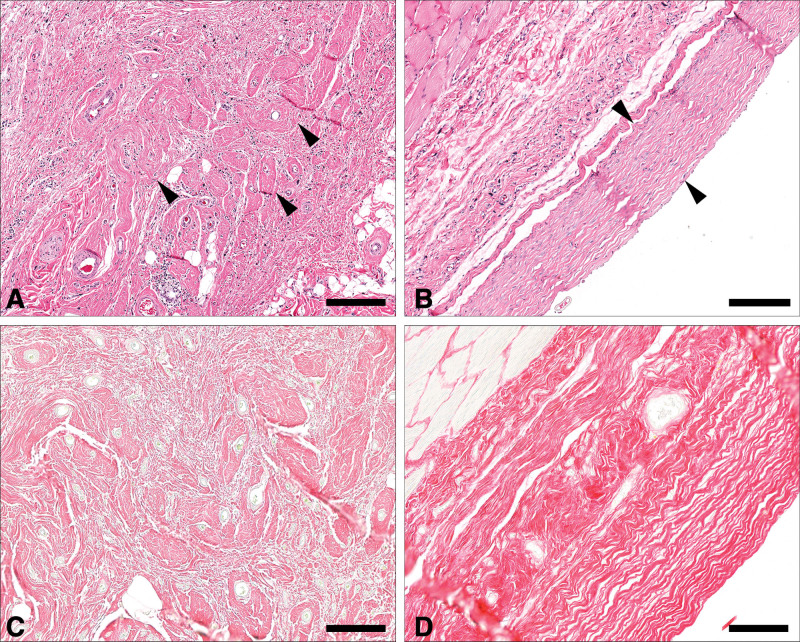
With time, peri-prosthetic capsules became almost 13 times denser (P = 0.02) (Table 2). Interestingly, fat graft was associated with almost a 70-fold reduction in capsular density overall (P = 0.001). Additionally, fat-grafted samples were 67 times more likely to have a lower fiber alignment score than the controls (P < 0.001). Capsular collagen fibers were statistically more organized and mature with time in both groups as evidenced by increased fiber alignment (P = 0.001) and greater dense collagen content at 12 weeks (P < 0.001), compared with values at three weeks (Fig. 9). Fat grafting resulted in 28.7% reduction in dense collagen (P = 0.001). This finding corresponded with a lower loose collagen content at 12 weeks when compared with three weeks (P < 0.001) (Fig. 10). Moreover, fat-grafted capsules displayed a 59% reduction in percentage of total collagen when compared with nonfat-grafted samples (eg, if the control group was 1% collagen, the fat graft group would be 0.41% collagen; (P < 0.001)(Fig. 11). Similar to dense collagen, %total collagen was 114% higher at 12 weeks when compared with 3 weeks' measurements irrespective of fat graft (P < 0.001). Morphologically, fat-grafted samples displayed disorganized collagen bands infiltrating into grated fat rather than dense collagen bands firmly adjacent to the implant in Controls (Fig. 12). The AUC for both fiber alignment and capsular density were more than 0.80, considered to be excellent discrimination11
Table 2.
Capsular Density and Collagen Fiber Alignment
| Control Week 3 |
Fat Graft Week 3 |
Control Week 12 |
Fat Graft Week 12 |
Significance | AUC | |
|---|---|---|---|---|---|---|
| Capsular density | 3 (1, 3) | 1 (1) | 3 (1, 3) | 1 (1) | S† | 0.82 |
| Collagen fiber alignment | 2 (2) | 1 (1) | 3 (3) | 2 (1, 2) | S‡ | 0.83 |
Data are expressed as the median, with the 25th percentile and the 75th percentile in parentheses.
Ordinal data (0 = Histology within normal limits; 1 = minimal alteration; 2 = mild alteration; 3 = moderate alteration; 4 = marked alteration) are expressed as the median, with the 25th percentile and the 75th percentile in parentheses; independent variables for each analysis were fat graft, time, and % collagen (% collagen was not an independent variable for the % collagen analysis). Control, week 3, n = 10; fat graft group, week 3, n = 7; control, week 12, n = 10; fat graft group, week 12, n = 9.
†No significant effect of % collagen; those with a fat graft were 69 times less likely to have a higher capsular density score than those without a fat graft (P = 0.001); those at 12 weeks were 12.9 times more likely to have a higher capsular density score than those at 3 weeks (P = 0.020).
‡No significant effect of % collagen; those with a fat graft were 67 times less likely to have a higher fiber alignment score than those without a fat graft (P < 0.001); those at 12 weeks were 15 times more likely to have a higher fiber alignment score than those at 3 weeks (P = 0.001).
AUC: Area under the curve; S: Significant finding (P < 0.05).
Fig. 9.
Dense collagen box plots. Dense collagen content in fat-grafted capsules was 28.7% lower than in controls (P = 0.001). Week 12 capsules had dense collagen values 119.0% higher than week 3 capsules (P < 0.001).
Fig. 10.
Loose collagen box plots. There was not a significant effect of fat grafting (P = 0.137). However, loose collagen content at week 12 was 18.7% lower than at week three (P < 0.001).
Fig. 11.
%Total collagen box plots. Fat-grafted capsules displayed 59% lower values for % total collagen than controls (P < 0.001). Additionally, the capsules had 114% higher % total collagen content at 12 weeks than at three weeks (P = 0.001).
Fig. 12.
Fiber alignment and collagen content in fat graft (A, C) and control (B, D) samples at 12 weeks. Collagen distribution in grafted samples (A) is haphazard, loose, and poorly organized, with individual bundles of collagen (arrowheads) interspersed with fat tissue and inflammatory cell infiltrates. In comparison, capsular collagen tended to be denser, with parallel-arranged bundles in an organized multilayered fashion (arrowheads) in nongrafted samples (B) (A, B, bar = 200 μm). Picrosirius red histochemical staining shows a more loosely arranged organization of thinner collagen fibers in fat-grafted samples (C) compared with control samples (D), in which collagen is denser, characterized by deeper staining characteristics, and organization in parallel bundles (bottom right and left, bar = 100 μm).
Synovial Metaplasia
Synovial metaplasia occurred in 0%–25% of peri-prosthetic capsules, but there was no significant difference amongst groups (Table 3).
Table 3.
Synovial Metaplasia
| Control Week 3 |
Fat Graft Week 3 |
Control Week 12 |
Fat Graft Week 12 |
Significance | |
|---|---|---|---|---|---|
| Synovial metaplasia | 0/8 (0%) | 4/16 (25.0%) | 1/9 (11.1%) | 1/10 (10.0%) | NS |
NS: no significant findings (P > 0.05).
DISCUSSION
Fat grafting is widely used in reconstructive surgery of the breast. With recent reintroduction of prepectoral implant placement, peri-prosthetic soft tissue enhancement by grafted fat appears of paramount importance. Interestingly, despite the initial hesitation stemming from historically high complication rates of subcutaneous reconstructions, outcomes of today’s prepectoral breast surgery have been similar or better than the traditional subpectoral or dual plane techniques.12,13 These favorable results, including lower than previously reported capsular contracture rates, are attributable to improved surgical techniques, preservation of skin envelope, application of acellular dermal matrix, and fat grafting.3 ADM has been shown to reduce the incidence of capsular contracture in prepectoral reconstruction.5 However, following the concept of bioengineered breast, the use of human dermis is frequently coupled with fat grafting, so likely both contribute.3,5 In a series of 305 direct to implant prepectoral reconstructions with ADM by Jones and Anthony, 0% and 0.9% of capsular contracture rates were observed by each author at the longest follow up of over 3.5 years.14 Importantly, the authors report that additional fat grafting was performed by each author secondarily in respectively 38% and 13.5% of their patients to address contour deformities. It is then possible that clinically low capsular contracture rates were achieved in breasts with inherently appropriate or surgically augmented adipose tissue layers. Our study offers a similar observation: the 8.1% increase in adipose thickness surrounding the implant statistically corresponded with 10% decrease in capsular collagen content at any time point. This finding emphasizes the importance of adequate soft tissue implant padding in prevention of peri-prosthetic collagen deposition.
The relationship between adipose tissue and collagen bundles has been studied before and appears quite fascinating. Ruben et al have shown that collagen beads are a favorite scaffold for adipose-derived stem cells to transform into fat.15 Clinically, fat grafting has been used to soften scars naturally composed of collagen bundles, resulting in improvement in scar appearance and pliability.16 Khouri et al advocated percutaneous aponeurotomy of scars and tight contractures before injection of fat graft achieving improvement in congenital bands, burns, or Dupuytren contracture.17,18 In the authors’ experience, systematic meshing of scars before lipofilling expands the area resulting in tissue gain.17 One can postulate that similar process occurred during fat grafting of the “peri-prosthetic scar” in our experiment. Mechanical disruption of collagen band structure by a cannula resulted in “meshing” of the capsule and expansion of the peri-prosthetic structure. Our histological analysis supports this presumption as collagen fibers appeared to be intertwined in fat graft in erratic and disrupted manner with significantly decreased collagen fiber alignment.
Reduction of collagen fiber alignment, collagen content, and capsular density observed with fat grafting in our experiment has direct clinical implications. Bui et al correlated histological morphology of breast capsules with Baker stage of capsular contracture and concluded that collagen fiber alignment is the key feature of capsular contracture: severely contracted capsules had highly aligned dense collagen fibers as compared with loose, multidirectional, and string-like fibers noted in soft capsules.19 The authors also emphasized that disruption of collagen fiber alignment and deposition consists the most important intervention to decrease the incidence and severity of capsular contracture. As demonstrated in our study, early fat grafting can achieve capsular fiber disruption and remodeling and thus prevent formation of dense and organized capsule. Capsular softening with fat grafting was also observed in a porcine model of peri-prosthetic fat grafting.20 In contrast to our study, the authors did not note reduction of collagen content at three months, which may stem from different animal models, fat grafting techniques, or methods of histological analysis. Additionally, fat grafting of the porcine capsules did not affect the capsular thickness, which was corroborated in our experiment.
The relationship of capsular thickness and capsular contracture is controversial. In histological analysis of 20 peri-prosthetic capsules after cosmetic augmentation, Bakker et al found that Baker IV capsules were 3.3 times thicker than Baker I capsules, suggesting direct positive relationship between advanced capsular contracture and thickness.7,21–23 Similarly, in morphological study of 25 capsules from augmented breasts, the capsules from Baker II–IV were found to be thicker than Baker I capsules.24 Conversely, in an overview of the available literature to date, Kang et al claimed no direct relationship between capsular thickness and capsular contracture though they admitted that some relationship may exist.2 Other authors postulated that capsular thickness increases with time and patient age.23 Our experiment offers opposite observation as capsular thickness decreased with time irrespective of fat grafting status. This is likely due to subsiding inflammatory changes, noted particularly after fat transfer. It is also possible that longer than 12-week observation period would be necessary to appreciate a difference in capsular thickness with peri-prosthetic fat enhancement.
The limitation of our study is a small sample size and relatively short observation period. Clinically, evaluation of peri-prosthetic capsules and potential intervention typically occurs after follow-up period longer than three months. However, the aim of this experiment was to demonstrate early changes of peri-prosthetic structure after fat grafting, which may play a critical role in subsequent capsular structure long term.17,23 Additionally, peri-prosthetic fat transfer applied in our rat model employs a loose-skin animal, which does not fully reflect the characteristics of human subdermal structure. However, as emphasized by Vieira et al, of all the existing animal experiments, the rat is the most appropriate model to investigate capsular contracture with accurate histological extrapolation to human tissue.25
CONCLUSIONS
Peri-prosthetic soft tissue enhancement with fat grafting reduces collagen content, density, and fiber alignment in implant capsules. These findings can be extrapolated to breast surgery offering positive alteration of the peri-prosthetic structure potentially resulting in decreased rates of capsular contracture with fat grafting.
ACKNOWLEDGMENTS
The authors thank Dr. Mark J. Hoenerhoff, DVM, PhD, DACVP (Associate Professor at In Vivo Animal Core, Unit for Laboratory Animal Medicine, University of Michigan Medical School, Ann Arbor, Mich.) for his expert histological analysis. Miniature tissue expanders were generously donated by Sientra, Inc and Specialty Surgical Products, Inc.
Footnotes
Published online 11 November 2021.
Disclosure: The authors have no financial interest in relation to the content of this article. This study was sponsored by Spectrum Health Office of Research Administration (SHORA) Research Grant.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Lesavoy MA, Trussler AP, Dickinson BP. Difficulties with subpectoral augmentation mammaplasty and its correction: the role of subglandular site change in revision aesthetic breast surgery. Plast Reconstr Surg. 2010;125:363–371. [DOI] [PubMed] [Google Scholar]
- 2.Kang SH, Sutthiwanjampa C, Heo CY, et al. Current approaches including bovel nano/microtechniques to reduce silicone implant-induced contracture with adverse immune responses. Int J Mol Sci. 2018;19:E1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahabedian MY. The bioengineered prosthetic breast reconstruction: advancements, evidence, and outcomes. Gland Surg. 2019;8:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg. 1981;67:312–317. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell GP, Gabriel A. Bioengineered breast: concept, technique, and preliminary results. Plast Reconstr Surg. 2016;137:415–421. [DOI] [PubMed] [Google Scholar]
- 6.Holland MC, Lentz R, Sbitany H. Surgical correction of breast animation deformity with implant pocket conversion to a prepectoral plane. Plast Reconstr Surg. 2020;145:632–642. [DOI] [PubMed] [Google Scholar]
- 7.Hammond DC, Schmitt WP, O’Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg. 2015;135:1540–1544. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulos S, Vidovic G, Neid M, et al. Using fat grafting to treat breast implant capsular contracture. Plast Reconstr Surg Glob Open. 2018;6:e1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolber PC, Spach MS. Picrosirius red staining of cardiac muscle following phosphomolybdic acid treatment. Stain Technol. 1987;62:23–26. [DOI] [PubMed] [Google Scholar]
- 10.Newson R. Parameters behind “nonparametric” statistics: Kendall’s tau, Somers’ D and median differences. Stata J. 2002;2(1):45–64 [Google Scholar]
- 11.Hosmer DW, Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed, Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 12.Chatterjee A, Nahabedian MY, Gabriel A, et al. Early assessment of post-surgical outcomes with pre-pectoral breast reconstruction: a literature review and meta-analysis. J Surg Oncol. 2018;117:1119–1130. [DOI] [PubMed] [Google Scholar]
- 13.Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;140:432–443. [DOI] [PubMed] [Google Scholar]
- 14.Jones G, Antony AK. Single stage, direct to implant pre-pectoral breast reconstruction. Gland Surg. 2019;8:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin JP, Bennett JM, Doctor JS, et al. Collagenous microbeads as a scaffold for tissue engineering with adipose-derived stem cells. Plast Reconstr Surg. 2007;120:414–424. [DOI] [PubMed] [Google Scholar]
- 16.Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610–1615. [DOI] [PubMed] [Google Scholar]
- 17.Khouri RK, Smit JM, Cardoso E, et al. Percutaneous aponeurotomy and lipofilling: a regenerative alternative to flap reconstruction? Plast Reconstr Surg. 2013;132:1280–1290. [DOI] [PubMed] [Google Scholar]
- 18.Kan HJ, Selles RW, van Nieuwenhoven CA, et al. Percutaneous Aponeurotomy and Lipofilling (PALF) versus limited fasciectomy in patients with primary dupuytren’s contracture: a prospective, randomized, controlled trial. Plast Reconstr Surg. 2016;137:1800–1812. [DOI] [PubMed] [Google Scholar]
- 19.Bui JM, Perry T, Ren CD, et al. Histological characterization of human breast implant capsules. Aesthetic Plast Surg. 2015;39:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roça GB, Graf R, da Silva Freitas R, et al. Autologous fat grafting for treatment of breast implant capsular contracture: a study in pigs. Aesthet Surg J. 2014;34:769–775. [DOI] [PubMed] [Google Scholar]
- 21.de Bakker E, van den Broek LJ, Ritt MJPF, et al. The histological composition of capsular contracture focussed on the inner layer of the capsule: an intra-donor baker-I versus baker-IV comparison. Aesthetic Plast Surg. 2018;42:1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast Reconstr Surg. 2007;120:275–284. [DOI] [PubMed] [Google Scholar]
- 23.Tasoulis MK, Iqbal FM, Cawthorn S, et al. Subcutaneous implant breast reconstruction: time to reconsider? Eur J Surg Oncol. 2017;43:1636–1646. [DOI] [PubMed] [Google Scholar]
- 24.Prantl L, Pöppl N, Horvat N, et al. Serologic and histologic findings in patients with capsular contracture after breast augmentation with smooth silicone gel implants: is serum hyaluronan a potential predictor? Aesthetic Plast Surg. 2005;29:510–518. [DOI] [PubMed] [Google Scholar]
- 25.Vieira VJ, D’Acampora A, Neves FS, et al. Capsular contracture in silicone breast implants: insights from rat models. An Acad Bras Cienc. 2016;88:1459–1470. [DOI] [PubMed] [Google Scholar]