Abstract
Barrett's esophagus (BE) is a premalignant condition in which cancer prevention is performed by endoscopic surveillance combined with Seattle protocol mucosal biopsies. The Seattle protocol has significant limitations, including a high rate of sampling error due to the focality of dysplasia/carcinoma, low endoscopist adherence to the protocol, and a high degree of variability in pathologic interpretation. These factors all contribute to a high incidence of cancers missed within 1 year of surveillance endoscopy. Wide-area transepithelial sampling with computer-assisted three-dimensional analysis (WATS3D) is a relatively new technique that minimizes sampling error by using a brush biopsy device that extensively samples “at risk” mucosa and helps pathologists diagnose dysplasia/neoplasia by generating three-dimensional images of whole crypts using a neural network-based software program. Several large prospective trials (involving both academic and community practices) have shown significantly increased rates of detection of dysplasia and intestinal metaplasia in both screening and surveillance in patients with BE when used as an adjunct to Seattle protocol-based forceps biopsies. The WATS3D diagnostic platform was included in the most recent American Society for Gastrointestinal Endoscopy Barrett's guideline as an adjunct to forceps biopsies (conditional recommendation and low quality of evidence). This review summarizes the scientific and pathologic basis of WATS3D technology, its potential impact on BE surveillance and management, and its limitations and future directions.
INTRODUCTION
Barrett's esophagus (BE) is a chronic inflammatory condition caused by gastroesophageal reflux disease that results in conversion of the normal squamous esophageal lining to one that is columnar with intestinal differentiation (1,2). The incidence of BE has increased dramatically in recent decades (3). It affects approximately 2%–7% of asymptomatic adults in Western countries (4). In patients with gastroesophageal reflux disease undergoing upper endoscopy, the prevalence rate approaches 15% (5).
BE is the only recognized precursor for esophageal adenocarcinoma (EAC). The incidence of EAC has been increasing at an alarming rate, showing a 6-fold increase in incidence in the past 30 years. It also currently represents the second deadliest cancer in White men (6). Despite its rising incidence and largely grim prognosis, EAC is a potentially preventable disease if early (mucosal) neoplasia is recognized during surveillance and managed appropriately with endotherapy. EAC in BE develops through a multistep, progressive sequence of morphologic and molecular events that begins with metaplasia and then progresses through various stages of dysplasia to adenocarcinoma (7). As a result, high-quality endoscopic screening and surveillance forms the basis of cancer prevention in BE. This process is highly dependent on the pathologist's ability to diagnose BE and grade its associated neoplastic precursor lesions with accuracy.
CURRENT METHOD OF BARRETT'S SURVEILLANCE AND THE SEATTLE PROTOCOL
The most common and well-accepted gold standard method of screening and surveillance in BE—and as recommended by all national gastroenterology (GI) societies—is the “Seattle protocol.” (8–10) After the esophagus and BE segment is examined in detail with high-definition white-light endoscopy and electronic chromoendoscopy (narrow-band imaging, Fuji intelligent color enhancement, or similar), 4 quadrant forceps biopsies (FBs) are performed every 1–2 cm of the BE segment. In the United States, intestinal metaplasia (IM), defined by the presence of goblet cells, is a required diagnostic criterion for BE (11).
SEATTLE PROTOCOL LIMITATIONS
The Seattle protocol has several limitations. The biopsy protocol is time-consuming, and thus adherence is quite low (20%–30%) (12,13). Even when strictly adhered to, the Seattle protocol is a relatively insensitive method for detection of neoplastic abnormalities in BE. Because the Seattle protocol only samples <5% of BE mucosa, there is a high degree of sampling error (2). In addition, dysplasia and early cancer may be subtle, focal, and patchy in distribution, hence easily missed (14). In a recent systematic review and meta-analysis of 24 cohort studies of patients with BE with low-grade dysplasia (LGD), the pooled miss rate of EAC detected within 1 year of surveillance endoscopy was 33.24% (15).
There are also significant limitations in the interpretation of standard FBs by pathologists because there is a high degree of variability in this regard (16,17). Even with extensive sampling, pathologists continue to struggle in recognizing and grading dysplasia, particularly with recently recognized rarer subtypes, such as foveolar dysplasia and crypt dysplasia (7). For instance, 1 study reported overdiagnosis of regenerating BE epithelium as LGD in 75% of cases reported by community pathologists (18).
In a recent published study of 263 GI practices involving 58,709 endoscopies in 53,561 patients, 27% of patients underwent endoscopy at 1- to 2-year intervals. This may be a reflection of a number of factors, such as a lack of confidence in the Seattle protocol and/or the pathologist's interpretation (19). Considerable research has been devoted to the development of novel and advanced imaging modalities to improve detection of dysplasia and early neoplasia in patients with BE, such as confocal laser endomicroscopy and volumetric laser endomicroscopy. However, as of today, none have found adoption in routine practice (20).
One recently developed technology has shown to reduce sampling error and increase diagnostic yield of BE and dysplasia when used adjunctively with the Seattle protocol (21). The technique known as wide-area transepithelial sampling with 3-dimensional (3D) computer-assisted analysis (WATS3D) (CDx Diagnostics, Suffern, NY) is increasingly used in GI practices in both academic and community settings. The WATS3D diagnostic platform was recently included as an adjunct to routine FB sampling in patients with BE, in the recently published guidelines of the American Society for Gastrointestinal Endoscopy (1).
The remainder of this review will focus on the WATS3D technology and how it affects both pathologists and gastroenterologists alike.
WATS3D TECHNOLOGY
Brush biopsy and specimen preparation
The WATS3D diagnostic platform consists of 2 specific and interrelated methodologies. The first is a mucosal (full mucosal thickness) cytology sampling brush that is used at endoscopy to sample a large circumferential area of BE mucosa (Figures 1 and 2). Physicians use 2 WATS3D brushes for every 5 cm of BE mucosa. The sample is collected by moving the brush in a sweeping motion, or moving the endoscope up and down, over the BE mucosa (Figure 3a,b). This can be performed either before or after acquisition of routine FBs with equal efficacy, as demonstrated in a randomized prospective cross-over trial (21). The WATS3D sampling instrument penetrates deep in the mucosa to collect small tissue fragments (akin to microbiopsies) measuring approximately 500 μm in depth. The tissue sampling brush contains long and stiff bristles that result in the acquisition of thick and compact sheets and large, cohesive disaggregated tissue fragments that retain their 3D crypt architecture when smeared and alcohol fixed onto a glass slide (Figure 4a,b). This is distinct from conventional soft brush cytology specimens which consists mainly of single cells and small clusters of exfoliated surface cells, without architectural structure, and which are retrieved only from the superficial layers of the epithelium. In over 150,000 procedures, only 2 adverse events have been reported. Both were esophageal perforations that occurred during sampling by a surgeon or a surgeon assistant with the WATS3D brush. One patient was managed surgically and the other endoscopically (22).
Figure 1.
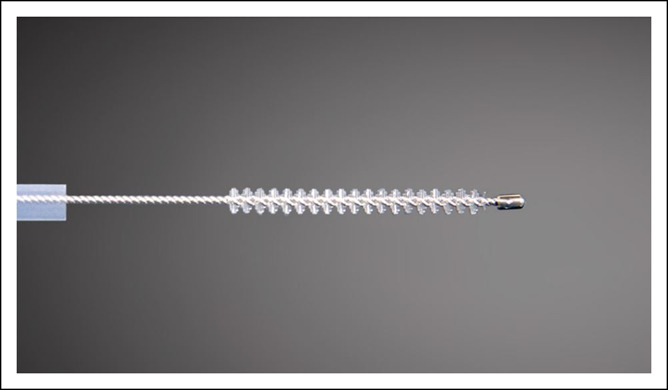
Close-up view of a WATS3D cytology brush. The long length and high level of stiffness of the bristles helps acquire aggregates of tissue from deep aspects of the mucosa.
Figure 2.
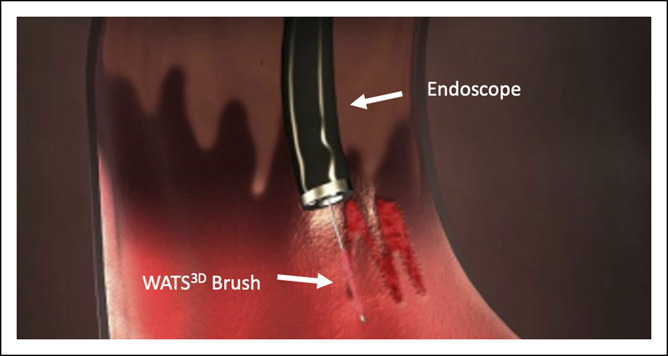
Simulated graphic showing WATS3D brush sampling of Barrett's mucosa.
Figure 3.
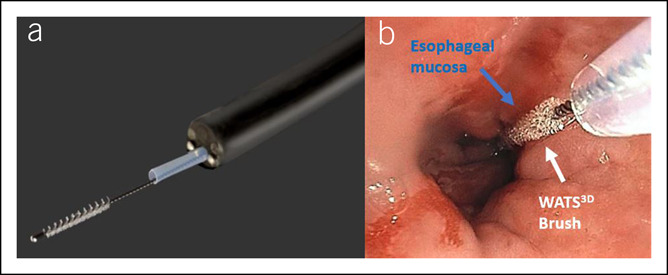
(a) Example of a WATS3D cytology brush passed through the accessory channel of the gastroscope. (b) WATS3D instrument sampling of a short segment of Barrett's esophagus.
Figure 4.
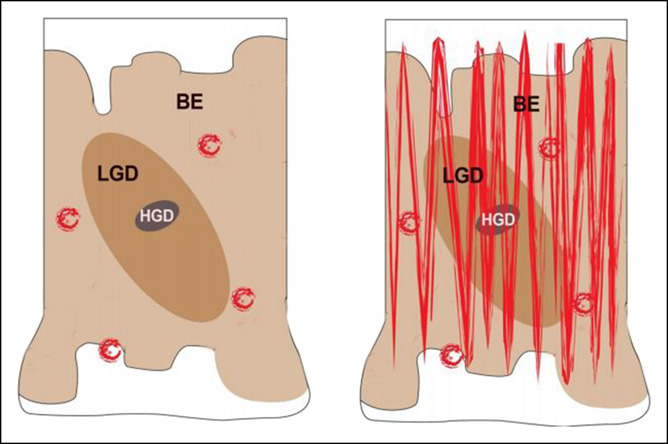
(a) Graphic illustration of a segment of BE showing the typical patchiness of dysplasia. (b) WATS3D brush in a sweeping manner samples more at risk mucosa including focal areas of dysplasia. BE, Barrett's esophagus; HGD, high-grade dysplasia; LGD, low-grade dysplasia.
WATS3D tissue processing and computer analysis
The WATS3D smear specimen, which is collected from the first brush, is stained with a modified Papanicolaou stain for subsequent computer and microscopic analysis by pathologists. The tissue collected with the second WATS3D brush is fixed in formalin, embedded in paraffin, cut into routine 4–5-μm-thick sections and stained with hematoxylin and eosin (H&E), similar to routine mucosal biopsies. This specimen, which consists mainly of strips of epithelium and small fragments of mucosa akin to minibiopsies, is also examined microscopically by pathologists and can be used for immunohistochemical staining when indicated. CDX and MUC2 can be used to help differentiate goblet cells from pseudogoblet cells, and molecular markers such as P53 and AMACR can be used to confirm the presence of dysplasia, but the latter are not currently used as predictive biomarkers of neoplastic progression.
THREE-DIMENSIONAL COMPUTER PROCESSING AND ANALYSIS OF WATS3D THROUGH NEURAL NETWORK AND EXTENDED DEPTH OF FIELD IMAGING
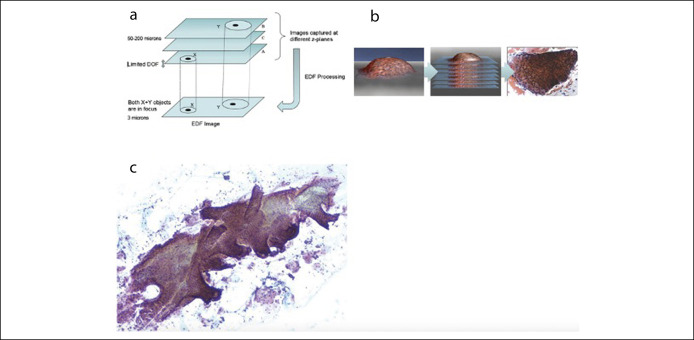
When analysis is attempted by a two-dimensional microscope with a standard 3 μm depth of field, abnormal cells located in the deep aspects of the specimen may not be recognized and much of the 3D structure of the tissue and the spatial relationship of the cells to each other are lost. Furthermore, other artifacts of routine processing and microscopic analysis can make diagnostic interpretation difficult. Another limitation is the amount of time required to analyze the specimen in its entirety for accurate identification of the most atypical cellular foci.
The WATS3D computer system helps solve these problems in several ways. First, the 150-μm thick tissue smear is scanned by an extended depth of field analysis (EDF) imaging system. The EDF system captures up to 50 separate optical slices throughout the full thickness of the cell aggregates at 3 μm intervals, and then, roughly analogous to a computer tomography scan, the computer integrates these multiple two-dimensional images into a single 3D image (Figure 5a). This synthesized 3D image is further processed and analyzed by an artificial intelligence–based neural network algorithm, which is then followed by a manual review of the annotated synthesized 3D images by the pathologist on a computer monitor (Figure 5b). The final image more accurately represents the true 3D architecture of the crypts (Figure 5c). This allows pathologists to visualize crypts in their more natural state.
Figure 5.
(a) Graphic representation of Z-stacking, performed by the WATS3D computer system. (b) Simplified version of this process in a graphic representation of a tissue fragment combined with the final computer-synthesized image on the far-right side. (c) A portion of columnar epithelium with a 3-dimensional–like representation of the surface epithelium and crypts created by computer Z-stacking. DOF, depth-of-field; EDF, extended depth of field analysis.
The neural network-based image analysis component of the WATS3D computer system then analyzes the resultant synthesized 3D image. This analysis is based on a training set of tens of thousands of synthesized 3D images of known normal and abnormal glandular cells. Tissue fragments are then prioritized and ranked according to their degree of atypicality and presented to the pathologist on a computer monitor at the time of sign-out. Analysis of the formalin-fixed H&E-stained slide, along with WATS3D-generated computer images of the tissue smear, has been shown to increase diagnostic accuracy (23).
PATHOLOGY OF WATS3D
Barrett's esophagus
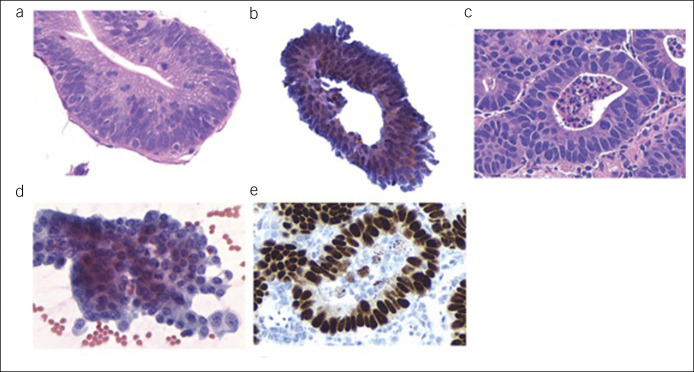
On H&E-stained slides from WATS3D samples, BE is diagnosed in small fragments of intact mucosa often containing at least 3–5 intact crypts (24). In the smear slide, nondysplastic BE appears as compact, variably sized sheets of cohesive columnar and goblet cells with small, regularly arranged and uniform-sized nuclei without loss of polarity, containing inconspicuous nucleoli and abundant cytoplasm (Figure 6a–c). The epithelium maintains a distinct honeycomb pattern, with crisp cell borders between individual cells, when visualized en face and particularly in whole crypts or collections of crypts which may appear 3D in the WATS3D images. Isolated goblet cells may be better visualized with EDF imaging as noted above.
Figure 6.
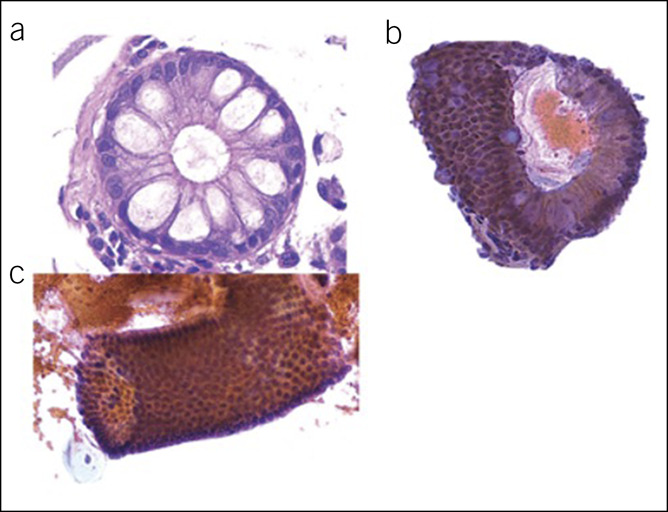
Cell block (a) and smear (b and c) of a case of nondysplastic Barrett's esophagus.
Low-grade and high-grade dysplasia
The histologic features of LGD and high-grade dysplasia (HGD) in the cell block of WATS3D samples are identical to FBs with the exception that architectural changes are occasionally more difficult to assess. LGD shows crypt cells with enlarged, hyperchromatic, often slightly irregular nuclei with mild stratification; increased nuclear–cytoplasmic ratio; and, if present, only slight loss of nuclear polarity and pleomorphism (Figure 7a,b). HGD shows many of the same features, but the degree of atypicality is greater. In the smear sample, the most characteristic feature of dysplasia of any grade is the complete loss of the normal honeycomb structure of the cell aggregates and crypts (Figure 7c,d). The aggregates usually show crowded overlapping enlarged cells containing nuclei with irregular nuclear membranes, clumpy chromatin, and peripheral condensation of chromatin. Differentiation from invasive cancer is performed similarly to FBs. The presence of marked pleomorphism, combined with abundant dissociated cells and a background tumor diathesis is characteristics of cancer. Immunostaining with p53 may also be present in neoplastic BE (Figure 7e).
Figure 7.
Cell block and smear examples of low-grade dysplasia (a and b) and high-grade dysplasia (c and d), and the latter also showing a positive p53 stain (e).
In a blinded interobserver study of 149 BE samples (109 without dysplasia and 49 with LGD, HGD, or EAC) evaluated by WATS3D, the overall kappa value for all 3 diagnoses between the 4 observers was 0.86. This is higher than previous interobserver studies of BE and associated dysplasia in FBs (range 0.24–0.65) (16,17,24). The kappa value for the indefinite/LGD group, a notoriously difficult and poorly reproducible category of dysplasia in FBs, was 0.74, which is considered excellent. One possible explanation for the higher level of agreement in WATS3D may be that the WATS3D neural network program chooses the 200 most atypical areas of interest from the brush smear samples, which helps to minimize misses and provides uniformity to areas examined by each pathologist. WATS3D specimens are currently diagnosed exclusively by WATS3D-trained CDX employed pathologists. Thus, future studies will be needed to determine whether high interobserver agreement levels can be achieved by community pathologists as well.
Crypt dysplasia
Several studies have provided strong evidence that dysplasia in BE begins in the crypt bases from progenitor stem cells. The exact mechanism of clonal dysplasia expansion is unknown. EDF imaging helps visualize the entire crypt from top to bottom and in all planes of depth. Crypt dysplasia shows cytologic features identical to conventional LGD. However, in the former, the surface is not involved (Figure 8).
Figure 8.
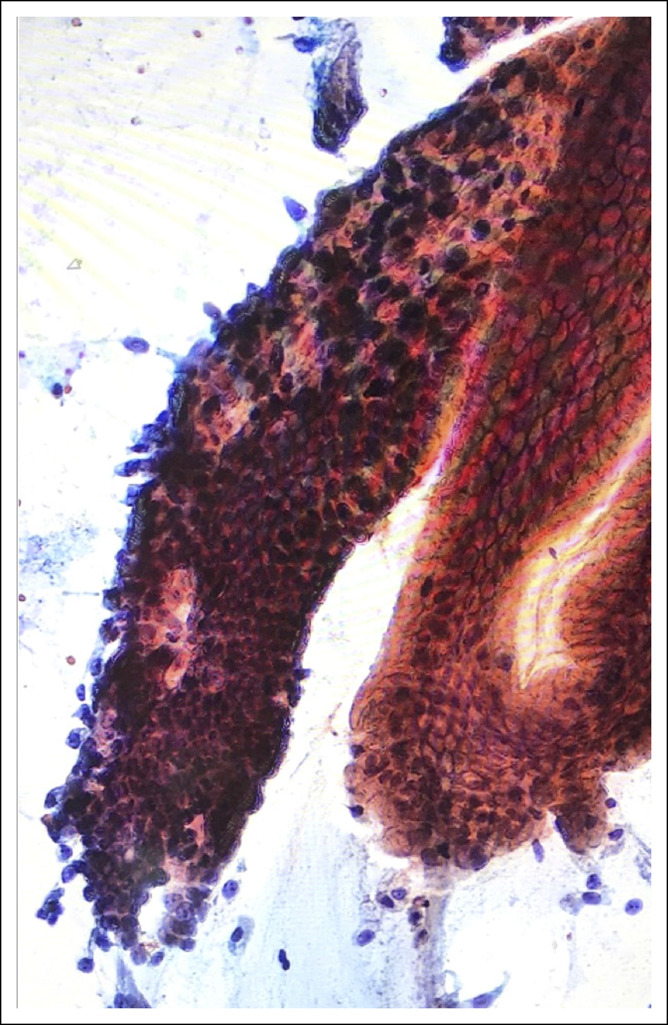
Smear sample of crypt dysplasia (CD). A medium-power magnification image showing 2 crypts connected at the surface, 1 showing CD (left side) and 1 nondysplastic (right side) but with mild reactive changes.
Two separate studies demonstrated crypt dysplasia progressed to HGD/carcinoma during the long-term follow-up in 25% and 26% of patients, respectively (25,26). These results were similar to a conventional LGD comparative group. Recently, in a large retrospective study of 43,145 WATS3D samples from patients with BE, progression of crypt dysplasia was significantly higher than progression of non-dysplastic BE (1.42% per patient-year vs 0.08% per patient year, respectively) (27). These data suggest crypt dysplasia is a potentially progressive neoplastic lesion and highlight the potential clinical importance of an accurate diagnosis to help guide further surveillance and treatment in this cohort of patients.
WATS3D LITERATURE REVIEW AND CLINICAL IMPACT
At least 6 studies have evaluated the use of WATS3D as an adjunct to routine FBs in patients with BE, with or without dysplasia/carcinoma (21,28–32) (Table 1). Of note, FBs were used for both visually targeted lesions and random 4 quadrant sampling, whereas WATS3D was used for only random sampling. These studies involved community (low risk) and academic (high risk) centers and included both screening and surveillance patient populations.
Table 1.
Summary of 5 studies that have evaluated the efficacy of WATS3D for detection of intestinal metaplasia and dysplasia/adenocarcinoma
| Study/Year | No. of Patients | Study design | Study population | Main results |
| Agha et al.32/2021 | 108 | Retrospective Observational |
Community-based | ↑ Barrett's 18.6% NNT to detect IM: 5 |
| Smith et al.31/2018 | 12,899 | Prospective Randomized |
Community-based | ↑ Barrett's 153% ↑ Dysplasia 242% |
| Gross et al.30/2018 | 4,203 | Prospective | Community-based | ↑ Barrett's 83% ↑ Dysplasia 89% |
| Vennalaganti et al.21/2018 | 160 16 medical centers |
Prospective Randomized |
Academic centers (high risk) | ↑ High-grade dysplasia 428% |
| Anandasabapathy et al.29/2011 | 151 4 medical centers |
Prospective | Prior dysplasia (high risk) | ↑ Dysplasia 42% |
| Johanson et al.28/2011 | 1,266 8 community practices |
Prospective | Low risk | ↑ Barrett's 40% |
IM, intestinal metaplasia; NNT, number needed to treat.
Two early prospective studies were performed with a first-generation endoscopic brush (2 mm) and computer software program (up to 30 μm of EDF) (28,29). More recently, 3 prospective studies used a second-generation brush (2.8 mm) and improved software (up to 150 μm of EDF) (21,30,31). The data from these studies show increased yield of detection of IM, ranging from 40% to 153%, and increased yield of detection of dysplasia/carcinoma, ranging from 42% to 428%. The highest added yields resulting from the addition of WATS3D to the Seattle protocol have been in the detection of HGD, likely because of the focality of HGD. In a recent randomized trial of 160 high-risk patients with BE undergoing surveillance at 16 academic medical centers in the United States, WATS3D detected additional 23 cases of HGD/EAC missed by the Seattle protocol (11 cases were classified as nondysplastic BE and 12 as LGD/indefinite for dysplasia by FBs), resulting in an overall added diagnostic yield of 428% (21). Of the 23 additional cases found by WATS3D, 9 had follow-up FBs after the study was completed, with 7 of the 9 confirming dysplasia (21).
In a recent multicenter prospective trial from 25 community practices involving 4,203 patients primarily screened for BE, the addition of WATS3D to FB increased the overall detection of BE by 83% and the overall yield of LGD by 88.5% (30). In this low-risk population, HGD was not detected in any patients by FBs; however, WATS3D detected a single case of EAC missed by FBs (30). A recent meta-analysis also strongly supported the adjunctive use of WATS3D to FB in BE screening and surveillance (33).
In the largest prospective study to date, the addition of WATS3D to FB increased the diagnostic yield of BE by 153% and of dysplasia by 242% in 12,899 patients undergoing BE screening or surveillance from 21 community practices (31). The results were equally significant in screening vs surveillance patients in this study. In an observational study of 138 patients with BE, the addition of WATS3D to advanced endoscopic imaging (high-definition white light imaging, narrow band imaging, and volumetric laser endomicroscopy), and random FBs, increased the yield of dysplasia detection by 34.3% (34).
A recent study highlighted that the adjunct use of WATS3D directly affected the management of 97.8% of patients with forceps-negative and WATS3D-positive results (35). Of 432 patients, 96% were enrolled in surveillance and 60% were either initiated on PPI therapy or had their PPI dose escalated based on WATS3D results. Endoscopic therapy was performed in 33.7% of patients with LGD and 70.6% of patients with HGD. The direct effect of a positive WATS3D result on patient management strongly supports its adjunctive use in clinical practice (35).
In a recent retrospective study of 108 patients, tissue sampling with FB and WATS3D demonstrated an 18.6% increase in yield compared to FB alone. WATS3D helped identify an additional 21 cases of nondysplastic BE, 1 case of LGD, and 1 case of EAC. The number needed to test with WATS3D was 5 (32).
CLINICAL APPLICATION AND PROCEDURE COSTS
Physicians who use WATS3D are supplied with kits by CDX that contain sampling devices and associated media and slides. Once the specimens are collected, the Papanicolaou-stained smear slide and the formalin bottle containing the brush tips are transported to CDX laboratories in preaddressed boxes for subsequent tissue processing and evaluation. Training regarding tissue acquisition, on site sample preparation, and submission is provided by clinical specialists. Diagnoses are rendered by WATS3D-certified pathologists, and the reports are sent to the physician electronically. The WATS3D technology is billed using existing histology and cytology codes; all of which are commonly accepted and routinely reimbursed by payers nationally as a covered benefit, such as Medicare and Centers for Medicare & Medicaid Services. Given the adjunctive yield for dysplasia detection, especially in high-risk individuals, and its ease of use, gastroenterologists should find this technology valuable and feasible to incorporate into their practice.
LIMITATIONS OF WATS3D
Despite the efficacy of WATS3D for detection of IM and dysplasia in BE, there are several potential limitations/outstanding questions that remain to be addressed. Currently, WATS3D is endorsed as an adjunct to the Seattle protocol FBs. Randomized controlled trials comparing outcomes of missed dysplasia and carcinoma between FB and WATS3D are necessary and will help further clarify the role of WATS3D sampling, particularly in low-risk populations. Although WATS3D helps detect crypt dysplasia, the clinical relevance and natural history of this neoplastic precursor needs to be investigated further in prospective trials. Furthermore, the efficacy of WATS3D needs to be tested in various risk settings, such as in patients with short-segment vs long-segment BE, in those with or without a hiatus hernia, and in postablation patients.
SUMMARY
The current method of screening and surveillance in patients with BE suffers from a high degree of sampling error. Several studies have shown a high incidence of missed dysplasia and carcinoma within a year of surveillance endoscopy in this specific patient population.
The WATS3D diagnostic platform, consisting of wide area transepithelial sampling of the Barrett's segment (including the deep mucosa) in conjunction with specialized 3D computer-based image analysis, is a promising technique that has been shown to increase the yield of detection of IM and dysplasia/carcinoma when used as an adjunct to the Seattle protocol, which helps to reduce sampling error and the incidence of undetected cancer. This increased yield of BE and dysplasia has implications for the surveillance and treatment of BE. Future prospective randomized control trials designed to test WATS3D and its utility in various BE clinical settings may further establish its role in the care of our patients.
CONFLICTS OF INTEREST
Guarantor of the article: Vivek Kaul, MD, FACG.
Specific author contributions: R.D.O., J.G., and V.K.: planning and drafting of manuscript. All authors have approved the final manuscript for submission.
Financial support: Open access publication charges supported by CDX Diagnostics.
Potential competing interests: R.D.O.: consultant for CDX Diagnostics. J.G.: consultant for CDX Diagnostics. V.K.: consultant for CDX Diagnostics, Steris, and Cook Medical.
Contributor Information
Robert D. Odze, Email: odzerobert@gmail.com.
John Goldblum, Email: goldblj@ccf.org.
REFERENCES
- 1.Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc 2019;90(3):335–59.e2. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ. Clinical practice. Barrett's esophagus. N Engl J Med 2002;346(11):836–42. [DOI] [PubMed] [Google Scholar]
- 3.Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett's esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am 2015;44(2):203–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayeck TJ, Kong CY, Spechler SJ, et al. The prevalence of Barrett's esophagus in the US: Estimates from a simulation model confirmed by SEER data. Dis Esophagus 2010;23(6):451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125(6):1670–7. [DOI] [PubMed] [Google Scholar]
- 6.Rustgi A, El-Serag HB. Esophageal carcinoma. N Engl J Med 2015;372(15):1472–3. [DOI] [PubMed] [Google Scholar]
- 7.Naini BV, Souza RF, Odze RD. Barrett's esophagus: A comprehensive and contemporary review for pathologists. Am J Surg Pathol 2016;40(5):e45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140(3):1084–91. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: Diagnosis and management of Barrett's esophagus. Am J Gastroenterol 2016;111(1):30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans JA, Early DS, Fukami N, et al. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76(6):1087–94. [DOI] [PubMed] [Google Scholar]
- 11.Salimian KJ, Waters KM, Eze O, et al. Definition of Barrett esophagus in the United States: Support for retention of a requirement for goblet cells. Am J Surg Pathol 2018;42(2):264–8. [DOI] [PubMed] [Google Scholar]
- 12.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett's esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009;7(7):736–42; quiz 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wani S, Williams JL, Komanduri S, et al. Endoscopists systematically undersample patients with long-segment Barrett's esophagus: An analysis of biopsy sampling practices from a quality improvement registry. Gastrointest Endosc 2019;90(5):732–41.e3. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P. Review article: Emerging techniques for screening and surveillance in Barrett's oesophagus. Aliment Pharmacol Ther 2004;20(Suppl 5):63–70; discussion 95–66. [DOI] [PubMed] [Google Scholar]
- 15.Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of missed esophageal adenocarcinoma after Barrett's esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology 2016;150(3):599–607.e7; quiz e514–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery E, Goldblum JR, Greenson JK, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: A follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol 2001;32(4):379–88. [DOI] [PubMed] [Google Scholar]
- 17.Kerkhof M, van Dekken H, Steyerberg EW, et al. Grading of dysplasia in Barrett's oesophagus: Substantial interobserver variation between general and gastrointestinal pathologists. Histopathology 2007;50(7):920–7. [DOI] [PubMed] [Google Scholar]
- 18.Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: Overdiagnosed and underestimated. Am J Gastroenterol 2010;105(7):1523–30. [DOI] [PubMed] [Google Scholar]
- 19.Wani S, Williams JL, Komanduri S, et al. Over-utilization of repeat upper endoscopy in patients with non-dysplastic Barrett's esophagus: A Quality Registry Study. Am J Gastroenterol 2019;114(8):1256–64. [DOI] [PubMed] [Google Scholar]
- 20.Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 2017;49(2):191–8. [DOI] [PubMed] [Google Scholar]
- 21.Vennalaganti PR, Kaul V, Wang KK, et al. Increased detection of Barrett's esophagus-associated neoplasia using wide-area trans-epithelial sampling: A multicenter, prospective, randomized trial. Gastrointest Endosc 2018;87(2):348–55. [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration. MAUDE—Manufacturer and User Facility Device Experience (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/results.cfm). Updated April 30, 2021.Accessed May 8, 2021. [Google Scholar]
- 23.Lomo LC, Blount PL, Sanchez CA, et al. Crypt dysplasia with surface maturation: A clinical, pathologic, and molecular study of a Barrett's esophagus cohort. Am J Surg Pathol 2006;30(4):423–35. [DOI] [PubMed] [Google Scholar]
- 24.Vennalaganti PR, Naag Kanakadandi V, Gross SA, et al. Inter-observer agreement among pathologists using wide-area transepithelial sampling with computer-assisted analysis in patients with Barrett's esophagus. Am J Gastroenterol 2015;110(9):1257–60. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava A, Coco DP, Sanchez CA, et al. Risk of conventional dysplasia and adenocarcinoma in patients with Barrett's esophagus and crypt dysplasia: A prospective follow-up study of 214 patients. Mod Pathol 2010;23:168A–9A. [Google Scholar]
- 26.Askari SLB, Niehans G, Odze RD. Long-term outcome study of Barrett's esophagus with basal crypt dysplasia. Mod Pathol 2014;27:163A. [Google Scholar]
- 27.Shaheen NJ, Smith MS, Odze RD. Progression of Barrett's esophagus, crypt dysplasia, and low-grade dysplasia diagnosed by wide-area transepithelial sampling with 3-dimensional computer-assisted analysis: a retrospective analysis. Gastrointestinal Endoscopy 2021, in press. 10.1016/j.gie.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Johanson JF, Frakes J, Eisen D. Computer-assisted analysis of abrasive transepithelial brush biopsies increases the effectiveness of esophageal screening: A multicenter prospective clinical trial by the EndoCDx Collaborative Group. Dig Dis Sci 2011;56(3):767–72. [DOI] [PubMed] [Google Scholar]
- 29.Anandasabapathy S, Sontag S, Graham DY, et al. Computer-assisted brush-biopsy analysis for the detection of dysplasia in a high-risk Barrett's esophagus surveillance population. Dig Dis Sci 2011;56(3):761–6. [DOI] [PubMed] [Google Scholar]
- 30.Gross SA, Smith MS, Kaul V. Increased detection of Barrett's esophagus and esophageal dysplasia with adjunctive use of wide-area transepithelial sample with three-dimensional computer-assisted analysis (WATS). United European Gastroenterol J 2018;6(4):529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MS, Ikonomi E, Bhuta R, et al. Wide-area transepithelial sampling with computer-assisted 3-dimensional analysis (WATS) markedly improves detection of esophageal dysplasia and Barrett's esophagus: Analysis from a prospective multicenter community-based study. Dis Esophagus 2019;32(3):doy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agha YH, Srinivasan S, Hyder J, et al. WATS(3D) versus forceps biopsy in screening for Barrett's esophagus: Experience in community endoscopy centers. Ann Gastroenterol 2021;34(2):164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suresh Kumar VC, Harne P, Patthipati VS, et al. Wide-area transepithelial sampling in adjunct to forceps biopsy increases the absolute detection rates of Barrett's oesophagus and oesophageal dysplasia: A meta-analysis and systematic review. BMJ Open Gastroenterol 2020;7(1):e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raphael KL, Stewart M, Sejpal DV, et al. Adjunctive yield of wide-area transepithelial sampling for dysplasia detection after advanced imaging and random biopsies in Barrett's esophagus. Clin Transl Gastroenterol 2019;10(12):e00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaul V, Gross S, Corbett FS, et al. Clinical utility of wide-area transepithelial sampling with three-dimensional computer-assisted analysis (WATS3D) in identifying Barrett's esophagus and associated neoplasia. Dis Esophagus 2020;33(12):doaa069. [DOI] [PubMed] [Google Scholar]