Abstract
Evidence on sunscreen use and cutaneous squamous cell carcinoma (cSCC) risk is limited. Most studies have not taken sun protection factor (SPF) into consideration and used nonusers of sunscreen as the reference group. Nonusers are likely a priori at lower cSCC risk than users. No study has investigated the effect of high- versus low-SPF sunscreens on cSCC, appropriately adjusting for time-varying confounding. Using data from the Norwegian Women and Cancer Study (1991–2016), we investigated whether use of SPF ≥15 versus SPF <15 sunscreens reduces cSCC risk. We used a marginal structural Cox proportional hazards model with inverse probability of treatment and censoring weights to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). During follow-up of 148,781 women (mean follow-up, 14.3 years), 653 women were diagnosed with cSCC. The effect on cSCC risk of sunscreens with SPF ≥15 versus SPF <15 was close to the null when used at any latitudes (HR = 1.02, 95% CI: 0.82, 1.27) and when used in lower-latitude settings (HR = 1.05, 95% CI: 0.84, 1.32). In conclusion, we found no indication that sunscreens with SPF ≥15 reduced Norwegian women’s cSCC risk more than sunscreens with SPF <15, suggesting that either there is no difference in their effects long-term or the difference is diluted by incorrect application.
Keywords: cohort study, cutaneous squamous cell carcinoma, inverse probability weighting, marginal structural model, sun protection factor, sunscreen, ultraviolet exposure
Abbreviations
- CI
confidence interval
- cSCC
cutaneous squamous cell carcinoma
- HR
hazard ratio
- NOWAC
Norwegian Women and Cancer
- RCT
randomized controlled trial
- SPF
sun protection factor
- UVR
ultraviolet radiation
Cutaneous squamous cell carcinoma (cSCC) is among the most common cancer types worldwide (1). National Norwegian cSCC data shows 24% increase in age-standardized incidence rates from 2010–2014 to 2015–2019 (2). Cumulative sun exposure is considered the main cSCC risk factor (1, 3), and sun protection recommendations include seeking shade, wearing protective clothing, and using sunscreens with sun protection factor (SPF) ≥15 (4, 5).
Evidence from randomized controlled trials (RCTs) shows that sunscreen use decreases the risk of actinic keratosis (6, 7), a known precursor of cSCC (1). The relationship between sunscreen use and cSCC risk has been investigated in 6 case-control studies (8–13), 1 cohort study (14), and 1 RCT (15, 16). One of the case-control studies (12) found an inverse association between cSCC risk and use of sunscreens with SPF 15–30 at ages 15–25 years, and the opposite for ages older than 25 years. The cohort study (14) and the other case-control studies (8–11, 13) found imprecise and inconsistent results. In the RCT, daily use of sunscreens with SPF ≥15 (specifically SPF 16) reduced cSCC tumor incidence during the trial period and primary cSCC incidence during extended follow-up (15, 16). Modern sunscreens have improved in performance compared with those used before the 21st century, when half of these studies were conducted (17, 18). Moreover, most of these studies did not take SPF into consideration and regarded nonusers of sunscreen as the reference group. Nonusers are likely a priori at lower cSCC risk than sunscreen users due to less sun exposure and less sun-sensitive phenotypic characteristics (19, 20). Furthermore, the majority of the studies were conducted in low-latitude, high–ambient ultraviolet radiation (UVR) settings where people experience mainly nonintentional sun exposure (21, 22). Studies from northern Europe, with lower ambient UVR and mainly intermittent and intentional sun exposure (23), are lacking.
An RCT randomizing participants to use of sunscreens with different SPFs would be ideal. Given enough follow-up time, and participants continuing using the same sunscreen throughout, a causal effect of higher-SPF versus low-SPF sunscreens on cSCC risk could be estimated. Realization of such an RCT is highly unlikely for ethical reasons (a control arm cannot be denied regular use of higher-SPF sunscreen) and the need long-term intervention and follow-up. Cohort studies are less constraining, and typically have long follow-up. However, they are still prone to bias arising from unmeasured confounding, informative censoring, and time-varying confounding (24, 25). Marginal structural models, popular in causal inference, can help with some of these issues and estimate causal effects from observational data with time-varying confounders (25–29).
In spite of the limited evidence on sunscreen use and cSCC, and because of the lack of studies with repeated information on sunscreen use and confounders, no observational study has yet used marginal structural models to investigate the causal effect of higher-SPF versus low-SPF sunscreens on cSCC risk. The population-based Norwegian Women and Cancer (NOWAC) cohort study holds unique information on host factors, sunscreen use, and history of sunburns and sun exposure, with up to 3 repeated measurements (19). Thus, based on NOWAC, we aimed to use marginal structural models to prospectively investigate whether use of sunscreens with SPF ≥15 versus SPF <15 reduces cSCC risk. We also applied standard methods for comparison.
METHODS
The NOWAC cohort study
The NOWAC cohort study has been described in detail elsewhere (19, 30). Briefly, women were selected randomly from the Norwegian Population Register and issued a questionnaire at study inclusion in 1991–2007. In total, 172,472 women, aged 31–70 years at inclusion, participated (response rate, 54%). First and second follow-up questionnaires were issued approximately every 5 years (response rates 80% and 79%, respectively). Participants of the NOWAC cohort study have all signed broad informed consent to study risk factors and cancer, and the cohort has been approved by the national Data Protection Authority and the Regional Committees for Medical Health Research Ethics of North Norway. Data were handled according to the permission given by the Data Protection Authority. This project received anonymous data only.
Sunscreen use
Participants were asked to report whether they used sunscreen within Norway or other northern places (hereafter high latitudes) and/or on sunbathing vacations in lower latitudes (typically southern European countries with latitudes of <45°, e.g., Spain or Greece) at the time of filling in the questionnaires. If sunscreen was used, participants were asked to report the SPF (19). Participants were classified as nonusers if they did not indicate sunscreen use or answered 0 to the SPF question. Users were classified as using sunscreens with SPF <15 or ≥15 based on the minimally recommended SPF level (4) considered sufficient to prevent sunburn if properly applied (31). We created a variable for sunscreen use in high- and lower-latitude settings combined (for high/lower: none/none, SPF <15/none or none/SPF <15, SPF <15/SPF <15, SPF ≥15 in at least 1 setting), and in high- and lower-latitude settings separately (none, SPF <15, SPF ≥15) (19). We used use of sunscreen with SPF <15/SPF <15 as the referent in high-/lower-latitude settings, and a referent of SPF <15 in high- and lower-latitude settings separately (19). Sunscreen use was assumed to be representative of the current use, as one would in an RCT (with intention-to-treat analysis).
Time-fixed covariates
Residential ambient UVR exposure was categorized based on mean ambient UVR hours of the region of residence (32) (latitudes 70°–58°) as low (northern Norway), medium-low (central Norway), medium (southwestern Norway), and highest (southeastern Norway) (33, 34). Participants reported education (in years: ≤10, 11–13, ≥14), smoking (never, former, current), hair color (black/dark brown, brown, blond/yellow, red), untanned skin color (color scale from 1 (very fair) to 10 (very dark); categorized as light (grades 1–3), medium (grades 4–5), dark (grades 6–8), very dark (grades 9–10)), and freckling when sunbathing (no, yes). Skin reactions to acute and chronic sun exposure were recorded for a subsample of the cohort.
Time-varying covariates
Annual number of sunburns that resulted in pain, blistering, and subsequent peeling (never, 1, 2–3, 4–5, ≥6), annual number of weeks spent on sunbathing vacations in high and/or lower latitudes (never, 1, 2–3, 4–6, ≥7), and history of use of indoor tanning devices (never, rarely, 1, 2, 3–4 times/month, >1 time/week) were recorded at study inclusion for childhood (<10 years), adolescence (10–19 years), and adulthood, and updated in follow-up questionnaires. Cumulative number of sunburns was calculated by converting reported frequencies for all age periods to a yearly amount and multiplying this by the number of years for the given period (33, 34). The cumulative number was categorized as none, lowest (1–30 sunburns), middle (31–53 sunburns), or highest (>53 sunburns) tertile. Cumulative number of weeks on sunbathing vacations was calculated similarly: never, lowest (1–73 weeks), middle (74–138 weeks), or highest (>138 weeks) tertile (33, 34). In the analyses, we further collapsed none/never with the lowest tertile of cumulative numbers of sunburns/sunbathing vacations due to low numbers of participants in those categories. Use of indoor tanning devices was categorized as never/ever.
Reproducibility of the NOWAC questionnaire was good (κ/intraclass correlation coefficient, 0.49–0.77) and independent of age, education, and skin color (35).
Follow-up
The cohort was linked to the Cancer Registry of Norway using the unique personal identification number of Norwegian residents for follow-up of cancer incidence and vital status (alive, emigrated, or dead) until December 31, 2016. cSCC cases were identified by the International Classification of Diseases, Seventh Revision, codes 191.0–191.9, including the International Classification of Diseases for Oncology, Third Edition, morphology codes 80703, 80713, 80763, 80953, 80513, 80723, and 80743. We excluded cases with code 191.4 (perineum, perianal) because they are unrelated to UVR exposure. The Cancer Registry of Norway does not routinely record information on basal cell carcinoma.
Study sample
Of the 172,472 women who returned questionnaires, a total of 150,073 received questions about sunscreen use either at study inclusion or in the first follow-up questionnaire (Figure 1). We excluded women with very dark skin (n = 290) and women diagnosed with cSCC (n = 114) or cutaneous melanoma (n = 865) before answering the sunscreen questions. We further excluded 23 women who emigrated or died before the date of the questionnaire return, resulting in 148,781 women, born 1927–1957.
Figure 1.
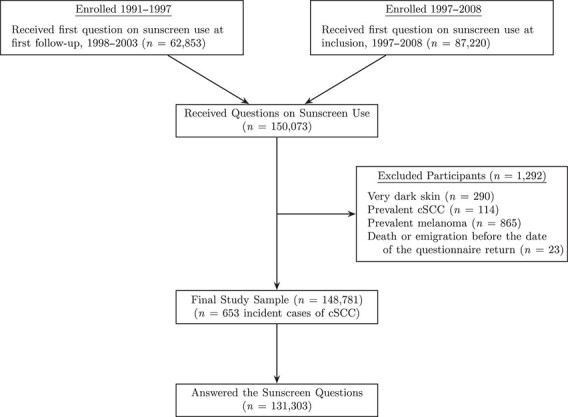
Selection of participants from enrollment into the study sample, Norwegian Women and Cancer Study, 1991–2016. cSCC, cutaneous squamous cell carcinoma.
Statistical analysis
The effect of sunscreen use on cSCC was estimated using a marginal structural Cox proportional hazards model, with hazard ratios (HRs) estimated using stabilized inverse probability of treatment and censoring weighting. Under assumptions of exchangeability, positivity, and consistency, weighting would mimic an RCT in which participants are randomized to sunscreen use with different SPFs and where censoring is random, allowing estimation of a causal effect also when exposure and confounders are time-varying (25–27, 36). The method has been described elsewhere (26, 36). For inverse probability of treatment weights, we used multinomial logistic regression (37) to estimate, at each time point, each participant’s probability of the observed level of sunscreen used, given their covariates. Similarly, for inverse probability of censoring weights, we used pooled logistic regression to estimate each participant’s probability of not being censored. (For details on weights estimation, see Web Appendix 1, available at https://doi.org/10.1093/aje/kwab216.) Covariates included in the models were based on assumptions in directed acyclic graphs (38, 39) (Web Figure 1A–B). Time-fixed covariates (recorded once in the first sunscreen questionnaire) included: age at return of the first sunscreen questionnaire, calendar year at recruitment to NOWAC (1991–1992, 1996–1997, 2003–2008), residential ambient UVR exposure, smoking status, hair color, and freckling when sunbathing. Sunscreen use and cumulative numbers of sunburns and sunbathing vacations were included as time-fixed (using only information recorded in the first sunscreen questionnaire) and as time-varying (using updated information from follow-up questionnaires) to assess the influence of time-varying confounding. Unweighted models were fitted (Cox regression, same covariates) to assess how weighting affected the results.
Analyses were conducted for sunscreen use in combined high-/lower-latitude settings, as well as high- and lower-latitude settings separately. The latter analyses were conducted in the subsample of women who spent at least 1 week of sunbathing vacation in lower latitudes. Participants contributed person-years of follow-up from receipt of the first sunscreen questionnaire (hereafter baseline) to first primary cSCC diagnosis, melanoma diagnosis (i.e., censoring at melanoma diagnosis), emigration, death, or end of follow-up (December 31, 2016), whichever occurred first. We used time-on-study as time scale, and we used robust variances to compute 95% confidence intervals (CIs) (40). All models (except marginal structural models with time-fixed covariates) were stratified by calendar year at inclusion. A likelihood ratio test was used to test for interaction between sunscreen use and cumulative number of sunburns (19).
In the study sample (n = 148,781), we had information on sunscreen use in 88% of women (n = 131,303, Figure 1), with up to 30% missing when combining covariates. To address this, we used multiple imputation with chained equations (41) to impute 40 data sets. In each data set, we conducted analyses using the models described above, and estimates were pooled using Rubin’s rule (42).
We conducted several complete-case sensitivity analyses. To investigate potential selection bias, we conducted analyses not excluding prevalent melanomas and cSCCs. To evaluate the assumption on the direction of the causal pathway between sunscreen use and sunburns, we conducted analyses based on directed acyclic graphs where the direction of this pathway was reversed (Web Figure 2A–B). To assess whether model choice affected the results, we also fitted a marginal structural Aalen additive hazards model (only high-/lower-latitude settings) (43). (For details on these and additional sensitivity analyses, see Web Appendix 2.) Statistical analyses were conducted using R, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Mean age at baseline for the 148,781 women included was 53.2 (standard deviation, 6.5) years. Mean follow-up was 14.3 (standard deviation, 3.8) years, during which 653 women were diagnosed with incident primary cSCC. This was the first diagnosis of any cancer for 519 women, second for 110, and the third to fifth cancer diagnosis for 24 women. Mean age at cSCC diagnosis was 68.3 years and similar among women with cSCC as their first (67.5 years) and second (68.1 years) cancer diagnosis, but slightly higher for women with cSCC as their third to fifth (70.7 years) cancer diagnosis. Head (n = 280) was the most common site, followed by trunk (includes neck, shoulders, and hips, n = 151) (Web Table 1).
Among the 131,303 who answered the sunscreen questions, a total of 111,159 (85%) reported using sunscreen (of any SPF) in high- and/or lower-latitude settings at the time of the first sunscreen questionnaire (Table 1). Users were younger than nonusers, and SPF ≥15 sunscreens were more common in women recruited in 2003–2008, living in areas with higher ambient UVR, with higher education, lighter hair and skin color, freckling when sunbathing, more sensitive skin, higher cumulative numbers of sunburns and sunbathing vacations, never/former smokers, and in women using indoor tanning.
Table 1.
Characteristics of Participants in a Study of Sunscreen Use and Cutaneous Squamous Cell Carcinoma Risk, Stratified by Sunscreen Use (n = 131,303), Norwegian Women and Cancer Study, 1991–2016
| Sunscreen Use in High-/Lower-Latitude Settings a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Total No. | None/None |
None/SPF <15 or
SPF <15/None |
SPF <15/SPF <15 | SPF ≥15 in at Least 1 Setting | ||||
| No. | % b | No. | % b | No. | % b | No. | % b | ||
| Participants | 20,144 | 15.3 | 32,267 | 24.6 | 43,175 | 32.9 | 35,717 | 27.2 | |
| Total person-years of follow-up | 289,081 | 489,274 | 630,759 | 472,620 | |||||
| Person-years of follow-upc | 14.4 (4.0) | 15.2 (3.7) | 14.6 (3.7) | 13.2 (3.5) | |||||
| Age at answering first sunscreen questions, yearsc | 55.6 (7.5) | 52.2 (6.5) | 52.3 (5.9) | 53.1 (5.7) | |||||
| Incident cSCC cases | 87 | 15.6 | 122 | 21.8 | 204 | 36.5 | 146 | 26.1 | |
| Age at diagnosis, yearsc | 71.8 (8.6) | 69.1 (8.9) | 66.7 (8.2) | 65.4 (8.7) | |||||
| Year at recruitment | 131,303 | ||||||||
| 1991–1992 | 5,474 | 13.1 | 13,584 | 32.5 | 15,207 | 36.4 | 7,523 | 18.0 | |
| 1996–1997 | 7,079 | 25.9 | 7,288 | 26.6 | 8,085 | 29.5 | 4,924 | 18.0 | |
| 2003–2008 | 7,591 | 12.2 | 11,395 | 18.3 | 19,883 | 32.0 | 23,270 | 37.4 | |
| Residential ambient UVR exposure | 131,303 | ||||||||
| Low (northern Norway) | 6,191 | 21.7 | 7,130 | 24.9 | 7,262 | 25.4 | 8,009 | 28.0 | |
| Medium-low (central Norway) | 2,132 | 15.1 | 3,854 | 27.2 | 4,883 | 34.5 | 3,275 | 23.2 | |
| Medium (southwestern Norway) | 2,971 | 12.0 | 5,986 | 24.2 | 8,471 | 34.3 | 7,273 | 29.4 | |
| Highest (southeastern Norway) | 8,850 | 13.9 | 15,297 | 24.0 | 22,559 | 35.3 | 17,160 | 26.9 | |
| Education, years | 124,561 | ||||||||
| ≤10 | 10,180 | 24.4 | 11,287 | 27.1 | 11,546 | 27.7 | 8,637 | 20.7 | |
| 11–13 | 4,178 | 11.2 | 9,362 | 25.1 | 13,691 | 36.7 | 10,060 | 27.0 | |
| ≥14 | 4,019 | 8.8 | 10,245 | 22.5 | 16,091 | 35.3 | 15,265 | 33.5 | |
| Smoking status at baseline | 125,503 | ||||||||
| Never | 6,974 | 15.8 | 11,333 | 25.6 | 13,463 | 30.5 | 12,434 | 28.1 | |
| Former | 5,532 | 11.9 | 10,256 | 22.0 | 16,394 | 35.2 | 14,389 | 30.9 | |
| Current | 6,439 | 18.5 | 9,116 | 26.2 | 11,435 | 32.9 | 7,738 | 22.3 | |
| Hair color | 127,360 | ||||||||
| Black/dark brown | 4,311 | 19.8 | 5,558 | 25.5 | 6,798 | 31.2 | 5,130 | 23.5 | |
| Brown | 7,413 | 14.3 | 12,934 | 24.9 | 17,666 | 34.1 | 13,858 | 26.7 | |
| Blond/yellow, red | 7,309 | 13.6 | 12,756 | 23.8 | 17,620 | 32.8 | 16,007 | 29.8 | |
| Untanned skin color | 120,376 | ||||||||
| Dark | 3,594 | 13.8 | 6,537 | 25.0 | 9,908 | 38.0 | 6,068 | 23.2 | |
| Medium | 5,639 | 12.3 | 11,503 | 25.2 | 16,350 | 35.8 | 12,212 | 26.7 | |
| Light | 7,338 | 15.1 | 11,758 | 24.2 | 14,299 | 29.4 | 15,170 | 31.2 | |
| Freckling when sunbathing | 124,227 | ||||||||
| No | 13,103 | 16.2 | 20,172 | 25.0 | 27,134 | 33.6 | 20,406 | 25.3 | |
| Yes | 4,737 | 10.9 | 10,424 | 24.0 | 14,239 | 32.8 | 14,012 | 32.3 | |
| Skin reaction to acute sun exposured | 65,833 | ||||||||
| Brown | 3,710 | 20.2 | 5,290 | 28.7 | 6,908 | 37.5 | 2,495 | 13.6 | |
| Red | 5,091 | 15.5 | 10,170 | 30.9 | 11,673 | 35.5 | 5,965 | 18.1 | |
| Red with pain | 1,750 | 15.1 | 3,881 | 33.4 | 3,360 | 29.0 | 2,613 | 22.5 | |
| Red with pain and blisters | 475 | 16.2 | 895 | 30.6 | 668 | 22.8 | 889 | 30.4 | |
| Skin reaction to chronic sun exposured | 64,793 | ||||||||
| Deep brown | 1,573 | 16.4 | 2,827 | 29.4 | 3,872 | 40.3 | 1,328 | 13.8 | |
| Brown | 5,753 | 15.5 | 11,462 | 30.8 | 13,725 | 36.9 | 6,285 | 16.9 | |
| Light brown | 3,070 | 18.3 | 5,327 | 31.7 | 4,545 | 27.1 | 3,853 | 22.9 | |
| Never brown | 487 | 41.5 | 267 | 22.8 | 106 | 9.0 | 313 | 26.7 | |
| Cumulative no. of sunburns | 104,829 | ||||||||
| None | 3,502 | 27.5 | 3,127 | 24.5 | 3,580 | 28.1 | 2,544 | 19.9 | |
| Lowest tertile | 4,233 | 13.1 | 8,163 | 25.2 | 10,954 | 33.8 | 9,084 | 28.0 | |
| Middle tertile | 3,232 | 10.8 | 8,050 | 27.0 | 10,657 | 35.7 | 7,897 | 26.5 | |
| Highest tertile | 2,666 | 8.9 | 6,672 | 22.4 | 10,474 | 35.1 | 9,994 | 33.5 | |
| Cumulative no. of weeks on sunbathing vacations | 112,998 | ||||||||
| None | 3,729 | 57.1 | 1,816 | 27.8 | 164 | 2.5 | 818 | 12.5 | |
| Lowest tertile | 5,772 | 16.5 | 11,230 | 32.1 | 10,068 | 28.8 | 7,924 | 22.6 | |
| Middle tertile | 3,081 | 8.6 | 8,779 | 24.6 | 12,702 | 35.6 | 11,102 | 31.1 | |
| Highest tertile | 2,502 | 7.0 | 6,082 | 17.0 | 15,149 | 42.3 | 12,080 | 33.7 | |
| Indoor tanning | 113,032 | ||||||||
| Never | 7,836 | 22.9 | 9,845 | 28.8 | 7,091 | 20.8 | 9,377 | 27.5 | |
| Ever | 7,090 | 9.0 | 18,024 | 22.8 | 31,261 | 39.6 | 22,508 | 28.5 | |
Abbreviations: cSCC, cutaneous squamous cell carcinoma; SPF, sun protection factor; UVR, ultraviolet radiation.
a Sunscreen use in high-/lower-latitude settings = sunscreen use in high- and lower-latitude settings combined.
b Percentages are row percentages. Because of rounding, percentages may not sum up to 100.
c Values are expressed as mean (standard deviation).
d Recorded in subsamples of the cohort.
In weighted analyses with multiple imputation and time-varying covariates (Table 2), the estimate of the causal effect on cSCC risk was close to the null in high-/lower-latitude settings for SPF ≥15 in at least 1 setting versus SPF <15/SPF <15 (HR = 1.02, 95% CI: 0.82, 1.27) and in lower-latitude settings for SPF ≥15 versus SPF <15 (HR = 1.05, 95% CI: 0.84, 1.32). There was higher cSCC risk in high-latitude settings for SPF ≥15 versus SPF <15 (HR = 1.33, 95% CI: 1.05, 1.67), although it was closer to the null in the complete-case analysis (HR = 1.16, 95% CI: 0.85, 1.58). Nonusers had lower cSCC risk in high-/lower-latitude settings compared with users of SPF <15/SPF <15 (HR = 0.71, 95% CI: 0.54, 0.94) (Table 2). Similar results were found in time-fixed models (Table 2). Confidence intervals included the null in all complete-case analyses. No indication of interaction was found between sunscreen use and cumulative number of sunburns (0.35 ≤ P for interaction ≤ 0.94).
Table 2.
Hazard Ratios for Sunscreen Use and Risk of Cutaneous Squamous Cell Carcinoma Among Participants in the Norwegian Women and Cancer Study, 1991–2016
| Complete-Case Analyses | Multiple Imputation Analyses a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sunscreen Use Variable | No. of Women b | % | No. of Cases b | Marginal Structural Model c | Unweighted Multivariable Model d | MarginalStructural Model c | Unweighted Multivariable Model d | ||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| SPF use in high-/lower-latitude settings | 94,594 | 312 | |||||||||
| Time-fixed covariates onlye | |||||||||||
| None/none | 11,612 | 12.3 | 35 | 0.72 | 0.46, 1.11 | 0.73 | 0.49, 1.08 | 0.71 | 0.53, 0.95 | 0.71 | 0.55, 0.92 |
| None/SPF <15, SPF <15/none | 23,109 | 24.4 | 68 | 0.77 | 0.56, 1.05 | 0.79 | 0.58, 1.06 | 0.79 | 0.62, 1.01 | 0.81 | 0.65, 1.00 |
| SPF <15/SPF <15 | 32,165 | 34.0 | 123 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| SPF ≥15 in at least 1 setting | 27,708 | 29.3 | 86 | 0.82 | 0.60, 1.11 | 0.95 | 0.72, 1.26 | 0.87 | 0.68, 1.13 | 0.97 | 0.78, 1.20 |
| Time-varyingf | |||||||||||
| None/none | 11,478 | 12.1 | 35 | 0.70 | 0.45, 1.07 | 0.73 | 0.49, 1.09 | 0.71 | 0.54, 0.94 | 0.76 | 0.58, 0.98 |
| None/SPF <15, SPF <15/none | 19,408 | 20.5 | 64 | 0.82 | 0.60, 1.13 | 0.86 | 0.63, 1.17 | 0.85 | 0.66, 1.08 | 0.86 | 0.68, 1.09 |
| SPF <15/SPF <15 | 30,137 | 31.9 | 113 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| SPF ≥15 in at least 1 setting | 33,571 | 35.5 | 100 | 0.91 | 0.69, 1.20 | 0.96 | 0.73, 1.26 | 1.02 | 0.82, 1.27 | 1.04 | 0.85, 1.28 |
| SPF use in high-latitude settings | 96,853 | 317 | |||||||||
| Time-fixed covariates onlye | |||||||||||
| None | 20,689 | 21.4 | 64 | 0.96 | 0.71, 1.30 | 0.91 | 0.68, 1.21 | 0.90 | 0.72, 1.11 | 0.85 | 0.69, 1.05 |
| SPF <15 | 62,420 | 64.4 | 203 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| SPF ≥15 | 13,744 | 14.2 | 50 | 1.09 | 0.77, 1.55 | 1.29 | 0.94, 1.76 | 1.16 | 0.88, 1.54 | 1.26 | 0.99, 1.59 |
| Time-varyingf | |||||||||||
| None | 21,278 | 22.0 | 67 | 0.91 | 0.67, 1.23 | 0.88 | 0.66, 1.18 | 0.88 | 0.72, 1.09 | 0.86 | 0.70, 1.06 |
| SPF <15 | 58,877 | 60.8 | 197 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| SPF ≥15 | 16,698 | 17.2 | 53 | 1.16 | 0.85, 1.58 | 1.13 | 0.83, 1.53 | 1.33 | 1.05, 1.67 | 1.28 | 1.03, 1.60 |
| SPF use in lower-latitude settingsg | 64,205 | 235 | |||||||||
| Time-fixed covariates onlye | |||||||||||
| None | 7,379 | 11.5 | 29 | 0.99 | 0.65, 1.53 | 1.01 | 0.67, 1.51 | 0.85 | 0.62, 1.17 | 0.87 | 0.64, 1.17 |
| SPF <15 | 36,334 | 56.6 | 138 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| SPF ≥15 | 20,492 | 31.9 | 68 | 0.97 | 0.71, 1.33 | 1.05 | 0.78, 1.41 | 0.96 | 0.74, 1.23 | 0.99 | 0.78, 1.25 |
| Time-varyingf | |||||||||||
| None | 7,452 | 11.6 | 33 | 1.08 | 0.72, 1.63 | 1.10 | 0.75, 1.63 | 0.99 | 0.73, 1.35 | 0.99 | 0.74, 1.33 |
| SPF <15 | 32,555 | 50.7 | 126 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| SPF ≥15 | 24,198 | 37.7 | 76 | 0.98 | 0.74, 1.31 | 1.01 | 0.76, 1.35 | 1.05 | 0.84, 1.32 | 1.06 | 0.85, 1.33 |
Abbreviations: CI, confidence interval; HR, hazard ratio; SPF, sun protection factor.
a Analyses with multiple imputation of missing data conducted using chained equations and a total of 40 imputed data sets, using the same models as in the complete-case analyses (n = 148,781; 653 cases).
b In the model with time-varying covariates, the numbers correspond to the category in which participants were at the end of follow-up.
c Marginal structural Cox proportional hazards model estimated using stabilized inverse probability of treatment weights and stabilized inverse probability of censoring weights. Weights were constructed using calendar year at study inclusion, age at baseline, residential ambient ultraviolet radiation exposure, smoking status, hair color, freckling when sunbathing, and cumulative numbers of sunburns and sunbathing vacations. In the models with time-varying covariates, time-fixed covariates were also included in the numerator of the weights and in the marginal structural model to further stabilize the weights.
d Unweighted Cox proportional hazards model, adjusted for age at baseline, residential ambient ultraviolet radiation exposure, smoking status, hair color, freckling when sunbathing, and cumulative numbers of sunburns and sunbathing vacations, stratified by calendar year at study inclusion.
e Using only information recorded at baseline.
f Sunscreen use as well as cumulative numbers of sunburns and sunbathing vacations as time-varying covariates.
g Analyses were conducted in a subsample of women who spent at least 1 week of sunbathing vacation in lower latitudes (n = 94,408; 435 cases).
Sensitivity analyses where prevalent melanomas and cSCCs were included (Web Table 2), those based on directed acyclic graphs where the direction of the causal pathway between sunscreen use and sunburns was reversed (Web Table 3), and those using the marginal structural Aalen additive hazards model (Web Figure 3) showed similar results. None of the sensitivity analyses described in Web Appendix 2 produced meaningful differences.
DISCUSSION
In this large, prospective study, we found no indication that SPF ≥15 sunscreens reduced cSCC risk more than SPF <15 sunscreens in high-/lower-latitude settings or lower-latitude settings. For sunscreen use in high-latitude settings, increased risk was found for SPF ≥15 versus SPF <15 in the multiple imputation analysis, although the effect was closer to the null in the complete-case analysis.
We conducted several sensitivity analyses to assess the impact of modeling choices on the estimates. Results were similar in models with time-fixed and time-varying covariates, as well as in the unweighted models, suggesting minimal time-varying confounding. Only 2.8% of women had information from 3 time points, which may explain these results. We found similar results in the marginal structural Aalen additive hazards model, indicating robustness of the estimates. Prevalent melanomas and cSCCs were excluded because of potential recall bias and bias due to changes in sun-protection behavior before the sunscreen questions were answered. Further, studies have found that skin cancer survivors have higher subsequent skin cancer risk than the general population (44, 45). However, our results were also similar in analyses not excluding prevalent melanomas and cSCCs.
NOWAC is a well-characterized cohort of women with complete follow-up and information about sunburns and sunbathing vacations from all decades of life. NOWAC is representative of Norwegian women aged 45–74 years with regard to total cancer incidence (30), with no major selection bias (46), and with almost no selection of participants from the recruitment questionnaire to the first follow-up questionnaire (30). Furthermore, 99.7% of cSCCs are morphologically verified (2), and all information was collected prior to cancer diagnosis, limiting the potential for recall bias. Exposure misclassification, inevitable in epidemiologic studies, is likely nondifferential in cohort studies, although differential misclassification can occur when forming categories (47).
To our knowledge, no other study on sunscreen use and cSCC used information collected during follow-up, or compared users of higher-SPF sunscreens with users of low-SPF sunscreens. The one RCT found a protective effect of daily sunscreen use on cSCC incidence, versus discretionary use of sunscreens (15, 16). However, this study was conducted in Australia, where UVR is much higher and sun exposure is likely nonintentional (21, 22), and the control group included nonusers of sunscreen.
The differences between multiple imputation analyses and complete-case analyses in high-/lower-latitude settings and high-latitude settings may indicate that some data were missing not at random (42). Moreover, a substantial amount of data were imputed (up to 22% for individual covariates), which could have influenced the results. Furthermore, in high-latitude settings, we do not know on what occasions the sunscreen was used, as opposed to lower-latitude settings where participants were asked about sunscreen use on sunbathing vacations specifically.
A causal interpretation of our results is relying on a number of assumptions not guaranteed in observational studies. We assumed that sunscreen use was a well-defined exposure, similar to an RCT. We did not have information on how/when participants applied sunscreen, so that within one category of sunscreen use, sunscreen exposure may be quite different. In addition, by design, only current use was recorded. Exposure was updated during follow-up, but we had no information on lifetime sunscreen use in the past, including in childhood and adolescence, nor did we have information on the number of hours spent outside, or on other sun protective behavior such as avoiding the sun or wearing protective clothing. Further, it has been suggested that sunscreen use may be connected to extended sun exposure, especially in regions with mainly intentional sun exposure, such as Norway (21–23). Cohort studies such as ours are prone to unmeasured/residual confounding. Sensitivity analyses with different adjustment strategies yielded similar results. Regarding nonusers, this group of women was previously reported to be more likely to have a less sun-sensitive phototype, live in areas of low ambient UVR, and to report no sunburns, sunbathing vacations, and use of indoor tanning devices (19). Thus, nonusers of sunscreen were a priori at lower cSCC risk than users.
The effectiveness of sunscreen depends not only on its SPF rating but also ultraviolet spectral absorption, amount applied, reapplication, duration of sun exposure, and coverage of sun-exposed parts. It has been reported that people use one-fifth to one-half of the recommended amount and do not reapply as recommended (21, 48), resulting in misclassification of sunscreen use in the direction of lower SPF (49). Participants in our reference group (SPF <15) were sunscreen users, thereby already screening a certain amount UVR and likely at lower risk for cSCC than nonusers. Thus, any difference in effect may have been attenuated. Finally, cumulative sun exposure is the main cSCC risk factor (1, 3). Although sunscreens are designed to protect against UVR, some radiation will always penetrate the skin (e.g., 10% for SPF 10, 6.3% for SPF 15, and 3.3% for SPF 30, with correct application) (50). This will be cumulative over time, potentially rendering any difference in effect between sunscreens marginal.
To our knowledge, this prospective study is the first to try to investigate the causal effect of use of sunscreens of different SPFs on cSCC risk. We found no indication that sunscreens with SPF ≥15 reduced Norwegian women’s cSCC risk more than sunscreens with SPF <15, suggesting that either there is no difference in their effect long term, or the difference is diluted by incorrect application. The importance of correct sunscreen application should therefore be emphasized.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Oslo Centre for Biostatistics and Epidemiology, Department of Biostatistics, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway (Simon Lergenmuller, Marit B. Veierød); Department of Research, Cancer Registry of Norway, Oslo, Norway (Reza Ghiasvand, Trude E. Robsahm, Eiliv Lund); Oslo Centre for Biostatistics and Epidemiology, Oslo University Hospital, Oslo, Norway (Reza Ghiasvand, Corina S. Rueegg); Population Health Department, QIMR Berghofer Medical Research Institute, Brisbane, Australia (Adele C. Green); Cancer Research UK Manchester and Faculty of Biology, Medicine and Health, University of Manchester, Manchester, United Kingdom (Adele C. Green); and Department of Public Health, Faculty of Health Sciences, University of Tromsø, Tromsø, Norway (Eiliv Lund).
This work was supported by the Institute of Basic Medical Sciences, University of Oslo (S.L.). No other funding, grants, or support was received.
Data availability statement: The data generated and/or analyzed in the current study can be accessed upon reasonable request to the originating cohort. Access will be conditional to adherence to local ethical and security policy. The R code used to conduct specific analyses will be shared on reasonable request (of the specific code) to the corresponding author.
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest: none declared.
REFERENCES
- 1. Green AC, Olsen CM. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol. 2017;177(2):373–381. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Registry of Norway . Cancer in Norway 2019—Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo, Norway: Institution of Population-Based Cancer Research; 2020. https://www.kreftregisteret.no/globalassets/cancer-in-norway/2019/cin_report.pdf. Accessed July 21, 2021. [Google Scholar]
- 3. Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379(4):363–374. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization, World Meteorological Organization, United Nations Environment Programme and International Commission on Non-Ionizing Radiation Protection . Global Solar UV Index: A Practical Guide. Geneva, Switzerland: World Health Organization; 2002. https://apps.who.int/iris/bitstream/handle/10665/42459/9241590076.pdf. Accessed July 21, 2021. [Google Scholar]
- 5. Robinson JK. Sun safety. JAMA Dermatol. 2018;154(3):380. [DOI] [PubMed] [Google Scholar]
- 6. Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med. 1993;329(16):1147–1151. [DOI] [PubMed] [Google Scholar]
- 7. Naylor MF, Boyd A, Smith DW, et al. High sun protection factor sunscreens in the suppression of actinic neoplasia. Arch Dermatol. 1995;131(2):170–175. [PubMed] [Google Scholar]
- 8. English DR, Armstrong BK, Kricker A, et al. Case-control study of sun exposure and squamous cell carcinoma of the skin. Int J Cancer. 1998;77(3):347–353. [DOI] [PubMed] [Google Scholar]
- 9. Rosso S, Joris F, Zanetti R. Risk of basal and squamous cell carcinomas of the skin in Sion, Switzerland: a case-control study. Tumori. 1999;85(6):435–442. [DOI] [PubMed] [Google Scholar]
- 10. Iannacone MR, Wang W, Stockwell HG, et al. Patterns and timing of sunlight exposure and risk of basal cell and squamous cell carcinomas of the skin—a case-control study. BMC Cancer. 2012;12(1):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sánchez G, Nova J. Risk factors for squamous cell carcinoma, a study by the National Dermatology Centre of Colombia. Actas Dermosifiliogr. 2013;104(8):672–678. [DOI] [PubMed] [Google Scholar]
- 12. Savoye I, Olsen CM, Whiteman DC, et al. Patterns of ultraviolet radiation exposure and skin cancer risk: the E3N-SunExp study. J Epidemiol. 2018;28(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Serna-Higuita LM, Harrison SL, Buttner P, et al. Modifiable risk-factors for keratinocyte cancers in Australia: a case-control study. Acta Derm Venereol. 2019;99(4):404–411. [DOI] [PubMed] [Google Scholar]
- 14. Grodstein F, Speizer FE, Hunter DJ. A prospective study of incident squamous cell carcinoma of the skin in the nurses' health study. J Natl Cancer Inst. 1995;87(14):1061–1066. [DOI] [PubMed] [Google Scholar]
- 15. Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354(9180):723–729. [DOI] [PubMed] [Google Scholar]
- 16. van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2546–2548. [DOI] [PubMed] [Google Scholar]
- 17. Green AC, Williams GM. Point: sunscreen use is a safe and effective approach to skin cancer prevention. Cancer Epidemiol Biomarkers Prev. 2007;16(10):1921–1922. [DOI] [PubMed] [Google Scholar]
- 18. Diffey B. Sunscreens and melanoma: the future looks bright. Br J Dermatol. 2005;153(2):378–381. [DOI] [PubMed] [Google Scholar]
- 19. Ghiasvand R, Weiderpass E, Green AC, et al. Sunscreen use and subsequent melanoma risk: a population-based cohort study. J Clin Oncol. 2016;34(33):3976–3983. [DOI] [PubMed] [Google Scholar]
- 20. Rueegg CS, Stenehjem JS, Egger M, et al. Challenges in assessing the sunscreen-melanoma association. Int J Cancer. 2019;144(11):2651–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Autier P, Boniol M, Doré JF. Sunscreen use and increased duration of intentional sun exposure: still a burning issue. Int J Cancer. 2007;121(1):1–5. [DOI] [PubMed] [Google Scholar]
- 22. Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol. 2009;161(suppl 3):40–45. [DOI] [PubMed] [Google Scholar]
- 23. Støle HS, Nilsen LTN, Joranger P. Beliefs, attitudes and perceptions to sun-tanning behaviour in the Norwegian population: a cross-sectional study using the health belief model. BMC Public Health. 2019;19(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hernán MA, Robins JM. Causal Inference: What If. Boca Raton, FL: Chapman & Hall/CRC; 2020. [Google Scholar]
- 26. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 27. Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glass TA, Goodman SN, Hernán MA, et al. Causal inference in public health. Annu Rev Public Health. 2013;34:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Williamson T, Ravani P. Marginal structural models in clinical research: when and how to use them? Nephrol Dial Transplant. 2017;32(suppl 2):ii84–ii90. [DOI] [PubMed] [Google Scholar]
- 30. Lund E, Dumeaux V, Braaten T, et al. Cohort profile: the Norwegian Women and Cancer Study—NOWAC—Kvinner og kreft. Int J Epidemiol. 2008;37(1):36–41. [DOI] [PubMed] [Google Scholar]
- 31. Pissavini M, Diffey B. The likelihood of sunburn in sunscreen users is disproportionate to the SPF. Photodermatol Photoimmunol Photomed. 2013;29(3):111–115. [DOI] [PubMed] [Google Scholar]
- 32. Edvardsen K, Veierød MB, Brustad M, et al. Vitamin D-effective solar UV radiation, dietary vitamin D and breast cancer risk. Int J Cancer. 2011;128(6):1425–1433. [DOI] [PubMed] [Google Scholar]
- 33. Ghiasvand R, Rueegg CS, Weiderpass E, et al. Indoor tanning and melanoma risk: long-term evidence from a prospective population-based cohort study. Am J Epidemiol. 2017;185(3):147–156. [DOI] [PubMed] [Google Scholar]
- 34. Lergenmuller S, Ghiasvand R, Robsahm TE, et al. Association of lifetime indoor tanning and subsequent risk of cutaneous squamous cell carcinoma. JAMA Dermatol. 2019;155(12):1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veierød MB, Parr CL, Lund E, et al. Reproducibility of self-reported melanoma risk factors in a large cohort study of Norwegian women. Melanoma Res. 2008;18(1):1–9. [DOI] [PubMed] [Google Scholar]
- 36. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20(1):18–26. [DOI] [PubMed] [Google Scholar]
- 38. Gran JM, Stigum H, Håberg SE, et al. Causal inference. In: Veierød MB, Lydersen S, Laake P, eds. Medical Statistics in Clinical and Epidemiological Research. 1st ed. Oslo, Norway: Gyldendal Akademisk; 2012:493–527. [Google Scholar]
- 39. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 40. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. 2nd ed. New York, NY: Springer; 2000. [Google Scholar]
- 41. van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2010;45(3):1–67. [Google Scholar]
- 42. van Buuren S. Flexible Imputation of Missing Data. 2nd ed. Boca Raton, FL: Chapman & Hall/CRC; 2018. [Google Scholar]
- 43. Aalen OO, Borgan Ø, Gjessing H. Survival and Event History Analysis: A Process Point of View. New York, NY: Springer; 2008. [Google Scholar]
- 44. Karagas MR, Stukel TA, Greenberg ER, et al. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin Cancer Prevention Study Group . JAMA. 1992;267(24):3305–3310. [PubMed] [Google Scholar]
- 45. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol. 2010;146(3):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lund E, Kumle M, Braaten T, et al. External validity in a population-based national prospective study—the Norwegian Women and Cancer Study (NOWAC). Cancer Causes Control. 2003;14(10):1001–1008. [DOI] [PubMed] [Google Scholar]
- 47. Flegal KM, Keyl PM, Nieto FJ. Differential misclassification arising from nondifferential errors in exposure measurement. Am J Epidemiol. 1991;134(10):1233–1244. [DOI] [PubMed] [Google Scholar]
- 48. Görig T, Schneider S, Seuffert S, et al. Does sunscreen use comply with official recommendations? Results of a nationwide survey in Germany. J Eur Acad Dermatol Venereol. 2020;34(5):1112–1117. [DOI] [PubMed] [Google Scholar]
- 49. Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30(2–3):96–101. [DOI] [PubMed] [Google Scholar]
- 50. Wilson BD, Moon S, Armstrong F. Comprehensive review of ultraviolet radiation and the current status on sunscreens. J Clin Aesthet Dermatol. 2012;5(9):18–23. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


