Abstract
Background
Obesity exacerbates age-related effects on body composition and physical and metabolic function. Which exercise mode is most effective in mitigating these deleterious changes in dieting older adults with obesity is unknown.
Methods
In a randomized controlled trial, we performed a head-to-head comparison of aerobic (AEX), resistance (REX), or combination (COMB) exercise during matched ~10% weight loss in 160 obese older adults. Prespecified analyses compared 6-month changes in intermuscular adipose tissue (IMAT) and visceral adipose tissue (VAT) assessed using MRI, insulin sensitivity index (ISI) by oral glucose tolerance test, physical function using Modified Physical Performance Test (PPT), VO2peak, gait speed, and knee strength by dynamometry.
Results
IMAT and VAT decreased more in COMB than AEX and REX groups (IMAT; −41% vs −28% and −23% and VAT: −36% vs −19% and −21%; p = .003 to .01); IMAT and VAT decreased in all groups more than control (between-group p < .001). ISI increased more in COMB than AEX and REX groups (86% vs 50% and 39%; p = .005 to .03). PPT improved more in COMB than AEX and REX groups, while VO2peak improved more in COMB and AEX than REX group (all p < .05). Knee strength improved more in COMB and REX than AEX group (all p < .05). Changes in IMAT and VAT correlated with PPT (r = −0.28 and −0.39), VO2peak (r = −0.49 and −0.52), gait speed (r = −0.25 and −0.36), and ISI (r = −0.49 and −0.52; all p < .05).
Conclusions
Weight loss plus combination aerobic and resistance exercise was most effective in improving ectopic fat deposition and physical and metabolic function in older adults with obesity.
Keywords: Clinical trials, Exercise, Functional performance, Obesity
Adipose tissue redistributes with aging and in older adults, there is a reduction in subcutaneous adipose tissue (SAT) and an increase in intermuscular adipose tissue (IMAT) and visceral adipose tissue (VAT) (1,2). Higher adiposity and, in particular, increased IMAT and VAT are associated with a range of poor physical and metabolic indicators. These include lower physical performance (3–5), impaired mobility (4,6,7), reduced muscle strength and quality (8,9), as well as increased cardiovascular risks (10,11), impaired glucose and lipid metabolism (12,13), and chronic inflammation and insulin resistance (14,15). Therefore, interventions in older adults which target a reduction in IMAT and VAT have the potential to benefit physical and metabolic function.
We conducted the Lifestyle Intervention Trial in Obese Elderly (LITOE) to determine whether aerobic and/or resistance exercise types could be used to preserve muscle and bone mass and improve physical function during a weight loss program in older adults (16). Losses of lean mass and bone mineral density during weight loss intervention were the least when resistance exercise was incorporated either alone or in combination with aerobic exercise, while physical function improved the most with a combination of aerobic and resistance exercise (16).
Little is known about the comparative efficacy of different exercise types in reducing ectopic fat deposition in older adults with obesity during weight loss therapy. Therefore, we performed a head-to-head comparison of aerobic or resistance exercise, or both, in dieting older adults with obesity to test the hypothesis that weight loss plus combination aerobic and resistance exercise would reduce IMAT and VAT more than weight loss plus aerobic exercise or weight loss plus resistance exercise. We also hypothesized that these reductions in regional body composition would correlate with improvements in physical and metabolic function in this population. The data reported in this article were obtained using the same subject group that participated in LITOE.
Method
Overview
The LITOE was a randomized controlled trial (RCT) with the main aim to evaluate the relative efficacy of several exercise modes in improving physical function and reversing frailty in older adults with obesity while dieting (ClinialTrials.gov number NCT01065636). The principal results showed that weight loss plus combination aerobic and resistance exercise was the most effective in improving the functional status of older adults with obesity (16). The present study reports secondary analyses of the trial examining changes in body composition including IMAT and VAT in relation to changes in physical and metabolic function, as prespecified in the protocol.
Participants
The methods of this study have been published previously (16). Briefly, the study was approved by the university’s institutional board and monitored by an independent data and safety monitoring board. Volunteers were recruited from Albuquerque, New Mexico through advertisements, and informed consent was obtained from each participant. All participants underwent a comprehensive medical examination. Persons were eligible if they were older (aged ≥65 years), obese (body mass index ≥30 kg/m2), sedentary (regular exercise <1 h/week), and had had a stable body weight (loss or gain of no more than 2 kg) and stable medication use for 6 months before enrollment. In addition, all participants had mild-to-moderate frailty, as defined by a score of 18–31 on the Modified Physical Performance Test (PPT) (scores range from 0 to 36 points, with higher scores indicating better performance) (17). Persons who had severe cardiopulmonary disease, musculoskeletal or neuromuscular impairments that precluded exercise training or cognitive impairments, or who used drugs affecting body composition were excluded. Volunteers were also excluded if they had diabetes requiring insulin therapy or uncontrolled diabetes (hemoglobin A1c of >8.5%).
Weight Loss and Exercise Intervention
In this 26-week RCT, participants were randomized with stratification for sex into one of 4 groups: (a) control group that did not participate in a weight management or exercise training (CON), (b) aerobic group that participated in a weight management program and aerobic exercise training (AEX), (c) resistance group that participated in a weight management program and resistance training (REX), and (d) combination group that participated in a weight management program and combination aerobic and resistance exercise training (COMB). All 3 exercise groups received the same weight management program of behavioral therapy sessions once per week including nutritional counseling to achieve a 500–750 kcal deficit in energy requirements per day and 1 g protein/kg body weight per day (18). The goal was to achieve a weight loss of approximately 10% at 6 months. Each of the exercise intervention groups also attended exercise sessions 3 times per week, lasting 60–90 minutes with 15 minutes of warm-up and cool down included. Aerobic exercise was performed on a treadmill, bike, or elliptical trainer at approximately 65% of their peak heart rate, which was gradually increased to 70%–85% of peak heart rate. Resistance exercise was performed on weight-lifting machines consisting of 9 upper-body and lower-body exercises. The initial sessions were 1–2 sets of 8–12 repetitions at 65% of the one-repetition maximum (1-RM; the maximum weight a participant can lift, in one attempt), which was increased progressively to 2–3 sets at approximately 85% of the 1-RM. All exercise sessions were supervised by exercise physiologists at our facility. The control group attended group educational sessions about a healthful diet during monthly visits and were asked not to participate in external weight loss or exercise programs.
Additional details about the interventions including compliance data, exercise adaptations, and adverse events have been reported in the primary paper (16).
Assessments of Body Composition, Physical Function, and Metabolic Function
Assessments were carried out at baseline and 6 months by personnel blinded to group assignment.
Body Composition Assessment
Measurements of fat mass and lean mass of the whole body were performed using dual-energy X-ray absorptiometry (Discovery A [Hologic] scanner or Lunar DPX [General Electric]) scanner, as described previously (19). Measurements of abdominal VAT, and SAT and thigh muscle, SAT, and IMAT volumes were performed using magnetic resonance imaging Magnetom Avanto (Siemens) scanner, as described previously (20–22). Briefly, for abdominal VAT and SAT, 10 serial 10 mm axial images were acquired, beginning at L1 (identified by the origin of the psoas muscle), and moving downward. Baseline and 6-month images were batched and analyzed using HIPPO software (version 1.3; Pisa, Italy) (23). Slice fat volumes were summed to give total abdominal VAT and SAT. For thigh muscle, SAT, and IMAT, 8 serial 10 mm images were acquired just superior to the patella. Baseline and 6-month images were batched and analyzed using Analyze Direct software (version 10.0; Mayo Clinic, Rochester, MN) (21,22), which distinguishes muscle from adipose tissue based on pixel brightness. IMAT was separated from SAT by drawing a line along the muscle fascia (epimysium) on the MR image. Cross-sectional areas for each slice were multiplied by slice thickness to obtain volume data. All thigh muscle and adipose tissue volumes are reported as the sum of the right and left thighs. The assessor of MRI images was blinded to group assignment.
Oral Glucose Tolerance Test
Two-hour, 75-g oral glucose tolerance tests (OGTTs) were performed in the morning after a 12-hour fast. Participants were advised to refrain from exercise for at least 48 hours before the OGTT. Blood samples were taken immediately prior to the glucose load and every 30 minutes after ingestion of glucose beverage. Plasma glucose was determined using the glucose oxidase method (YSI Stat Plus; YSI, Yellow Springs, OH). Plasma insulin was measured with chemiluminescent immunoassay (Siemens Immulite, Malvern, PA). Insulin sensitivity index (ISI) was calculated according to Matsuda and DeFronzo (24) based on all measures of glucose and insulin made during the OGTT. This index has been validated against the gold standard of hyperinsulinemic–euglycemic clamp technique (24). Total areas under the curve (AUC) for glucose and insulin were calculated by using the trapezoid method.
Muscle Strength Testing
Knee extension and knee flexion strength were evaluated using a Biodex System 3 dynamometer (Shirley, NY) while participants were seated with their back supported and hips placed at 120o as previously described (25). All tests were performed on the right leg. Tests were performed at an angular velocity of 60°/second. The best of 3 maximal voluntary efforts was used as the measure of muscle strength.
Physical Function
The 9-item Modified PPT was used to assess the degree of physical frailty as previously described (16,17,19). Peak oxygen consumption (VO2peak) was assessed during graded treadmill walking by indirect calorimetry (True Max 2400; Parvo Medics), as previously described (19). Fast gait speed was assessed by the time needed to walk 25 feet (16).
Statistical Analysis
The same statistical methodologies used in the parent RCT were applied. Briefly, intention-to-treat analyses were performed with SAS software (SAS version 9.4, Cary, NC) by analyzing data from all participants originally randomized. Baseline characteristics were compared by using analyses of variance or Fisher’s exact test. Longitudinal changes between groups were tested with the use of mixed-model repeated-measures analyses of covariance. Change from baseline was used as the dependent variable with group, visit, and group × visit as independent effects and baseline values and sex as covariates. The primary focus of these analyses was on the significance of the interaction between group and time point. Within the framework of the mixed model, when the overall p value for the interaction was less than .05, prespecified contrast statements were used to test 5 hypotheses: that changes in the AEX group would differ from those in the CON group, that changes in the REX group would differ from those in the CON group, that changes in the AEX group would differ from those in the REX group, and that changes in the COMB group would differ from those in the AEX group and from those in the REX group. Analysis testing for within-group changes was also performed using mixed-model repeated-measures analyses of variance. Partial correlation analyses (adjusting for sex) were performed to examine relations between changes in IMAT and VAT and changes in indicators of physical and metabolic function.
Sensitivity analyses that validated the statistical results obtained included multiple imputation for missing fitness data (which confirmed the same pattern of results). The SAS procedures computed 50 complete databases by replacing the missing values with randomly assigned values based on the mean and covariance structure estimated from the original database. The results computed from the 50 imputations (databases) were then combined to obtain one set of valid estimates and p values. Additional analyses included logistic regression that verified that data were consistent with an assumption that data were missing at random. Data are presented as least-square means (SE) unless otherwise specified. P values of less than .05 were considered to indicate statistical significance.
Results
The Consolidated Standards of Reporting Trials diagram has been reported previously (16). Briefly, 160 participants were randomized and 141 (88%) completed the study. Nineteen participants discontinued the intervention (4 CON, 5 AEX, 5 REX, and 5 in COMB) due to personal or medical reasons and were included in the intention-to-treat analyses. The mean ± SD age of participants was 70 ± 5 years. Just below 4% of participants identified as Black and 31% identified as Hispanic or Latino. Baseline characteristics of the study participants are presented in Table 1. There were no appreciable differences in baseline characteristics among any of the groups.
Table 1.
Baseline Characteristics of the Participants
| Characteristic | CON (n = 40) | AEX (n = 40) | REX (n = 40) | COMB (n = 40) |
|---|---|---|---|---|
| Age, years | 70 ± 5 | 70 ± 4 | 70 ± 5 | 70 ± 5 |
| Sex, number (%) | ||||
| Male | 12 (30) | 14 (35) | 15 (37) | 16 (40) |
| Female | 28 (70) | 26 (65) | 25 (63) | 24 (60) |
| Race, number (%)* | ||||
| White | 36 (90) | 36 (90) | 33 (83) | 35 (88) |
| Black | 1 (3) | 0 (0) | 3 (7) | 2 (5) |
| Other | 3 (7) | 4 (10) | 4 (10) | 3 (7) |
| Ethnicity, number (%)* | ||||
| Hispanic or Latino | 13 (33) | 13 (33) | 12 (30) | 12 (30) |
| Not Hispanic or Latino | 27 (67) | 27 (67) | 27 (67) | 28 (70) |
| Unknown | 0 (0) | 0 (0) | 1 (3) | 0 (0) |
| PPT score | 28.6 ± 3.0 | 29.3 ± 2.0 | 28.8 ± 2.3 | 27.9 ± 2.6 |
| VO2peak (mL/kg/min) | 17.0 ± 3.5 | 17.6 ± 3.0 | 17.0 ± 3.6 | 17.2 ± 3.9 |
| Weight (kg) | 97.9 ± 18.5 | 96.9 ± 15.0 | 101.8 ± 18.5 | 99.0 ± 18.1 |
| Height (cm) | 163.4 ± 11.6 | 164.8 ± 15.3 | 166.5 ± 11.9 | 165.6 ± 9.2 |
| Body mass index (kg/m2) | 36.7 ± 5.0 | 35.9 ± 4.4 | 36.7 ± 5.8 | 35.8 ± 4.5 |
| Fat mass (kg) | 43.0 ± 9.4 | 41.9 ± 8.0 | 44.3 ± 9.3 | 42.5 ± 10.3 |
| Lean body mass (kg) | 54.9 ± 14.5 | 55.0 ± 12.0 | 58.1 ± 14.8 | 56.5 ± 1.16 |
| Chronic diseases (no.)† | 3.2 ± 3.4 | 2.6 ± 2.7 | 3.6 ± 3.9 | 2.7 ± 2.0 |
| Chronic medications (no.)‡ | 5.5 ± 4.3 | 5.7 ± 3.4 | 6.2 ± 5.7 | 5.7 ± 4.0 |
Notes: Groups: CON = control; AEX = weight management and aerobic training; REX = weight management and resistance training; COMB = weight management and combined aerobic and resistance training; PPT = Modified Physical Performance Test; VO2peak = peak oxygen consumption. Plus–minus values are means ± SD. There were no significant differences among groups (all p > .05).
*Race and ethnic group were reported by participants.
†Chronic diseases included hypertension, diabetes, coronary artery disease, congestive heart failure, arthritis, and chronic lung disease.
‡Routine medications included antihypertensive, antidiabetic, antidyspeptic, antianginal, diuretics, antiarthritic, antilipidemic, and antidepressants.
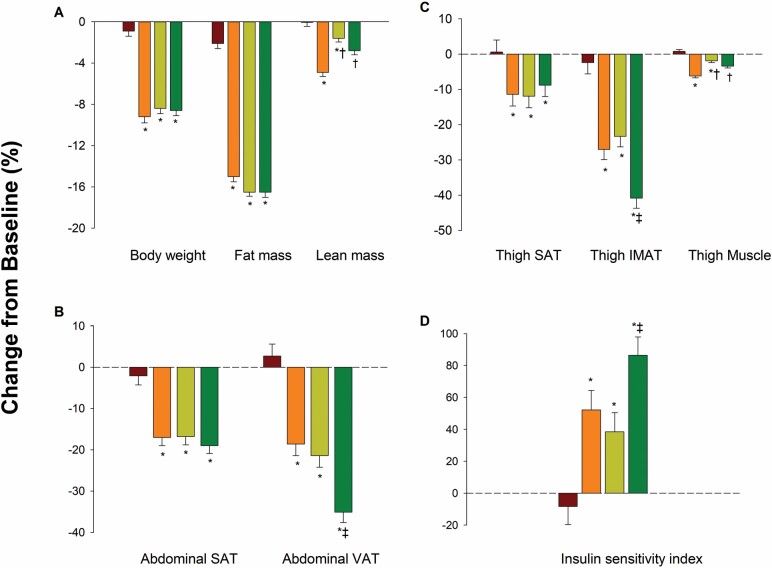
As reported, body weight decreased similarly in the AEX group (−9.0 ± 0.6 kg [9% decrease]), REX group (−8.5 ± 0.5 kg [9% decrease]), and COMB group (−8.5 ± 0.5 kg [9% decrease]) but not in the CON group (Figure 1A) (16). However, despite equal weight loss, lean body mass decreased less in the REX group (−1.0 ± 0.3 kg [2% decrease]) and COMB group (−1.7 ± 0.3 kg [3% decrease]) than in the AEX group (−2.7 ± 0.3 kg [5% decrease]) group. The score in the Modified PPT increased more in the COMB group (5.5 ± 0.5 points [21% increase]) than in the AEX group (3.9 ± 0.4 points [14% increase]) and REX group (3.9 ± 0.4 points [14% increase]), while VO2peak increased more in the COMB group (3.1 ± 0.3 points [17% increase]) and AEX group (3.3 ± 0.3 points [18% increase]) than in the REX group (1.3 ± 0.3 points [8% increase]) (16).
Figure 1.
Mean percentage changes in body weight and composition (A), abdominal subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) volumes (B), thigh SAT, thigh intermuscular adipose tissue (IMAT), and thigh muscle volumes (C), and insulin sensitivity index (D) in the control (red bars), aerobic (orange bars), resistance (olive green bars), and combination (darker green bars) groups. Percentage changes are presented as least-squares adjusted means; T bars indicate standard errors. The asterisk indicates p < .05 for the comparison with the control group, the dagger p < .05 for the comparison with the aerobic group, and the double dagger p < .05 for the comparison with the resistance group. Error bars represent the least-squares adjusted SE from repeated-measures analysis of variance.
Abdominal VAT decreased more in the COMB group (−507 ± 40 cm3 [36% decrease]) than in the AEX group (−269 ± 44 cm3 [19% decrease]) and REX group (−308 ± 44 cm3 [21% decrease]), while losses of abdominal SAT did not significantly differ among the 3 exercise groups (Table 2 and Figure 1B). Likewise, thigh IMAT was reduced more in the COMB group (−172 ± 13 cm3 [41% decrease]) than in the AEX group (−105 ± 13 cm3 [28% decrease]) and REX group (−101 ± 14 cm3 [23% decrease]), while losses of thigh SAT did not significantly differ among the 3 exercise groups (Table 2 and Figure 1C). VAT and IMAT decreased in all exercise groups more than in the CON group. Thigh muscle volume decreased less in the REX group (−23 ± 7 cm3 [2% decrease]) and COMB group (−40 ± 7 cm3 [3% decrease]) than in the AEX group (−77 ± 7 cm3 [6% decrease]).
Table 2.
Effect of Specific Exercise Modes Added to Diet-induced Weight Loss on Abdominal Fat, Thigh Fat and Muscle, and Physical Function
| p Value* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Variables | CON | AEX | REX | COMB | Group–Time Interaction | AEX vs CON | REX vs CON | AEX vs REX | COMB vs AEX | COMB vs REX |
| Abdominal VAT (cm3) | ||||||||||
| Baseline | 1347 ± 106 | 1443 ± 131 | 1466 ± 93 | 1405 ± 97 | ||||||
| Change at 6 months | 38 ± 45 | −269 ± 44† | −308 ± 44† | −507 ± 40† | <.001 | <.001 | <.001 | .62 | .003 | .01 |
| Abdominal SAT (cm3) | ||||||||||
| Baseline | 3639 ± 208 | 3505 ± 215 | 3456 ± 242 | 3302 ± 163 | ||||||
| Change at 6 months | −78 ± 77 | −596 ± 73† | −598 ± 75† | −633 ± 67† | <.001 | <.001 | <.001 | .97 | .89 | .92 |
| Thigh IMAT (cm3) | ||||||||||
| Baseline | 375 ± 27 | 372 ± 23 | 433 ± 30 | 422 ± 31 | ||||||
| Change at 6 months | −9 ± 14 | −105 ± 13† | −101 ± 14† | −172 ± 13† | <.001 | .001 | <.001 | .82 | .007 | .01 |
| Thigh SAT (cm3) | ||||||||||
| Baseline | 1398 ± 118 | 1328 ± 92 | 1415 ± 99 | 1362 ± 109 | ||||||
| Change at 6 months | 3 ± 32 | −149 ± 32‡ | −167 ± 31† | −132 ± 30‡ | .03 | .02 | .01 | .71 | .80 | .53 |
| Thigh muscle (cm3) | ||||||||||
| Baseline | 1302 ± 63 | 1234 ± 62 | 1190 ± 48 | 1186 ± 66 | ||||||
| Change at 6 months | 10 ± 7 | −77 ± 7† | −23 ± 7‡ | −40 ± 7† | <.001 | <.001 | .008 | <.001 | .005 | .21 |
| Knee extension (ft-lb) | ||||||||||
| Baseline | 68 ± 5 | 66 ± 4 | 66 ± 4 | 71 ± 4 | ||||||
| Change at 6 months | −1 ± 2 | −4 ± 2 | 13 ± 2‡ | 12 ± 2† | <.001 | .42 | <.001 | <.001 | <.001 | .70 |
| Knee flexion (ft-lb) | ||||||||||
| Baseline | 43 ± 3 | 41 ± 2 | 44 ± 3 | 44 ± 3 | ||||||
| Change at 6 months | −1 ± 1 | −1 ± 1 | 6 ± 1‡ | 7 ± 1† | <.001 | .84 | .001 | .002 | .001 | .81 |
| Gait speed (m/min) | ||||||||||
| Baseline | 75.0 ± 2.6 | 74.6 ± 2.0 | 74.3 ± 2.3 | 68.8 ± 2.2 | ||||||
| Change at 6 months | −0.5 ± 1.3 | 8.1 ± 1.3† | 9.3 ± 1.3† | 12.1 ± 1.3† | <.001 | <.001 | <.001 | .56 | .03 | .09 |
Notes: Groups: CON = control; AEX = weight management and aerobic training; REX = weight management and resistance training; COMB = weight management and combined aerobic and resistance training; VAT = visceral adipose tissue; SAT = subcutaneous adipose tissue; IMAT = intermuscular adipose tissue. Plus–minus values for the change scores are the least-squares adjusted means ± SE from the repeated-measures analyses of variance; plus–minus values for the baseline values are the observed means ± SE.
*p Values for the comparison among the groups of changes from baseline to 6 months were calculated with the use of mixed-model repeated-measures analyses of variance (with baseline values and sex as covariates). Secondary analyses included a comparison between the combination group and the control group; all p values were less than .05.
† p < .001 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
‡ p < .01 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
Knee extension strength and knee flexion strength increased in the REX group (13 ± 2 ft lb [20% increase] and 6 ± 1 ft lb [14% increase], respectively) and COMB group (12 ± 2 ft lb [20% increase] and 7 ± 1 ft lb [16% increase], respectively), whereas they did not change in the AEX group (Table 2). Gait speed improved more in the COMB group (12.1 ± 1.3 m/min [17% increase]) than in the AEX group (8.1 ± 1.3 m/min [11% increase]).
The ISI increased more in the COMB group (1.9 ± 0.2 [86% increase]) than in the AEX group (1.2 ± 0.2 [28% increase]) and REX group (1.0 ± 0.2 [39% increase]) (Table 3 and Figure 1D). Improvements in fasting glucose, 2-hour glucose, glucose AUC, and insulin AUC during the OGTT did not differ between the exercise groups, although all changes in glucometabolic profile in each group were significantly different from those in the CON group.
Table 3.
Effect of Specific Exercise Modes Added to Diet-Induced Weight Loss on Oral Glucose Tolerance Variables
| p Value* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Variables | CON | AEX | REX | COMB | Group–Time Interaction | AEX vs CON | REX vs CON | AEX vs REX | COMB vs AEX | COMB vs REX |
| Insulin sensitivity index | ||||||||||
| Baseline | 2.4 ± 0.2 | 2.3 ± 0.2 | 2.6 ± 0.2 | 2.2 ± 0.1 | ||||||
| Change at 6 months | −0.2 ± 0.2 | 1.2 ± 0.2† | 1.0 ± 0.2† | 1.9 ± 0.2† | <.001 | <.001 | <.001 | .57 | .02 | .004 |
| Fasting glucose (mg/dL) | ||||||||||
| Baseline | 99.6 ± 2.7 | 99.6 ± 3.0 | 101.8 ± 2.8 | 99.4 ± 1.9 | ||||||
| Change at 6 months | 2.7 ± 1.4 | −3.3 ± 1.4‡ | −7.0 ± 1.4‡ | −4.8 ± 1.4† | .009 | .04 | .001 | .18 | .67 | .36 |
| 2-h glucose (mg/dL) | ||||||||||
| Baseline | 143.8 ± 9.0 | 159.0 ± 6.2 | 163.0 ± 163.0 | 149.5 ± 9.4 | ||||||
| Change at 6 months | 3.8 ± 4.4 | −18.6 ± 4.7‡ | −25.7 ± 4.8‡ | −28.0 ± 4.5† | .001 | .007 | .001 | .43 | .44 | .98 |
| Glucose AUC (mg/dL per 2 h) | ||||||||||
| Baseline | 18 877 ± 908 | 20 100 ± 562 | 19 806 ± 1073 | 19 190 ± 771 | ||||||
| Change at 6 months | 130 ± 371 | −2240 ± 413† | −3013 ± 401† | −2538 ± 13† | <.001 | .001 | <.001 | .37 | .88 | .45 |
| Insulin AUC (µU/mL per 2 h) | ||||||||||
| Baseline | 13 859 ± 1319 | 13 524 ± 1101 | 12 029 ± 1075 | 13 032 ± 1217 | ||||||
| Change at 6 months | 543 ± 481 | −3355 ± 528† | −2482 ± 512† | −4025 ± 486† | <.001 | .001 | .01 | .70 | .53 | .09 |
Notes: Groups: CON = control; AEX = weight management and aerobic training; REX = weight management and resistance training; COMB = weight management and combined aerobic and resistance training; AUC = area under the curve. Plus–minus values for the change scores are the least-squares adjusted means ± SE from the repeated-measures analyses of variance; plus–minus values for the baseline values are the observed means ± SE.
*p Values for the comparison among the groups of changes from baseline to 6 months were calculated with the use of mixed-model repeated-measures analyses of variance (with baseline values and sex as covariates). Secondary analyses included a comparison between the combination group and the control group; all p values were less than .05.
† p < .001 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
‡ p < .01 for the comparison of the value at the follow-up time with the baseline value within the group, as calculated with the use of mixed-model repeated-measures analysis of variance.
Controlling for sex, changes in thigh IMAT and abdominal VAT were significantly correlated with changes in PPT score, VO2peak, gait speed, and ISI, with ISI demonstrating the strongest correlations with thigh IMAT (r = −0.49) and VAT (r = −0.52; Table 4).
Table 4.
Correlations Between Changes in Intermuscular and Visceral Adipose Tissue With Changes in Physical and Metabolic Function Indicators
| Indicator | Intermuscular Adipose Tissue (cm3) | Visceral Adipose Tissue (cm3) |
|---|---|---|
| PPT score | r = −0.28, p = .02 | r = −0.39, p < .01 |
| VO2peak (mL/kg/min) | r = −0.49, p = .045 | r = −0.52, p < .001 |
| Gait speed (m/min) | r = −0.25, p = .04 | r = −0.36, p < .01 |
| Insulin sensitivity index | r = −0.49, p < .001 | r = −0.52, p < .001 |
Notes: PPT = Modified Physical Performance Test; VO2peak = peak oxygen consumption. Partial correlation analysis was used to control for the effect of sex.
Discussion
Our comparative efficacy RCT in frail adults 65 years and older with obesity indicated that during matched weight loss, aerobic and resistance exercise had independent effects in reducing ectopic fat, such that their combination resulted in additive effects that translated into mitigation of aging- and obesity-related physical and metabolic dysfunction. Specifically, in this at-risk population, we provide evidence that the COMB exercise was superior to either mode independently for reducing IMAT in addition to VAT. These changes were associated with the largest improvements in PPT score, VO2peak, gait speed, and ISI while losing weight. Until recently, the appropriate management of obesity in older adults has been controversial owing to the weight loss-induced reduction of lean body mass that might worsen frailty (18,26). The findings of positive effects on regional body composition in the current RCT reinforce the findings from our previous report that functional impairments or limitations with obesity can now be addressed safely through weight loss plus COMB exercise, so that older adults identified as obese may be considered for such interventions (27). Additionally, the benefits of resistance exercise alone (REX) in attenuating the loss of lean body mass should be noted. This mode of exercise may be a suitable option for those unable to complete aerobic or combined exercise protocols.
There are limited data from RCTs regarding the effect of diet-induced weight loss plus exercise training on IMAT or VAT in older adults with obesity (28–32), none of which has directly compared the independent and combined effects of aerobic and resistance exercise within an RCT. For example, Santanasto et al. (29,32) reported that a 6- and 12-month weight loss plus primarily an aerobic exercise program compared to aerobic exercise alone reduced IMAT by approximately 16% and VAT by approximately 18% which, similar to our results, correlated with improvements in physical function (as assessed by the Short Physical Performance Battery). Manini et al. (30) demonstrated that a 6-month weight loss plus combined walking, resistance, and flexibility program compared to education control reduced IMAT by approximately 6%−8% at the thigh and calf, with the IMAT reduction at the calf associated with faster walking speed. On the other hand, Nicklas et al. (31) showed that while a 6-month resistance training plus weight loss program compared to resistance training alone similarly reduced IMAT by about 6%, the addition of weight loss improved mobility. However, as in most prior studies, the study population was relatively high functioning, which contrasts with the frailer population in the current study. Our results showed about a 20%−40% reduction in IMAT which is a larger reduction than in prior studies likely because of the higher starting levels of IMAT in our frailer, older participants with obesity (PPT score 18–31) (17). It may also be due to the greater weight loss (~10%) achieved by our participants and the additive effects of aerobic and resistance exercise in the COMB group. The novel finding in our head-to-head comparative RCT is that among the methods tested, COMB with weight loss was the most effective in improving regional fat deposition (ie, ectopic fat) and thereby also the most effective in improving physical and metabolic function.
With respect to underlying physiologic mechanisms, we found that changes in IMAT and VAT correlated not only with changes in physical function such as physical performance, VO2peak, and gait speed, but also with changes in metabolic function such as ISI. Older adults who are also obese are particularly susceptible to developing type 2 diabetes (T2D), because of the additive adverse effects of obesity and aging on insulin sensitivity (33). Therefore, the additive effects of REX and AEX in the COMB group in improving ISI are the most likely to reduce the risk, or delay the progression, of T2D (34). Accordingly, we found that OGTT parameters (eg, fasting and 2-h glucose, and glucose and insulin AUCs) also improved in the AEX and REX groups, although without additive effects in the COMB group possibly due to most participants having normal or only mildly impaired glucose tolerance.
It is possible that the improved insulin action contributed to the improved physical function, for example, via an increase in insulin-mediated muscle blood flow (35) and muscle protein synthesis (MPS) (36) as well as improved myocellular quality (37). Indeed, we have recently reported that the combination of aerobic and resistance exercise in this study population was superior to either mode independently for improving MPS and myocellular quality, thereby maintaining muscle mass during weight loss therapy, which was accompanied by preservation of myocyte-enhancing factor, MEF2A (38). However, it is likely that multiple mechanisms may be involved that underlie the largest improvement in physical function associated with the largest reduction in IMAT and VAT in the COMB group including improved mitochondrial function associated with the improved VO2peak (39,40) and decreased local and systemic inflammation (41,42). In this regard, we have reported that the expression of mitochondrial stress regulators (eg, FIS1, LONP1, and AFG3L2) and markers of macrophage infiltration (eg, TLR2, CD68, and CCL2) in skeletal muscles were reduced in the COMB group compared to the other groups (38). Other potential mechanisms, especially given the proximity of IMAT to the muscle fiber, may include increased muscle activation and/or improved muscle fiber orientation with IMAT reduction (43,44), which warrant examination in future studies.
Strengths of our study included the head-to-head comparative RCT design, the comprehensive lifestyle programs and assessments of physical and metabolic outcomes, the high degree of compliance resulting in matched weight loss that allowed for unbiased comparisons across groups, and the well-characterized, unique but increasing population of frail older adults with obesity (19). We used MRI to directly measure changes in IMAT, which has a higher sensitivity than computerized tomography (CT) for identifying fatty replacement in muscle and because it is not density based, provides better anatomical details of soft tissue than CT (45). Potential limitations included that we did not have a weight loss alone group. The decision was made for ethical reasons given that we have shown that weight loss without exercise results in significant bone and muscle loss (46). Moreover, weight loss plus exercise provides greater improvement in physical function than either intervention alone (27); thus, all weight loss interventions in this study were combined with exercise. The principal aim of the parent RCT was to compare the effectiveness of AEX, REX, and COMB in reversing frailty and preserving muscle and bone mass during weight loss in older adults with obesity (16). Another potential limitation was that our study was not large enough to examine potential sex differences in regional body composition changes. We controlled for the effect of sex by including it as a covariate in the mixed-model repeated-measures analyses of covariance and as a variable held constant in the partial correlation analyses. It was once commonly thought that it may be difficult to change ingrained, lifelong diet and activity habits in older adults (47). However, we have found that most participants looked forward to the weekly group meetings and adhered well to the regular exercise sessions, thus embracing lifestyle change (16,27). Nonetheless, a possible limitation is the feasibility of maintaining long-term lifestyle change in the “real world.” We have previously reported that older adults who participated in our lifestyle intervention trial can adhere to lifestyle changes 30 months following a 12-month intervention (48). Findings from other studies also suggest that older adults may be more successful in achieving long-term weight loss than younger adults (49,50). Additional studies are needed to determine long-term adherence and whether the beneficial effects of lifestyle intervention therapy can prevent the institutionalization of older adults with obesity.
In conclusion, our findings demonstrated that in the at-risk population of older adults with obesity, weight loss plus the combination of aerobic and resistance exercise is the most effective in reducing ectopic fat deposition and in improving aging- and obesity-related physical and metabolic complications. These findings suggest that this specific lifestyle approach may be the most useful in helping to maintain functional independence in this population.
Funding
This work was supported by the National Institutes of Health (RO1-AG031176, UL1-TR000041, and P30-DK020579).
Conflict of Interest
None declared.
Acknowledgments
The contents do not represent the views of the US Department of Veterans Affairs or the US Government. We thank the participants for their cooperation, Brandy Martinez and Erik Faria for exercise training, and Ronni Farris and Reed Vawter for weight loss training. We also thank the members of the Alkek Foundation for their support. The findings reported in this article are the result of work supported with resources and the use of facilities at the New Mexico VA Health Care System and Michael E. DeBakey VA Medical Center.
Author Contributions
Study concept and design: D.T.V., R.A.-V., and C.Q.; acquisition and analyses of data: D.T.V., D.L.W., L.A., B.G., D.R.S., K.F., G.G., R.A.V., and C.Q.; drafting of the manuscript: D.T.V. and D.L.W.; critical revision of the manuscript for important intellectual content: D.T.V., D.L.W., L.A., B.G., D.R.S., K.F., G.G., R.A.V., and C.Q.; statistical analyses: C.Q. and D.T.V.; administrative, technical, and material support: D.T.V., D.L.W., L.A., B.G., D.R.S., K.F., G.G., R.A.V., and C.Q.
References
- 1. Waters DL. Intermuscular adipose tissue: a brief review of etiology, association with physical function and weight loss in older adults. Ann Geriatr Med Res. 2019;23:3–8. doi: 10.4235/agmr.19.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757–767. doi: 10.1016/s0531-5565(02)00014-1 [DOI] [PubMed] [Google Scholar]
- 3. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x [DOI] [PubMed] [Google Scholar]
- 4. Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344. doi: 10.2522/ptj.20080079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arsenault BJ, Lachance D, Lemieux I, et al. Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med. 2007;167:1518–1525. doi: 10.1001/archinte.167.14.1518 [DOI] [PubMed] [Google Scholar]
- 6. Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res. 2012;2012:629637. doi: 10.1155/2012/629637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy RA, Reinders I, Register TC, et al. Associations of BMI and adipose tissue area and density with incident mobility limitation and poor performance in older adults. Am J Clin Nutr. 2014;99:1059–1065. doi: 10.3945/ajcn.113.080796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985). 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 9. Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res. 2012;2012:172957. doi: 10.1155/2012/172957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 11. Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond). 2007;31:1400–1405. doi: 10.1038/sj.ijo.0803621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubé MC, Lemieux S, Piché ME, et al. The contribution of visceral adiposity and mid-thigh fat-rich muscle to the metabolic profile in postmenopausal women. Obesity (Silver Spring). 2011;19:953–959. doi: 10.1038/oby.2010.348 [DOI] [PubMed] [Google Scholar]
- 13. Durheim MT, Slentz CA, Bateman LA, Mabe SK, Kraus WE. Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am J Physiol Endocrinol Metab. 2008;295:E407–E412. doi: 10.1152/ajpendo.90397.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koster A, Stenholm S, Alley DE, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring). 2010;18:2354–2361. doi: 10.1038/oby.2010.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goss AM, Gower BA. Insulin sensitivity is associated with thigh adipose tissue distribution in healthy postmenopausal women. Metabolism. 2012;61:1817–1823. doi: 10.1016/j.metabol.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376:1943–1955. doi: 10.1056/NEJMoa1616338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–M355. doi: 10.1093/gerona/55.6.m350 [DOI] [PubMed] [Google Scholar]
- 18. Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923 [DOI] [PubMed] [Google Scholar]
- 19. Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111 [DOI] [PubMed] [Google Scholar]
- 20. Arif H, Racette SB, Villareal DT, Holloszy JO, Weiss EP. Comparison of methods for assessing abdominal adipose tissue from magnetic resonance images. Obesity (Silver Spring). 2007;15:2240–2244. doi: 10.1038/oby.2007.266 [DOI] [PubMed] [Google Scholar]
- 21. Murphy JC, McDaniel JL, Mora K, Villareal DT, Fontana L, Weiss EP. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. J Appl Physiol (1985). 2012;112:79–85. doi: 10.1152/japplphysiol.00355.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol (1985). 2007;102:634–640. doi: 10.1152/japplphysiol.00853.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lancaster JL, Ghiatas AA, Alyassin A, Kilcoyne RF, Bonora E, DeFronzo RA. Measurement of abdominal fat with T1-weighted MR images. J Magn Reson Imaging. 1991;1:363–369. doi: 10.1002/jmri.1880010315 [DOI] [PubMed] [Google Scholar]
- 24. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 25. Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006;291:E1003–E1008. doi: 10.1152/ajpendo.00100.2006 [DOI] [PubMed] [Google Scholar]
- 26. Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: a review of the controversy. Exp Gerontol. 2013;48:1054–1061. doi: 10.1016/j.exger.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prior SJ, Joseph LJ, Brandauer J, Katzel LI, Hagberg JM, Ryan AS. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J Clin Endocrinol Metab. 2007;92:880–886. doi: 10.1210/jc.2006-2113 [DOI] [PubMed] [Google Scholar]
- 29. Santanasto AJ, Glynn NW, Newman MA, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011;1–10.doi: 10.1155/2011/516576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manini TM, Buford TW, Lott DJ, et al. Effect of dietary restriction and exercise on lower extremity tissue compartments in obese, older women: a pilot study. J Gerontol A Biol Sci Med Sci. 2014;69:101–108. doi: 10.1093/gerona/gls337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101:991–999. doi: 10.3945/ajcn.114.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santanasto AJ, Newman AB, Strotmeyer ES, Boudreau RM, Goodpaster BH, Glynn NW. Effects of changes in regional body composition on physical function in older adults: a pilot randomized controlled trial. J Nutr Health Aging. 2015;19:913–921. doi: 10.1007/s12603-015-0523-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738 [DOI] [PubMed] [Google Scholar]
- 34. Kahn SE, Larson VG, Beard JC, et al. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol. 1990;258(6 Pt 1):E937–E943. doi: 10.1152/ajpendo.1990.258.6.E937 [DOI] [PubMed] [Google Scholar]
- 35. Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–E754. doi: 10.1152/ajpendo.00271.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring). 2011;19:312–318. doi: 10.1038/oby.2010.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colleluori G, Aguirre L, Phadnis U, et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab. 2019;30:261–273.e6. doi: 10.1016/j.cmet.2019.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huffman KM, Koves TR, Hubal MJ, et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia. 2014;57:2282–2295. doi: 10.1007/s00125-014-3343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zoico E, Rossi A, Di Francesco V, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65:295–299. doi: 10.1093/gerona/glp155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22:1004–1007. doi: 10.1016/j.orthres.2004.02.009 [DOI] [PubMed] [Google Scholar]
- 44. Yoshida Y, Marcus RL, Lastayo PC. Intramuscular adipose tissue and central activation in older adults. Muscle Nerve. 2012;46:813–816. doi: 10.1002/mus.23506 [DOI] [PubMed] [Google Scholar]
- 45. Lemos T, Gallagher D. Current body composition measurement techniques. Curr Opin Endocrinol Diabetes Obes. 2017;24:310–314. doi: 10.1097/MED.0000000000000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–2859. doi: 10.1002/jbmr.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elia M. Obesity in the elderly. Obes Res. 2001;9(Suppl. 4):244S–248S. doi: 10.1038/oby.2001.126 [DOI] [PubMed] [Google Scholar]
- 48. Waters DL, Vawter R, Qualls C, Chode S, Armamento-Villareal R, Villareal DT. Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J Nutr Health Aging. 2013;17:3–7. doi: 10.1007/s12603-012-0421-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Svetkey LP, Clark JM, Funk K, et al. Greater weight loss with increasing age in the weight loss maintenance trial. Obesity (Silver Spring). 2014;22:39–44. doi: 10.1002/oby.20506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Apolzan JW, Venditti EM, Edelstein SL, et al. Long-term weight loss with metformin or lifestyle intervention in the diabetes prevention program outcomes study. Ann Intern Med. 2019;170:682–690. doi: 10.7326/M18-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]