Abstract
Aerobic exercise is a promising intervention to attenuate frailty, but preclinical studies have used only male animals. We investigated the impact of voluntary aerobic exercise on frailty, biological age (FRailty Inferred Geriatric Health Timeline [FRIGHT] clock), predicted life expectancy (Analysis of FRAIlty and Death [AFRAID] clock), and mortality in both sexes and determined whether exercise was associated with changes in inflammation. Older (21–23 months) male (n = 12) and female (n = 22) C57Bl/6 mice matched for baseline frailty scores were randomized into exercise (running wheel) and sedentary (no wheel) groups. Frailty index scores were measured biweekly (13 weeks), and 23 serum cytokines were measured at midpoint and end point. Exercise levels varied between mice but not between the sexes. Exercise had no effect on mortality, but it attenuated the development of frailty in both sexes (female = 0.32 ± 0.04 vs 0.21 ± 0.01; p = .005; male = 0.30 ± 0.02 vs 0.22 ± 0.02; p = .042) and reduced frailty in older females after 10 weeks. FRIGHT scores were unaffected by exercise but increased with time in sedentary males indicating increased biological age. Exercise prevented the age-associated decline in AFRAID scores in older females such that exercised females had a longer life expectancy. We investigated whether aerobic exercise was associated with changes in systemic inflammation. Cytokine levels were not affected by exercise in males, but levels of pro-inflammatory cytokines were positively correlated with the frequency of exercise in females. Despite increases in systemic inflammation, exercise reduced frailty and increased life span in older females. Thus, voluntary aerobic exercise, even late in life, has beneficial effects on health in both sexes but may be especially helpful in older females.
Keywords: Biological age, Inflammation, Life expectancy, Sex differences
Frailty is a state of elevated risk for negative health outcomes that can be quantified in many ways (1). One common clinical approach creates a frailty index (FI) that measures health-deficit accumulation (2). This concept has been reverse-translated for use in preclinical models (3), where scores from mice and humans exhibit similarities including higher FI scores in females than males (4,5). Mouse FI data have been used to create FRailty Inferred Geriatric Health Timeline (FRIGHT) and Analysis of FRAIlty and Death (AFRAID) clocks to estimate biological age and life expectancy, respectively (6). Together, this provides a powerful translational approach to explore the biology of frailty.
The FI can evaluate the efficacy of interventions, like aerobic exercise, on the degree of frailty. The limited data from older humans suggest aerobic exercise can reverse frailty (7–9). Still, little is known about effects of exercise on frailty, especially in women who are underrepresented in exercise research (10). This sex bias has translated to preclinical work. Studies of aerobic exercise and frailty in aging rodents have typically used only males (11,12), although short-term higher intensity exercise has benefits in both sexes (13–15). Thus, effects of aerobic exercise on frailty in older females are unclear; whether exercise can affect FRIGHT or AFRAID scores has not been investigated in either sex.
Chronic inflammation is implicated in the pathogenesis of frailty (16). Indeed, serum levels of pro-inflammatory cytokines increase with frailty in mice and this differs between the sexes (5). Regular exercise can positively affect chronic inflammation, although its impact varies based on age, sex, exercise intensity, and intervention length (17–19). Whether aerobic exercise may reduce frailty, in part by modifying age-related inflammation, is unclear.
Our objectives were to determine, in both sexes, whether: (i) voluntary aerobic exercise attenuated frailty in older mice; (ii) aerobic exercise affected biological age and predicted life expectancy assessed with FRIGHT and AFRAID clocks; (iii) effects of exercise were associated with changes in inflammation.
Method
Animals and Exercise
Male (n = 12) and female (n = 22) C57Bl/6 mice (Charles River, St. Constant, QC, Canada) were aged in individually ventilated cages in the Dalhousie Animal Care Facility (12-hour light–dark cycle, 21°C, 35% humidity) until ages 21–23 months. They were then transferred to individual cages with or without a running wheel (Clocklab, ActiMetrics, model: ACT-551-MS-SS: circumference 34.56 cm) and voluntary running was tracked as wheel rotations. Twenty-four-hour activity data were collected on a computer and analyzed with Clocklab Analysis software version 6. Animals had free access to food (ProLab RMH3000, LabDiet, St. Louis, MO) and water throughout. Experiments were approved by the Dalhousie University Committee on Laboratory Animals and followed Canadian Council on Animal Care guidelines.
Frailty Scoring
Frailty was assessed weekly with the mouse FI, as described (3). Briefly, mice were placed in a clean cage in a quiet room to acclimatize (5 minutes) and then weighed and body surface temperature was measured. They were scored for health deficits, where 0 = no deficit, 0.5 = mild deficit, and 1 = severe deficit. For body weight and body surface temperature, deficits were scored between 0 and 1 based on deviation from group means (3). Values for each deficit were summed and divided by 31 to produce an FI score. Mice were randomly assigned to exercise or sedentary groups based on similar FI scores. FRIGHT and AFRAID scores were calculated from FI items as described (6).
Cytokines
Mice were moved to cages without running wheels for at least 2 hours before blood was collected (facial vein at midpoint or the aorta at end point). After blood clotted (30 minutes, room temperature), it was centrifuged (211 G, 4°C, 12 minutes) and the supernatant (serum) collected and frozen (−20°C). Serum cytokines were analyzed with a bead-based multiplex assay (BioRad, M60009RDPD, Hercules, CA). Cytokine concentrations below the limit of detection (LOD) were replaced with LOD/2 as this imparts a smaller bias than the LOD (20). Of note, 16 of 23 cytokines (70%) required no substitutions, 5 required 1 substitution, and 2 (interleukin [IL]-2, IL-1β) required slightly more.
Statistics
Data were expressed as mean ± SEM unless otherwise stated. Analyses were done with SigmaPlot (version 14, Systat Software, Erkrath, Germany) or Prism (version 8.3, GraphPad Software, San Diego, CA). Frailty data were analyzed with a mixed-effects model comparing sedentary and exercised mice by sex over time (Fisher’s least significant difference post hoc test); this model accounts for missing data due to mortality. The proportion of mice with deficits was analyzed with Fisher’s Exact Test. Frailty data from only those mice that survived the entire study were also analyzed separately. Mortality data were analyzed with a Log-rank test. “Bouts” of exercise represent >5 wheel rotations separated by less than a 5-minute gap and were analyzed with a t test. Relationships between cytokines and exercise were analyzed with Pearson’s correlation; in these correlations, each data point represents an individual mouse. P < .05 was considered statistically significant.
Results
Exercise
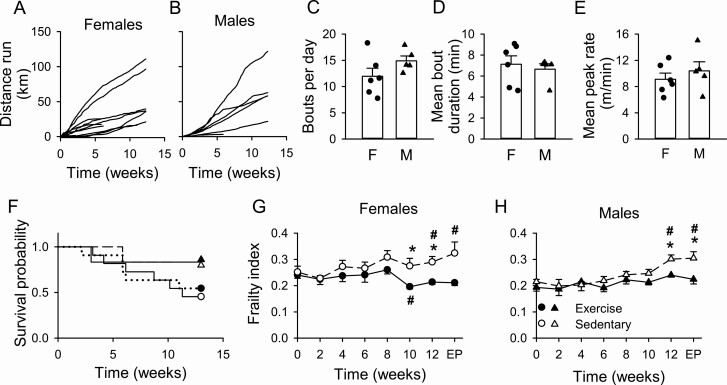
Exercise levels in aged male and female mice demonstrated marked interindividual variation in cumulative distance run (Figure 1A and B) with no sex differences (p = .34). We also compared periodic stretches of exercise (“bouts”) in exercised mice. The number of bouts/day (Figure 1C, p = .14), mean bout duration (Figure 1D, p = .63), and average peak speed (Figure 1E, p = .47) were similar in both sexes. Thus, exercise levels were similar in both sexes in these older mice.
Figure 1.
Voluntary exercise had no impact on mortality but reduced frailty in aging male and female mice. Cumulative distance run for females (A, n = 11) and males (B, n = 6) illustrates heterogeneity in the total distance run over the course of the study in both sexes. Mean ± SEM exercise parameters including bouts/day (C), bout duration (D), and peak rate (E) were similar in both sexes (n = 6 female and 5 male mice; analyzed with a Student’s t test). Exercise did not affect the probability of survival assessed with a Log-rank test regardless of sex (F). Voluntary aerobic exercise attenuated the development of frailty in females (G, exercise n = 11–6, sedentary n = 11–5) and males (H, exercise n = 6–5, sedentary n = 6–5). Group differences were evaluated with a mixed-effects model. (F = female, M = male, EP = end point; * denotes significant difference between sedentary and exercise groups; # denotes significant effect of time; p < .05).
Mortality and Frailty
We investigated whether voluntary exercise affected mortality. Interestingly, mortality was similar in sedentary and exercised mice (females: p = .77; males: p = .95). Mortality was also similar when all 4 groups were considered together (Figure 1F, p = .38). We next explored whether voluntary exercise modified frailty in aging mice. FI scores increased over time in sedentary mice in both sexes and exercise prevented this increase (Figure 1G and H); this became statistically significant after 10 weeks in females and 12 weeks in males. In addition, exercise reduced frailty in females, as FI scores were lower than baseline after 10 weeks (Figure 1G). This effect was not seen in males (Figure 1H). Baseline frailty scores also were higher in females than males (p = .03). However, this sex difference did not persist as there were no end point sex differences for either sedentary or exercised mice (Figure 1G and H). As some mice died during this study, we completed a separate analysis of only mice that survived and found similar effects of exercise on frailty (Supplementary Figure 1). FI scores were not correlated with exercise volume (Supplementary Table 1). Thus, although voluntary exercise did not affect mortality, it attenuated the development of frailty in both sexes, and reduced frailty in older females.
We assessed whether the impact of exercise on frailty was dominated by effects on a few deficits, or whether it was apparent across many deficits. Supplementary Figure 2 shows the proportion of mice with specific deficits at baseline and at end point for all mice in this study. Only a few deficits showed significant differences between groups, and only in females (Supplementary Figure 2). There were no significant differences in individual deficits in males (Supplementary Figure 2). When we considered only mice that survived, we found virtually identical results (Supplementary Figure 3). Thus, cumulative effects of many small changes in a range of health deficits resulted in lower frailty scores in the exercised animals when compared to sedentary controls.
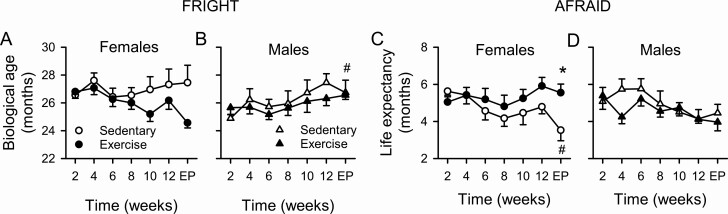
Frailty Clocks
In aging mice of both sexes, we explored effects of voluntary exercise on biological age (FRIGHT clock; Figure 2A and B) and predicted life span (AFRAID clock; Figure 2C and D). FRIGHT scores declined over time in exercised females compared to sedentary females, although this was not statistically significant (Figure 2A). In contrast, FRIGHT scores increased with time in males, and this was statistically significant for sedentary mice at the end point (Figure 2B). AFRAID scores declined in sedentary but not exercised females, with sedentary females exhibiting a shorter life expectancy than exercised mice at end point (Figure 2C). In contrast, AFRAID scores were not affected by time or by exercise in aging males (Figure 2D). FRIGHT and AFRAID scores were not correlated with exercise volume (Supplementary Table 1). Together these findings indicate both that exercise increased predicted life expectancy in aging females but not in males and that biological age increased in sedentary males.
Figure 2.
Impact of voluntary exercise on FRailty Inferred Geriatric Health Timeline (FRIGHT) and Analysis of FRAIlty and Death (AFRAID) scores in aging male and female mice. FRIGHT scores (predicted biological age) declined over time in exercised compared to sedentary females, although this was not statistically significant (A). By contrast, FRIGHT scores increased with time in males and this effect was statistically significant for sedentary mice at the end point (B). AFRAID scores (estimated life expectancy) showed a significant decline with age in females (C), an effect that was not seen in males (D). Group differences were evaluated with a mixed-effects model. EP = end point. (* denotes significant difference between sedentary and exercise groups; # denotes significant effect of time; p < .05).
Cytokines
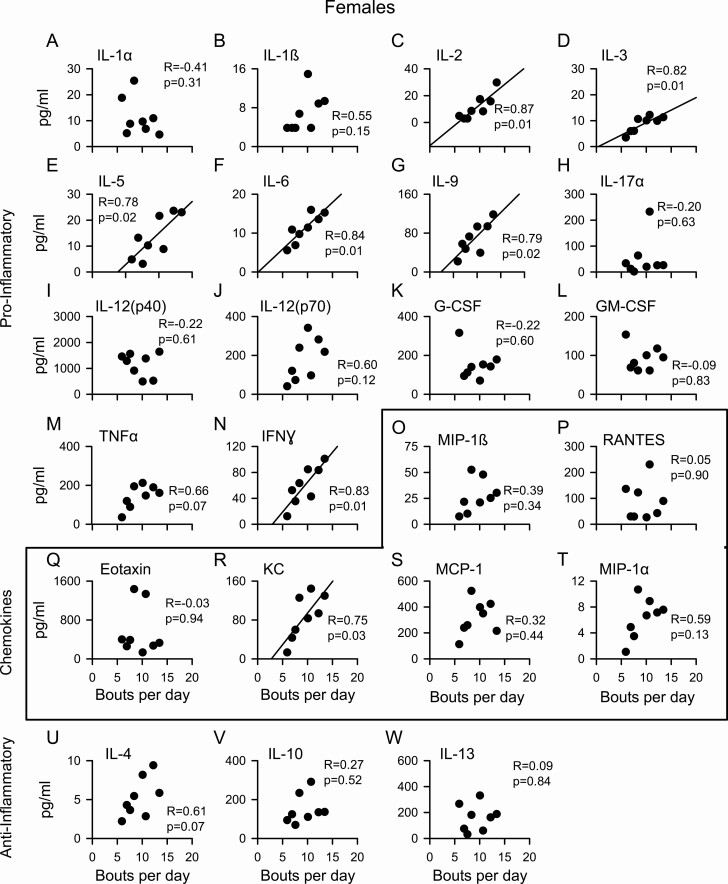
We next investigated whether there was a relationship between serum cytokines and volume of exercise performed. Cytokine concentrations were plotted as a function of bouts/day and fitted with linear regression. Figure 3 shows a clear positive linear relationship between many cytokine levels and the volume of exercise performed in aging females (Figure 3A–W). Levels of pro-inflammatory cytokines (IL-2, IL-3, IL-5, IL-6, IL-9, interferon γ) and a chemokine (KC) increased as the volume of exercise increased in females (Figure 3). By contrast, there was no relationship between serum cytokines and exercise volume in aging male mice (Supplementary Figure 4A–W). Thus, aerobic exercise was associated with high levels of pro-inflammatory cytokines in aging females, a relationship that was not seen in males.
Figure 3.
Cytokine levels were graded by exercise volume in aging female mice. Serum cytokines (n = 14) were categorized into pro-inflammatory cytokines (A–N), chemokines (O–T), and anti-inflammatory cytokines (Q–W). Values were plotted as a function of exercise (bouts/day), using the average bouts/day for the 5 previous days before serum collection. Data were analyzed with a Pearson’s correlation and a linear regression line was drawn only if p < .05.
Discussion
This study investigated whether aerobic exercise affected frailty, biological age, and/or life expectancy in older mice of both sexes, and whether effects of exercise were associated with changes in inflammation. We found that, despite interindividual variation in overall distances, there were no sex differences in the volume of exercise performed. Exercise had no impact on mortality, but it attenuated the development of frailty in both sexes and reduced FI scores in older females. FRIGHT scores (biological age) were not affected by exercise although they increased over time in sedentary males. AFRAID scores showed that exercised females, but not males, had a higher life expectancy than sedentary controls. Cytokine levels were not affected by aerobic exercise in males, but levels of pro-inflammatory cytokines increased as exercise volume increased in females. These findings suggest that aerobic exercise is beneficial in both sexes, even when started late in life, and may be particularly beneficial in older females. Despite increased chronic inflammation in older exercised females, frailty was substantially lower in this group than in sedentary controls.
Prior studies of the influence of voluntary exercise on frailty in preclinical models reported beneficial effects of exercise on frailty/health span, but have used only male animals (11,12,21). A major novel finding in our study is that voluntary exercise, even when introduced late in life, prevented the development of frailty in both sexes and reduced frailty in older females with no impact on mortality. This observation suggests that exercised mice in our study died healthier than sedentary controls, an attractive idea that is gaining traction in the clinical literature (22). We also demonstrated that aerobic exercise reduced frailty levels in females, an effect not seen in males. As human and animal studies (including this study) show that females have higher frailty levels than males (4,5,23), it is critically important to investigate strategies to mitigate frailty in older women. Our data strongly suggest that aerobic exercise is a powerful intervention to prevent the development of frailty and even reduce preexisting frailty in older females and it can be initiated in late life to great benefit.
FI scores from male mice have been used to create FRIGHT and AFRAID clocks to estimate biological age and life expectancy, respectively (6). These clocks are responsive to pharmacological and dietary interventions in aging male mice (6). Here we show, for the first time, that these clocks can detect important effects in females and they can be used to assess effects of an exercise intervention in both sexes. Indeed, we found that FRIGHT and AFRAID clocks can identify sex-specific differences in effects of exercise, with exercise increasing life expectancy in females and sedentary behavior increasing biological age in males. It is now clear that these clocks can add value to studies of interventions to modify frailty in both sexes.
Chronic systemic inflammation plays a key role in the pathogenesis of frailty (16). As aerobic exercise can reduce pro-inflammatory cytokines in younger humans and animals (24), a new clinical trial is exploring whether exercise reduces inflammation associated with frailty (25). Conversely, while aerobic exercise reduces circulating tumor necrosis factor α (TNFα) in young male rats, levels are unaffected by exercise in older animals (18). Here, we show that levels of 23 different serum cytokines (including TNFα) did not change in response to exercise in aging male mice. Further, many pro-inflammatory cytokines increased in proportion to exercise volume in aging females. Interestingly, we found that exercise reduced frailty in older females even though it increased IL-6, IL-9, and IFNγ, cytokines that are associated with higher FI scores in aging female mice (5). Whether aerobic exercise increases serum cytokines in older women has not yet been investigated, although levels of pro-inflammatory cytokines are increased by exercise in skeletal muscle from older women (19). Additional studies of the relationship between systemic inflammation, exercise, and frailty in older women are warranted.
There are limitations to this work. The beneficial effects we observed reflect a 13-week voluntary exercise program that was initiated late in life. Other interventions (eg, lifelong exercise) may produce different results. The survival analysis also was initiated at older ages, and we saw no significant differences within the study time frame. It is possible that differences in exercise effects on survival, including sex differences, might emerge if a longer time frame was investigated.
Funding
This work was supported by the Canadian Institutes of Health Research (grant numbers PGT 162462, MOP 97973) and the Heart and Stroke Foundation of Canada (grant number G-19-0026260) awarded to S.E.H. S.H.-M. is supported by the Izaak Walton Killam Predoctoral Scholarship, Nova Scotia Health Research Foundation’s Scotia Scholars Award, the Dalhousie Medical Research Foundation’s MacDonald Graduate Studentship, the Canadian Institutes of Health Research Doctoral Research Award, and Dalhousie University’s President’s Award.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the excellent technical assistance of Peter Nicholl.
Author Contributions
S.E.H. and E.S.B. contributed to the conception or design of the work; S.E.H., E.S.B., S.H.-M., and S.A.G. contributed to the acquisition, analysis, or interpretation of data for the work; S.E.H. and E.S.B drafted the work; S.E.H., E.S.B., S.H.-M., and S.A.G. revised it critically for important intellectual content; S.E.H., E.S.B., S.H.-M., and S.A.G. approved the final submission and are accountable for the work.
References
- 1. Martin FC, O’Halloran AM. Tools for assessing frailty in older people: general concepts. Adv Exp Med Biol. 2020;1216:9–19. doi: 10.1007/978-3-030-33330-0_2 [DOI] [PubMed] [Google Scholar]
- 2. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69(6):621–632. doi: 10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon EH, Hubbard RE. Differences in frailty in older men and women. Med J Aust. 2020;212(4):183–188. doi: 10.5694/mja2.50466 [DOI] [PubMed] [Google Scholar]
- 5. Kane AE, Keller KM, Heinze-Milne S, Grandy SA, Howlett SE. A murine frailty index based on clinical and laboratory measurements: links between frailty and pro-inflammatory cytokines differ in a sex-specific manner. J Gerontol A Biol Sci Med Sci. 2019;74(3):275–282. doi: 10.1093/gerona/gly117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultz MB, Kane AE, Mitchell SJ, et al. Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat Commun. 2020;11(1):4618. doi: 10.1038/s41467-020-18446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50(12):1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x [DOI] [PubMed] [Google Scholar]
- 8. Bray NW, Smart RR, Jakobi JM, Jones GR. Exercise prescription to reverse frailty. Appl Physiol Nutr Metab. 2016;41(10):1112–1116. doi: 10.1139/apnm-2016-0226 [DOI] [PubMed] [Google Scholar]
- 9. Llamas-Velasco S, Villarejo-Galende A, Contador I, Lora Pablos D, Hernández-Gallego J, Bermejo-Pareja F. Physical activity and long-term mortality risk in older adults: a prospective population based study (NEDICES). Prev Med Rep. 2016;4:546–550. doi: 10.1016/j.pmedr.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in sports and exercise medicine research? Eur J Sport Sci. 2014;14(8):847–851. doi: 10.1080/17461391.2014.911354 [DOI] [PubMed] [Google Scholar]
- 11. Graber TG, Ferguson-Stegall L, Liu H, Thompson LV. Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci. 2015;70(9):1045–1058. doi: 10.1093/gerona/glu163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, et al. A new frailty score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J Gerontol A Biol Sci Med Sci. 2017;72(7):885–891. doi: 10.1093/gerona/glw337 [DOI] [PubMed] [Google Scholar]
- 13. Seldeen KL, Lasky G, Leiker MM, Pang M, Personius KE, Troen BR. High intensity interval training improves physical performance and frailty in aged mice. J Gerontol A Biol Sci Med Sci. 2018;73(4):429–437. doi: 10.1093/gerona/glx120 [DOI] [PubMed] [Google Scholar]
- 14. Seldeen KL, Redae YZ, Thiyagarajan R, Berman RN, Leiker MM, Troen BR. High intensity interval training improves physical performance in aged female mice: a comparison of mouse frailty assessment tools. Mech Ageing Dev. 2019;180:49–62. doi: 10.1016/j.mad.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graber TG, Fandrey KR, Thompson LV. Novel individualized power training protocol preserves physical function in adult and older mice. Geroscience. 2019;41(2):165–183. doi: 10.1007/s11357-019-00069-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bisset ES, Howlett SE. The biology of frailty in humans and animals: understanding frailty and promoting translation. Aging Med (Milton). 2019;2(1):27–34. doi: 10.1002/agm2.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rose GL, Skinner TL, Mielke GI, Schaumberg MA. The effect of exercise intensity on chronic inflammation: a systematic review and meta-analysis. J Sci Med Sport. 2021;24(4):345–351. doi: 10.1016/j.jsams.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 18. Moon MK, Cho BJ, Lee YJ, et al. The effects of chronic exercise on the inflammatory cytokines interleukin-6 and tumor necrosis factor-α are different with age. Appl Physiol Nutr Metab. 2012;37(4):631–636. doi: 10.1139/h2012-039 [DOI] [PubMed] [Google Scholar]
- 19. Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation in women. J Appl Physiol (1985). 2020;129(6):1493–1504. doi: 10.1152/japplphysiol.00655.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hewett P, Ganser GH. A comparison of several methods for analyzing censored data. Ann Occup Hyg. 2007;51(7):611–632. doi: 10.1093/annhyg/mem045 [DOI] [PubMed] [Google Scholar]
- 21. Garcia-Valles R, Gomez-Cabrera MC, Rodriguez-Mañas L, et al. Life-long spontaneous exercise does not prolong lifespan but improves health span in mice. Longev Healthspan. 2013;2(1):14. doi: 10.1186/2046-2395-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Annas GJ, Galea S. Dying healthy: public health priorities for fixed population life expectancies. Ann Intern Med. 2018;169(8):568–569. doi: 10.7326/M18-1609 [DOI] [PubMed] [Google Scholar]
- 23. Baumann CW, Kwak D, Thompson LV. Sex-specific components of frailty in C57BL/6 mice. Aging (Albany NY). 2019;11(14):5206–5214. doi: 10.18632/aging.102114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suzuki K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int J Sports Exerc Med. 2019;5:122. doi: 10.23937/2469-5718/1510122 [DOI] [Google Scholar]
- 25. Petrella M, Aprahamian I, Mamoni RL, et al. The effect of a multicomponent exercise protocol (VIVIFRAIL©) on inflammatory profile and physical performance of older adults with different frailty status: study protocol for a randomized controlled trial. BMC Geriatr. 2021;21(1):83. doi: 10.1186/s12877-021-02030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.