Abstract
Background
Monitoring trajectories of intrinsic capacity (IC) in older adults has been suggested by the World Health Organization as a means to inform prevention to avoid or delay negative health outcomes. Due to a lack of longitudinal studies, it is currently unclear how IC changes over time and whether repeatedly measured IC predicts negative health outcomes.
Methods
Based on 4 751 repeated observations of IC (range = 0–100) during 21 years of follow-up among 754 older adults 70 and older, we assessed longitudinal trajectories of IC, and whether time-varying IC predicted the risk of chronic activities of daily living disability, long-term nursing home stay, and mortality using joint models.
Results
Average IC declined progressively from 77 to 11 points during follow-up, with substantial heterogeneity between older adults. Adjusted for sociodemographics and chronic diseases, a 1-point lower IC value was associated with a 7% increase in the risk of activities of daily living disability, a 6% increase in the risk of a nursing home stay, and a 5% increase in mortality. Accuracy for 5- and 10-year predictions based on up to 3 repeated measurements of IC ranged between moderate and good (area under the receiver operating characteristic curve = 0.76–0.82).
Conclusions
Our study indicates that IC declines progressively and that it predicts negative health outcomes among older adults. Therefore, regular monitoring of IC could work as an early warning system informing preventive efforts.
Keywords: Epidemiology, Longitudinal, Normative aging, Successful aging
In a shift from a disease-oriented to a functioning- and life-course-oriented approach of healthy aging, the World Health Organization (1) proposed the construct of intrinsic capacity (IC), which is defined as a composite measure of a person’s physical and mental capacities. IC is conceptualized as a dynamic construct (2) composed of 5 domains (vitality, locomotor, sensory, cognitive, and psychological) (3). Based on fine-grained measurements and regular monitoring, it may be possible to detect declines in IC ahead of clinical manifestations. This could facilitate the development and testing of preventive interventions to avoid or delay the onset of geriatric syndromes and subsequent health care utilization.
Although there is solid evidence (4–6) that indicators associated with IC domains such as grip strength (vitality) or gait speed (locomotor) predict adverse health outcomes, few studies to date (7–10) have assessed the predictive ability of the overall IC construct. Moreover, these studies were either cross-sectional (7) or assessed only the association of between-person differences in IC at baseline with the incidence of disability in activities of daily living (ADL) (8–10) and mortality (10). Thus, it is currently unclear how IC changes over time and whether longitudinal monitoring of IC is informative with regard to the prediction of negative health outcomes. This knowledge is required if IC is to act as an early warning system informing preventive efforts. The aim of this study was therefore to measure changes in IC longitudinally and to assess whether repeatedly measured IC predicts 3 negative health outcomes (ADL disability, nursing home stay, and mortality) among community-dwelling older adults.
Method
Data
In the Yale Precipitating Events Project Study (11), monthly telephone interviews and comprehensive home-based face-to-face assessments at 18-month intervals have been conducted since 1998 among 754 community-dwelling health plan members from greater New Haven, Connecticut. Eligible participants were aged 70 years or older and without ADL disability at baseline. For the current study, we used follow-up data through June 2019 (21 years).
Variables
Intrinsic capacity
IC was monitored based on the 18-month assessments, which amounted to a total of 4 751 repeated observations (6.5 observations per person on average). IC was measured via its 5 domains. (a) Vitality was operationalized via muscle strength and respiratory functioning. Muscle strength was assessed by mean handgrip strength (kg) over 3 readings with a hand-held Chatillon 100 dynamometer. Respiratory functioning was assessed with the maximum peak expiratory flow value (liter/min) over 3 attempts measured with a Mini-Wright meter. (b) Locomotor was assessed with gait speed (in seconds) tested across a 20-foot walk with a turn, the time required to perform 3 stands in the chair-rising test (in seconds), and the balance test (tandem, semitandem, side-by-side, 0–4 points) from the Short Physical Performance Battery. (c) The sensory domain included near-vision acuity, which was measured with a Jaeger chart (impairment in %), and hearing impairment, which was measured with an Audioscope (impairment in %). (d) Cognition was measured with the Mini-Mental State Examination (range = 0–30), and (e) the psychological domain was measured with the 11-item Center for Epidemiologic Studies—Depression scale (transformed range = 0–60). Input variables were rescaled after stratification by sex with the percent of maximum possible method (12), so that values were comparable longitudinally (13), and ranged from 0 (minimum possible) to 100 (maximum possible). We computed IC in each wave by first calculating a mean score for domains with multiple indicators (vitality, locomotor) before calculating a mean score over all 5 domains. Finally, the obtained mean score values of IC were again rescaled so that they ranged from 0 to 100.
Internal consistency reliability and construct validity of IC were assessed with confirmatory factor analysis (CFA), where we tested a second-order and a bifactor model (8,9), with the latter providing the better model fit. We removed hearing impairment due to low correlation (r < 0.10) to all other IC input variables which led to problematic negative variances in the CFA. Without hearing impairment, maximum-likelihood estimation with robust standard errors via R-package lavaan (v0.6-7) yielded a very good fit for the bifactor model: for example, at baseline χ 2 (16) = 23.56, Root Mean Square Error of Approximation (RMSEA) = 0.03, Comparative Fit Index (CFI) = 0.99, Tucker–Lewis index (TLI) = 0.99, Standardized Root Mean Square Residual (SRMR) = 0.02. Average reliability of the IC as a general factor (hierarchical ω) was 0.82 (range = 0.71–0.88) across waves. The correlation between the extracted IC factor scores and the IC mean scores that were used in the further analysis was high (r = 0.91–0.97) throughout the follow-up.
Negative health outcomes
Negative health outcomes included (a) onset of chronic ADL disability (14) (need for personal assistance in either dressing, bathing, walking inside the house, or transferring from a chair) lasting at least 3 months, (b) long-term (3+ months) nursing home stay (NHS), and (c) mortality (MOR). ADL and NHS were assessed via monthly telephone interviews, MOR was determined from local obituaries and informants. Participant retention was high (11), and follow-up data were 95% complete for ADL and NHS and 100% complete for mortality. For each outcome, a timeline was created, with the first interview marking the beginning of the observation period until either the time of the outcome, the end of follow-up, or dropout for other reasons, whatever came first.
Additional predictor variables
These included sex (men/women), 12 or more years of education (no/yes), age at baseline (years), ethnicity (White/non-White), and the number of chronic diseases at baseline (range = 0–9).
Statistical Analysis
To predict the negative health outcomes, we used joint models for longitudinal and time-to-event data (15), an advanced statistical approach where effects of time-varying predictors on time-to-event outcomes are estimated based on their longitudinal characteristics captured with mixed-effects regression models. In contrast to the extended Cox models for time-dependent predictors, joint models are better suited to handle both incomplete follow-up data and noisy information in time-varying covariates. In the mixed regression submodel, we first compared different specifications of time according to goodness-of-fit measures (Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), Likelihood Ratio Test (LRT) Test) to correctly characterize population- and individual-level IC trajectories (16). The best fit was obtained with a restricted natural cubic spline with one internal knot placed at the median of the follow-up time capturing nonlinear population- and individual-level trajectories of IC.
The survival regression submodel was formulated as:
where the risk of an adverse health outcome h for individual i at time t depends on the baseline hazard function h0 estimated with a cubic spline (5 knots), the regression coefficients γ 1−5 of the time-constant predictors, and the association parameter α. For α, we used the current value parametrization, that is, the “true” value of IC based on the individual-level growth model at t as a time-varying predictor. To assess the predictive performance, we calculated the time-dependent area under the receiver operating characteristic curve (AUC) values using information on IC up to the third year and medium (5 years) and long-term (10 years) prediction windows. AUC values were corrected for overfitting with an internal validation procedure using bootstrap (17). Joint models were fitted using R-package JMbayes (v0.8-85). All calculations were done in R: A language and environment for statistical computing (v4.0.3).
Results
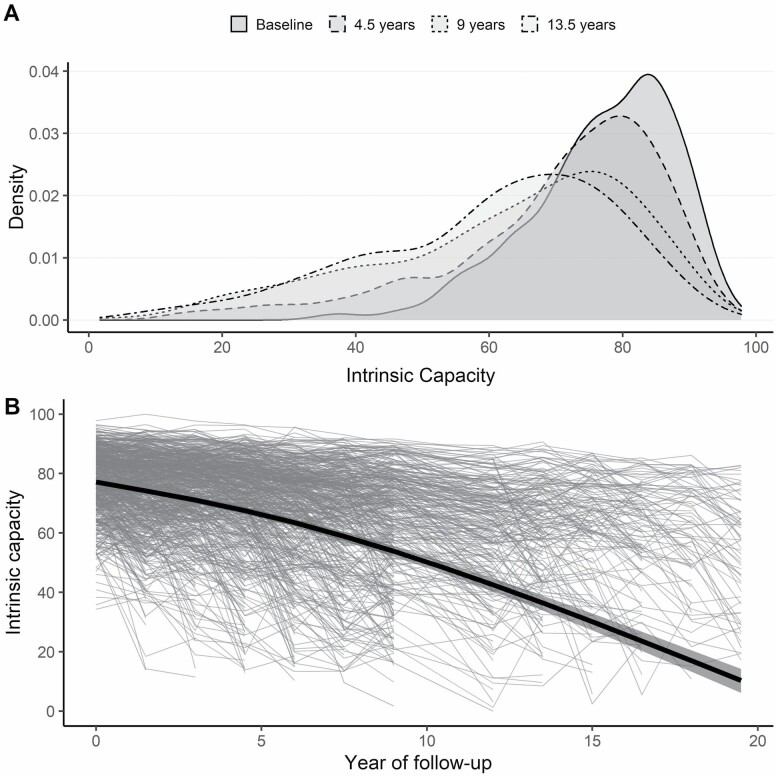
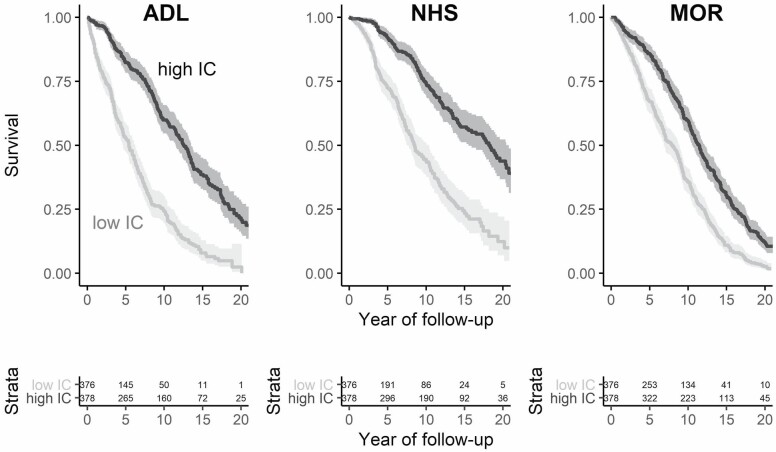
At baseline, the average age was 78.4 (SD = 5.3, range = 70–96) years; 67.1% of the sample were women, 89.5% identified as White, 68.3% had 12 or more years of education, and the median number of chronic diseases reported was 2 (interquartile range [IQR] = 1). The distribution of IC values at baseline was skewed to the left, that is, most older adults had initially high levels of IC (Figure 1A). The mean IC at baseline was 77 (SD = 11), which declined progressively to 11 points at the end of follow-up (Figure 1B). Figure 1 also shows that there was a substantial variance between participants (thin lines), which increased during follow-up: for example, IQR at baseline = 14, after 6 years = 21, and after 12 years = 23. Older adults with below-average IC at baseline had a higher risk of negative health outcomes compared to those with above-average baseline IC (Figure 2).
Figure 1.
Distribution and trajectory of intrinsic capacity (IC) over time. (A) Smoothed histograms of the distribution of values of IC at 4 selected points in time: baseline, 4.5, 9, and 13.5 years. (B) Thin gray lines show raw longitudinal observations of IC for 754 participants, which illustrate the broad variety of IC levels at baseline and of IC trajectories throughout follow-up (no measurement was available at 10.5 years of follow-up due to lack of funding). Thick black line shows the estimated average trajectory of IC from the mixed regression submodel. Shaded areas indicate 95% confidence intervals.
Figure 2.
Baseline intrinsic capacity (IC) and negative health outcomes. Kaplan–Meier plots for survival probability (ie, the probability to not experience the respective health outcome) by IC category (high = above-median IC, low = below-median IC) at baseline. Shaded areas indicate 95% confidence intervals. Below, the absolute number of participants at risk by a group is shown. ADL = incidence of disability in activities of daily living; NHS = nursing home stay; MOR = mortality.
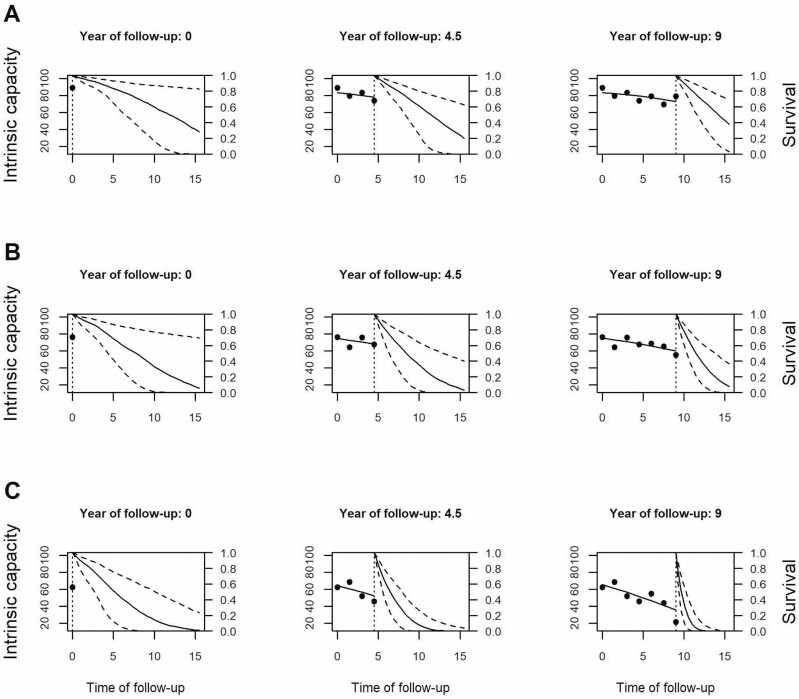
During follow-up, 61.4% of the participants reported the onset of chronic ADL disability after a median of 5.5 years (IQR = 7.1), 42.7% were admitted to a nursing home after a median of 7.2 (IQR = 7.0) years, and 93.1% eventually died after a median of 9.4 9.1 (IQR = 8.0) years. The results from the joint models, adjusted for sociodemographics and chronic diseases (Supplementary Table 1), indicate that a 1-point lower IC (on scale 0–100) was associated with a 7% (=1/0.94) increase in the risk for ADL (95% confidence interval [CI]: 1.06–1.07), a 6% increase in the risk for NHS (95% CI: 1.05–1.07), and a 5% increase in the risk of death (95% CI: 1.04–1.05). The AUC values indicate that IC, together with information on sociodemographics and chronic diseases, has good discriminative power predicting future negative health outcomes (5-year AUC: ADL = 0.81, NHS = 0.80, MOR = 0.76; 10-year AUC: ADL = 0.82, NHS = 0.81, MOR = 0.76). Figure 3 shows how individual predictions are updated as additional values of IC become available. To illustrate, we selected 3 female participants aged 74, 81, and 77 years at baseline, of whom B and C developed chronic ADL disability after 11 and 12 years of follow-up, respectively. Their baseline IC values and their IC trajectories varied considerably, as did their predicted outcome probabilities at different time points. The sharp decline of IC in Participant C over time, for example, results in an ever more certain and lower probability to remain free from ADL disability.
Figure 3.
Dynamic predictions of ADL-free status for 3 selected participants. Trajectories of intrinsic capacity (IC) and associated ADL-free survival probabilities for 3 selected participants (A, B, and C) after 1, 4, and 7 repeated IC measurements based on joint model. The x-axis shows the time of follow-up in years, the y-axis on the left side represents IC, the y-axis on the right side indicates the estimated probability to remain ADL-free. Points are raw observations of IC, the dotted vertical line indicates the last IC measurement, solid lines left of the dotted vertical line represent IC trajectories (middle and right column), solid lines right of the dotted vertical line refer to trajectories of survival probability to remain ADL-free, and dashed lines show 95% prediction intervals.
Discussion
IC has been suggested as a fine-grained, comprehensive measure of overall health status to monitor minor health declines in older individuals and populations in order to intervene and subsequently avoid, attenuate, or delay further major health declines. Based on extensive longitudinal data covering more than 20 years among 754 older adults (70+), we showed in this report that IC declined progressively in later life, and that regular monitoring of IC can predict future negative health outcomes, particularly the onset of chronic ADL disability and NHS, a proxy for severe long-term care needs. These results suggest that monitoring could help doctors to target older persons with a low or declining IC for preventive interventions years before the onset of care dependency.
In this study, we found that, on average, IC declined progressively over time. However, it is important to keep the substantial between-person heterogeneity around this average trajectory in mind: participants entered the study with broadly varying levels of IC, and IC trajectories during follow-up varied from stability and even improvements to drastic declines within a few years. What is more, the between-person variance in IC increased during follow-up. Plans to develop normative trajectories of IC—to identify individual deviations from normality—for preventive actions (2,9) should pay attention to this increasing heterogeneity. In our analysis, we measured IC up to 13 times per person at 18-month intervals. Assessments of IC among older adults during annual health checkups could provide even more timely information to better tailor diagnostic and therapeutic decisions without additionally taxing routine medical care.
Our study is the first attempt to predict negative health outcomes based on repeatedly measured IC. Whereas previous studies relied on one-time assessments of IC (7–10), we could show that IC declines progressively and the current (or last) IC value predicts future negative health outcomes. The IC therefore could be used to predict probabilities for medium- and long-term negative health outcomes dynamically and to update prognoses, which may act as an early warning system and support medical decision making. In addition, the IC could also be a relevant research outcome in itself.
IC has been discussed (2,8) as a concept complementary to or leading up to phenotypic frailty (18). However, there is a potentially problematic overlap in some of the indicators of frailty and IC (ie, gait speed, grip strength, items on exhaustion from Center for Epidemiologic Studies—Depression scale), which precluded the inclusion of (pre-)frailty as a negative health outcome in our study. IC also shares a number of characteristics with the other main model of frailty, namely the health deficit accumulation approach and its frailty index (FI) (19). Both FI and IC are multidimensional—that is, including information about physical, cognitive, and mental health—and continuous indicators of overall health that can be measured repeatedly to assess longitudinal trajectories in the general older population (20). Interestingly, the FI, which is based on 30+ dichotomized health deficits, typically exhibits a right-skewed distribution in community-dwelling samples of older adults, whereas IC values—which are based on fewer but continuous measurements of functioning—exhibited a left-skew in our study. Future studies should compare the predictive performance of the FI and IC for negative health outcomes explicitly, considering the more established conceptual framework and more straightforward calculation of the FI which previously yielded similar discrimination accuracy (20).
In our analysis, we used joint models for longitudinal and time-to-event data which have 2 major benefits over extended Cox-regression models. First, the mixed regression submodel provides unbiased population-level estimates of IC trajectories by using all available observations. Second, the joint model approach can handle measurement errors in the time-varying predictor. A recent simulation study (16) showed that when there is measurement error in the time-varying predictor, as found in our study by ω-coefficients ranging between “acceptable” and “good,” joint models provide more accurate estimates compared to Cox-regression models.
Strengths of this analysis include the long follow-up period and the high number of repeated observations of IC, the use of continuous indicators and assessment of reliability and construct validity of IC, and the statistical approach that accounted for nonlinear trajectories and measurement error in IC, and which yield population-level and individual-level predictions. However, there are also several limitations to this research. First, there is no consensus yet on how to measure IC, with regard both to the selection of indicators (7) and how to calculate, rescale, or validate the summary measure of IC. In this study, we used similar indicators—although no biomarkers were available—and also a bifactor model as Beard et al. (8,9) to operationalize IC. However, hearing impairment would not fit into our model, and thus, the domain sensory included only near-vision acuity. Also, our analysis suggests that about a fifth of the variance of IC is random measurement error or noise, which should be improved upon before implementation by further clarification of the conceptual model and/or the measurement strategy. Second, we focused on the overall construct of IC in this study, although our understanding of changes in IC and of potential targets for prevention and treatment could also benefit from assessing the coevolution of the constituting dimensions of IC over time. Also, different domains likely exhibit different dynamics, for example, mood likely changes faster or fluctuates compared to more gradual declines in sensory functioning. Third, our data did not include young older adults (50–70 years old), who might be a more appropriate target population for long-term prevention efforts. Fourth, we did not adjust the models for income, which might be relevant for (affordability of) NHS. Fifth, the study participants were members of a single regional health plan 70 years or older who were initially nondisabled. Hence, our results might not be generalizable to the older US population.
In conclusion, our study indicates that IC declines progressively and that regular monitoring of IC can predict negative health outcomes among older adults. IC could therefore work as an early warning system informing preventive efforts. However, more research is needed to improve the conceptual foundations and measurement of IC before routine monitoring can be implemented.
Supplementary Material
Acknowledgments
We thank Denise Shepard, Andrea Benjamin, Barbara Foster, and Amy Shelton for assistance with data collection; Geraldine Hawthorne and Evelyne A. Gahbauer for assistance with data entry and data management; Peter Charpentier for design and development of the study database and participant tracking system; and Joanne McGloin for leadership and advice as the Project Director.
Funding
This work was supported by a grant from the National Institute on Aging (grant number R01AG017560). T.M.G. is supported by the Yale Claude D. Pepper Older Americans Independence Center (grant number P30AG021342).
Conflict of Interest
None declared.
Author Contributions
E.S. planned the study, performed all statistical analyses, and wrote the article. T.M.G. contributed to the planning of the study, provided access to the data, and critically reviewed the manuscript. H.M. contributed to the CFA and critically reviewed the manuscript. W.F. contributed to the planning of the study and critically reviewed the article. R.R.-W. contributed to the interpretation of results and critically reviewed the article.
Data Availability
Requests for access to data from the PEP Study for meritorious analyses from qualified investigators should be directed to T.M.G. (thomas.gill@yale.edu). The R-Markdown code reproducing all analyses, results, and this manuscript are available online (https://osf.io/h7kyp/).
References
- 1. WHO. World Report on Ageing and Health. World Health Organization; 2015. [Google Scholar]
- 2. Belloni G, Cesari M. Frailty and intrinsic capacity: two distinct but related constructs. Front Med (Lausanne). 2019;6:133. doi: 10.3389/fmed.2019.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol A Biol Sci Med Sci. 2018;73(12):1653–1660. doi: 10.1093/gerona/gly011 [DOI] [PubMed] [Google Scholar]
- 4. Gill TM, Williams CS, Richardson ED, Tinetti ME. Impairments in physical performance and cognitive status as predisposing factors for functional dependence among nondisabled older persons. J Gerontol A Biol Sci Med Sci. 1996;51(6):M283–M288. doi: 10.1093/gerona/51a.6.m283 [DOI] [PubMed] [Google Scholar]
- 5. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charles A, Buckinx F, Locquet M, et al. Prediction of adverse outcomes in nursing home residents according to intrinsic capacity proposed by the World Health Organization. J Gerontol A Biol Sci Med Sci. 2020;75(8):1594–1599. doi: 10.1093/gerona/glz218 [DOI] [PubMed] [Google Scholar]
- 7. Gutiérrez-Robledo LM, García-Chanes RE, González-Bautista E, et al. Validation of two intrinsic capacity scales and its relationship with frailty and other outcomes in Mexican community-dwelling older adults. J Nutr Health Aging. 2021;25:33–40. doi: 10.1007/s12603-020-1555-5 [DOI] [PubMed] [Google Scholar]
- 8. Beard JR, Jotheeswaran AT, Cesari M, Araujo de Carvalho I. The structure and predictive value of intrinsic capacity in a longitudinal study of ageing. BMJ Open. 2019;9(11):e026119. doi: 10.1136/bmjopen-2018-026119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beard JR, Yafei S, Liu Z, et al. Intrinsic capacity: validation of a new WHO concept for healthy ageing in a longitudinal Chinese study. J Gerontol A Biol Sci Med Sci. 2022;77(1):94–100. doi: 10.1093/gerona/glab226 [DOI] [PubMed] [Google Scholar]
- 10. Zeng X, Shen S, Xu Let al. The impact of intrinsic capacity on adverse outcomes in older hospitalized patients: a one-year follow-up study. Gerontology. 2021;67:267–275. doi: 10.1159/000512794 [DOI] [PubMed] [Google Scholar]
- 11. Gill TM, Han L, Gahbauer EA, Leo-Summers L, Murphy TE. Cohort profile: the Precipitating Events Project (PEP Study). J Nutr Health Aging. 2020;24(4):438–444. doi: 10.1007/s12603-020-1341-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen P, Cohen J, Aiken LS, et al. The problem of units and the circumstance for POMP. Multivar Behav Res. 1999;34:315–346. doi: 10.1207/S15327906MBR3403_2 [DOI] [Google Scholar]
- 13. Moeller J. A word on standardization in longitudinal studies: don’t. Front Psychol. 2015;6:1389. doi: 10.3389/fpsyg.2015.01389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gill TM, Gahbauer EA. Overestimation of chronic disability among elderly persons. Arch Intern Med. 2005;165(22):2625–2630. doi: 10.1001/archinte.165.22.2625 [DOI] [PubMed] [Google Scholar]
- 15. Rizopoulos D. Joint Models for Longitudinal and Time-to-Event Data: With Applications in R. CRC Press; 2012. [Google Scholar]
- 16. Arisido MW, Antolini L, Bernasconi DP, Valsecchi MG, Rebora P. Joint model robustness compared with the time-varying covariate Cox model to evaluate the association between a longitudinal marker and a time-to-event endpoint. BMC Med Res Methodol. 2019;19(1):222. doi: 10.1186/s12874-019-0873-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrinopoulou E-R, Harhay MO, Ratcliffe SJ, et al. Reflections on modern methods: dynamic prediction using joint models of longitudinal and time-to-event data. Int J Epidemiol. 2021. doi: 10.1093/ije/dyab047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 19. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 20. Stolz E, Hoogendijk EO, Mayerl H, Freidl W. Frailty changes predict mortality in 4 longitudinal studies of aging. J Gerontol A Biol Sci Med Sci. 2021;76(9):1619–1626. doi: 10.1093/gerona/glaa266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to data from the PEP Study for meritorious analyses from qualified investigators should be directed to T.M.G. (thomas.gill@yale.edu). The R-Markdown code reproducing all analyses, results, and this manuscript are available online (https://osf.io/h7kyp/).