Abstract
17-α-Estradiol (17aE2) treatment from 4 months of age extends life span in male mice and can reduce neuroinflammatory responses in the hypothalamus of 12-month-old males. Although 17aE2 improves longevity in males, female mice are unaffected, suggesting a sexually dimorphic pattern of life-span regulation. We tested whether the sex-specific effects of 17aE2 on neuroinflammatory responses are affected by gonadal removal and whether hypothalamic changes extend to other brain regions in old age. We show that sex-specific effects of 17aE2 on age-associated gliosis are brain region specific and are partially dependent on gonadectomy. 17aE2 treatment started at 4 months of age protected 25-month-old males from hypothalamic inflammation. Castration before 17aE2 exposure reduced the effect of 17aE2 on hypothalamic astrogliosis in males. In contrast, sex-specific inhibition of microgliosis generated by 17aE2 was not significantly affected by castration. In the hippocampus, gonadectomy influenced the severity of gliosis and the responsiveness to 17aE2 in a region-dependent manner. The male-specific effects of 17aE2 correlate with increases in hypothalamic estrogen receptor alpha expression, specifically in gonadally intact males, consistent with the idea that 17aE2 might act through this receptor. Our results indicate that neuroinflammatory responses to 17aE2 are partially controlled by the presence of sex-specific gonads. Loss of gonadal function and age-associated neuroinflammation could, therefore, influence late-life health and disease onset, leading to sexual dimorphism in both aging and in response to drugs that modify the pace of aging.
Keywords: 17-α-Estradiol, Aging, Castration, Hypothalamus, Longevity, Neuroinflammation, Sexual dimorphism
Aging is characterized by increased neuroinflammatory responses in various brain regions, contributing to age-associated cognitive impairment, memory loss, neurodegenerative diseases, and metabolic imbalance. These neuroinflammatory responses are associated with a marked increase in the number of activated glial cells, specifically the astrocytes and the microglia (1). During the aging process, activated microglia induce the formation of neurotoxic reactive astrocytes that can contribute to neuronal loss and drive the progression of neurodegeneration (2). With age, astrocytes become more proinflammatory and contribute to the inflammatory responses in the aging brain. Our recent studies have demonstrated that age-associated gliosis is reduced in the long-lived mouse models, suggesting that a reduction in neuroinflammatory responses may attenuate the aging process (3).
Targeting brain immune responses and inflammatory glial cells holds promise for the treatment of neurological diseases and the aging brain. Recent studies have provided evidence that pharmacological interventions that extend mouse life span also reduce age-associated neuroinflammation. Treatment with acarbose (ACA), an antidiabetes drug, or 17-α-estradiol (17aE2), an optical isomer of 17-β-estradiol (4), causes a consistent extension in male life span, but life-span changes in females are either smaller (ACA) or nonexistent (17aE2). Similarly, we have recently demonstrated that ACA and 17aE2 significantly reduce neuroinflammatory responses in males but not in females in the hypothalamus of adult 12-month-old animals, the brain region responsible for metabolic regulation and energy homeostasis (5). The causes for these sexually dimorphic neuroinflammatory responses to drug treatment are not clear.
There is substantial sexual dimorphism in the number of glia cells in the brain, detectable during brain development and throughout the adult life span. Microglial number, morphology, and neuroinflammatory responses are dependent on age and brain region, as well as on hormonal and environmental factors (6,7). Microglia are likely to be responsive to male and female sex hormones (8). In particular, it is established that immune functions and inflammatory responses of microglia are significantly mitigated by estrogens (9). Similarly, astrocytes exhibit sex-specific gene expression profiles in different brain regions and are implicated in estrogen-dependent neuroprotection (10). Given the emerging evidence that hypothalamic inflammation and microglia activity can influence systemic metabolism and aging (11), these sex differences in glial abundance and activity could lead to sexual dimorphism in metabolic responses outside of the brain.
Recently, Garratt et al. have demonstrated that male-specific metabolic responses associated with 17aE2 treatment are specifically dependent on the presence of intact male gonads (12). Male castration before treatment initiation eliminated the insulin and glucose sensitivity induced by drug treatment (12). These hormonally dependent sex-specific effects of 17aE2 for males suggest a potential mechanism of 17aE2 action that requires male gonadal hormone production. These effects could still involve the estrogen signaling pathway, and possible interaction with the estrogen receptor (ER), but could be linked to steroid metabolism, as 17aE2 leads to a male-specific increase in other estrogen steroids in the liver, particularly sulfated forms of estriol (13). 17aE2 and estriol can bind to ER, but with lower affinity than 17βE2 in some cell types (14). In support, it has been recently demonstrated that 17aE2 inhibits inflammation via ERα in cultured primary preadipocyte cells (15).
Recently, Stout et al. (16) demonstrated reductions in inflammatory markers in adipose tissue in male mice treated with 17aE2, suggesting that 17aE2 may be a useful pharmacological intervention to treat metabolic imbalance and inflammation with aging. Moreover, in a subsequent study, it was shown that some of the short-term metabolic responses to 17aE2, particularly feeding behavior on a high-fat diet, are dependent on the functional presence of pro-opiomelanocortin (POMC) neurons (17), a subset of neurons found in the arcuate nucleus of the hypothalamus (ARC). Additionally, these metabolic benefits are dependent on the expression of ERα, as high-fat diet-fed male mice with global deletion of ERα do not show improved metabolic responses to 17aE2 treatment (18). Altogether, these results suggest that 17aE2 responses are linked partly to hypothalamic actions and require ERα expression, at least in the context of obesity, in addition to the requirement of intact male gonads. In this study, we evaluated the effect of 17aE2 and gonadectomy on neuroinflammatory responses in old 25-month-old mice, with a specific focus on brain regions that are sensitive to age-related neurological changes and metabolic imbalance. We further assess changes in ERα abundance with 17aE2 and gonadectomy in these brain regions, in an effort to explain why male-specific responses to 17aE2 are dependent on both male gonads and ERα signaling.
Method
Animals
Procedures involved in this study were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA). UM-HET3 mice were produced as previously described (5). All animals were fed LabDiet 5LG6 (67.1% carbohydrate, 21.9% protein, and 10.9% fat) until 4 months of age. Animals were maintained under temperature- and light-controlled conditions.
Surgical Procedures
At 3 months of age, all animals went through castration, ovariectomy, or a sham procedure (19). After surgical preparation, an incision was made in the caudal end of each scrotal sac, the testicle was pulled through the incision by gentle traction, and the blood vessels, vas deferens, and deferential vessels were clamped and sutured. The incision was closed with tissue adhesive. For ovariectomy an incision was made on the left side perpendicular to the vertebral column approximately midway between the iliac crest and the last rib. The ovarian fat pad was grasped and exteriorized. The pedicle under the ovarian blood vessels and the fat pad under the ovary were grasped and crushed, the pedicle cut on the ovary side and the ovary removed. The abdominal wall was closed with absorbable suture and the skin was closed with staples. The procedure was then repeated on the opposite side. For sham ovariectomy, animals underwent the same surgical procedure, but the ovary and fat pad were exteriorized and replaced without being excised.
Experimental Diets
17aE2 was purchased from Steraloids, Inc. (Newport, RI) and mixed at a dose of 14.4 mg/kg diet (14.4 ppm). LabDiet 5LG6 (67.1% carbohydrate, 21.9% protein, and 10.9% fat) supplemented with 17aE2 (14.4 ppm). Animals from each surgical type were randomly allocated to control or 17aE2 treatment. Control animals were maintained on 5LG6, whereas the treatment group was fed the 17aE2 diet continuously from 4 months of age.
Perfusion and Immunolabeling
Brains were fixed with 4% paraformaldehyde, dehydrated, and then sectioned coronally (30 µm) using a sliding microtome, followed by immunofluorescent analysis as previously described (5). Brain sections were washed with phosphate-buffered saline (PBS) several times; blocked for 2 hr in 0.3% Triton X-100 with 3% normal donkey serum in PBS; and then stained with the following primary antibodies overnight: rabbit anti-glial fibrillary acidic protein (GFAP; 1:1000; Millipore) and rabbit anti-Iba1 (1:1000; Wako) sheep anti-S100b (1:200; Invitrogen). For rabbit anti-ERα (1:1000; Santa Cruz) and rabbit anti pS6 (1:100; Cell signaling) immunostaining, sections were pretreated for 20 minutes in 0.5% NaOH and 0.5% HR2ROR2R in PBS. Sections were incubated with AlexaFluor-conjugated secondary antibodies for 2 hours (Invitrogen). Microscopic images were obtained using an Olympus FluoView 500 Laser Scanning Confocal Microscope (Olympus) equipped with 10×, 20×, and 40× objectives.
Quantification
For quantification of immunoreactive cells, images of matched brain areas were taken from at least “three-four” sections containing the hypothalamus for each brain between bregma −0.82 mm and −2.4 mm (according to the Franklin mouse brain atlas). Brain slices were taken at the same distance from bregma. Serial brain sections across the hypothalamus were made at 30 μm thickness, and every fifth section was used for staining and cell counting. Iba1, GFAP, S100β-positive cells, and cells stained for ERα, and pS6 protein were counted using ImageJ software with DAPI (nuclear stain). The average of the total number of cells/field of view was used for statistical analysis as described previously (5).
Statistical Analysis
For each measured parameter, we first tested whether there was a significant effect of sex, treatment, and interaction between these factors in data generated from sham-operated (intact) animals. The aim was to determine whether there were sex-specific neuroinflammatory responses to treatment that could be linked to the life-span effects in intact animals. We conducted a 2-factor analysis of variance (ANOVA), using the general linear model function and a full factorial model, which included an effect of treatment (comparing control to 17aE2 treatment), an effect of sex (male or female), and an interaction between sex and treatment. To help understand the underlying causes for sex-specific responses to the 17aE2 treatment, we then conducted 2 sets of follow-up analyses (within males or females), using the data from both sham-operated (the same data used to determine sex-specific effects of treatment in the first analysis) and gonadectomized animals. Within males, we tested whether there was a significant interaction between 17aE2 and surgery status (sham or castrated), which could indicate that the male response to 17aE2 is influenced by the presence of male gonads. In females, we tested whether there was a significant interaction between 17aE2 and surgery status (sham or ovariectomized), which could indicate that the female response to 17aE2 is influenced by the presence of ovaries. Where interaction terms were significant we followed up with a Student’s t-test, focusing on the effect of 17aE2 within one sex and surgery type (eg, sham males). Results are analyzed using Statistica software (version 10).
Results
Effect of 17aE2 on Astrogliosis in 25-Month-Old Mice
Our previous research has shown that 17aE2, started at age 4 months, increases male median life span by 12% (20) and reduces hypothalamic astrogliosis and microgliosis in male, but not in female 12-month-old mice (5). This previous work found effects of 17aE2 on inflammation only in the hypothalamus but did not assess changes in older animals, where inflammatory processes are expected to be greater and lead to impaired metabolism and physical function. We, therefore, first assessed the effects of 17aE2 on gliosis in the hypothalamus and the hippocampus of 25-month-old mice of both sexes, testing whether neuroinflammatory responses to 17aE2 are still evident, are sex specific, and extend to other brain regions.
Astrogliosis is correlated with increased expression of the GFAP (5). In 25-month-old mice treated with 17aE2 from 4 months of age, we observed sex-specific changes in hypothalamic astrogliosis with 17aE2 treatment, as can be seen by sex × drug interaction terms for GFAP+ astrocytes in the hypothalamus (Table 1; p = .02). Intact males (who underwent a sham surgery at 3 months old) responded to 17aE2 treatment with reduced numbers of GFAP+ astrocytes in the hypothalamus, with numbers after 17aE2 treatment comparable to either control or 17aE2-treated 25-month-old females (Figure 1A and B).
Table 1.
Two-way ANOVA statistical analysis.
| Sex × Treatment Interaction | Surgery × Treatment Interaction | ||
|---|---|---|---|
| Castration | Ovarectomy | ||
| GFAP+ cells in the ARC | p = .021 | p = .004 | p = .383 |
| GFAP+ cells in the CA3 | p = .016 | p = .036 | p = .036 |
| GFAP+ cells in the DG | p = .001 | p = .018 | p = .370 |
| Iba1+ cells in the ARC | p = .005 | p = .806 | p = .886 |
| Iba1+ cells in the CA3 | p = .017 | p = .059 | p = .774 |
| Iba1+ cells in the DG | p = .014 | p = .661 | p = .702 |
| ERα cells in ARC | p = .001 | p = .034 | p = .295 |
| pS6+ cells in ARC | p = .275 | p = .041 | p = .838 |
| pS6+ cells in CA3 | p = .242 | p = .511 | p = .235 |
| pS6+ cells in DG | p = .434 | p = .265 | p = .450 |
| s100b+ cells in CA3 | p = .001 | p = .001 | p = .001 |
| s100b+ cells in DG | p = .001 | p = .006 | p = .397 |
Statistical significances p < .05 are shown in bold.
Figure 1.
Astrogliosis in 17-α-estradiol (17aE2)-treated mice as measured by GFAP+ cells. Brain sections of 25-mo-old male and female mice were analyzed for hypothalamic and hippocampus astrocytes. (A) Representative images showing immunostaining in the arcuate nucleus of the hypothalamus (ARC), CA3, and dentate gyrus (DG) hippocampus areas of chow-fed control and 17aE2-treated mice. Scale bars: 200 μm. 3V, third ventricle. (B) Numbers of cells immunoreactive for GFAP in the ARC (B), CA3 (C), and DG (D) from indicated male and female mice; error bars show SEM for n = 5–4 mice of each. Images of matched brain areas from each mouse were taken from at least “three-four” sections containing the hypothalamus and hippocampus. Data were analyzed by 2-factor ANOVA and further analyzed with the Newman–Keuls post hoc test (***p < .001;****p < .0001).
Animal and human work has demonstrated that inflammatory changes in CA3 and dentate gyrus (DG) regions of the hippocampus are linked to age-related memory disorders (21). In our previous work, we did not detect an effect of 17aE2 on astrogliosis in the hippocampus of the 12-month-old drug-treated mice (5). In contrast, an assessment of GFAP+ astrocytes in CA3 of 25-month-old mice revealed that 17aE2 treatment reduced astrogliosis in males at this age, without a significant effect in females (sex × drug interaction p = .016; Figure 1C and D). A similar male-specific reduction in GFAP+ astrocytes was observed in the DG after 17aE2 treatment (sex × drug interaction; Figure 1A and C), without a change in females. We further verified these findings using an additional marker for astrocytes, S100β, a Ca2+ binding peptide abundant in the cytoplasm and nucleus of astrocytes (22,23), which can provide additional coverage of astrocyte populations in the hippocampus (23). S100β and GFAP are colocalized in the same cells (Supplementary Figure 1) and demonstrate a significant effect of 17aE2 treatment on astrogliosis in the CA3 and DG in males, but not in females (sex × drug interaction p = .01; Supplementary Figure 2).
Effect of 17aE2 on Microgliosis in 25-Month-Old Mice
Using immunostaining for the microglia-specific ionized calcium-binding adaptor molecule 1 (Iba1), we next analyzed the number of microglial cells in the hypothalamus and the hippocampus (CA3 and DG). Consistent with the astrocyte data, the microgliosis was higher in the ARC of control males than females and was reduced by 17aE2 treatment, with a significant interaction between sex and drug treatment compared to control (p = .005; Figure 2A and B). The effect of the 17aE2 treatment was highly significant in males (p = .001) but was not significant in females. In the CA3 and DG, there was a similar sex-specific effect of 17aE2 treatment on astrogliosis in male but not female mice (sex × drug interaction in the CA3, p = .017, in the DG, p = .014), with males showing a significant reduction in Iba1+ microglia in both hippocampal regions (Figure 2A, C, and D).
Figure 2.
Microgliosis in 17-α-estradiol (17aE2)-treated mice as measured by Iba1+ cells. (A) Representative images showing immunostaining in the arcuate nucleus of the hypothalamus (ARC), CA3, and DG of control and 17aE2-treated mice. Scale bars: 200 μm. 3V, third ventricle. (B) Numbers of cells immunoreactive for Iba1 in the ARC (B), CA3 (C), and DG (D) from indicated male and female mice; error bars show SEM for n = 5-4 mice of each type. Images were taken from at least “three-four” sections containing the hypothalamus and hippocampus. Data were analyzed by 2-factor ANOVA and further analyzed with the Newman–Keuls post hoc test (**p < .01; ***p < .001).
Effect of 17aE2 on Hypothalamic ERα Expression
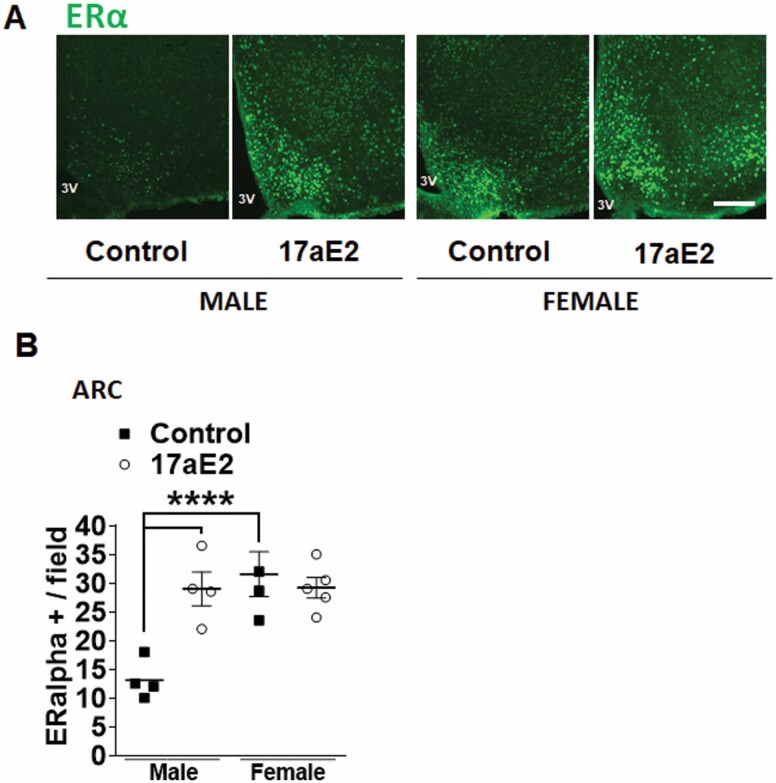
In mice, ERα is a predominant steroid receptor expressed in the hippocampus and hypothalamus, although its expression levels are reduced during aging, particularly in the hippocampus (24). ERα promotes neuronal survival and is more effective than ERβ in mediating ligand-independent transcription (25). It was recently demonstrated that 17aE2 suppresses inflammation in adipocytes through its effects on the ERα and NF-κB pathways (15), and in ERα knockout mice, 17aE2 fails to elicit metabolic responses to treatment (26). Therefore, the mechanism of action by which 17aE2 modulates neuroinflammatory responses in the hypothalamus could involve ERα activation. As seen in Figure 3, there is a male-specific increase in ERα expression in the ARC of 17aE2-treated males, but not females. ERα expression in males was elevated to the levels similar to those of chow-fed or 17aE2-treated females (Figure 3), and there was significant sex × treatment interaction (p = .001), indicating a different response in hypothalamic ERα expression to 17aE2 in males and females. We were not able to detect ERα in the hippocampus of old mice, in contrast to previous studies assessing hippocampal ERα signals in younger animals (27). This could suggest that ERα expression decreases in this area with age and that reduced inflammation in this brain region does not occur as a consequence of cell-autonomous ERα-dependent processes, but involves additional mechanisms.
Figure 3.
Hypothalamic ERα protein expression in 17-α-estradiol (17aE2)-treated mice. Brain sections of 25-mo-old male and female mice were analyzed for hypothalamic ERα protein expression. (A) Representative images showing immunostaining in the arcuate nucleus of the hypothalamus (ARC) of control and 17aE2-treated mice. Scale bars: 200 μm. 3V, third ventricle. (B) Quantification of ERα protein in the ARC from indicated male and female mice; error bars show SEM for n = 4 mice of each type. Images were taken from at least “three-four” sections containing the hypothalamus and hippocampus. Data were analyzed by 2-factor ANOVA and further analyzed with the Newman–Keuls post hoc test (****p < .0001).
Consequences of Gonadectomy for Changes in Gliosis in 17aE2-Treated Mice
To test whether the effects of 17aE2 on age-associated neuroinflammation are dependent on the presence of male or female gonads, we assessed gliosis in the hypothalamus and in the hippocampus of 25-month-old mice that had undergone surgical gonadal removal and then were treated with 17aE2 or control diet. Mice underwent surgeries at 3 months of age and were treated with 17aE2 from 4 to 25 months of age, that is, over the same period as the sham-operated animals presented above. Sham-operated mice and those subjected to gonadectomy were produced, aged, and treated in parallel (12). Following our previous approach for testing whether metabolic responses to 17aE2 are responsive to the presence of male or female gonads (12), we tested whether either castration or ovariectomy influenced the response to 17aE2. We tested whether the response to 17aE2 seen in sham-operated animals was observed in gonadectomized individuals of the same sex. For each comparison, we used a 2-way ANOVA to test whether the response to 17aE2 treatment in one sex significantly differed in sham-operated or gonadectomized animals (ie, whether there was a significant interaction between surgery status and drug treatment).
In males, castration significantly influenced the response to 17aE2 for GFAP+ astrocytes in the ARC (surgery by treatment interaction: p = .004), when compared with the sham surgery male responses (ie, also shown in Figures 1–3). Although sham males showed a reduction in GFAP+ astrocytes in the ARC with 17aE2 treatment, there was no change with 17aE2 treatment in castrated males (Figure 4A and Supplementary Figure 3). Castrated males also failed to show a significant reduction in either of the astrocyte markers we measured in the hippocampus in response to 17aE2. There was a significant surgery by treatment interaction for GFAP+ astrocytes in both the DG (p = .018) and CA3 (p = .036) of the hippocampus, indicating a different response to treatment in these regions (Figure 4B and C and Supplementary Figure 3). The additional astrocyte marker S100β also showed a significant surgery by treatment interaction in both regions, further supporting an effect of gonadal hormones on astrocyte responses to 17aE2 in the hippocampus of males (Supplementary Figure 4).
Figure 4.
Quantification of GFAP+ cells in the arcuate nucleus of the hypothalamus (ARC) (A), CA3 (B), and DG (C); quantification of Iba1+ cells in the ARC (D), CA3 (E), and DG (F); and quantification of ERα protein expression (G) obtained from 25-mo-old male and female control and 17-α-estradiol (17aE2)-treated mice, castrated, or ovariectomized; error bars show SEM for n = 5–4 mice of each type. Images were taken from at least “three-four” sections containing the hypothalamus and hippocampus. Data were analyzed by 2-factor ANOVA and further analyzed with the Newman–Keuls post hoc test (**p < .01; ***p < .001; ****p < .0001).
For females, ovariectomy did not influence astrocyte responses to 17aE2 in either the ARC of the hypothalamus or the DG of the hippocampus. OVX females showed no change in GFAP and/or S100β-positive cells in response to 17aE2 treatment, and there was no significant interaction between surgery and treatment in marker scores in this region (although OVX increased GFAP+ cells in the ARC in both control and 17aE2-treated females; Figure 4A–C and Supplementary Figure 3 and 4). Only in the CA3 was there evidence that OVX influenced female responses to 17aE2 treatment, with OVX females having higher levels of astrocyte markers in this region and 17aE2 significantly reducing this (Figure 4A and B and Supplementary Figure 3). This indicates that female ovarian hormones influence the response to 17aE2 in this brain region.
Although there was evidence that some sex-specific astrocyte responses to 17aE2 were influenced by the presence of gonads (particularly male gonads), the strong-sex-specific effects of 17aE2 on microglia abundance appeared completely unaffected by the removal of gonads in either sex (Table 1, Figure 4D–F, and Supplementary Figure 5). Castrated males showed the same response to 17aE2 treatment as sham-operated individuals in each brain region studied, as indicated by the lack of a significant interaction between surgery and treatment for Iba1+ microglia. Female ovariectomy elevated Iba1+ microglia in the ARC in both control and 17aE2-treated females, supporting the proposed role of estrogen on hypothalamic microglia inflammatory responses (9,28). However, there was no interaction between surgery status and drug treatment, with ovariectomized females also showing no change in Iba1+ microglia counts in the ARC with 17aE2 treatment (Table 1, Figure 4D, and Supplementary Figure 5).
Sex-Specific Changes in ERα Expression Are Altered by Gonadectomy
The increase in hypothalamic ERα expression with 17aE2 in intact males is not observed in castrated males, with intact and castrated males showing a significantly different response to 17aE2 treatment as demonstrated by the surgery by treatment interaction in male mice (p = .034). Castration itself did not alter ERα expression on the control diet, as there is no significant difference in astrogliosis between sham and castrated males in the ARC. In females, there was no interaction between surgery and 17aE2 treatment (p = .295), with neither sham-operated nor ovariectomized females showing an increase in ERα expression. We note that there was a main effect of surgery in the 2-way ANOVA (p = .001), indicating that females show a decrease in ERα expression in response to ovariectomy regardless of 17aE2 treatment (Figure 4G and Supplementary Figure 6).
Effect of 17aE2 and Gonadectomy on Hypothalamic mTOR Signaling
mTOR signaling is involved in sex differences in aging and metabolism (29). Reduced mTORC1 signaling extends life span in female mice (30), whereas reduced mTORC2 signaling reduces male life span without a significant effect on female life span (31). Interestingly, 17aE2 treatment elevates hepatic mTORC2 activity in males, without a significant effect on mTOR1 signaling in either male or female mice (12). In the ARC, mTORC1 signaling can mediate estrogen’s anorectic effects on energy homeostasis (32). We assessed phosphorylation of S6, one of the downstream substrates of mTORC1, in the ARC of 25-month-old mice. Consistent with the liver data (12), S6 phosphorylation in the ARC did not significantly change with the 17aE2 treatment, and there was no significant effect of sex (Figure 5). On the other hand, castration increased phosphorylation levels of pS6 in both chow and 17aE2-treated males, although there was surgery by drug interaction (p = .041; Figure 5), because 17aE2 treatment reduced pS6 specifically in castrated male mice (p = .001). There was no overall effect of ovariectomy on pS6 and no interaction between ovariectomy and drug treatment within females. We did not detect a significant effect of 17aE2 treatment on phosphorylation levels of pS6 in the CA3 or DG in either sham-operated or castrated animals of both sexes (Figure 5).
Figure 5.
pS6 Protein expression in the hypothalamus and hippocampus of control and 17-α-estradiol (17aE2)-treated mice. Brain sections of 25-mo-old male and female mice were analyzed for pS6 protein expression in sham-treated, castrated, and ovariectomized mice. (A) Representative images showing immunostaining in the arcuate nucleus of the hypothalamus (ARC), CA3, and dentate gyrus (DG) of chow-fed control and 17aE2-treated mice, castrated, or ovariectomized. Scale bars: 200 μm. 3V, third ventricle. Quantification of pS6 in the ARC (B), CA3 (C), and DG (D) from indicated male and female mice; error bars show SEM for n = 5–4 mice of each type. Images were taken from at least “three-four” sections containing the hypothalamus and hippocampus. Data were analyzed by 2-factor ANOVA and further analyzed with the Newman–Keuls post hoc test (****p < .0001).
Discussion
Our results show that sex-specific effects of 17aE2 on life span are reflected in reduced age-associated neuroinflammation specifically in males, within brain regions that are sensitive to neurodegeneration and metabolic imbalance. These sexually dimorphic neuroinflammatory responses are brain region specific and are partially, but not entirely, dependent on intact gonads. We provide evidence that the male-specific effects of 17aE2 are correlated with male-specific changes in ERα expression in the ARC, responses that are not observed in females or castrated males.
Castrated males do not show the decline in age-associated gliosis produced by 17aE2 treatment in intact males and do not respond to 17aE2 with an increase in ERα expression in the ARC, as seen in intact males. Male gonads, therefore, contribute to the sexually dimorphic responses elicited by 17aE2, with castrated males showing the lack of drug effect that is observed in sham females. We note here that males were castrated at 3 months of age and end points studied at 25 months of age, so it is not possible to determine the time course over which the presence of male gonads is important for treatment responses. Similarly, we do not know which gonadally derived factors are important in mediating drug responses, although testes-dependent androgen production would be a likely candidate. These effects of gonadectomy may also be indirect and reflect alterations or changes in other hormonal pathways due to gonadectomy. For example, in response to gonadectomy, changes in corticosterone pulsatile patterns and glucocorticoid signaling in the brain might also be associated with enhanced neuroinflammation (33,34). Because the production and circulating concentration of gonadal hormones vary over life, it is not possible to mimic these with reapplication of specific hormones (eg, testosterone) across the extended treatment period used in this study because this would require repeated injections or pellet reapplication across 2 years at varying doses. However, to build on our current findings, future studies with shorter-term application of hormones like testosterone in castrated males under 17aE2 treatment may provide a fruitful approach, which could provide an insight into whether testosterone is required for specific treatment responses (eg, increased ERα expression in the ARC) that might be elicited over a shorter time course.
Ovariectomy activates age-associated hypothalamic gliosis in females that are otherwise protected from hypothalamic inflammation. A similar increase in age-associated gliosis is observed in CA3 after ovariectomy, but this hormonal effect is not apparent in DG. Each of these areas of the hippocampus has a distinctive function that is differentially affected by brain aging (35). Indeed striking structural and functional sex differences are observed in the CA3 pyramidal cells in this brain region of adult rats, differences that are sensitive to gonadectomy (36), which may explain why an effect of OVX is observed in this area of the hippocampus. Interestingly, in the CA3, 17aE2 also reduced gliosis in OVX females, indicating the importance of both male and female gonads in the sex-specific responses to 17aE2, depending on the brain region. Neuroinflammation has been reported to take place before overt neuron loss in various animal models of age-related neurodegeneration (37), raising the possibility that the early use of drugs that can delay neuroinflammation could play a critical role in mitigating disease progression. In support, it has been suggested that 17aE2 could be effective in the protection against oxidative stress, amyloid toxicity, and Parkinson’s and Alzheimer’s diseases (38).
We could not detect the expression of ERα in the hippocampus of old brains of male and female mice. Although no report has specifically examined the effect of age on the expression of ERα in the hippocampus of mice, aging led to an 80% decline in ERα expression in rats (39), which may explain why ERα was below the threshold for detection in this brain region in our study. Because knockout of ERα expression in mice is associated with a decrease in the expression of genes critical for hippocampal function during aging (40), the loss of detectable ERα in these brain regions during aging could contribute to impairing brain function stemming from this region. Indeed, 17-β-estradiol’s effects on cognition are reduced with aging, and it has been suggested that this could be caused by altered expression of ERα in the hippocampus (41).
Our previous study did not detect a significant effect of 17αE2 on neuroinflammatory responses in the hippocampus of 12-month-old mice (5). However, we now show that by 25 months of age, both astrogliosis and microgliosis are significantly reduced by 17αE2 in CA3 and DG in males, though not in females. Since we do not detect ERα in these regions at this time point, our results suggest that either other hormone receptors/signaling processes are involved in causing changes in this brain area or ERα mediates the signaling effects of 17αE2, but effects in the hippocampus are indirect and occur as a consequence of ERα activation occurring in a different brain region. Alternatively, it is possible that the effects are mediated by ERα, but take place at an earlier age, an age at which ERα is more active in CA3 or DG in males.
ERα is strongly expressed in the ARC and plays an important role in metabolic regulation. Female but not male mice with ERα selectively deleted in POMC neurons in the ARC are hyperphagic and develop modest obesity (42). Our data show that in response to 17aE2 treatment, ERα expression levels are elevated in the ARC of male mice and become similar to the ERα levels in chow-fed or 17aE2-treated females. Notably, this increase did not occur in castrated male mice treated with 17aE2, potentially explaining why 17aE2 responses, which have been explored in various contexts, may be dependent on both ERα expression (26) and the presence of male gonads (18). ERα is expressed in astrocytes and can activate several neuroprotective mechanisms including the control of neuroinflammation (28). These data could potentially imply that the ability of 17aE2 to decrease neuroinflammatory responses in the hypothalamus depends on interactions with ERα, a hypothesis worthy of future investigation. Similarly, 17aE2 suppressed inflammation in primary adipocytes through their effects on ERα in a sex-specific manner with males being more sensitive to its effects (15).
A recent study suggested that the central actions of estradiol on energy balance are at least partially mediated by the selective modulation of the mTOR pathway through ERα in the ARC (43). We show that castration reduced the expression levels of hypothalamic ERα in 17aE2-treated males, and significantly increased mTORC1 signaling in the ARC, without an effect in the hippocampus (in CA3 or DG). Although the molecular mechanisms underlying this relationship require further investigation, such an interaction could be related to the altered activity of mTORC2 signaling in the ARC neurons (44). Understanding the effect of hypothalamic mTOR signaling in the control of neuroinflammatory responses may provide significant insight into the causes of sex-specific differences in aging.
Our previous work has shown that a variety of other metabolic and antiaging responses to 17aE2 occur in males but not in females and that these sex-specific responses are not seen in castrated males (12). Improvements in glucose tolerance and enhanced hepatic mTORC2 signaling with 17aE2 treatment are observed in male mice only, and these effects are inhibited in castrated males. Furthermore, the 17aE2 treatment improves male grip strength and capacity to balance on a rotarod, and these improvements are not seen in castrated male mice treated with 17aE2 (18). The hypothalamus is a major central regulator of peripheral metabolism, and the regulation of metabolism via this region is influenced by inflammatory responses (11). Thus, it is possible that reduced hypothalamic inflammation by 17aE2 could promote the metabolic and health benefits of this drug observed in other regions of the body.
It has previously been shown that some responses to 17aE2, particularly changes in feeding behavior, are dependent on the presence of functional POMC neurons (17), although other metabolic responses to 17aE2 remain intact. The ARC of the hypothalamus, where we observe changes in the expression of ERα and reduced inflammation with 17aE2, contains POMC neurons. This could suggest a connection between activation of these neurons via ERα, altered feeding behavior, and also changes in the abundance of astrocytes and microglial nearby. The ARC also contains additional neuronal populations involved in metabolic homeostasis, many of which express ERα (45), which are also reasonable candidates through which 17aE2 may elicit its metabolic effects. Ultimately, hypothalamic processes are strongly implicated in the control of systemic aging, particularly in response to inflammation (11). Therefore, careful dissection of the central signaling pathways and neuronal populations through which 17aE2 influences metabolic homeostasis, and ultimately life span, could provide a key insight into life-span control. The alternative hypothesis is that 17aE2 acts at peripheral sites like the liver, muscle, or adipose tissue, potentially helping to maintain metabolic regulation and glucose control, and this reduces hypothalamic inflammation. For example, it was recently demonstrated that 17aE2 signals through ERα to modulate hepatic function and systemic metabolism, thereby potentially contributing to the life span–extending effects of 17aE2. Similarly, hypothalamic administration of 17aE2 also was able to modulate hepatic gluconeogenesis, suggesting that the liver and hypothalamus are 2 important sites of action for the regulation of metabolic parameters by 17aE2 (26). Distinguishing the central and peripheral signaling targets of 17aE2, and the receptors involved, would be crucial in the development of targeted androgen and estrogen pathway modulators that help to slow aging while minimizing adverse health outcomes.
Supplementary Material
Funding
This study was supported by American Diabetes Association (grant no. 1-lB-IDF-063). M.S. was supported by Glenn Foundation for Medical Research, as well as R.A.M. received grants from National Institutes of Health (AG022303 and AG024824). M.G. acknowledges support from the Michigan Society of Fellows.
Author Contributions
L.K.D. and H.S.M.J. carried out the research. R.A.M. provided animal models, reviewed, and revised the manuscript. M.S. and M.G. designed the study, analyzed the data, wrote the manuscript, and are responsible for the integrity of this work. All authors approved the final version of the manuscript.
Conflict of Interest
None declared.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Valles SL, Iradi A, Aldasoro M, et al. Function of glia in aging and the brain diseases. Int J Med Sci. 2019;16(11):1473–1479. doi: 10.7150/ijms.37769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sadagurski M, Landeryou T, Cady G, et al. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell. 2015;14(6):1045–1054. doi: 10.1111/acel.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhurova EA, Zhurov VV, Chopra D, Stash AI, Pinkerton AA. 17Alpha-estradiol × 1/2 H2O: super-structural ordering, electronic properties, chemical bonding, and biological activity in comparison with other estrogens. J Am Chem Soc. 2009;131(47):17260–17269. doi: 10.1021/ja906057z [DOI] [PubMed] [Google Scholar]
- 5. Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. 2017;16(4):652–660. doi: 10.1111/acel.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grabert K, Michoel T, Karavolos MH, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19(3):504–516. doi: 10.1038/nn.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett FC, Bennett ML, Yaqoob F, et al. A combination of ontogeny and CNS environment establishes microglial identity. Neuron. 2018;98(6):1170–1183.e8. doi: 10.1016/j.neuron.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kodama L, Gan L. Do microglial sex differences contribute to sex differences in neurodegenerative diseases? Trends Mol Med. 2019;25(9):741–749. doi: 10.1016/j.molmed.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37(4):372–402. doi: 10.1210/er.2016-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Acaz-Fonseca E, Sanchez-Gonzalez R, Azcoitia I, Arevalo MA, Garcia-Segura LM. Role of astrocytes in the neuroprotective actions of 17β-estradiol and selective estrogen receptor modulators. Mol Cell Endocrinol. 2014;389(1–2):48–57. doi: 10.1016/j.mce.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 11. Zhang G, Li J, Purkayastha S, et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. 2013;497(7448):211–216. doi: 10.1038/nature12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garratt M, Bower B, Garcia GG, Miller RA. Sex differences in lifespan extension with acarbose and 17-α estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell. 2017;16(6):1256–1266. doi: 10.1111/acel.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garratt M, Lagerborg KA, Tsai YM, Galecki A, Jain M, Miller RA. Male lifespan extension with 17-α estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell. 2018;17(4):e12786. doi: 10.1111/acel.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin AH, Li RW, Ho EY, et al. Differential ligand binding affinities of human estrogen receptor-α isoforms. PLoS One. 2013;8(4):e63199. doi: 10.1371/journal.pone.0063199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santos RS, de Fatima LA, Frank AP, Carneiro EM, Clegg DJ. The effects of 17 alpha-estradiol to inhibit inflammation in vitro. Biol Sex Differ. 2017;8(1):30. doi: 10.1186/s13293-017-0151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stout MB, Steyn FJ, Jurczak MJ, et al. 17α-Estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J Gerontol A Biol Sci Med Sci. 2017;72(1):3–15. doi: 10.1093/gerona/glv309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steyn FJ, Ngo ST, Chen VP, Bailey-Downs LC, Xie TY, Ghadami M, et al. 17α-estradiol acts through hypothalamic pro-opiomelanocortin expressing neurons to reduce feeding behavior. Aging Cell. 2018;17:e12703. doi: 10.1111/acel.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mann SN, Hadad N, Nelson Holte M, et al. Health benefits attributed to 17alpha-estradiol, a lifespan-extending compound, are mediated through estrogen receptor alpha. Elife. 2020;9. doi: 10.1101/2020.06.02.130674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–282. doi: 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lana D, Iovino L, Nosi D, Wenk GL, Giovannini MG. The neuron-astrocyte-microglia triad involvement in neuroinflammaging mechanisms in the CA3 hippocampus of memory-impaired aged rats. Exp Gerontol. 2016;83:71–88. doi: 10.1016/j.exger.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 21. Morquette P, Verdier D, Kadala A, et al. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat Neurosci. 2015;18(6):844–854. doi: 10.1038/nn.4013 [DOI] [PubMed] [Google Scholar]
- 22. Sakatani S, Seto-Ohshima A, Shinohara Y, et al. Neural-activity-dependent release of S100B from astrocytes enhances kainate-induced gamma oscillations in vivo. J Neurosci. 2008;28(43):10928–10936. doi: 10.1523/JNEUROSCI.3693-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Z, Ma Z, Zou W, et al. The appropriate marker for astrocytes: comparing the distribution and expression of three astrocytic markers in different mouse cerebral regions. Biomed Res Int. 2019;2019:9605265. doi: 10.1155/2019/9605265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22(4):656–669. doi: 10.1002/hipo.20935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coleman KM, Dutertre M, El-Gharbawy A, Rowan BG, Weigel NL, Smith CL. Mechanistic differences in the activation of estrogen receptor-alpha (ER alpha)- and ER beta-dependent gene expression by cAMP signaling pathway(s). J Biol Chem. 2003;278(15):12834–12845. doi: 10.1074/jbc.M212312200 [DOI] [PubMed] [Google Scholar]
- 26. Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146(7):3070–3079. doi: 10.1210/en.2004-1515 [DOI] [PubMed] [Google Scholar]
- 27. Vegeto E, Belcredito S, Etteri S, et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA. 2003;100(16):9614–9619. doi: 10.1073/pnas.1531957100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garratt M, Nakagawa S, Simons MJ. Comparative idiosyncrasies in life extension by reduced mTOR signalling and its distinctiveness from dietary restriction. Aging Cell. 2016;15(4):737–743. doi: 10.1111/acel.12489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. doi: 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamming DW, Mihaylova MM, Katajisto P, et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. 2014;13(5):911–917. doi: 10.1111/acel.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu F, Xu Y, Liu F. Hypothalamic roles of mTOR complex I: integration of nutrient and hormone signals to regulate energy homeostasis. Am J Physiol Endocrinol Metab. 2016;310(11):E994–E1002. doi: 10.1152/ajpendo.00121.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol. 2004;16(12):989–998. doi: 10.1111/j.1365-2826.2004.01258.x [DOI] [PubMed] [Google Scholar]
- 33. Bellavance MA, Rivest S. The HPA – immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol. 2014;5:136. doi: 10.3389/fimmu.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dillon SE, Tsivos D, Knight M, et al. The impact of ageing reveals distinct roles for human dentate gyrus and CA3 in pattern separation and object recognition memory. Sci Rep. 2017;7(1):14069. doi: 10.1038/s41598-017-13853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scharfman HE, MacLusky NJ. Sex differences in hippocampal area CA3 pyramidal cells. J Neurosci Res. 2017;95(1-2):563–575. doi: 10.1002/jnr.23927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tabuchi K, Chen G, Südhof TC, Shen J. Conditional forebrain inactivation of nicastrin causes progressive memory impairment and age-related neurodegeneration. J Neurosci. 2009;29(22):7290–7301. doi: 10.1523/JNEUROSCI.1320-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dykens JA, Moos WH, Howell N. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann N Y Acad Sci. 2005;1052:116–135. doi: 10.1196/annals.1347.008 [DOI] [PubMed] [Google Scholar]
- 38. Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056(1):22–35. doi: 10.1016/j.brainres.2005.06.073 [DOI] [PubMed] [Google Scholar]
- 39. Han X, Aenlle KK, Bean LA, et al. Role of estrogen receptor α and β in preserving hippocampal function during aging. J Neurosci. 2013;33(6):2671–2683. doi: 10.1523/JNEUROSCI.4937-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol. 2009;21(7):640–647. doi: 10.1111/j.1365-2826.2009.01882.x [DOI] [PubMed] [Google Scholar]
- 41. Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. doi: 10.1016/j.cmet.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. González-García I, Martínez de Morentin PB, Estévez-Salguero Á, et al. mTOR signaling in the arcuate nucleus of the hypothalamus mediates the anorectic action of estradiol. J Endocrinol. 2018;238(3):177–186. doi: 10.1530/JOE-18-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chellappa K, Brinkman JA, Mukherjee S, et al. Hypothalamic mTORC2 is essential for metabolic health and longevity. Aging Cell. 2019;18(5):e13014. doi: 10.1111/acel.13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Garratt M, Leander D, Pifer K, et al. 17-α Estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell. 2019;18(2):e12920. doi: 10.1111/acel.12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Herber CB, Krause WC, Wang L, et al. Estrogen signaling in arcuate Kiss1 neurons suppresses a sex-dependent female circuit promoting dense strong bones. Nat Commun. 2019;10(1):163. doi: 10.1038/s41467-018-08046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.