Abstract
The link between survival and reproductive function is demonstrated across many species and is under both long-term evolutionary pressures and short-term environmental pressures. Loss of reproductive function is common in mammals and is strongly correlated with increased rates of disease in both males and females. However, the reproduction-associated change in disease rates is more abrupt and more severe in women, who benefit from a significant health advantage over men until the age of menopause. Young women with early ovarian failure also suffer from increased disease risks, further supporting the role of ovarian function in female health. Contemporary experiments where the influence of young ovarian tissue has been restored in postreproductive-aged females with surgical manipulation were found to increase survival significantly. In these experiments, young, intact ovaries were used to replace the aged ovaries of females that had already reached reproductive cessation. As has been seen previously in primitive species, when the young mammalian ovaries were depleted of germ cells prior to transplantation to the postreproductive female, survival was increased even further than with germ cell-containing young ovaries. Thus, extending reproductive potential significantly increases survival and appears to be germ cell and ovarian hormone-independent. The current review will discuss historical and contemporary observations and theories that support the link between reproduction and survival and provide hope for future clinical applications to decrease menopause-associated increases in disease risks.
Keywords: Germ cell, Health, Hormone, Menopause, Ovarian
Biological changes that occur coincident with an advancing chronological timeline are termed aging. Two people may have the same chronological age, but have very different biological ages. One biological function that significantly influences biological age is reproductive function. Evidence over the past decade indicates that an individual’s reproductive status is associated with an increased risk of developing chronic health conditions (1). One study documented an association between shorter life spans and reproductive failure for a cohort of men (2). The association is even more striking in women. Cardiovascular disease (CVD) is rare in premenopausal women, but ovarian failure increases CVD sharply at menopause, and in young women with premature ovarian failure (POF) (3–5). Insulin resistance and bone loss both increase at menopause and two thirds of Americans with Alzheimer’s disease are women (6–8). A basic understanding of chronological aging, reproductive aging, and the events affecting both is required to develop a reasonable understanding of the dynamic relationship between reproduction, aging and survival, and the specific influence of female reproductive aging on health and longevity.
Chronological Aging
Chronological age is often defined as the amount of time that has passed from birth to the current date and is the primary way people define their age. Life span is a measure of chronological age at death. Life expectancy is a statistically derived expectation of life span from a given point in time, usually reported as life expectancy from birth, and can be used as an alternative measure of life span without needing to wait for death to occur. Life expectancy from birth has increased exponentially in the last century. Current life expectancy in the United States at birth is 78.5 years (76.0 years for men, 81.0 years for women; https://www.worldlifeexpectancy.com). Under specific conditions, most species display gender differences in life expectancy, but these differences are often contradicted. In one study, the commonly used C57BL/6 female mice were shown to live 24% longer than males (9), while in a different location (10), males lived 8% longer (although in this and the previous study, maximum life spans were established in females). Female short-finned pilot whales live almost twice as long as male pilot whales, and in a species of vesper bats (Brandt’s bat), which can live past 40 years of age, all individuals older than the age of 20 years have been males (11,12).
Humans are the one species where a consistent, gender-specific survival advantage exists. Life span in women is on average longer than the life span in men. The difference between the life expectancy of men and women is 6.4% at birth, 4.3% at age 50, and less than 1% at age 85. While women consistently live longer than men, women generally appear to be in poorer health than men throughout adult life. This phenomenon, seen only in humans, is termed the “mortality–morbidity paradox” (13–15). Contemporary extensions of chronological life span have often been accredited to modern social and medical advances. However, increasing life expectancies tend to be accompanied with increasing morbidity. Taking an antibiotic drug could prevent you from dying from a current infection (decrease mortality), but does little to improve the function of your immune system to combat future challenges. Chronological age is simply a measure of time and does not care if you just ran a marathon or are in a hospital on life support, chronological time marches on and so does your chronological age, independent of your current health status. Often, chronological age is often the only data type available for epidemiology studies and is therefore considered the major risk factor for the development of chronic diseases. Both chronological aging and biological aging contribute to substantial changes in functional status, resilience, and health. However, biological processes linked to aging, in some cases may be more indicative of an individual’s current health status than chronological age; therefore, chronological age is not always a reliable indicator of biological age. We cannot modify chronological age, but biological aging can be manipulated to promote favorable function in aged individuals. An important link to biological function that can be manipulated is reproductive function.
Reproductive Function
Indeterminate growth species continue to grow throughout their entire life with increasing reproductive fitness and no traditional signs of aging and no reproductive decline with age. In indeterminate growth species such as reptiles, most fish, and many mollusks, reproductive output generally increases over time until some external cause intervenes to injure or kill the animal. Organisms with indeterminate growth can support increased reproductive competency over time and tend to have little to no postreproductive life span. Semelparous species, such as the Pacific salmon, are also said to have no postreproductive life span as they reproduce only once, which leads to their death (semelparous species have a single reproductive episode before death; iteroparous species have multiple reproductive cycles over the life span; some species can be iteroparous or semelparous, depending on environmental factors). In the postreproductive life span, the form of aging demonstrated can range from the short and dramatic degeneration displayed by the Pacific salmon to the more gradual aging seen in rodents and humans and the insignificant aging seen in crustaceans such as the lobster, which increases its size and fecundity with negligible aging or deterioration until death.
Indeterminate growth species, the transition to the sexually mature or reproductively competent state, in most cases, involves changes in the rate of mortality and aging. Once the tools required for reproduction are acquired, using these tools can have costs. Reproduction is often thought of as negatively influencing survival. Among mammals, high fecundity has historically been associated with short life spans (16). Repeated breeding by pairs of Sprague Dawley rats caused early induction of many age-related diseases, including adrenal hypertrophy and diabetes, compared with virgin rats. These “repeated breeding” rats died prematurely (17,18).
Opposition to the concept that reproduction negatively influences survival comes from eusocial mole rats, where increased sexual activity and/or reproduction doubles life expectancy in the long-lived rodent, Fukomys mechowii. Bimodal aging occurs naturally in these African mole rats. These animals are characterized by a subdivision into breeders, which can reach the age of 20 years or more, and nonbreeders, which usually die before the age of 10 (19). In this instance, “bimodal aging” refers to the divergence of survival probabilities between breeders and nonbreeders. This “breeder” influence can have gender effects. In the naked mole rat Heterocephalus glaber, a life-span advantage of breeders over nonbreeders was reported in females, but not males (20).
In eusocial animals, dominant breeding females can suppress reproduction in subordinate females (21). In the dominant females, the occurrence of reproductive cessation may be an evolutionary adaptation to allow subordinate females, with potentially superior genetics to replace the dominant female and reproduce sooner than otherwise possible. The menopausal transition in humans may reflect a switch from a “breeder” to a “nonbreeder” and bring with it the negative effects on individual survival seen in nonbreeder females.
Puberty in mammals can be thought of as a process to accelerate reproductive maturation, which may provide an evolutionary advantage in environments with high extrinsic mortality. While not universal, this early-life advantage is thought to be linked to detrimental events later in life, such as degenerative aging. In contrast to indeterminate species, in most mammals, when an individual becomes reproductively mature is the time when they possess their greatest reproductive potential, which then decreases until reproductive failure or death. Several researchers have reported that the age of puberty generally occurs at a younger age in obese girls than in nonobese girls. In adolescent and young women, the age at onset of obesity and that of menstrual irregularities was significantly correlated (22). Data suggest that the association with menstrual disorders may be more frequent in girls with the onset of excess body weight during puberty, than in those who were obese during infancy (23).
Female Reproductive Aging
Mammalian males tend to display a slow, gradual reproductive decline with age. Many female mammals show a more abrupt change, including a major-to-complete loss of fecundity at midlife, living the often-substantial remaining life span in a postreproductive state. This loss of fecundity, as documented in laboratory rodents, domestic animals, and several primates, is often thought to result mainly from a single cause, the irreversible age-related loss of ovarian oocytes, the number having been fixed during development (24). The loss of oocytes leads to a reduction in cyclic ovarian steroids and to many pathological changes, including osteoporosis, CVD, and neurodegeneration. The decrease in ovarian steroids is often associated with reproductive tissue atrophy (25), and decreased ovarian hormone levels can contribute to the development of epilepsy, a condition known as catamenial epilepsy, supporting a link between ovarian hormones and disease (26).
Similar to the influence of obesity during puberty, in adults, approximately 43% of women affected by various menstrual disorders, infertility, and recurrent miscarriages were either overweight or obese (27). Obese women had a twofold increase in the risk of infertility (increased from 1.3 in nonobese women to 2.7 in obese women) (28) and a fourfold increase in the risk of miscarriage (29). Analysis of data also suggests that the onset of ovarian failure and increased production of the follicle-stimulating hormone at menopause occurred several years earlier in obese women, compared with nonobese women (30). Conversely, inadequate caloric intake combined with high energy expenditures results in an energy deficit that stimulates compensatory mechanisms, subsequently causing suppression of reproductive function or amenorrhea (31). Increasing caloric intake alone can reverse this amenorrhea without the need to decrease energy expenditures (32). Prolonged negative energy balance or inadequate nutritional status can have permanent effects on pre- and peripubertal females, although no negative, long-term reproductive effects are normally observed in healthy adult women subjected to inadequate caloric intake.
The major increases in life span in women during the twentieth century have not translated to an increased age at menopause. The approximately 30-year increase in the life expectancy of women in developed countries during the twentieth century does not translate into an increased reproductive life span, with live births in women after age 50 still relatively rare (33). However, there is an association between childbearing in women approaching the age of 50 and increased longevity. Extended reproductive fitness may influence the rate of aging and susceptibility to age-related diseases. An example of the association of ovarian function with longevity in humans is seen in female centenarians, who have a fourfold greater likelihood of having children after the age of 40 versus females who die by the age of 73 (34). Each additional year of maternal age is associated with a 5% increase in the odds for exceptional longevity (35).
Cardiovascular disease is the leading cause of death in men and women (36,37), but it is relatively uncommon in premenopausal women who enjoy a significantly reduced risk of CVD, compared with similarly aged men. On average, women develop CVD 10–15 years later in their life span than men (38). Unfortunately, this advantage disappears after menopause as the incidence of CVD increases rapidly in women after transitioning to a postreproductive state. The incidence of CVD might be expected to increase simply due to increasing age, independent of reproductive status, but women who undergo premature menopause display an increased risk for CVD at an earlier age than women with normally occurring menopause, suggesting an age-independent reproductive link. Results from observational studies which suggest that hormone replacement therapy (HRT) provides cardioprotective effects, while well supported in the literature, have struggled to be confirmed by clinical trials. Some researchers who conducted clinical trials have even suggested an increased risk of CVD in HRT users (39) and have advised that HRT be eliminated as a tool to fight increasing rates of CVD in postmenopausal women (40). The benefits and risks of HRT manifest very differently depending on the regime of therapy used, and consistent results will likely continue to be a moving target.
Premature ovarian failure before 40 years of age sharply increased mortality (41,42). The age-adjusted odds ratio of death in women with natural menopause before age 40 was twofold greater than women reporting natural menopause at ages 50–54 (43). Women who experience early menopause (<age 45 years) increase these risks further with considerable public health impact (44). As mentioned above, insulin resistance and bone loss increase at menopause and almost two thirds of Americans with Alzheimer’s disease are women (6–8). Genetic effects on menopause include influences on the initial oocyte pool and its rate of loss through atresia during aging, as well as on hypothalamic controls that may be responsive to environmental effects, such as stress and nutrition (45).
Ovarian Function and Aging
In most reproductively mature females, the oocytes within the ovaries orchestrate a milieu of microenvironments throughout the body with the goal of supporting reproductive function. Prior to puberty and after menopause, the ovary is not yet or is no longer tasked with supporting reproduction, although it is still an integral part of female physiology. Prepubertally, the ovaries are continually recruiting oocytes and the accompanying somatic cells toward follicular development and their typical fate is atresia. The prepubertal female is not normally capable of producing offspring, but her ovaries have significant reproductive and survival potential. Her ovaries have all the oocytes and somatic cells necessary for a lifetime of offspring production and prepubertal females are rarely diagnosed with aging-associated types of diseases.
The fixed stock of postmitotic ovarian oocytes is slowly lost at a relatively constant rate during much of mammalian adult reproductive life. A minority of oocytes are ovulated, with most oocyte losses attributed to atresia (46). In humans, as menopause approaches, an accelerated loss of oocytes is observed coincident with an increase in pituitary follicle-stimulating hormone (47). Other ovarian cells, not just the oocytes, display age-related changes as well. When cultured in vitro, granulosa cells collected from women younger than 40 years of age produced significantly higher levels of hormones, than cells from women 40 years or older (48).
The formation of new oocytes through oogonial mitotic division ceases before or within a few weeks of birth in placental mammals (49,50) with a few rare exceptions in prosimians (51,52). In lorisoid primates, oogenesis persists into adult life and involves the synthesis of DNA. Oogonia in all stages of mitosis, primary oocytes in meiotic prophase, as well as germ cells undergoing atresia have been observed in the population of germ cell nests in all adult lorisoids studied (53), although it has been difficult to find adequate evidence to support the view that the newly formed germ cells become transformed into definitive oocytes (54). It has been postulated that putative oogonial stem cells exist in adult ovarian cortex tissues and that these mitotically active cells can be used to produce new oocytes (55,56). This concept is still very controversial. However, germline stem cells exist in the testes of adult human males (spermatogonia) and in the adult ovaries of many organisms. The exceptions in prosimians, when combined with recent efforts to define germinal stem cells in the adult ovary, suggest that the potential for postnatal oogenesis cannot be excluded (57,58). Therefore, it is logical to anticipate that “new” oocytes may potentially be derived from the tissues of adult ovaries for therapeutic use in the future. However, the occurrence of menopause implies that if oogonial stem cells are indeed present, they do not divide at a rate that supports maintenance of the oocyte pool.
At menopause, most of the oocytes have been exhausted along with the accompanying somatic cells, leaving a relatively quiescent ovary behind. However, the menopausal ovary still possesses reproductive and survival potential. Like the prepubertal female, the menopausal female is not normally capable of producing offspring, but her quiescent ovaries still maintain a slight reproductive potential, demonstrated by the ability to reproduce under laboratory conditions. And while menopausal women are often diagnosed with aging-associated diseases, the “depleted” ovary is only depleted from a fertility perspective, as it still provides significant health benefits. This is clearly demonstrated by the observation that women who undergo natural menopause have a significant health advantage over women who undergo surgical menopause (59). Elective ovariectomy, performed before the age of 65, is associated with an increased risk of death from heart disease and is associated with an increased risk of death from lung cancer for women of all ages. Heart disease is the primary cause of death among pre- and postmenopausal women in the United States, followed closely by cancer (60). The observation that menopause due to surgery is associated with an increased risk of disease, compared with natural menopause suggests that even postreproductive, germ cell-exhausted ovaries provide a health advantage, independent of the ovarian components necessary for reproduction, active germ cells (60–62). The role of ovarian function in disease is further emphasized by the observation that the beneficial effects of dietary restriction on glucose and triglyceride metabolism present in ovary-intact female rodents, do not appear in the tissues of ovariectomized rodents, supporting a central role for the ovary in female metabolic health (63). Based on these observations, disease risk in females is negatively correlated with reproductive potential throughout the life span. As reproductive potential decreases, disease risk increases.
Observations in support of this concept are seen in instances where we are able to decrease or increase the reproductive potential of an individual, independent of chronological age. Currently, ovarian tissue transplants are used to restore fertility in many types of patients. Approximately 70% of patients who underwent ovarian tissue transplantation (OTT) had restoration of ovarian and endocrine function, and pregnancy was reported with 52% of the patients. OTT is a useful approach to restore hormonal function, endocrine balance, and fertility in patients who are predisposed to lose their fertility or diagnosed with POF, as well as women undergoing cancer treatments. In a 2018 study (64), 360 OTT procedures in 318 women from 21 different countries reported less than 3% of cancer recurrence. Renewed ovarian endocrine function was reported in 95% of the women. Half of all children born following OTT resulted from natural conception. Newborns were reported to be healthy (with the exception of one newborn with a familial chromosome anomaly). In the future, tissue and/or cell transplantation procedures may be utilized, in addition to extending fertility, to improve health and decrease aging/menopause-associated increases in disease risks.
Manipulation of Reproductive Function
Decreasing Reproductive Potential
Probably the most easily understood and widely known manipulation of reproduction involves castration or removal of the gonads. Castration eliminates the potential to reproduce in the castrated individual. Individuals who suffer from early reproductive failure/loss of reproductive potential often suffer from an early onset of many “aging-related” diseases when they themselves are not considered “old.” The removal of reproductive potential through castration can have divergent gender- and species-specific effects on survival. In mice, castration can have a negative influence on females, but a positive influence on males (65). In dogs, females hold a longevity advantage over males, which is erased if the females are castrated within the first 4 years of life (66). Castration in dogs can increase aging-associated disease in both genders, and when followed to natural death, intact dogs of both genders can outlive their castrated counterparts (67,68). In humans, studies on castration are very difficult to conduct. There is much controversy surrounding the speculation that castration in males extends life span, with most of the information coming from historical accounts of prisoners and eunuchs. There is little confirmable data from the many studies of eunuchs indicating that castration has any effect on the life span of men (69,70). However, more recent studies suggest that androgen-depletion therapy, used to treat prostatic hyperplasia, appears to accelerate aging in men.
In women, castration is often considered around the time of menopause as prophylaxis to eliminate the chances of developing ovarian cancer or breast cancer or in conjunction with hysterectomy (removal of the uterus). Oophorectomy (surgical removal of an ovary or ovaries), also called ovariectomy, is often done at this time and eliminates the chances of developing ovarian cancer, but also significantly increases all-cause, long-term mortality (59). As mentioned previously, naturally menopausal women hold a significant health advantage over surgically menopausal women. It can be argued that surgically menopausal women often became menopausal at an earlier age or had an underlying cause for removal of the ovaries, but the age-adjusted data still support a beneficial role for postmenopausal ovaries in health and survival. The increase in disease risk from ovariectomy is not limited to women who have ovariectomy performed before menopause; an impact on survival is seen for surgeries performed up to the age of 65 (71), long after the normal time of reproductive cessation. Interestingly, women with primary ovarian insufficiency (POI) suffer from increased mortality whereas women with polycystic ovary syndrome (PCOS) often experience normal life spans. Women diagnosed as having PCOS can have normal life spans with no increased risk of cancer or heart disease. However, diabetes later in life is more common in women with PCOS, likely due to dihydrotestosterone-induced hyperglycemia (72). A major difference between PCOS and POI groups is the loss of reproductive potential with POI (accelerated follicle exhaustion) and the preservation of reproductive potential (in most cases) with PCOS (anovulation, most often with follicle preservation).
Increasing Reproductive Potential
A common method for the extension of reproductive potential is nutritional restriction. This “extension” could also be called the “delay” of reproductive potential because it often incorporates a period of ovarian inactivity or anovulation. The female’s nutrition is simply restricted to a level that decreases or eliminates ovarian cycling, thereby preserving ovarian oocytes and somatic cells for later use. In mice, caloric restriction increases the preservation of the ovarian primordial follicular reserve and extends reproductive potential (73). During restriction, the animal normally has decreased fecundity, but upon a return to ad libitum feeding, can produce offspring at an age when fully fed animals have ceased reproduction. By extending or delaying the reproductive potential to later in the life span, survival is often concomitantly increased/extended in nutritionally restricted animals.
Another method for extending reproductive potential (often unintentional) is exercise-induced anovulation. In this scenario, high levels of exercise cause ovarian cycling to decrease or cease entirely. This is not an uncommon occurrence in elite athletes. These high levels of exercise can also cause delayed puberty in young females. It can be difficult to discern if there are any health benefits from exercise-induced anovulation because of the very high levels of health these women attain, compared with the general population. Extending ovarian function can also be accomplished through selective breeding. In the female medfly Ceratitis capitata, age-related changes in the rate of egg laying predict subsequent mortality. Flies that were selected for laying eggs at advanced ages displayed extended longevity. Extending reproductive potential lowered the subsequent mortality (74). A similar phenomenon occurs in humans, where late childbearing (after age 40) is associated with increased survival among very old women (75).
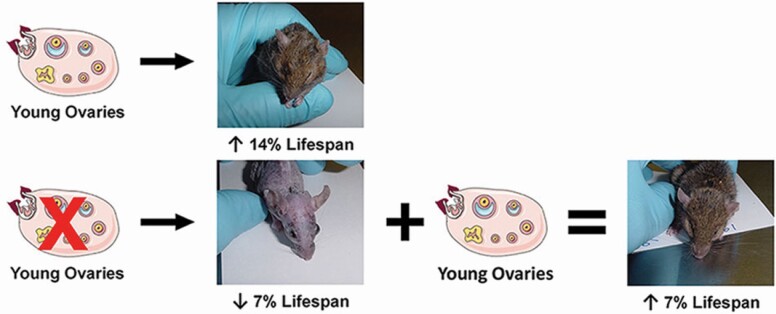
Reproductive potential has also been extended/delayed in an experimental model used previously in our laboratory. In these experiments, mice were ovariectomized prepubertally. When these ovariectomized mice reached the age of normal reproductive cessation (11 months), young, 60-day-old ovaries were transplanted back to their ovarian bursae (the mouse ovary is enclosed within a bursal sack). In this way, these mice began their reproductive life span at 11 months of age and benefited from a delayed reproductive potential. These mice demonstrated increased survival, compared with sham-operated and control mice. However, these mice also suffered the consequences of having no ovaries for the first 11 months of their life and, as mentioned previously, ovariectomy generally has a negative influence on an animal’s health and survival. In this animal model, the positive effects of extended reproductive potential were partially offset by the negative effects of early-life ovariectomy (Figure 1 (76)).
Figure 1.
Increasing and decreasing reproductive potential have opposing survival influences. Mice ovariectomized at 21 days displayed a 7% decrease in life span. Intact mice that received young replacement ovaries at 335 days displayed a 14% increase in life span. Mice ovariectomized at 21 days and that then received young ovaries at 335 days had a 7% increase in life span. Images were taken within 7 days of natural death for each mouse.
Experiments that preceded this research included transplanting young ovaries to mice at various ages (77). These experiments revealed a “dose–response” to the transplanted ovaries in reproductive-aged recipients. A mouse that received a young ovary at 8 months of age would demonstrate reduced chronological life span benefit, compared to a mouse that received a young ovary at 11 months of age. The remaining life expectancy at 11 months of age was 231 days in ovariectomized mice, 246 days for mice that received new ovaries at 5 months of age, 286 days for mice that received new ovaries at 8 months of age, and 367 days for mice that received new ovaries at 11 months of age (77). However, this dose–response was lost after the time of normal reproductive cessation (approximately 11–12 months in CBA/J females), as mice that received young ovaries at 17 or 18 months of age demonstrated the same life span extension (within 2%) as mice that received young ovaries at 11 months of age (78).
An increase in reproductive potential, in contrast to the previous “extension/delay” of reproductive potential, was accomplished using very similar surgical techniques. In the 2003 study by Cargill et al. (77), ovariectomized mice displayed a decreased life expectancy, compared with intact control mice. The decreased life-expectancy effects observed in ovariectomized mice in the study by Cargill et al., combined with the lack of life-span benefit in gonadectomized Caenorhabditis elegans (79), suggested that a gonad-intact transplant model might display increased longevity, compared with an ovariectomized transplant model. The study by Mason et al. (76) included ovarian transplantations in mice that were gonad-intact at the time of transplant. Essentially, these mice experienced 2 reproductive life spans back-to-back. In this study, only 29% of transplant females died in the 450-day period following ovarian transplantation, whereas 90% of the postreproductive sham-treated females died during this same period. These results demonstrate that increasing reproductive potential in old mice enhanced longevity. Additional analysis with this model revealed that replacing aged ovaries in postreproductive mice with young, actively cycling ovaries restored many health benefits, including restoration of cardiovascular health (80), orthopedic health (81), decreased sarcopenia (82,83), improved immune and renal function (84), improved cognitive behavior and sensory function (85), reduced inflammation (78), improved glucose metabolism (86), and extended life span (76,78).
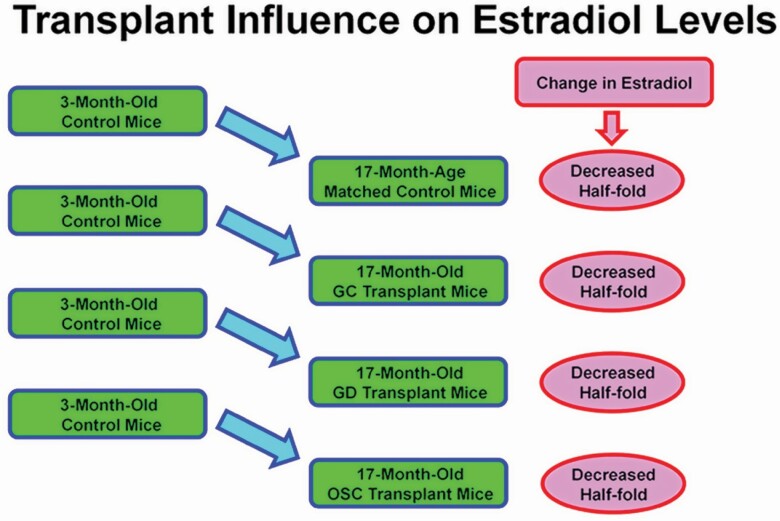
The observation that replacement of the aged ovaries of postreproductive female mice with young ovaries significantly increased health span is robust. However, the question remained; what were the factors responsible for this ovary-dependent enhancement of health? We originally hypothesized that this phenomenon was driven by germ cell-stimulated ovarian hormone production from the new, transplanted ovaries. The well-established supportive role of ovarian hormones in many aspects of female health implicates the loss of hormone production from actively cycling germ cells, as the principal cause of increased disease risks at menopause. While the value of ovarian hormones in female health is unquestionable, efforts to replace the hormonal milieu of actively cycling ovaries in peri- and postmenopausal women have struggled to reliably restore the health benefits enjoyed by young women with young ovaries. Preliminary data from our laboratory suggest that estrogen and androgen levels are not changed in recipients of new ovaries, compared with age-matched controls (Figure 2).
Figure 2.
Extension of health and life span is not estradiol-dependent. Estradiol levels decreased by approximately 50% from young (3 months) control mice to postreproductive controls (17 months). In all transplant recipients, estradiol was not different from age-matched control mice (±4%). GC = received germ cell-containing 60 day ovaries at 13 months; GD = received germ cell-depleted 60 day ovaries at 13 months; OSC = received isolated somatic cells from 60 day ovaries at 13 months. (Estradiol was collected at the diestrus stage of the estrous cycle in cycling mice.).
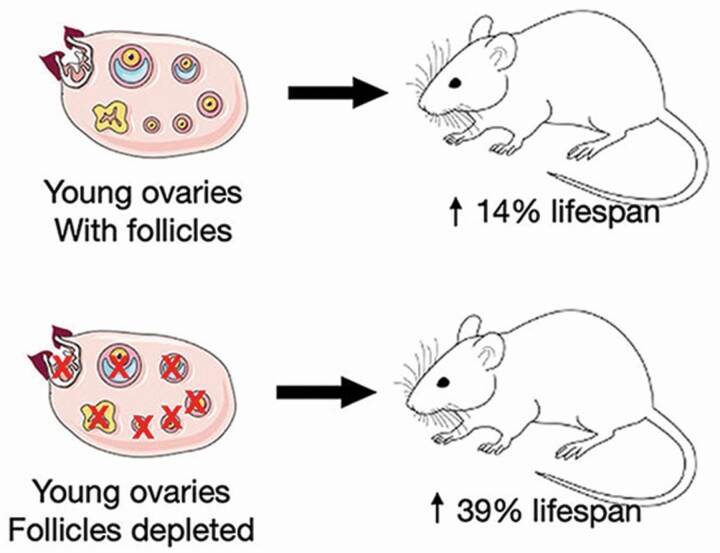
Results from castration experiments are influenced by the removal of ovarian-produced hormones, but also by the removal of the influence of the somatic gonad. In humans, at menopause, germ cell-exhausted ovaries still possess health-promoting attributes, as removal of the postreproductive ovaries further increases rates of mortality (59,60). If the mechanisms influencing longevity are evolutionarily conserved, evidence from model organisms argues against a germ cell/estrogen-only explanation for health span extension in mammals. In both worms and flies, gonadal germ cells act in the adult to negatively influence life span. In the hermaphroditic worm C. elegans, removal of the germ cells, while leaving the somatic gonad intact, results in increased life span, but removal of the entire gonad (castration), including the somatic tissue, yields no change (87). Similar results are found in the lesser fruit fly, Drosophila melanogaster (88). In both species, the somatic gonad promotes health span, whereas the germ components of the gonad act to suppress it. We addressed this phenomenon in a mammalian model by chemically depleting ovarian germ cells in prepubertal ovaries prior to transplanting the young ovaries to postreproductive mice (89). Germ cell depletion did not diminish the health benefits of the young, transplanted ovaries and further extended the longevity benefits past that of transplanted young, germ cell-containing ovaries (Figure 3, 880 vs 790 days, respectively). The surgical transplantation of germ cell-depleted ovaries provided young ovarian somatic cells, but no cyclic hormone supply. These transplanted germ cell-depleted ovaries did not go through the normal ovarian aging process (no follicular cycling), but did “reset” the health and life span clocks and extended life span to a greater degree than with the germ cell-containing ovaries (78). These experiments demonstrated that the female health advantage was not entirely germ cell/follicle hormone-dependent and that it was “transplantable.”
Figure 3.
Germ cell depletion extended life span. Depleting germ cells prior to transplantation of young ovaries extended life span 39% past controls and 25% past mice that received germ cell-containing ovary transplants. GC = received germ cell-containing 60 day ovaries at 17 months; GD = received germ cell-depleted 60 day ovaries at 17 months.
Alzheimer’s disease is the sixth leading cause of death in the United States and, as mentioned previously, two thirds of Americans with Alzheimer’s disease are women (8). The early symptoms of Parkinson’s disease and Lewy body dementia resemble those found in Alzheimer’s disease and often include an increased occurrence of tremors (90). Estrogen replacement therapy has been reported to improve the symptoms of Alzheimer’s disease (91). In contrasting studies, postmenopausal hormone use was shown to increase Parkinson’s disease risk (92). In our ovarian transplant recipient mice, tremor amplitude was decreased at most frequencies in all mice that received young ovarian tissue transplants, both with and without germ cells.
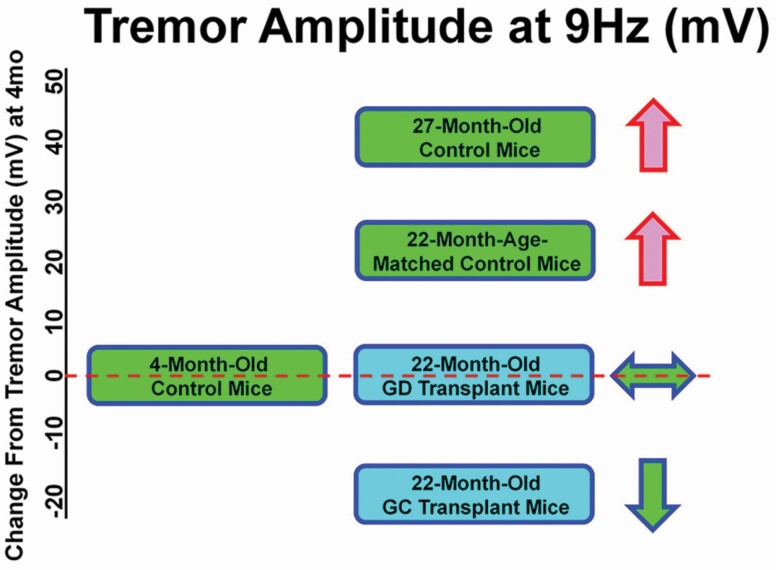
Peak tremor amplitude occurred at a frequency of 9 Hz in most groups and was significantly decreased in transplant recipients, compared with age-matched controls in both C57BL/6 and CBA/J strain female mice (unpublished data, Figure 4). This suggests a hormone-independent mechanism for the development of tremors in aged female mice. In these experiments, only the ovary was surgically changed.
Figure 4.
Age and treatment changes in tremor amplitude. Tremor amplitude increased with age in age-matched controls (22 months) and increased further in old (27 months) mice, but did not increase in 22 months transplant recipients at most frequencies. Young control = 3 months; Age-matched controls = 22 months; Old control = 27 months; GC = 22 months, received 60 day germ cell-containing ovaries at 17 months; GD = 22 months, received 60 day germ cell-depleted ovaries at 17 months.
The life span benefit in germ cell-depleted C. elegans requires DAF-16, a forkhead transcription factor known to be a target of insulin-like signaling (79). While only one FOXO gene (DAF-16) exists in C. elegans, 4 FoxO genes are present in mice and humans (FoxO1, 3, 4, and 6). These 4 transcription factors are involved in multiple cellular pathways, and several studies have consistently identified FoxO1 and FoxO3a as “longevity genes” in humans (93,94). Interestingly, in the study by Li et al. (94), FoxO1 was a significant factor in female, but not male longevity. Increased expression of FoxO3a is necessary for the extension of life span with dietary restriction (95). Increased FoxO1 activity is responsible for protection against oxidative and genotoxic stress (96). The expression of FoxO1 in gonadal somatic cells appears to be highly conserved, suggesting an important functional role for FoxO1 in somatic cells (97). Both FoxOs have been shown to benefit health span, albeit differentially. The observation that FoxO3 has been reported to be expressed in granulosa cells rather than oocytes in human ovaries and that FoxO1 is expressed in mouse somatic cells, but not oocytes, suggests a potential role for FoxO1 in the extension of health and longevity in germ cell-depleted models (97).
The ovary interacts with many, if not most body systems, leaving us guessing as to the mechanisms of the “transplantable” female health advantage. Our results with ovarian germ cell depletion in a mammalian model reflect what was seen previously in primitive species (C. elegans and D. melanogaster) and suggest that a conserved survival mechanism closely links reproduction, health, and survival. This research also provides promise for the currently irreversible increase of disease rates at menopause.
Conclusions
The average age of first-time mothers in developed countries is 31, the same age when the fall in fecundity in women is estimated to start. In addition, the probability of having a healthy baby decreases by 3.5% per year after the age of 30. Combining both of these age effects, the chance of a woman at the age of 35 of having a healthy baby is about half that of a woman aged 25 (98). Extending reproductive potential has the potential to improve fertility and health for the woman and to improve the health of her baby.
In summary, female reproductive aging is not universal, is manifested differently in many different organisms, and can be manipulated both positively and negatively. In addition, the female health advantage appears to be germ cell and ovarian hormone-independent and transplantable. Ovarian tissue is currently benefiting many women for resumption of fertility, and it may also possess the translational potential to extend the human life span and decrease menopause-associated disease risks.
Funding
This work was supported by the National Institute on Aging of the National Institutes of Health (grant number R15AG061795 to J.B.M.); the San Antonio Nathan Shock Center Pilot Funding Program; the Oklahoma Nathan Shock Center Pilot Funding Program; the Utah Agricultural Experiment Station, Utah State University; and by the School of Veterinary Medicine, Department of Animal, Dairy and Veterinary Sciences, Utah State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Dr. Aaron Olsen, Mrs. Lisa DeSoi, and Kate Parkinson for help with the mice. Additionally, the authors thank the Utah Science Technology and Research Initiative, Utah State University, School of Veterinary Medicine, and the Department of Animal, Dairy, and Veterinary Sciences.
References
- 1. National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Fertility and Infertility Branch. New Research Priorities. Fertility status as a marker of overall health. https://www.nichd.nih.gov/about/org/der/branches/fib. [Google Scholar]
- 2. Eisenberg ML, Li S, Behr B, et al. . Semen quality, infertility and mortality in the USA. Hum Reprod. 2014;29(7):1567–1574. doi: 10.1093/humrep/deu106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thom T, Haase N, Rosamond W, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600 [DOI] [PubMed] [Google Scholar]
- 4. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65(2):161–166. doi: 10.1016/j.maturitas.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52(4):303–307. doi: 10.1016/s0895-4356(98)00170-x [DOI] [PubMed] [Google Scholar]
- 6. Kulaksizoglu M, Ipekci SH, Kebapcilar L, et al. . Risk factors for diabetes mellitus in women with primary ovarian insufficiency. Biol Trace Elem Res. 2013;154(3):313–320. doi: 10.1007/s12011-013-9738-0 [DOI] [PubMed] [Google Scholar]
- 7. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4 [DOI] [PubMed] [Google Scholar]
- 8. Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32(4):604–613. doi: 10.1016/j.neurobiolaging.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheney KE, Liu RK, Smith GS, Leung RE, Mickey MR, Walford RL. Survival and disease patterns in C57BL/6J mice subjected to undernutrition. Exp Gerontol. 1980;15(4):237–258. doi: 10.1016/0531-5565(80)90029-7 [DOI] [PubMed] [Google Scholar]
- 10. Tanaka S, Segawa T, Tamaya N, Ohno1 T. Establishment of an aging farm of F344/N rats and C57BL/6 mice at the National Institute for Longevity Sciences (NILS). Arch Gerontol Geriatr. 2000;30(3):215–223. doi: 10.1016/s0167-4943(00)00053-4 [DOI] [PubMed] [Google Scholar]
- 11. Podlutsky AJ, Khritankov AM, Ovodov ND, Austad SN. A new field record for bat longevity. J Gerontol A Biol Sci Med Sci. 2005;60(11):1366–1368. doi: 10.1093/gerona/60.11.1366 [DOI] [PubMed] [Google Scholar]
- 12. Kasuya T, Marsh H. Life history and reproductive biology of the short-finned pilot whale, Globicephala macrorhynchus, off the Pacific coast of Japan. Rep Int Whal Comm (Special Issue). 1984;6:259–310. [Google Scholar]
- 13. Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–1208. doi: 10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Macintyre S, Ford G, Hunt K. Do women ‘over-report’ morbidity? Men’s and women’s responses to structured prompting on a standard question on long standing illness. Soc Sci Med. 1999;48(1):89–98. doi: 10.1016/s0277-9536(98)00292-5 [DOI] [PubMed] [Google Scholar]
- 15. Verbrugge LM. Women, men, and osteoarthritis. Arthritis Care Res. 1995;8(4):212–220. doi: 10.1002/art.1790080404 [DOI] [PubMed] [Google Scholar]
- 16. Finch CE. Longevity, Senescence, and the Genome. University of Chicago Press; 1990. doi: 10.1002/ajhb.1310070517 [DOI] [Google Scholar]
- 17. Wexler BC, Iams SG, Judd JT. Arterial lesions in repeatedly bred spontaneously hypertensive rats. Circ Res. 1976;38(6):494–501. doi: 10.1161/01.res.38.6.494 [DOI] [PubMed] [Google Scholar]
- 18. Wexler BC, McMurtry JP. Genetically mediated resistance to naturally occurring aortic sclerosis in spontaneously hypertensive as against Sprague-Dawley and Wistar-Kyoto breeder rats. Br J Exp Pathol. 1982;63(1):66–81. PMID: 7066185; PMCID: PMC2040749. [PMC free article] [PubMed] [Google Scholar]
- 19. Sahm A, Platzer M, Koch P, et al. . Increased longevity due to sexual activity in mole-rats is associated with transcriptional changes in the HPA stress axis. eLife. 2021. ;10:e57843. PMID: 33724179; PMCID: PMC8012063. doi: 10.7554/eLife.57843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruby JG, Smith M, Buffenstein R. Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age. eLife. 2018;7:e31157. doi: 10.7554/eLife.31157.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toor I, Edwards PD, Kaka N, et al. . Aggression and motivation to disperse in eusocial naked mole-rats, Heterocephalus glaber. Anim Behav. 2020;168:45–58. doi: 10.1016/j.anbehav.2020.07.022 [DOI] [Google Scholar]
- 22. Pasquali R, Casimirri F. The impact of obesity on hyperandrogenism and polycystic ovary syndrome in premenopausal women. Clin Endocrinol (Oxf). 1993;39(1):1–16. doi: 10.1111/j.1365-2265.1993.tb01744.x [DOI] [PubMed] [Google Scholar]
- 23. Lake JK, Power C, Cole TJ. Women’s reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432–438. doi: 10.1038/sj.ijo.0800424 [DOI] [PubMed] [Google Scholar]
- 24. Harman SM, Talbert GB. Reproductive aging. In: Finch CE, Schneider EL, eds. The Handbook of the Biology of Aging. 2nd ed. Van Nostrand; 1985:457–510. [Google Scholar]
- 25. Steger RW, Huang H, Meites J. Reproduction. In: Massoro EJ, ed. Handbook of Physiology in Aging. CRC Press; 1981:333–382. [Google Scholar]
- 26. Hajszan T, MacLusky NJ. Neurologic links between epilepsy and depression in women: is hippocampal neuroplasticity the key? Neurology. 2006;66(6suppl 3):S13–S22. doi: 10.1212/wnl.66.66_suppl_3.s13 [DOI] [PubMed] [Google Scholar]
- 27. Rogers J, Mitchell GW Jr. The relation of obesity to menstrual disturbances. N Engl J Med. 1952;247(2):53–55. doi: 10.1056/NEJM195207102470204 [DOI] [PubMed] [Google Scholar]
- 28. Rich-Edwards JW, Goldman MB, Willett WC, et al. . Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171–177. doi: 10.1016/0002-9378(94)90465-0 [DOI] [PubMed] [Google Scholar]
- 29. Bellver J, Rossal LP, Bosch E, et al. . Obesity and the risk of spontaneous abortion after oocyte donation. Fertil Steril. 2003;79(5):1136–1140. doi: 10.1016/s0015-0282(03)00176-6 [DOI] [PubMed] [Google Scholar]
- 30. Norman RJ, Clark AM. Obesity and reproductive disorders: a review. Reprod Fertil Dev. 1998;10(1):55–63. doi: 10.1071/r98010 [DOI] [PubMed] [Google Scholar]
- 31. McLean JA, Barr SI. Cognitive dietary restraint is associated with eating behaviors, lifestyle practices, personality characteristics and menstrual irregularity in college women. Appetite. 2003;40(2):185–192. doi: 10.1016/s0195-6663(02)00125-3 [DOI] [PubMed] [Google Scholar]
- 32. Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab. 2001;86(11):5184–5193. doi: 10.1210/jcem.86.11.8024 [DOI] [PubMed] [Google Scholar]
- 33. Brody JA, Grant MD, Frateschi LJ, Miller SC, Zhang H. Reproductive longevity and increased life expectancy. Age Ageing. 2000;29(1):75–78. doi: 10.1093/ageing/29.1.75 [DOI] [PubMed] [Google Scholar]
- 34. Perls TT. Centenarians prove the compression of morbidity hypothesis, but what about the rest of us who are genetically less fortunate? Med Hypotheses. 1997;49(5):405–407. doi: 10.1016/s0306-9877(97)90086-4 [DOI] [PubMed] [Google Scholar]
- 35. Sun F, Sebastiani P, Schupf N, et al. . Extended maternal age at birth of last child and women’s longevity in the Long Life Family Study. Menopause. 2015;22(1):26–31. doi: 10.1097/GME.0000000000000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349(9063):1436–1442. doi: 10.1016/S0140-6736(96)07495-8 [DOI] [PubMed] [Google Scholar]
- 37. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossouw JE. Hormones, genetic factors, and gender differences in cardiovascular disease. Cardiovasc Res. 2002;53(3):550–557. doi: 10.1016/s0008-6363(01)00478-3 [DOI] [PubMed] [Google Scholar]
- 39. Ballard VL, Edelberg JM. Harnessing hormonal signaling for cardioprotection. Sci Aging Knowledge Environ. 2005;2005(51):re6. doi: 10.1126/sageke.2005.51.re6 [DOI] [PubMed] [Google Scholar]
- 40. Dull P. Hormone replacement therapy. Prim Care. 2006;33(4):953–963. doi: 10.1016/j.pop.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 41. Kalantaridou SN, Davis SR, Nelson LM. Premature ovarian failure. Endocrinol Metab Clin North Am. 1998;27(4):989–1006. doi: 10.1016/s0889-8529(05)70051-7 [DOI] [PubMed] [Google Scholar]
- 42. Finch CE, Kirkwood TBL.. Chance, Development, and Aging. Oxford University Press; 2000:94–97. [Google Scholar]
- 43. Snowdon DA, Kane RL, Beeson WL, et al. . Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79(6):709–714. doi: 10.2105/ajph.79.6.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tunstall-Pedoe H. Myth and paradox of coronary risk and the menopause. Lancet. 1998;351(9113):1425–1427. doi: 10.1016/S0140-6736(97)11321-6 [DOI] [PubMed] [Google Scholar]
- 45. Wise PM, Smith MJ, Dubal DB, Wilson ME, Krajnak KM, Rosewell KL. Neuroendocrine influences and repercussions of the menopause. Endocr Rev. 1999;20(3):243–248. doi: 10.1210/edrv.20.3.0364 [DOI] [PubMed] [Google Scholar]
- 46. vom Saal F, Finch C. Reproductive senescence: phenomena and mechanisms in mammals and selected vertebrates. In: Knobil E, Neill J, Pfaff D, eds. Physiology of Reproduction. Raven Press; 1988:2351–2413. [Google Scholar]
- 47. Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition—evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231 [DOI] [PubMed] [Google Scholar]
- 48. Pellicer A, Simón C, Remohí J. Effects of aging on the female reproductive system. Hum Reprod. 1995;10(suppl 2):77–83. doi: 10.1093/humrep/10.suppl_2.77 [DOI] [PubMed] [Google Scholar]
- 49. Tokarz RR. Oogonial proliferation, oogenesis, and folliculogenesis in non-mammalian vertebrates. In: Jones RE, ed. The Vertebrate Ovary: Comparative Biology and Evolution. Plenum; 1978:145–179. [Google Scholar]
- 50. Tilly JL. Apoptosis and ovarian function. Rev Reprod. 1996;1(3):162–172. doi: 10.1530/ror.0.0010162 [DOI] [PubMed] [Google Scholar]
- 51. Butler H, Juma MB. Oogenesis in an adult prosimian. Nature. 1970;226(5245):552–553. doi: 10.1038/226552a0 [DOI] [PubMed] [Google Scholar]
- 52. Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev. 1986;66(1):71–117. doi: 10.1152/physrev.1986.66.1.71 [DOI] [PubMed] [Google Scholar]
- 53. Kumar TC. Oogenesis in adult prosimian primates. Contrib Primatol. 1974;3:82–96. PMID: 4610679. [PubMed] [Google Scholar]
- 54. Bukovsky A, Svetlikova M, Caudle MR. Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol. 2005;3:17. doi: 10.1186/1477-7827-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi: 10.1038/nature02316 [DOI] [PubMed] [Google Scholar]
- 56. Martin JJ, Woods DC, Tilly JL. Implications and current limitations of oogenesis from female germline or oogonial stem cells in adult mammalian ovaries. Cells. 2019;8:93. doi: 10.3390/cells8020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421. doi: 10.1038/nm.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med. 2012;18(3):353–354. doi: 10.1038/nm.2699 [DOI] [PubMed] [Google Scholar]
- 59. Parker WH, Feskanich D, Broder MS, et al. . Long-term mortality associated with oophorectomy compared with ovarian conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121(4):709–716. doi: 10.1097/AOG.0b013e3182864350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parker WH, Broder MS, Chang E, et al. . Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113(5):1027–1037. doi: 10.1097/AOG.0b013e3181a11c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157–161. doi: 10.7326/0003-4819-89-2-157 [DOI] [PubMed] [Google Scholar]
- 62. Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316(18):1105–1110. doi: 10.1056/NEJM198704303161801 [DOI] [PubMed] [Google Scholar]
- 63. Casalino SM, Linares JA, Goldraij A. Different effect of a restricted diet on isolated uteri of ovariectomized and non-ovariectomized rats. Influence of indomethacin and prostaglandins. Prostaglandins Leukot Essent Fatty Acids. 1994;51(1):41–45. doi: 10.1016/0952-3278(94)90176-7 [DOI] [PubMed] [Google Scholar]
- 64. Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding Andersen C. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018;35(4):561–570. doi: 10.1007/s10815-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Benedusi V, Martini E, Kallikourdis M, Villa A, Meda C, Maggi A. Ovariectomy shortens the life span of female mice. Oncotarget. 2015;6(13):10801–10811. doi: 10.18632/oncotarget.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waters DJ, Kengeri SS, Clever B, et al. . Exploring mechanisms of sex differences in longevity: lifetime ovary exposure and exceptional longevity in dogs. Aging Cell. 2009;8(6):752–755. doi: 10.1111/j.1474-9726.2009.00513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Torres de la Riva G, Hart BL, Farver TB, et al. . Neutering dogs: effects on joint disorders and cancers in golden retrievers. PLoS One. 2013;8(2):e55937. doi: 10.1371/journal.pone.0055937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hart RW, Turturro A. Evolution and dietary restriction. Exp Gerontol. 1998;33(1–2):53–60. doi: 10.1016/s0531-5565(97)00063-6 [DOI] [PubMed] [Google Scholar]
- 69. Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24(3):313–340. doi: 10.1210/er.2003-0005 [DOI] [PubMed] [Google Scholar]
- 70. Corona G, Rastrelli G, Monami M, et al. . Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687–701. doi: 10.1530/EJE-11-0447 [DOI] [PubMed] [Google Scholar]
- 71. Erekson EA, Martin DK, Ratner ES. Oophorectomy: the debate between ovarian conservation and elective oophorectomy. Menopause. 2013;20(1):110–114. doi: 10.1097/gme.0b013e31825a27ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Caldwell ASL, Edwards MC, Desai R, et al. . Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2017;114(16):E3334–E3343. doi: 10.1073/pnas.1616467114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Garcia D, Saccon T, Rincon J, et al. . Effect of caloric restriction and rapamycin on ovarian aging in mice. Innov Aging. 2019;3(suppl 1):S103. doi: 10.1007/s11357-019-00087-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Müller HG, Carey JR, Wu D, Liedo P, Vaupel JW. Reproductive potential predicts longevity of female Mediterranean fruitflies. Proc Biol Sci. 2001;268(1466):445–450. doi: 10.1098/rspb.2000.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yi Z, Vaupel J. Association of late childbearing with healthy longevity among the oldest-old in China. Popul Stud (Camb). 2004;58(1):37–53. doi: 10.1080/0032472032000175437 [DOI] [PubMed] [Google Scholar]
- 76. Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci. 2009;64(12):1207–1211. doi: 10.1093/gerona/glp134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cargill SL, Carey JR, Müller HG, Anderson G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2(3):185–190. doi: 10.1046/j.1474-9728.2003.00049.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Habermehl TL, Parkinson KC, Hubbard GB, et al. . Extension of longevity and reduction of inflammation is ovarian-dependent, but germ cell-independent in post-reproductive female mice. Geroscience. 2019;41(1):25–38. doi: 10.1007/s11357-018-0049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399(6734):362–366. doi: 10.1038/20694 [DOI] [PubMed] [Google Scholar]
- 80. Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR. Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell. 2011;10(3):448–456. doi: 10.1111/j.1474-9726.2011.00691.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mason JB, Terry BC, Merchant SS, Mason HM, Nazokkarmaher M. Manipulation of ovarian function significantly influenced trabecular and cortical bone volume, architecture and density in mice at death. PLoS One 2015;10:15. doi: 10.1371/journal.pone.0145821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Peterson RL, Parkinson KC, Mason JB. Manipulation of ovarian function significantly influenced sarcopenia in postreproductive-age mice. J Transplant. 2016;2016:4570842. doi: 10.1155/2016/4570842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Habermehl TL, Mason JB. Decreased sarcopenia in aged females with young ovary transplants was preserved in mice that received germ cell-depleted young ovaries. J Clin Med. 2019b;8(1):40. doi: 10.3390/jcm8010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Peterson RL, Parkinson KC, Mason JB. Restoration of immune and renal function in aged females by re-establishment of active ovarian function. Reprod Fertil Dev. 2017;29(10):2052–2059. doi: 10.1071/RD16333 [DOI] [PubMed] [Google Scholar]
- 85. Parkinson KC, Peterson RL, Mason JB. Cognitive behavior and sensory function were significantly influenced by restoration of active ovarian function in postreproductive mice. Exp Gerontol. 2017;92:28–33. doi: 10.1016/j.exger.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 86. Tyler KA, Habermehl TL, Mason JB. Manipulation of ovarian function influenced glucose metabolism in CBA/J mice. Exp Gerontol. 2019;126:110686. doi: 10.1016/j.exger.2019.110686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295(5554):502–505. doi: 10.1126/science.1065768 [DOI] [PubMed] [Google Scholar]
- 88. Flatt T, Min KJ, D’Alterio C, et al. . Drosophila germ-line modulation of insulin signaling and life span. Proc Natl Acad Sci U S A. 2008;105(17):6368. doi: 10.1073/pnas.0709128105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda). 2016;31(4):250–257. doi: 10.1152/physiol.00057.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Louis ED, Benito-Leon J, Faust PL. Essential tremor seems to be a risk factor for Parkinson’s disease. Parkinsonism Relat Disord. 2016;26:82–83. doi: 10.1016/j.parkreldis.2016.02.026 [DOI] [PubMed] [Google Scholar]
- 91. Song YJ, Li SR, Li XW, et al. . The effect of estrogen replacement therapy on Alzheimer’s disease and Parkinson’s disease in postmenopausal women: a meta-analysis. Front Neurosci. 2020;14:157. doi: 10.3389/fnins.2020.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Popat RA, Van Den Eeden SK, Tanner CM, et al. . Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson’s disease. Neurology. 2005;65(3):383–390. doi: 10.1212/01.wnl.0000171344.87802.94 [DOI] [PubMed] [Google Scholar]
- 93. Anselmi CV, Malovini A, Roncarati R, et al. . Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12(2):95–104. doi: 10.1089/rej.2008.0827 [DOI] [PubMed] [Google Scholar]
- 94. Li Y, Wang WJ, Cao H, et al. . Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet. 2009;18(24):4897–4904. doi: 10.1093/hmg/ddp459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shimokawa I, Komatsu T, Hayashi N, et al. . The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell. 2015;14(4):707–709. doi: 10.1111/acel.12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yamaza H, Komatsu T, Wakita S, et al. . FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell. 2010;9(3):372–382. doi: 10.1111/j.1474-9726.2010.00563.x [DOI] [PubMed] [Google Scholar]
- 97. Tarnawa ED, Baker MD, Aloisio GM, Carr BR, Castrillon DH. Gonadal expression of Foxo1, but Not Foxo3, is conserved in diverse mammalian species. Biol Reprod. 2013;88(4):103, 1–11. doi: 10.1095/biolreprod.112.105791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. van Noord-Zaadstra BM, Looman CW, Alsbach H, Habbema JD, te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ. 1991;302(6789):1361–1365. doi: 10.1136/bmj.302.6789.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]