Abstract
Most human immunodeficiency virus (HIV) drug susceptibility studies have involved subtype B strains. Little information on the impact of viral diversity on natural susceptibility to antiretroviral drugs has been reported. However, the prevalence of non-subtype-B (non-B) HIV type 1 (HIV-1) strains continues to increase in industrialized countries, and antiretroviral treatments have recently become available in certain developing countries where non-B subtypes predominate. We sequenced the protease and reverse transcriptase (RT) genes of 142 HIV-1 isolates from antiretroviral-naive patients: 4 belonged to group O and 138 belonged to group M (9 subtype A, 13 subtype B, 2 subtype C, 5 subtype D, 2 subtype F1, 9 subtype F2, 4 subtype G, 5 subtype J, 2 subtype K, 3 subtype CRF01-AE, 67 subtype CRF02-AG, and 17 unclassified isolates). No major mutations associated with resistance to nucleoside reverse transcriptase inhibitors (NRTIs) or protease inhibitors were detected. Major mutations linked to resistance to non-NRTI agents were detected in all group O isolates (A98G and Y181C) and in one subtype J virus (V108I). In contrast, many accessory mutations were found, especially in the protease gene. Only 5.6% of the 142 strains, all belonging to subtype B or D, had no mutations in the protease gene. Sixty percent had one mutation, 22.5% had two mutations, 9.8% had three mutations, and 2.1% (all group O strains) had four mutations. In order of decreasing frequency, the following mutations were identified in the protease gene: M36I (86.6%), L10I/V (26%), L63P (12.6%), K20M/R (11.2%), V77I (5.6%), A71V (2.8%), L33F (0.7%), and M46I (0.7%). R211K, an accessory mutation associated with NRTI resistance, was also observed in 43.6% of the samples. Phenotypic and clinical studies are now required to determine whether multidrug-resistant viruses emerge more rapidly during antiretroviral therapy when minor resistance-conferring mutations are present before treatment initiation.
Human immunodeficiency virus (HIV) replication is markedly inhibited by highly active antiretroviral drug combinations. Drugs belonging to three different classes—nucleoside analogue reverse transcriptase (RT) inhibitors (NRTIs), non-NRTIs (NNRTIs), and protease inhibitors (PIs)—are currently used in various combinations to treat HIV-infected patients (3, 14, 20, 43). Replication of drug-resistant HIV type 1 (HIV-1) during combination therapy is considered a major cause of treatment failure (18, 43). Drug resistance arises from mutations in the genes that encode the molecular targets for the drugs, i.e., the RT and protease pol gene products. This viral polymorphism is due to the high rate of HIV-1 replication and the low fidelity of RT (19, 30, 45). The emergence of amino acid substitutions associated with resistance to RT and PIs has been extensively characterized (18, 34; http://hivdb.stanford.edu/hiv/), and these substitutions can be classified into major and accessory (modifying) mutations. Major mutations lead to a severalfold decrease in sensitivity to one or more antiretroviral drugs (18). Accessory mutations may not result in a significant decrease in sensitivity but are associated with an increase in viral fitness (replication capacity) (14, 18). Thus, the appearance of a major mutation in a genome already containing accessory mutations could influence the speed with which highly resistant viruses are selected during therapy.
Genetic characterization and phylogenetic analysis of HIV-1 isolates from different geographic localities have revealed that HIV-1 can be divided into at least three distinctive groups, designated M (major), N (new or non-M, non-O), and O (outlier) (38). Group M comprises most of the HIV-1 strains responsible for the AIDS pandemic (15) and can be further subdivided into subtypes (subtypes A to K) (8, 42). Recombination events among sequences of different genetic subtypes of HIV-1 group M have frequently been identified (32, 33). Some of these mosaic HIV-1 genomes are unique, but others play a major role in the AIDS pandemic and are named circulating recombinant forms (CRFs) (8). They are designated, according to new nomenclature proposals (33a), by an identifying number and letters indicating the source subtypes, e.g., CRF01-AE (initially env subtype E) and CRF02-AG (AG-IBNG-like viruses) (9, 16).
Most HIV-1 isolates in North America and Europe belong to subtype B. Therefore, anti-HIV drug testing and characterization of drug resistance mutations that confer resistance have been done in studies with subtype B isolates, but subtype B isolates are the cause of only a limited proportion of infections worldwide (15). The efficacy of antiretroviral treatment can be influenced by the viral subtype. Like HIV-2, HIV-1 group O viruses are naturally resistant to NNRTIs (11, 28, 31). Within group M, some subtype F samples are less susceptible to the tetrahydroimidazo [4,5,1-jk] [1,4]-benzodiazepin-2-(1H)-one and -thione (TIBO) derivative, an NNRTI, and some subtype G strains have decreased susceptibility to PIs (2, 12).
With the increasing frequency of non-subtype-B (non-B) isolates in Europe (1, 4, 13) and the recent introduction of antiretroviral drugs in developing countries, there is a need to test the efficacies of existing and new drugs against non-B strains and to monitor resistance. Natural mutations that confer drug resistance have been described in drug-naive patients infected with subtype B strains before the drugs were first used in the relevant population (6, 7, 24, 26, 36), but the prevalence of such mutations has not been routinely studied in untreated individuals infected with non-B strains (10, 29, 39). In this study, we analyzed the protease and RT sequences of isolates from 129 treatment-naive patients infected with non-B HIV-1 strains for the presence of natural mutations linked to resistance to antiretroviral drugs.
MATERIALS AND METHODS
Patients.
One hundred forty-two antiretroviral drug-naive, HIV-1-infected patients were studied. Samples were collected between 1995 and 1999 from patients from four distinct sites: 62 from Cameroon, 38 from Senegal, 18 from the Democratic Republic of Congo (DRC; formerly Zaire), and 24 from a hospital in southern France. Among the 24 patients recruited in France, 9 were infected in Europe, 2 were infected in Cambodia, and 13 were infected in Africa (Ivory Coast [n = 3], Guinea Conakry [n = 1], Central African Republic [CAR; n = 3], Burkina Faso [n = 1], and Cameroon [n = 1]); the country of infection was unknown for the other 4 patients).
RNA and DNA extraction, cDNA synthesis, and PCR.
For samples from DRC and Cameroon, proviral DNA was extracted from uncultured peripheral blood mononuclear cells and from cultured peripheral blood mononuclear cells, respectively, with the QIAamp Blood and Tissue kit (QIAGEN, Courtaboeuf, France). For samples from Senegal and France, viral RNA was isolated from plasma with the QIAamp Viral RNA Mini kit (QIAGEN).
Viral RNA was transcribed to cDNA by using Expand RT (Boehringer Mannheim, Germany) with a reverse primer (primer IN3 [5′-TCTATBCCATCTAAAAATAGTACTTTCCTGATTCC-3′] in RT fragment at position 4261) (44). Reverse transcription was carried out in a final volume of 20 μl containing 1× Expand RT buffer, 10 mM dithiothreitol, 1 mM each deoxynucleoside triphosphate, 40 pmol of reverse primer, and 50 U of Expand RT at 42°C for 60 min and 92°C for 2 min. A 2,200-bp fragment encompassing the protease and RT genes was amplified from DNA or cDNA with the Expand Long Template PCR system (Boehringer, Mannheim, Germany) by a seminested PCR method with outer primers G25REV (5′-GCAAGAGTTTTGGCTGAAGCAATGAG-3′; at position 1873) and IN3 and inner primers AV150 (5′-GTGGAAAGGAAGGACACCAAATGAAAG-3′; at position 2042) and IN3 (44). PCRs were carried out in final volumes of 50 μl (first round) and 100 μl (second round). The reaction mixture consisted of 1× PCR buffer with 0.75 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 20 pmol of each primer, and 2.5 U of Expand Long Template enzyme mixture. The PCR conditions were 92°C for 5 min, followed by 39 cycles at 92°C for 20 s, 50°C for 30 s, and 72°C for 2 min, with a final extension step at 72°C for 10 min.
Sequencing reactions.
The amplified fragments were purified with the QIAquick gel extraction kit (QIAGEN) and were directly sequenced with the ABIPRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit with AmpliTaq DNA polymerase (FS; Perkin-Elmer, Roissy, France) on an automated sequencer (373A stretch; Applied Biosystems).
Phylogenetic and sequence analyses.
The genetic subtypes were determined by phylogenetic tree analysis. The new nucleotide sequences and the sequences of the protease and RT genes from reference strains representing the different genetic subtypes were aligned with the CLUSTAL W program, bearing in mind the protein sequences (40). Phylogenetic trees constructed by the neighbor-joining method and the reliability of the branching orders obtained by the bootstrap approach were implemented with the CLUSTAL W program. Genetic distances were calculated by the two-parameter method of Kimura (22). To determine whether the viruses were recombinants in the sequenced region, several additional analyses were performed. Diversity plots, obtained with the DIVERT program (available online [http://193.50.234.246/∼beudoin/anrs/Diversity.html]) were used to determine the percent diversity between selected pairs of sequences by moving a window of 300 bp along the genome alignment in 20-bp increments. The divergence values for each pairwise comparison were plotted at the midpoint of the 300-bp segment. Simplot, version 2.5, software (http://www.med.jhu.edu/deptmed/sray/download) was used to calculate bootstrap plots by bootscanning the neighbor-joining trees with SEQBOOT, DNADIST (by the two-parameter method of Kimura [22] and with a transition/transversion ratio of 2.0), NEIGHBOR, and CONSENSUS from the Phylip package for a 300-bp window moving along the alignment in increments of 20 bp. We evaluated 100 replicates for each phylogenetic analysis. The bootstrap values for the sequences studied were plotted at the midpoint of each window.
The amino acid sequences of the protease and RT genes, deduced from the nucleic acid sequences, were compared to a subtype B consensus sequence from the Stanford HIV RT and Protease Sequence database (37; http://hivdb.stanford.edu/hiv/) and analyzed for mutations associated with reduced sensitivity to antiretroviral drugs. A consensus pol (protease and RT) sequence was calculated for each subtype with VESPA (Viral Epidemiologic Signature Pattern Analysis) software (23), and signatures specific to each subtype were deduced relative to subtype B.
Nucleotide sequence accession numbers.
The protease and RT sequences are available in GenBank (EMBL) with the following accession numbers: AJ286930 to AJ286979, AJ286133 to AJ286135, AJ286137 to AJ286140, AJ286142, AJ286143, AJ249236, AJ249237, and AJ249239 for sequences from Cameroon; AJ286980 to AJ287014, AJ286136, AJ286141, and AJ251057 for sequences from Senegal; AJ287015 to AJ287031 and AJ249235 for sequences from DRC; and AJ287032 to AJ287054 and AJ249238 for sequences from France.
RESULTS
Phylogenetic analysis of the protease and RT sequences.
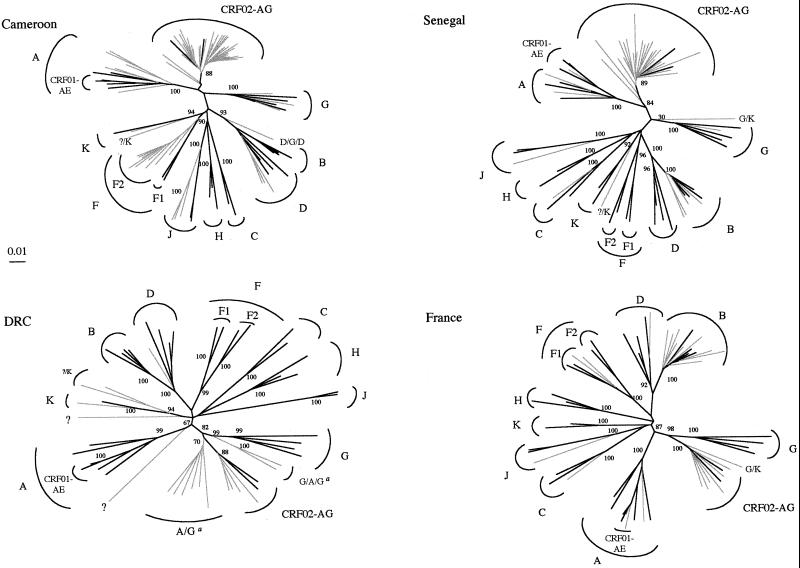
Table 1 summarizes the genetic subtypes in the pol region, and Fig. 1 shows the phylogenetic trees for the HIV-1 group M sequences from Senegal, Cameroon, DRC, and France. Phylogenetic tree analysis of all the isolates together showed that no laboratory contamination had occurred (data not shown). Four of the 142 isolates tested belonged to group O (1 isolate from Cameroon and 3 isolates from Senegal). Among the remaining 138 group M isolates, the overall subtype distribution was as follows: 9 subtype A, 13 subtype B, 2 subtype C, 5 subtype D, 2 subtype F1, 9 subtype F2, 4 subtype G, 5 subtype J, 2 subtype K, 3 subtype CRF01-AE, and 67 subtype CRF02-AG. The subtypes of 17 HIV-1 strains could not be clearly determined, as they did not cluster with any of the known subtypes.
TABLE 1.
Distribution of genetic subtypes in protease and RT regions of 142 treatment-naive patients
| Originating country | No. of strains
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Group M nonrecombinant forms
|
Group M recombinant forms
|
Group O | ||||||||||||||||
| A | B | C | D | F1 | F2 | G | J | K | CRF02-AG | CRF01-AE | A/G | G/A/G | G/K | D/G/D | ?/K | Ua | |||
| Cameroon | 62 | 6 | 3 | 9 | 3 | 3 | 1 | 34 | 1 | 1 | 1 | ||||||||
| Senegal | 38 | 2 | 4 | 1 | 1 | 1 | 23 | 1 | 1 | 1 | 3 | ||||||||
| DRC | 18 | 1 | 1 | 4 | 6 | 2 | 2 | 2 | |||||||||||
| France | 24 | 1 | 9 | 1 | 1 | 2 | 1 | 6 | 2 | 1 | |||||||||
| Total | 142 | 9 | 13 | 2 | 5 | 2 | 9 | 4 | 5 | 2 | 67 | 3 | 6 | 2 | 2 | 1 | 4 | 2 | 4 |
U, did not cluster with any of the known subtypes.
FIG. 1.
Unrooted phylogenetic trees of protease and RT nucleotide sequences (1,600 bp) from 138 group M isolates (in gray) and from reference strains (in black) representing the different subtypes. The trees were generated as described in Materials and Methods. The reference sequences (8) used in the trees were as follows: A.U455, A.Q2317, A.92UG037.1, B.OYI, B.HXB2, B.JRFL, B.RF, D.NDK, D.ELI, D.84ZR085, D.94UG114.1, C.ETH2220, C.92BR025.8, F1.93BR020.1, F1.FIN9363, G.SE6165, G.92NG083, G(A).92NG003, G.HH8793.1.1, H.VI991, H.VI997, H.90CF056.1, J.SE9173.3, J.SE9280.9, CRF01-AE.CM240, CRF01-AE.93TH253.3, CRF01-AE.90CF402.1, CRF02-AG.IBNG, and CRF02-AG.DJ263. The reference sequences for the K and F2 subtypes (41, 42) were used either as references or as samples (K.96CAM.MP535, K.97ZR.EQTB11, F2.95CM.MP255, F2.95CM.MP257). For sequences that did not cluster with high bootstrap values with any of the known subtypes or CRFs, the results of complementary analyses indicating their recombinant nature are shown. a, the isolates designated A/G and G/A/G are intersubtype A/G recombinant viruses in which the breakpoints between A and G are different from those for the prototype CRF02-AG strain. The numbers at the branch points indicate bootstrap values as percentages.
More detailed analysis of the 17 unclassified strains by bootscanning and with the Divert program showed that 15 were recombinants in the pol region studied. Six isolates from DRC were A/G recombinants (but different from the CRF02-AG strains) and formed a distinct cluster in the phylogenetic tree; another two isolates from DRC were G/A/G recombinants. Two isolates (one isolate from Senegal and one isolate from a patient from Burkina Faso attending a hospital in France) were G/K recombinants. One Cameroonian isolate was a D/G/D recombinant in the studied region. Four isolates (one isolate from Cameroon, one isolate from Senegal, and two isolates from DRC) had a similar recombinant profile, with an unclassified protease and an unclassified 5′ end of the RT gene and with the 3′ end of the RT being subtype K. Finally, two samples from DRC did not cluster with any of the known subtypes over the entire protease and RT regions.
Isolates of almost all known subtypes and CRFs were represented in our study. CRF02-AG was predominant, representing most of the viruses circulating in Senegal and Cameroon and non-B isolates in France. These strains are subtype G in the protease gene and at the 5′ end of the RT gene and subtype A at the 3′ end of the RT gene.
Protease sequence variability in isolates from untreated non-B HIV-1-infected patients.
The amino acid sequence of each strain was compared to the subtype B consensus amino acid sequence (Stanford HIV protease sequence database) for mutations associated with resistance to protease inhibitors (37; http://hivdb.stanford.edu/hiv/). No major mutations (D30N, G48V, I50V, V82A/T/F, I84V, or L90M) were seen in any of the subtype B or non-B strains from our sample. In contrast, many minor or accessory mutations were found at the following positions, in order of decreasing frequency: M36I (n = 123 [86.6%]), L10I/V (n = 37 [26%]), L63P (n = 18 [12.6%]), K20M/R (n = 16 [11.2%]), V77I (n = 8 [5.6%]), A71V (n = 4 [2.8%]), L33F (n = 1 [0.7%]), and M46I (n = 1 [0.7%]). No accessory mutations were seen at positions F53L, G73S, and N88D. Table 2 summarizes the frequencies of the different mutations according to subtype. No mutations specific for a given subtype were noted, but the M36I mutation was present in 123 of the 129 non-B strains but was not present in any of the subtype B strains. In general, subtype B strains (n = 13) bore few mutations (only L63P [n = 6] and V77I [n = 3]). Group O strains bore the resistance-conferring minor mutations L10I/V, M36I, L63P (n = 3), and A71V, with the last mutation being specific for group O viruses.
TABLE 2.
Amino acid substitutions in the HIV-1 protease sequences of isolates from 142 treatment-naive patients at key positions associated with resistance to available drugs
| Subtype | No. of strains | Mutation(s) in the protease at key positions in the following wild-type amino acids and positions:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L10 | K20 | L33 | M36 | M46 | L63 | A71 | V77 | V82 | ||
| A | 9 | I3a, V1 | R1, I1 | I9 | P1, Q1 | |||||
| B | 13 | P6, S1, H1 | I3 | |||||||
| C | 2 | R1 | I2 | T1, S1 | ||||||
| D | 5 | V1 | I3 | P1, S1 | ||||||
| F1 + F2 | 11 | V4 | R8, I1 | I11 | I1 | P3, T2, S1, V1 | ||||
| G | 4 | I1 | I4 | I4 | P1 | I4 | ||||
| K | 2 | I1, V1 | R2 | I2 | C1 | |||||
| J | 5 | I2, V1 | I3 | T2, I1 | I1 | |||||
| CRF02-AG | 67 | I3, V6 | M1, I63, V2 | F1 | I67 | P2, S3, F1, V1, I3, N1 | I4 | I3 | ||
| CRF01-AE | 3 | V1 | I3 | C1 | ||||||
| A/G | 6 | I2 | I6 | I6 | S1, I1, N1 | |||||
| G/A/G | 2 | I2 | I2 | M1 | I2 | |||||
| G/K | 2 | I2 | I2 | P1 | ||||||
| D/G/D | 1 | I1 | ||||||||
| ?/K | 4 | I2, V2 | R1 | I3 | S1 | |||||
| Ub | 2 | I1, V1 | R2 | I1 | T1, V1 | |||||
| Group O | 4 | I2, V2 | C4 | I4 | P3, T1 | V4 | ||||
| Total | 142 | I17, V20 | M1/R15, I79, V2/C4 | F1 | I123 | I1 | P18, Other32 | V4 | I8 | I9 |
The letter represents the amino acid substitution, and the number indicates the number of strains with this mutation. Amino acids in boldface type are associated with resistance to PIs.
U, unknown subtypes.
Considering all the minor mutations related to resistance, only 8 (5.6%) of the 142 strains had no mutations, and they were all of subtype B or D. Eighty-five strains (60%) had one mutation, 32 (22.5%) had two mutations, 14 (9.8%) had three mutations, and 3 (2.1%) (all group O) had four mutations.
Amino acid changes associated with resistance to protease inhibitors (ABT378 and DMP450) currently being tested in clinical trials were also identified: G16E (n = 27) and D60E (n = 8) (17).
In addition, at some key positions (i.e., positions known for amino acid mutations associated with drug resistance), amino acid substitutions different from those linked to PI resistance were observed. Eighty-five strains (59.8%) had an amino acid different from lysine (K) at position 20: K20I (n = 79), K20C (n = 4), and K20V (n = 2). The K20I substitution was found in 63 of the 67 CRF02-AG strains, in all subtype G strains, in all A/G and G/A/G recombinant strains from DRC, and in the G/K recombinants. Thirty-two strains showed a polymorphism other than L63P. For all the subtype G strains, the G/A/G recombinants, and the three CRF02-AG viruses, the valine (V) at position 82 was replaced by an isoleucine (I).
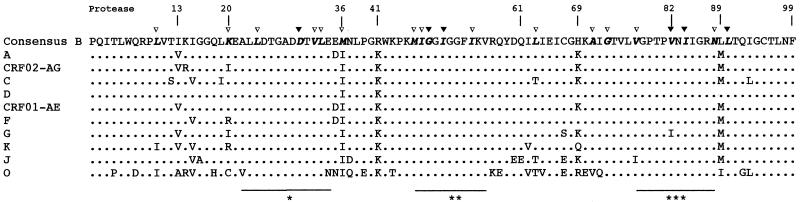
Figure 2 shows the alignment of the amino acid consensus sequences of the protease gene for each subtype compared with a subtype B consensus sequence from the database. The subtype B consensus sequence from the isolates from the 13 HIV-1 subtype B-infected patients in our study corresponds to the subtype B consensus sequence from the database. Overall, the three functional sites in which major mutations are located (except L90M) were highly conserved among all the subtypes. Leucine (L) was replaced by a methionine (M) at position 89 of all group M subtype isolates (except isolates of subtypes B and D) and by an isoleucine (I) in group O isolates. In the nonfunctional sites of protease, more amino acid substitutions were seen in the group O sequences than in the group M sequences. Other mutations specific to certain subtypes were seen in other regions of the protease gene by using the VESPA program (80% threshold): 13V for subtypes A, G, and CRF02-AG; 15V for subtype K; 35D for subtypes A, CRF01-AE, and F; 41K for all the subtypes except J; 61F and 67C for subtype J; 69K for all the subtypes except F and K; 89M for all the subtypes except D and K; and 93L for subtype C.
FIG. 2.
Amino acid alignment of protease consensus sequences for each subtype of group M and for group O. The sequences were aligned against a subtype B consensus sequence from the database; dots indicate homology. The amino acid positions associated with drug resistance are depicted in boldface italics; the major mutations (D30N, G48V, I50V, V82A/F/T, I84V, L90M) are marked at the top of the consensus sequence (▾), as are the minor mutations (L10I/V, K20M/R, L24I, V32I, L33F, M36I, M46I/L, I47V, I54L/V, L63P, A71T/V, G73S, V77I, N88D) (▿). The functional domains of the protease (∗, active site [amino acids 22 to 34]; ∗∗, flap region [amino acids 47 to 56]; ∗∗∗, substrate binding site [amino acids 78 to 88]) are shown under the consensus sequence.
Sequence variability in RT region of isolates from untreated non-B HIV-1-infected patients.
The mutations leading to resistance to NRTIs and NNRTIs are well defined and differ between the two classes of RT inhibitors (18, 34).
None of the strains had major mutations associated with NRTI resistance. Only one patient, a European infected with a subtype B isolate from a zidovudine (AZT)-treated partner, had the accessory mutation M41L, along with T215D, which represents a transition to the T215Y/F major mutation associated with resistance to AZT (37; http://hivdb.stanford.edu/hiv/). Accessory mutations were also observed in the RT gene: 62 (43.6%) of the strains, representing all subtypes, had the R211K mutation, and 5 strains had the G333E mutation.
In some strains, polymorphisms were observed at key positions but were not associated with resistance, namely, L210Y (n = 4) and L210Q (n = 4; all group O), R211S (n = 10), and R211N (n = 2).
Major mutations associated with NNRTI resistance were seen: V108I in one subtype J isolate and A98G and Y181C in the four group O isolates (18; http://hivdb.stanford.edu/hiv/). Other amino acid changes not associated with drug resistance were observed at key positions in a few strains, namely, A98S (n = 8) and K101R (n = 1).
Amino acid changes associated with resistance to NNRTIs being tested in clinical trials were also identified, namely, V106I (n = 3) (HBY097), V179D (n = 1) (trovirdine, QM96521, UC-10, ADAMII, L-697,661, and TIBO R82913), V179E (n = 6) (L-697,661), and V189I (n = 1) (HBY097) (17).
DISCUSSION
We analyzed the protease and RT gene-coding regions of 142 HIV-1 isolates from treatment-naive patients. Most strains were isolated in Africa, where access to antiretroviral drugs is very limited. More than 90% of the strains were non-B, and isolates of all genetic subtypes with the exception of subtype H and two CRFs were represented. Isolates of subtype CRF02-AG, which is predominant in west and west-central Africa (27), represented 47% of the isolates.
Overall, the protease gene region was less conserved than the RT gene region. No major mutations conferring resistance to PIs were seen, but more than 34% of the strains had two or more minor mutations associated with PI resistance; only 5.6% of strains (mainly subtype B strains) had no minor mutations. M36I, the predominant minor mutation, was observed in 87% of the strains overall and in 95% of the non-B strains. Amino acid substitutions associated with PI resistance have been reported as natural variants in treatment-naive patients (5, 6, 10, 24, 25, 29, 35, 36, 39), but the prevalence of substitutions determined from our data are significantly higher than those from previous studies with subtype B isolates (10, 24, 37, 39). Several mutations not classically associated with resistance were observed, and their biological consequences remain to be studied. Accessory mutations are not always associated with a decrease in in vitro susceptibility, in contrast to major mutations (18, 43). For example, the protease sequences of subtype C strains isolated in Zimbabwe had several accessory mutations, but in vitro susceptibility tests with a subset of these samples showed no decrease in sensitivity (35). Minor mutations can compensate for the reduced fitness of resistant mutants, and in contrast to major mutations, which are different for each protease inhibitor, compensatory mutations are similar for many PIs (14, 18, 43). The efficacy of a switch from one PI to another might therefore be compromised when the virus has had the opportunity to develop compensatory mutations. One consequence of preexisting accessory mutations might be the faster emergence of viruses resistant to PIs. Preliminary data suggest that prior M36I and L10I/V mutations are associated with a more rapid fall in sensitivity during treatment (C. F. Perno, A. D'Arminio-Monforte, A. Cozzi-Lepri, C. Balotta, F. Forbici, A. Bertoli, P. Pezzotti, G. Facchi, L. Monno, G. Angarano, P. Bottura, V. Vullo, A. Cargnel, M. Capobianchi, G. Ippolito, and M. Moroni, 7th Conference on Retroviruses and Opportunistic Infections, 2000).
No major mutations conferring resistance to NRTIs were seen, but two accessory mutations—R211K (43.6%) and G333E (3.5%)—were found. These mutations facilitate dual resistance to AZT and lamivudine (3TC) in association with M184V and other AZT resistance mutations (17, 21). In contrast to NRTIs, only major mutations associated with resistance to NNRTIs were seen. A pol subtype J isolate from one patient originally from CAR had a mutation (V108I) associated with resistance to nevirapine and efavirenz. In keeping with previous reports, major mutations to NNRTIs were present in all four group O isolates.
In conclusion, the prevalence of major mutations associated with resistance to NRTIs, NNRTIs, and PIs is very low among non-B group M HIV-1 isolates of African origin, whereas many minor or accessory mutations related to resistance to NRTIs and PIs are present as natural variants. Phenotypic and clinical studies are necessary to determine whether, when antiretroviral therapy is started, these viruses generate multidrug-resistant strains more rapidly.
ACKNOWLEDGMENTS
This study was cosponsored by grants from the Agence Nationale de Recherche contre le SIDA (ANRS; project SIDAK), the European Union (INCO-DC; grant IC18CT97-0216), and SIDACTION.
REFERENCES
- 1.Alaeus A, Leitner T, Lidman K, Albert J. Most HIV-1 genetic subtypes have entered Sweden. AIDS. 1997;11:199–202. doi: 10.1097/00002030-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Apetrei C, Descamps D, Collin G, Loussert-Ajaka I, Damond F, Duca M, Simon F, Brun-Vezinet F. Human immunodeficiency virus type 1 subtype F reverse transcriptase sequence and drug susceptibility. J Virol. 1998;72:3534–3538. doi: 10.1128/jvi.72.5.3534-3538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arts E J, Wainberg M A. Mechanisms of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1996;40:527–540. doi: 10.1128/aac.40.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barin F, Courouce A M, Pillonel J, Buzelay L. The Retrovirus Study Group of the French Society of Blood Transfusion: increasing diversity of HIV-1 M serotypes in French blood donors over a 10-year period (1985–1995) AIDS. 1997;11:1503–1508. doi: 10.1097/00002030-199712000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Birk M, Sönnerborg A. Variations in HIV-1 pol gene associated with reduced sensitivity to antiretroviral drugs in treatment-naive patients. AIDS. 1998;12:2369–2375. doi: 10.1097/00002030-199818000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bossi P, Mouroux M, Yvon A, Bricaire F, Agut H, Huraux J M, Katlama C, Calvez V. Polymorphism of the human immunodeficiency virus type 1 (HIV-1) protease gene and response of HIV-1-infected patients to a protease inhibitor. J Clin Microbiol. 1999;37:2910–2912. doi: 10.1128/jcm.37.9.2910-2912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brindeiro R, Vanderborght B, Caride E, Correa L, Oravec R M, Berro O, Stuyver L, Tanuri A. Sequence diversity of the reverse transcriptase of human immunodeficiency virus type 1 from untreated Brazilian individuals. Antimicrob Agents Chemother. 1999;43:1674–1680. doi: 10.1128/aac.43.7.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr J K, Foley B T, Leitner T, Salminen M, Korber B, McCutchan F. Human retroviruses and AIDS: part III. Los Alamos, N.M: Los Alamos National Laboratory; 1998. Reference sequences representing the principal genetic diversity of HIV-1 in the pandemic; pp. 10–17. [Google Scholar]
- 9.Carr J K, Salminen M O, Albert J, Sanders-Buell E, Gotte D, Birx D L, McCutchan F E. Full genome sequences of human immunodeficiency virus type 1 subtypes G and A/G intersubtype recombinants. Virology. 1998;247:22–31. doi: 10.1006/viro.1998.9211. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen M, van den Burg R, Zorgdrager F, Lukashov V, Goudsmit J. pol gene diversity of five human immunodeficiency virus type 1 subtypes: evidence for naturally occurring mutations that contribute to drug resistance, limited recombination patterns, and common ancestry for subtypes B and D. J Virol. 1997;71:6348–6358. doi: 10.1128/jvi.71.9.6348-6358.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Descamps D, Collin G, Letourneur F, Apetrei C, Damond F, Loussert-Ajaka I, Simon F, Saragosti S, Brun-Vezinet F. Susceptibility of human immunodeficiency virus type 1 group O isolates to antiretroviral agents: in vitro phenotypic and genotypic analyses. J Virol. 1997;71:8893–8898. doi: 10.1128/jvi.71.11.8893-8898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Descamps D, Apetrei C, Collin G, Damond F, Simon F, Brun-Vezinet F. Naturally occurring decreased susceptibility of HIV-1 subtype G to protease inhibitors. AIDS. 1998;12:1109–1111. [PubMed] [Google Scholar]
- 13.Dietrich U, Ruppach H, Gehring S, Knecthen H, Knickmann M, Jager H, Wolf E, Husak R, Orfanos C E, Brede H D, Rubsamen-Waigman H, von Briesen H. Large proportion of non-B HIV-1 subtypes and presence of zidovudine resistance mutations among German seroconvertors. AIDS. 1997;11:1532–1533. [PubMed] [Google Scholar]
- 14.Erickson J W, Gulnik S V, Markowitz M. Protease inhibitors: resistance, cross-resistance, fitness and the choice of initial and salvage therapies. AIDS. 1999;13(Suppl. A):S189–S204. [PubMed] [Google Scholar]
- 15.European Commission and the Joint United Nations Programme on HIV/AIDS. HIV-1 subtypes: implications for epidemiology, pathogenicity, vaccines and diagnostics. Workshop Report AIDS. 1997;11:UNAIDS17–UNAIDS36. [PubMed] [Google Scholar]
- 16.Gao F, Robertson D L, Morrison S G, Hui H, Craig S, Decker J, Fultz P N, Girard M, Shaw G M, Hahn B H, Sharp P M. The heterosexual human immunodeficiency virus type 1 epidemic in Thailand is caused by an intersubtype (A/E) recombinant of African origin. J Virol. 1996;70:7013–7029. doi: 10.1128/jvi.70.10.7013-7029.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond J, Calef C, Larder B, Schinazi R, Mellors J W. Human retroviruses and AIDS: part III. Los Alamos, N.M: Los Alamos National Laboratory; 1998. Mutations in retroviral genes associated with drug resistance; pp. 36–79. [Google Scholar]
- 18.Hirsch M S, Conway B, D'Aquila R T, Johnson V A, Brun-Vezinet F, Clotet B, Demeter L M, Hammer S M, Jacobsen D M, Kuritzkes D R, Loveday C, Mellors J W, Vella S, Richman D D. Antiretroviral drug resistance testing in adults with HIV infection. JAMA. 1998;279:1984–1991. doi: 10.1001/jama.279.24.1984. [DOI] [PubMed] [Google Scholar]
- 19.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 20.Joly V, Yeni P. Non nucleoside reverse transcriptase inhibitors. AIDS Rev. 1999;1:37–44. [Google Scholar]
- 21.Kemps S D, Shi C, Bloor S, Harrigan P R, Mellors J W, Larder B A. A novel polymorphism at codon 333 of human immunodeficiency virus type 1 reverse transcriptase can facilitate dual resistance to zidovudine and l-2′,3′-dideoxy-3′-thiacytidine. J Virol. 1998;72:5093–5098. doi: 10.1128/jvi.72.6.5093-5098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 23.Korber B, Myers G. Signature pattern analysis: a method for assessing viral sequence relatedness. AIDS Res Hum Retrovir. 1992;8:1549–1560. doi: 10.1089/aid.1992.8.1549. [DOI] [PubMed] [Google Scholar]
- 24.Kozal M J, Shah N, Shen N, Yang R, Fucini R, Merignan T C, Richman D D, Morris D, Hubbell E, Chee M, Gingeras T R. Extensive polymorphisms observed in HIV-1 clade B protease gene using high-density oligonucleotide arrays. Nat Med. 1996;2:753–759. doi: 10.1038/nm0796-753. [DOI] [PubMed] [Google Scholar]
- 25.Lech W J, Wang G, Yang Y L, Chee Y, Dorman K, McCrae D, Lazzeroni L C, Erickson J W, Sinsheimer J S, Kaplan A H. In vivo sequence diversity of the protease of human immunodeficiency virus type 1: presence of protease inhibitor-resistant variants in untreated subjects. J Virol. 1996;70:2038–2043. doi: 10.1128/jvi.70.3.2038-2043.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magiorkinis E, Paraskevis D, Lazanas M, Kiosses V G, Gargalianos P, Hatzakis A. Identification of reverse transcriptase mutations associated with HIV-1 drug resistance mainly against non-nucleoside reverse transcriptase inhibitors in treatment-naive patients. AIDS. 1999;13:1276–1278. doi: 10.1097/00002030-199907090-00020. [DOI] [PubMed] [Google Scholar]
- 27.Montavon C, Toure-Kane C, Liegois F, Mpoudi E, Bourgeois A, Vergne L, Perret J L, Boumah A, Saman E, Mboup S, Delaporte E, Peeters M. The majority of env and gag subtype A HIV-1 viruses circulating in West and West Central Africa are recombinant AG-IBNG like strains. J Acquir Immune Defic Syndr. 2000;23:363–374. doi: 10.1097/00126334-200004150-00001. [DOI] [PubMed] [Google Scholar]
- 28.Pauwels R, Andries K, Desmyter J, Schols D, Kukla M J, Breslin H J, Raeymaeckers A, Van Gelder J, Woestenborghs R, Heykants J, Schellekens K, Janssen M, De Clercq E, Janssens P. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature. 1990;343:470–474. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- 29.Pilcher C D, Perkins M D, Fiscus S A, Johnston D M, Dietze R, Duque U H, Zago A M, Assad-Antunes F, Eron J J. Genotypic resistance and the treatment of HIV-1 infection in Espirito Santo, Brazil. J Infect Dis. 1999;179:1259–1263. doi: 10.1086/314709. [DOI] [PubMed] [Google Scholar]
- 30.Preston B D, Poiesz B J, Loeb L A. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- 31.Quinones-Mateu M E, Albright J L, Mas A, Soriano V, Arts E J. Analysis of pol gene heterogeneity, viral quasispecies, and drug resistance in individuals infected with group O strains of human immunodeficiency virus type 1. J Virol. 1998;72:9002–9015. doi: 10.1128/jvi.72.11.9002-9015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson D L, Hahn B H, Sharp P M. Recombination in AIDS. J Mol Evol. 1995;40:249–259. doi: 10.1007/BF00163230. [DOI] [PubMed] [Google Scholar]
- 33.Robertson D L, Gao F, Hahn B H, Sharp P M. Human retroviruses and AIDS: part III. Los Alamos, N.M: Los Alamos National Laboratory; 1997. Intersubtype recombinant HIV-1 sequences; pp. 25–30. [Google Scholar]
- 33a.Robertson D L, Anderson J P, Bradac J A, Carr J K, Foley B, Funkhouser R K, Gao F, Hahn B H, Kalish M L, Kuiken C, Learn G H, Leitner T, McCutchan F, Osmanov S, Peeters M, Pieniazek D, Salminen M, Sharp P M, Wolinsky S, Korber B. HIV-1 nomenclature proposal: a reference guide to HIV-1 classification. In: Kuiken C L, Foley B, Hahn B H, Korber B, McCutchan F, Marx P A, Mellors J W, Mullins J I, Sodroski J, Wolinsky S, editors. Human retroviruses and AIDS 1999: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.M: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1999. pp. 492–505. [Google Scholar]
- 34.Schinazi R F, Larder B A, Mellors J W. Mutations in retroviral genes associated with drug resistance. Int Antivir News. 1997;5:129–142. [Google Scholar]
- 35.Shafer R W, Chuang T K, Hsu P, Bodley White C, Katzenstein D A. Sequence and drug susceptibility of subtype C protease from human immunodeficiency virus type 1 seroconverters in Zimbabwe. AIDS Res Hum Retrovir. 1999;15:65–69. doi: 10.1089/088922299311727. [DOI] [PubMed] [Google Scholar]
- 36.Shafer R W, Hsu P, Patick A K, Craig C, Brendel V. Identification of biased amino acid substitution patterns in human immunodeficiency virus type 1 isolates from patients treated with protease inhibitors. J Virol. 1999;73:6197–6202. doi: 10.1128/jvi.73.7.6197-6202.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafer R W, Stevenson D, Chan B. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 1999;27:348–352. doi: 10.1093/nar/27.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon F, Mauclere P, Roques P, Loussert-Ajaka I, Müller-Trutwin M C, Saragosti S, Georges-Courbot M C, Barre-Sinoussi F, Brun-Vezinet F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat Med. 1998;4:1032–1037. doi: 10.1038/2017. [DOI] [PubMed] [Google Scholar]
- 39.Tanuri A, Vicente A C P, Otsuki K, Ramos C A, Ferreira O C, Schecther M, Janini L M, Pieniazek D, Rayfield M A. Genetic variation and susceptibilities to protease inhibitors among subtype B and F isolates in Brazil. Antimicrob Agents Chemother. 1999;43:253–258. doi: 10.1128/aac.43.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson J, Higgins D, Gibson T. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Triques K, Bourgeois A, Saragosti S, Mpoudi-Ngole E, Nzilambi N, Apetrei C, Ekwalanga M, Delaporte E, Peeters M. High diversity of HIV-1 subtype F strains in Central Africa. Virology. 1999;259:99–109. doi: 10.1006/viro.1999.9720. [DOI] [PubMed] [Google Scholar]
- 42.Triques K, Bourgeois A, Vidal N, Mpoudi-Ngole E, Mulanga-Kabeya C, Nzila N, Torimiro N, Saman E, Delaporte E, Peeters M. Near-full length genome sequencing of divergent African HIV-1 subtype F viruses leads to the identification of a new HIV-1 subtype designated K. AIDS Res Hum Retrovir. 2000;16:139–151. doi: 10.1089/088922200309485. [DOI] [PubMed] [Google Scholar]
- 43.Vandamme A M, Van Laethem K, De Clercq E. Managing resistance to anti-HIV drugs: an important consideration for effective disease management. Drugs. 1999;57:337–361. doi: 10.2165/00003495-199957030-00006. [DOI] [PubMed] [Google Scholar]
- 44.Vandamme A M, Witvrouw M, Pannecouque C, Balzarini J, Van Laethem K, Schmit J C, Desmyter J, De Clercq E. Evaluating clinical isolates for their phenotypic and genotypic resistance against anti-HIV drugs. In: Kinchington D, Schinazi R F, editors. Methods in molecular medicine: antiviral methods and protocols. Totowa, N.J: Humana Press; 1999. [DOI] [PubMed] [Google Scholar]
- 45.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]