Abstract
Background
Little is known about long-term lipid variability in young adulthood in relation to cognitive function and brain integrity in midlife.
Method
We studied 3 328 adults from the Coronary Artery Risk Development in Young Adults. We defined low- and high-density lipoprotein (LDL and HDL) variability as the intraindividual standard deviation of lipid measurements over 20 years of young adulthood (1985–2005). Cognitive tests were administered in 2010. Brain scans were performed in 2010 on 714 participants. To facilitate comparison, cognitive tests and brain metrics were z-scored.
Results
Mean age at baseline was 25.4 years. Higher 20-year LDL variability was associated with worse verbal memory in midlife (β = −0.25, 95% CI: −0.42, −0.08), adjusted for important covariates. Higher 20-year HDL variability was associated with worse processing speed in midlife (β = −0.80, 95% CI: −1.18, −0.41) and brain integrity, for example, smaller total brain volume (β = −0.58, 95% CI: −0.82, −0.34) and worse total brain fractional anisotropy (β = −1.13, 95% CI: −1.87, −0.39).
Conclusions
Higher long-term lipid variability in adulthood was associated with worse cognition and brain integrity in midlife, in a relatively young cohort.
Keywords: Brain, Cognition, Epidemiology, Homeostasis, Lipid
Given the contribution of vascular disease in the development of Alzheimer’s disease and other dementias (1), there has been great interest in investigating the associations between vascular risk factors, cognition, and metrics of brain integrity. Abnormal cholesterol levels are associated with greater risk of carotid atherosclerosis and stroke (2), which in turn are associated with greater risk of dementia (3,4). Elevated total and low-density lipoprotein (LDL) cholesterol may contribute to the development of cognitive disorders through dysregulation of amyloid generation and clearance in the central nervous system (5). Thus, control of lipid levels represents a potential target for intervention to attenuate the risk of cognitive impairment.
Several epidemiologic studies have associated abnormal levels of serum total, LDL, and high-density lipoprotein (HDL) cholesterol levels with worse white matter (WM) integrity, cognitive impairment, and dementia (6–9). However, these studies only examined mean lipid levels without consideration of intrapersonal variability over multiple measurements. Increased variability in vascular risk factor levels may reflect deficits in homeostatic mechanisms (10,11). Recent work has shown that variability in blood pressure and heart rate is associated with worse cognition (12–17). Yet, very little research has been conducted to examine how lipid level variability over time influences cognitive function and brain structural integrity (18–20). Recent findings from the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) trial showed that higher visit-to-visit LDL and HDL variability were associated with worse cognition (18,19) and greater WM lesion load (16). Other studies have related visit-to-visit LDL variability with various outcomes other than cognition, including myocardial infarction and stroke, or mortality in patients with a history of cardiovascular disease (21–23). Most of these studies are post-hoc analyses of clinical trials, thus, results may not be generalizable to the general population. Additionally, there is growing evidence that exposure to vascular risk factors earlier in the lifecourse affects brain health outcomes in midlife and late life (24). Therefore, more research on lipid variability and brain health from large epidemiologic studies using a lifecourse approach is warranted.
In the present study, we leverage data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, an ongoing prospective study since 1985 and with active follow-up data on a cohort of young to middle-aged adults. We examined the associations of 20-year LDL and HDL variability during young adulthood with subsequent cognitive function and markers of brain integrity in midlife.
Method
Study Population
We studied participants enrolled in the CARDIA study, a prospective cohort study of cardiovascular disease risk in young to middle-aged adults. Details of the CARDIA Study have been previously published (25). Briefly, a total of 5 114 adults aged 18–30 years old at baseline (1985–1986) were recruited from 4 field centers: the University of Alabama at Birmingham (Birmingham, AL), the University of Minnesota (Minneapolis, MN), Northwestern University (Chicago, IL), and Kaiser Permanente (Oakland, CA). Recruitment was balanced within center by sex, age, and education. Participants were examined at baseline and at follow-up examinations 2, 5, 7, 10, 15, 20, and 25 years after baseline. Informed consent was obtained from study participants, and the study was approved by the institutional review boards from each field center and the coordinating center. The present analysis was also approved by the Publications & Presentations committee of the CARDIA study.
Twenty-Year Lipid Variability Throughout Adulthood (1985–2005)
Lipid levels were measured from blood samples drawn after an overnight fast, stored in ethylenediaminetetraacetic acid (EDTA) tubes, kept at −70 °C, and sent to Northwest Lipid Research Laboratories at the University of Seattle, Washington, as previously described (26). HDL was obtained using precipitation by dextran sulfate–magnesium chloride. LDL was calculated by Friedewald equation. Lipid levels were measured repeatedly across all study visits. For the present study, we used lipid levels measured at the baseline visit (year 1985), then at years 2, 5, 7, 10, 15, and 20 years after baseline (year 2005).
For each participant, we then calculated visit-to-visit LDL (or HDL) variability as the intraindividual standard deviation of LDL (or HDL) measurements over the 20 years. Participants were included if they had at least 3 repeated measures of lipids. More than 75% of the participants had 5 or more repeated measurements. 20-year LDL and HDL variability were treated as the primary predictors and analyzed separately. For each participant, we also calculated mean LDL (or HDL) as the average of LDL (or HDL) levels over the 20 years, and average change in LDL (or HDL) over the 20 years as the slope coefficient in the person-specific linear regression of LDL (or HDL) on time (in years).
Midlife Cognitive Function (2010): Primary Outcome
In 2010 (year 25 after baseline), CARDIA participants were administered a cognitive battery that included 3 cognitive tests. The Rey Auditory-Verbal Learning Test (RAVLT, range 0–15) measures verbal memory and assesses the ability to memorize and retrieve words, with higher score (in words) indicating better performance (27). The Digit Symbol Substitution Test (DSST, range 0–133) is a subtest of the Wechsler Adult Intelligence Scale and measures performance on speed test, with higher score (in symbols) indicating better performance (28). The interference score on the Stroop test (executive skills) measures the additional amount of processing needed to respond to one stimulus while suppressing another. The test was scored by seconds to spell out color words printed in a different color plus number of errors, thus higher score (seconds + errors) indicates worse performance (29).
Midlife Brain Magnetic Resonance Imaging Markers (2010): Secondary Outcome
In 2010, the CARDIA MRI Ancillary Study which included 3 of the 4 CARDIA sites, Birmingham, AL, Minneapolis, MN, and Oakland, CA, enrolled a total of 719 participants. The procedures for the CARDIA MRI Ancillary Study have been previously described (30). Since magnetic resonance imaging (MRI) markers were only available in a small subset of participants, we considered these analyses exploratory and MRI markers as secondary outcome.
To explore associations across a variety of tissues, we chose to examine both measures of gray matter (GM) structure and WM microstructure. Briefly, brain MRI was acquired on 3-T scanners located proximal to each CARDIA site. MRIs were sent, quality controlled, and analyzed at the MRI Reading Center by the Section of Biomedical Image Analysis (Department of Radiology, University of Pennsylvania). We chose to examine the following markers because they represent different tissues and possible etiologies, and thereby may help inform mechanisms. Normal total intracranial, cerebral, GM, WM, and hippocampal volumes were measured from sagittal 3D T1 images (31–33) using an automated algorithm that classifies supratentorial brain tissue into GM, WM, and cerebrospinal fluid. Microstructural integrity of these tissues was measured by calculating fractional anisotropy (FA) measures from diffusion tensor images (34). All volumes were z-scored to facilitate comparison across estimates. For both volume and FA measures, negative values indicate worse brain aging.
Other Covariates
CARDIA participants reported their age, sex, race, years of education completed, and smoking status (current, previous, and never). Participants reported the amount of time per week spent in 13 categories of physical activity over the past year, and then the total amount in exercise units was calculated. Body mass index (BMI in kg/m2) was calculated using measured weight and height. Systolic blood pressure (SBP) was measured while seated using a standard automated blood pressure measurement monitor. Fasting glucose was measured from blood samples drawn after an overnight fast. At each visit, participants also reported whether they were taking any lipid medications (yes/no). Use of lipid medication between baseline and year 20 was classified into: always, ever (on/off), or never on medications.
Statistical Analysis
Analytical samples
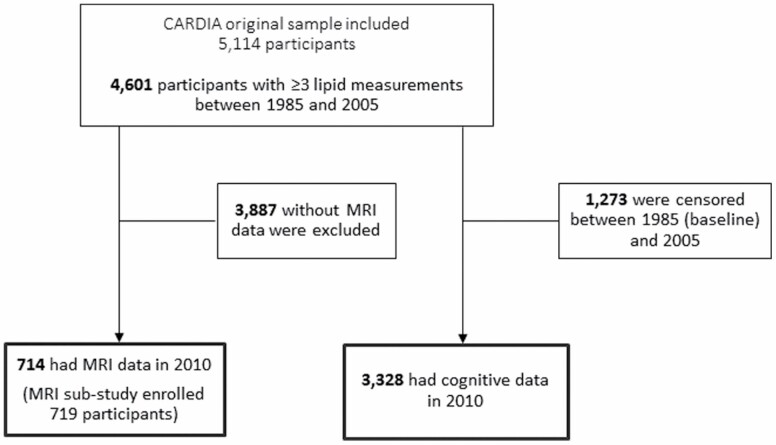
Of the 4 601 participants with 3 or more lipid measurements over the 20-year period between 1985 and 2005, a total of 3 328 had cognitive data in 2010. Similarly, of the 4 601 participants with 3 or more lipid measurements over the 20-year period, 714 participants had MRI data available in 2010 (the MRI substudy enrolled a total of 719 participants) (see Figure 1).
Figure 1.
Flow chart of the CARDIA study leading to the cognitive and MRI analytical samples. MRI = magnetic resonance imaging.
Using the cognitive analytical sample, we first assessed participant characteristics at baseline across tertiles of 20-year LDL and HDL variability. Linear regression models were used to examine the associations of 20-year LDL or HDL variability (as intraindividual standard deviation) in young adulthood with midlife domain-specific cognitive function and midlife brain MRI markers (tissue volumes and FA). To get at pure lipid variability, in our minimally adjusted models, we adjusted for 20-year mean and 20-year change (ie, slope) LDL (or HDL). In our fully adjusted models, we additionally adjusted for the following baseline covariates: age, race, sex, education, smoking status, BMI, physical activity, fasting glucose, and SBP. Adjustment for those covariates was made based on a priori hypotheses and their relationships with both lipids and cognition. In models for volumetric MRI markers, total intracranial volume (ICV) was additionally added as a covariate to adjust for differences in head size, except in models of total hippocampal volume where we instead adjusted for the ratio of GM volume/ICV.
We performed 3 sensitivity analyses. First, we performed an analysis in which we excluded participants who ever used any lipid medications any time during the study. Second, to examine the influence of selective attrition over the study period (1985–2010), we computed inverse probability of censoring weights (35) modeling the probability of having cognitive function in 2010, and then applied those weights to the analysis of LDL and HDL variability with midlife cognition. Third, we examined the relationship between LDL (HDL) variability and cognitive function adjusting for covariates at time of cognition instead of baseline. All statistical analyses were performed with STATA version 14 (STATACorp, College Station, TX). Significance testing was 2-sided with 5% significance level.
Results
Participants included in the cognitive sample were more likely to be older, female, White, and a current smoker, as well as more likely to have less than or equal to high school education, higher physical activity, lower SBP, lower fasting glucose, higher lipid variability, and a higher prevalence of ever being on lipid medication on average, compared to those who were not in the cognitive sample (Supplementary Table 1). Similarly, participants with MRI data differed, on various characteristics, from those without MRI data. Participants who were in the MRI sample were more likely to be White, more likely to have less than or equal to high school education, be current smokers, but have lower mean BMI, lower mean SBP, and greater mean physical activity on average compared to those who were not in the MRI sample (Supplementary Table 1).
Table 1 describes the baseline demographic and clinical characteristics of our study sample across tertiles of 20-year LDL and HDL variability. Participants with higher LDL variability were more likely to be male, current smokers, and to have ever used lipid medications. They were also more likely to have higher 20-year mean LDL, mean BMI, fasting glucose, and SBP. Participants with higher HDL variability were less likely to be male or ever using lipid medications, but were more likely to be Black and current smokers.
Table 1.
Sample Characteristics at Baseline, Stratified by Category of 20-Year LDL and HDL Variability Throughout Young Adulthood (N = 3 328)
| Tertiles of LDL Variability, 1985–2005 | Tertiles of HDL Variability, 1985–2005 | |||||
|---|---|---|---|---|---|---|
| Lowest (n = 1 059) | Middle (n = 1 155) | Highest (n = 1 114) | Lowest (n = 1 124) | Middle (n = 1 119) | Highest (n = 1 085) | |
| Age, y | 25.1 (3.7) | 25.1 (3.6) | 25.1 (3.6) | 25.2 (3.6) | 25.0 (3.6) | 25.1 (3.6) |
| Male | 38.9% | 44.9% | 47.2% | 56.7% | 42.0% | 32.2% |
| Black | 49.8% | 43.8% | 44.7% | 42.4% | 46.1% | 49.6% |
| Less than or equal to high school education | 18.7% | 19.5% | 22.2% | 18.2% | 19.6% | 22.7% |
| Current smoker | 21.7% | 26.2% | 29.4% | 20.8% | 26.0% | 30.9% |
| BMI, kg/m2 | 24.4 (5.1) | 24.3 (4.8) | 24.7 (4.8) | 25.3 (5.3) | 24.3 (4.6) | 23.8 (4.7) |
| Physical activity, exercise units | 409.3 (282) | 426.5 (293) | 424.1 (314) | 422.3 (293) | 409.2 (290) | 429.4 (308) |
| SBP, mm Hg | 108.9 (10.3) | 110.0 (10.8) | 110.9 (11.1) | 111.3 (10.7) | 109.3 (10.7) | 109.2 (10.6) |
| Fasting glucose, mg/dL | 81.2 (8.5) | 81.6 (9.0) | 82.8 (13.1) | 82.5 (10.4) | 81.4 (8.1) | 81.7 (12.5) |
| Ever on lipid medication | 2.6% | 4.9% | 18.3% | 10.5% | 8.8% | 6.6% |
| 20-y mean LDL, mmol/L | 2.5 (0.6) | 2.8 (0.6) | 3.2 (0.7) | — | — | — |
| 20-y mean HDL, mmol/L | — | — | — | 1.2 (0.3) | 1.3 (0.3) | 1.6 (0.3) |
| 20-y LDL slope | 0.00 (0.02) | 0.01 (0.03) | −0.01 (0.06) | — | — | — |
| 20-y HDL slope | — | — | — | −0.00 (0.01) | −0.00 (0.01) | −0.00 (0.03) |
| 20-y LDL variability, mmol/L | 0.2 (0.1) | 0.4 (0.0) | 0.7 (0.2) | — | — | — |
| 20-y HDL variability, mmol/L | — | — | — | 0.1 (0.02) | 0.2 (0.02) | 0.3 (0.1) |
Notes: BMI = body mass index; HDL = high-density lipoprotein; LDL = low-density lipoprotein. Continuous variables presented as mean (SD). LDL variability: lowest tertile is 0.04–0.31 mmol/L, middle tertile is 0.31–0.46 mmol/L, and highest tertile is 0.46–2.52 mmol/L. HDL variability: lowest tertile is 0.0–0.13 mmol/L, middle tertile is 0.13–0.20 mmol/L, and highest tertile is 0.20–1.23 mmol/L.
Twenty-Year HDL and LDL Variability and Midlife Cognitive Function
Higher 20-year LDL variability was significantly associated with worse midlife RAVLT performance (β [95% CI] = −0.25 [−0.42, −0.08]), in the fully adjusted model. LDL variability was not associated with DSST or Stroop performance. Higher 20-year HDL variability was significantly associated with worse midlife DSST performance (β [95% CI] = −0.80 [−1.18, −0.41]), in the fully adjusted model. HDL variability was not associated with performance on the RAVLT or the Stroop test in the adjusted models (Table 2).
Table 2.
Associations Between 20-Year Lipid Variability Throughout Young Adulthood and Midlife Cognitive Function (N = 3 328)
| RAVLT | DSST | Stroop | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| LDL | ||||||
| Variability | −0.29** (−0.46, −0.11) | −0.25** (−0.42, −0.08) | −0.10 (−0.24, 0.11) | −0.02 (−0.18, 0.14) | 0.09 (−0.09, 0.27) | 0.06 (−0.11, 0.23) |
| Mean | −0.06* (−0.11, 0.01) | −0.01 (−0.06, 0.04) | −0.06* (−0.11, −0.01) | 0.01 (−0.04, 0.06) | −0.06* (−0.12, −0.01) | −0.03 (−0.08, 0.02) |
| Slope | −0.05 (−0.91, 0.81) | −0.02 (−0.83, 0.80) | 0.55 (−0.31, 1.40) | 0.42 (−0.37, 1.20) | 1.02* (0.16, 1.9) | 0.33 (−0.50, 1.17) |
| HDL | ||||||
| Variability | −0.56* (−0.99, −0.14) | −0.01 (−0.41, 0.39) | −1.39** (−1.81, −0.97) | −0.80** (−1.18, −0.41) | −0.66** (−1.09, −0.23) | −0.20 (−0.61, 0.21) |
| Mean | 0.31** (0.19, 0.44) | 0.01 (−0.11, 0.14) | 0.56** (0.43, 0.68) | 0.23** (0.10, 0.35) | 0.22** (0.10, 0.35) | 0.12 (−0.01, 0.25) |
| Slope | 4.84** (2.92, 6.78) | 2.29* (0.49, 4.09) | 2.29* (0.38, 4.20) | 0.18 (−1.54, 1.91) | 0.98 (−0.95, 2.92) | 0.18 (−1.66, 2.03) |
Notes: DSST = Digit Symbol Substitution Test; HDL = high-density lipoprotein; LDL = low-density lipoprotein; RAVLT = Rey Auditory-Verbal Learning Test. Cognitive scores are z-scored to facilitate comparison across estimates and Stroop scores were additionally reverse coded. Model 1 is adjusted for 20-y intraindividual mean and slope LDL (or HDL). Model 2 is additionally adjusted for baseline age, sex, race, education, smoking status, body mass index, fasting glucose, systolic blood pressure, physical activity, and lipid medication use.
*p < .05. **p < .01.
In sensitivity analyses, associations of LDL and HDL variability with cognitive function remained similar when we excluded 288 participants who ever used any lipid-lowering medications (data not shown). Furthermore, in analyses accounting for selective attrition using inverse probability of censoring weights, inferences for cognitive function were largely unchanged. Generally, effect estimates became slightly stronger than in the original unweighted analysis (Supplementary Table 2). Finally, inferences remained largely the same in analyses adjusting for covariates at time of cognition instead of baseline (Supplementary Table 3).
Twenty-Year HDL and LDL Variability and Midlife MRI Markers
Twenty-year LDL variability was not associated with volumetric measures or FA measures. On the other hand, higher 20-year HDL variability was significantly associated with smaller total brain volume (β [95% CI] = −0.58 [−0.82, −0.34]), GM volume (β [95% CI] = −0.70 [−1.06, −0.35]),WM volume (β [95% CI] = −0.41 [−0.69, −0.12]), and hippocampus volume (β [95% CI] = −0.83 [−1.57, −0.09]), in fully adjusted models, including adjusted for ICV. In addition, higher 20-year HDL variability was significantly associated with worse FA in total brain (β [95% CI] = −1.13 [−1.87, −0.39]) and in WM (β [95% CI] = −1.36 [−2.14, −0.58]), in fully adjusted models. Associations of 20-year LDL and HDL variability with brain MRI markers remained similar when we excluded 59 participants who ever used any lipid-lowering medications (data not shown) (Table 3).
Table 3.
Associations Between 20-Year Lipid Variability Throughout Young Adulthood and Midlife Brain Structure (N = 714)
| Total Cerebral Volume | Total GM Volume | Total WM Volume | Total Hippocampal Volumea | |||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| LDL | ||||||||
| Variability | −0.04 (−0.16, 0.08) | −0.03 (−0.15, 0.09) | −0.02 (−0.19, 0.16) | −0.02 (−0.19, 0.16) | −0.06 (−0.19, 0.08) | −0.04 (−0.18, 0.10) | 0.16 (−0.23, 0.55) | 0.19 (−0.18,0.55) |
| Mean | −0.02 (−0.05, 0.02) | −0.01 (−0.05, 0.02) | −0.01 (−0.06, 0.04) | 0.00 (−0.05, 0.05) | −0.02 (−0.06, 0.02) | −0.03 (−0.07, 0.01) | 0.15** (0.04, 0.26) | 0.09 (−0.01, 0.20) |
| Slope | 0.45 (−0.15, 1.04) | 0.20 (−0.41, 0.81) | 0.92* (0.05, 1.78) | 0.53 (−0.37, 1.43) | −0.07 (−0.75, 0.60) | −0.16 (−0.86, 0.54) | 4.01** (2.08, 5.95) | 2.44** (0.61, 4.28) |
| HDL | ||||||||
| Variability | −0.58** (−0.82, −0.34) | −0.58** (−0.82, −0.34) | −0.75** (−1.10, −0.40) | −0.70** (−1.06, −0.35) | −0.36* (−0.63, −0.08) | −0.41* (−0.69, −0.12) | −0.54 (−1.33, 0.26) | −0.83* (−1.57, −0.09) |
| Mean | 0.07 (−0.02, 0.15) | 0.04 (−0.05, 0.13) | 0.08 (−0.04, 0.20) | 0.05 (−0.08, 0.18) | 0.05 (−0.05, 0.14) | 0.02 (−0.08, 0.12) | −0.50** (−0.77, −0.23) | −0.02 (−0.29, 0.26) |
| Slope | −1.69** (−2.91, −0.48) | −1.84** (−3.07, −0.62) | −2.81** (−4.60, −1.03) | −3.20** (−5.02, −1.39) | −0.42 (−1.82, 0.99) | −0.31 (−1.74, 1.13) | −2.06 (−6.12, 2.00) | 0.47 (−3.33, 4.27) |
| FA in Total Cerebral Volume | FA in Total GM Volume | FA in Total WM Volume | FA in Total Hippocampal Volume | |||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| LDL | ||||||||
| Variability | −0.37 (−0.77, 0.02) | −0.22 (−0.59, 0.14) | −0.24 (−0.64, 0.16) | −0.11 (−0.45, 0.23) | −0.37 (−0.76, 0.02) | −0.24 (−0.63, 0.15) | −0.20 (−0.61, 0.20) | −0.12 (−0.49, 0.26) |
| Mean | 0.05 (−0.07, 0.16) | 0.02 (−0.08, 0.13) | 0.05 (−0.07, 0.16) | 0.04 (−0.06, 0.13) | 0.04 (−0.07, 0.15) | 0.02 (−0.09, 0.13) | 0.01 (−0.11, 0.12) | −0.00 (−0.11, 0.11) |
| Slope | −0.63 (−2.60, 1.33) | −0.28 (−2.13, 1.56) | 0.19 (−1.78, 2.17) | 0.84 (−0.88, 2.57) | −0.67 (−2.60, 1.25) | −0.86 (−2.82, 1.09) | −0.36 (−1.63, 2.36) | 0.87 (−1.04, 2.78) |
| HDL | ||||||||
| Variability | −1.66** (−2.46, −0.86) | −1.13** (−1.87, −0.39) | −1.12* (−1.93, −0.32) | −0.63 (−1.32, 0.07) | −1.82** (−2.59, −1.04) | −1.36** (−2.14, −0.58) | −1.06* (−1.87, −0.25) | −0.55 (−1.32, 0.21) |
| Mean | 0.13 (−0.14, 0.41) | −0.03 (−0.30, 0.24) | 0.09 (−0.18, 0.37) | −0.03 (−0.29, 0.22) | 0.16 (−0.10, 0.43) | 0.01 (−0.27, 0.29) | 0.19 (−0.10, 0.47) | −0.01 (−0.28, 0.27) |
| Slope | −0.76 (−4.87, 3.34) | −1.24 (−5.00, 2.52) | −2.03 (−6.18, 2.12) | −1.56 (−5.11, 1.98) | −0.43 (−4.43, 3.57) | −1.95 (−5.93, 2.03) | −4.15 (−8.33, 0.04) | −4.62* (−8.52, −0.71) |
Notes: FA = fractional anisotropy; GM = gray matter; HDL = high-density lipoprotein; LDL = low-density lipoprotein; WM = white matter. All volumes were z-scored to facilitate comparison of estimates. Model 1 is adjusted for 20-y intraindividual mean and slope LDL (or HDL). Model 2 is additionally adjusted for baseline age, sex, race, education, smoking status, body mass index, fasting glucose, systolic blood pressure, physical activity, lipid medication use, and total intracranial volume (ICV).
aAdjusted for total brain GM volume/ICV.
*p < .05. **p < .01.
Discussion
Our findings showed that higher 20-year HDL variability throughout young adulthood was associated with worse processing speed, smaller normal brain volumes, and worse microstructural brain integrity, all measured as early as midlife. On the other hand, higher LDL variability was only associated with worse verbal memory, but not with other cognitive domains, volumetric measures, or microstructural measures. Overall, our findings suggested that maintenance of lipids, especially that of HDL, in young adulthood may aid in preserving cognitive function and brain integrity with age.
Evidence for the associations of cardiovascular risk factors with brain aging has largely relied on studies utilizing a one-time biomarker measure as the main exposure. However, there has been growing interest in the intraindividual variability of these biomarkers over time, as these longitudinal patterns may better reflect homeostatic dysfunction. For example, increased variability in blood pressure and decreased variability in heart rate have been consistently related to structural brain aging and cognitive outcomes (12–16,36). Yet little work has been done to examine the association of lipid variability on brain health. Current research on lipid variability has focused primarily on vascular disease outcomes and mortality in patients with a history of cardiovascular disease (21–23). To our knowledge, only 2 prior studies examined such associations using data from the PROSPER trial (18,19). Thus, the present study utilizing data from an ongoing population-based cohort study fills a much-needed gap in the literature.
Despite differences in study design and population, our findings regarding cognitive function are generally consistent with those from the PROSPER trial, which showed that higher LDL-C and HDL-C variability were associated with worse cognitive performance (18,19). Inconsistent with PROSPER, we found that higher HDL, but not LDL, variability was associated with smaller normal tissue volumes and smaller FA (ie, less tissue integrity).
There are several potential mechanisms through which lipid variability may influence cognition and brain tissue integrity. First, variability in LDL may result in the instability of atherosclerotic plaques (31,32) as well as endothelial dysfunction (33), resulting in cerebrovascular damage that could affect cognitive function. This is consistent with our results showing that higher LDL variability is related to worse verbal memory function. Second, lipid-lowering treatment, especially nonadherence, may result in increased LDL variability. However, our findings were robust and unchanged when we excluded individuals who ever used lipid medication at any time during the study period. Third, we found significant associations of HDL variability, which is less likely to be influenced by lipid lowering medication, with cognition and metrics of brain integrity (34). To our knowledge, only one previous study has examined HDL variability in relation to brain health. As such, the mechanism of how HDL variability affects brain health is underexplored. Variability in HDL may reflect worse antiatherogenic function, since HDL is known to mediate excess cholesterol removal from macrophages that act to induce atherosclerosis (37). Greater intima-media thickness, a subclinical marker of atherosclerosis, has been related to worse processing speed in other cohorts, so these findings are complementary, but more work should be done to confirm these findings (38). In addition, cholesterol may impact neurodegenerative processes, given its known role in amyloid-beta regulation (5). A previous PROSPER study only examined cognition and did not examine neuroimaging outcomes in association with HDL variability, so further work is needed to confirm our findings (19). Fourth, lipid variability may reflect other underlying pathways that are also associated with cognitive function, such as inflammation and homeostatic dysfunction in parallel systems. Lastly, further work is warranted to examine how variability in lipids may interact with markers of neurodegeneration, such as in vivo imaging of amyloid or tau pathology, to impact cognition.
Our study has limitations that are worth noting. Cognitive function was not administered until year 25 after baseline, so we could not rule out the possibility that any lipid variability is a consequence of cognitive change or ongoing brain changes. Further, only 719 CARDIA participants were enrolled in the MRI substudy, yet any resulting bias would likely lead to more conservative estimates as mean LDL and HDL variability were statistically equivalent among those included versus excluded in the MRI substudy. We also acknowledge that the cognitive test battery is limited. In future studies, it is important to have more comprehensive batteries assessing cognitive domains with multiple tests. While we acknowledge that repeated lipid measurements might be subject to some measurement error, we anticipate it to be the same throughout the visits. Use of lipid medication was self-reported; however, this has been shown to be fairly accurate (39). While we adjusted for potential confounders, we cannot rule out the possibility of residual confounding in the observed associations. Further, while we examined the relationship of lipid variability with cognition, examining ways to reduce variability is beyond the scope of this analysis. Finally, most prior work on lipid variability and cognition has focused on variability in older age—so further work is needed to confirm our findings in younger age.
Despite these limitations, this study has significant strengths that contribute to a sparse literature on the relationship between lipid variability and brain health. This is among the few studies to our knowledge that have examined such associations among middle-aged adults (mean age of 45.3 years). Our study included repeated lipid measurements over 20 years of adulthood which enabled us to examine intrapersonal variability using a lifecourse approach. Our study also included brain imaging studies in addition to neuropsychological cognitive testing. The nature of the study design enabled us to examine the associations prospectively. Our data come from a large and ongoing cohort of middle-aged adults which included a rich collection of well-established risk factors of cognitive function such as behavioral and cardiovascular disease risk factors. Thus, results from this study are more generalizable compared to post-hoc analyses of clinical trials data, since our inclusion and exclusion criteria are less stringent. Finally, given the longitudinal nature of the study, we addressed attrition during the study period using inverse probability of attrition weights.
In conclusion, our findings suggested that maintenance of lipids, especially that of HDL, in young adulthood may aid in preserving cognitive function and brain integrity with age. Further investigations replicating our findings, how lipid variability is maintained, and investigations into the mechanisms mediating these associations are needed to inform potential interventions that may help attenuate the risk of cognitive impairment and brain aging.
Funding
This work was supported by the National Institutes of Health, National Institute on Aging (K01AG047273), and National Institute of Neurological Disorders and Stroke (F30NS103462). The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgment
This manuscript has been reviewed by CARDIA for scientific content.
Author Contributions
A.Z.A.H. contributed to the conception of the research question, statistical analysis, interpretation of the results, drafting of manuscript, and review of manuscript for scientific content. M.R.C. contributed to the interpretation of the results, drafting of manuscript, and review of manuscript for scientific content. N.J. contributed to the review of manuscript for scientific content. L.G. contributed to the interpretation of the results, drafting of manuscript, and review of manuscript for scientific content. T.E. contributed to the interpretation of the results and review of manuscript for scientific content. M.C.O. contributed to the interpretation of the results and review of manuscript for scientific content. C.W. contributed to the interpretation of the results and review of manuscript for scientific content. M.E. contributed to the interpretation of the results and review of manuscript for scientific content. L.L. contributed to the interpretation of the results and review of manuscript for scientific content. K.Y. contributed to the interpretation of the results and review of manuscript for scientific content.
References
- 1. Gardener H, Wright CB, Rundek T, Sacco RL. Brain health and shared risk factors for dementia and stroke. Nat Rev Neurol. 2015;11(11): 651–657. doi: 10.1038/nrneurol.2015.195 [DOI] [PubMed] [Google Scholar]
- 2. Amarenco P, Labreuche J, Touboul PJ. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis. 2008;196(2): 489–496. doi: 10.1016/j.atherosclerosis.2007.07.033 [DOI] [PubMed] [Google Scholar]
- 3. Carcaillon L, Plichart M, Zureik M, et al. Carotid plaque as a predictor of dementia in older adults: the Three-City Study. Alzheimers Dement. 2015;11(3): 239–248. doi: 10.1016/j.jalz.2014.07.160 [DOI] [PubMed] [Google Scholar]
- 4. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11): 1006–1018. doi: 10.1016/S1474-4422(09)70236-4 [DOI] [PubMed] [Google Scholar]
- 5. Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6(4): 345–351. doi: 10.1038/nn0403-345 [DOI] [PubMed] [Google Scholar]
- 6. Elias PK, Elias MF, D’Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med. 2005;67(1): 24–30. doi: 10.1097/01.psy.0000151745.67285.c2 [DOI] [PubMed] [Google Scholar]
- 7. Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010;67(12): 1491–1497. doi: 10.1001/archneurol.2010.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Bruijn RF, Akoudad S, Cremers LG, et al. Determinants, MRI correlates, and prognosis of mild cognitive impairment: the Rotterdam Study. J Alzheimer Dis. 2014;42(suppl. 3):S239–249. doi: 10.3233/jad-132558. [DOI] [PubMed] [Google Scholar]
- 9. Warstadt NM, Dennis EL, Jahanshad N, et al. ; Alzheimer’s Disease Neuroimaging Initiative (ADNI) . Serum cholesterol and variant in cholesterol-related gene CETP predict white matter microstructure. Neurobiol Aging. 2014;35(11): 2504–2513. doi: 10.1016/j.neurobiolaging.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parati G, Torlasco C, Pengo M, Bilo G, Ochoa JE. Blood pressure variability: its relevance for cardiovascular homeostasis and cardiovascular diseases. Hypertens Res. 2020;43(7): 609–620. doi: 10.1038/s41440-020-0421-5 [DOI] [PubMed] [Google Scholar]
- 11. Yano Y. Visit-to-visit blood pressure variability—what is the current challenge? Am J Hypertens. 2017;30(2): 112–114. doi: 10.1093/ajh/hpw124 [DOI] [PubMed] [Google Scholar]
- 12. Sabayan B, Wijsman LW, Foster-Dingley JC, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. Br Med J. 2013;347:f4600. doi: 10.1136/bmj.f4600 [DOI] [PubMed] [Google Scholar]
- 13. Britton A, Singh-Manoux A, Hnatkova K, Malik M, Marmot MG, Shipley M. The association between heart rate variability and cognitive impairment in middle-aged men and women. The Whitehall II cohort study. Neuroepidemiology. 2008;31(2): 115–121. doi: 10.1159/000148257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeki Al Hazzouri A, Haan MN, Deng Y, Neuhaus J, Yaffe K. Reduced heart rate variability is associated with worse cognitive performance in elderly Mexican Americans. Hypertension (Dallas, Tex: 1979). 2014;63(1):181–187. doi: 10.1161/hypertensionaha.113.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu C, Shlipak MG, Stawski RS, et al. Visit-to-visit blood pressure variability and mortality and cardiovascular outcomes among older adults: the Health, Aging, and Body Composition Study. Am J Hypertens. 2017;30(2): 151–158. doi: 10.1093/ajh/hpw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeki Al Hazzouri A, Elfassy T, Carnethon MR, Lloyd-Jones DM, Yaffe K. Heart rate variability and cognitive function in middle-age adults: the Coronary Artery Risk Development in Young Adults. Am J Hypertens. 2017;31(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yano Y, Reis JP, Levine DA, et al. Visit-to-visit blood pressure variability in young adulthood and hippocampal volume and integrity at middle age: the CARDIA Study (Coronary Artery Risk Development in Young Adults). Hypertension (Dallas, Tex: 1979). 2017;70(6):1091–1098. doi: 10.1161/HYPERTENSIONAHA.117.10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smit RA, Trompet S, Sabayan B, et al. Higher visit-to-visit low-density lipoprotein cholesterol variability is associated with lower cognitive performance, lower cerebral blood flow, and greater white matter hyperintensity load in older subjects. Circulation. 2016;134(3): 212–221. doi: 10.1161/CIRCULATIONAHA.115.020627 [DOI] [PubMed] [Google Scholar]
- 19. Grasset L, Smit RAJ, Caunca MR, et al. Association of high-density lipoprotein cholesterol with cognitive function: findings from the PROspective Study of Pravastatin in the Elderly at Risk. J Aging Health. 2020;32:1267–1274. doi: 10.1177/0898264320916959 [DOI] [PubMed] [Google Scholar]
- 20. Chung HS, Lee JS, Kim JA, et al. Variability in total cholesterol concentration is associated with the risk of dementia: a nationwide population-based cohort study. Front Neurol. 2019;10:441. doi: 10.3389/fneur.2019.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH; TNT Steering Committee and Investigators . Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol. 2015;65(15): 1539–1548. doi: 10.1016/j.jacc.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 22. Bangalore S, Fayyad R, Messerli FH, et al. Relation of variability of low-density lipoprotein cholesterol and blood pressure to events in patients with previous myocardial infarction from the IDEAL trial. Am J Cardiol. 2017;119(3): 379–387. doi: 10.1016/j.amjcard.2016.10.037 [DOI] [PubMed] [Google Scholar]
- 23. Boey E, Gay GM, Poh KK, Yeo TC, Tan HC, Lee CH. Visit-to-visit variability in LDL- and HDL-cholesterol is associated with adverse events after ST-segment elevation myocardial infarction: a 5-year follow-up study. Atherosclerosis. 2016;244:86–92. doi: 10.1016/j.atherosclerosis.2015.10.110 [DOI] [PubMed] [Google Scholar]
- 24. Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129(15): 1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 26. Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6(3):235–245. doi: 10.1016/1047-2797(96)00005-1 [DOI] [PubMed] [Google Scholar]
- 27. Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol. 1984;40(3): 785–787. doi: [DOI] [PubMed] [Google Scholar]
- 28. Weschler D. Wechsler Adult Intelligence Scale-III (WAIS-III). San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 29. MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109(2): 163–203. doi: 10.1037/0033-2909.109.2.163 [DOI] [PubMed] [Google Scholar]
- 30. Launer LJ, Lewis CE, Schreiner PJ, et al. Vascular factors and multiple measures of early brain health: CARDIA Brain MRI Study. PLoS ONE. 2015;10(3): e0122138. doi: 10.1371/journal.pone.0122138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Z, Ichetovkin M, Kurtz M, et al. Cholesterol in human atherosclerotic plaque is a marker for underlying disease state and plaque vulnerability. Lipids Health Dis. 2010;9(6): 61. doi: 10.1186/1476-511X-9-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103(7):926–933. doi: 10.1161/01.cir.103.7.926 [DOI] [PubMed] [Google Scholar]
- 33. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 suppl. 1):III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 34. Arsenault BJ, Boekholdt SM. Clinical and biological relevance of statin-mediated changes in HDL metabolism. Curr Atheroscler Rep. 2014;16(7): 379. doi: 10.1007/s11883-013-0379-8 [DOI] [PubMed] [Google Scholar]
- 35. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67(1): 564–569. doi: 10.1001/archneurol.2010.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125(15): 1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeki Al Hazzouri A, Vittinghoff E, Sidney S, Reis JP, Jacobs DR Jr., Yaffe K. Intima-media thickness and cognitive function in stroke-free middle-aged adults: findings from the Coronary Artery Risk Development in Young Adults Study. Stroke. 2015. doi: 10.1161/STROKEAHA.115.008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4): 470–482. doi: 10.1007/s13142-015-0315-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.