Abstract
Background
The Ambient Intelligent Geriatric Management (AmbIGeM) system augments best practice and involves a novel wearable sensor (accelerometer and gyroscope) worn by patients where the data captured by the sensor are interpreted by algorithms to trigger alerts on clinician handheld mobile devices when risk movements are detected.
Methods
A 3-cluster stepped-wedge pragmatic trial investigating the effect on the primary outcome of falls rate and secondary outcome of injurious fall and proportion of fallers. Three wards across 2 states were included. Patients aged ≥65 years were eligible. Patients requiring palliative care were excluded. The trial was registered with the Australia and New Zealand Clinical Trials registry, number 12617000981325.
Results
A total of 4924 older patients were admitted to the study wards with 1076 excluded and 3240 (1995 control, 1245 intervention) enrolled. The median proportion of study duration with valid readings per patient was 49% ((interquartile range [IQR] 25%-67%)). There was no significant difference between intervention and control relating to the falls rate (adjusted rate ratio = 1.41, 95% confidence interval [0.85, 2.34]; p = .192), proportion of fallers (odds ratio = 1.54, 95% confidence interval [0.91, 2.61]; p = .105), and injurious falls rate (adjusted rate ratio = 0.90, 95% confidence interval [0.38, 2.14]; p = .807). In a post hoc analysis, falls and injurious falls rate were reduced in the Geriatric Evaluation and Management Unit wards when the intervention period was compared to the control period.
Conclusions
The AmbIGeM system did not reduce the rate of falls, rate of injurious falls, or proportion of fallers. There remains a case for further exploration and refinement of this technology given the post hoc analysis findings with the Geriatric Evaluation and Management Unit wards.
Clinical Trials Registration Number: 12617000981325
Keywords: Hospital related, Morbidity, Preventative health care
Falls in hospital are a major contributor to adverse events leading to deaths, with the incidence increasing by 14% over the decade between 2006/2007 and 2015/2016 (1). Falls remain common in Australian hospitals, occurring in more than 34 000 separations at a rate of 3.2 per 1000 separations in the 2015/2016 financial year (2). Falls are costly resulting in additional hospital costs of AU$6669 (95% confidence interval [CI], $3888–$9450) (3). Importantly, falls also have functional and psychological consequences, resulting in loss of independence and premature institutionalization (4,5).
Research evidence for effective interventions to reduce falls among older people in hospitals remains limited (6). In the most recent Cochrane review, multifactorial interventions were shown to have stronger evidence of effect than single interventions, and there was stronger evidence in subacute compared to acute hospital wards (6). Further, there remains an evidence gap for falls prevention for hospitalized patients with dementia (7), despite a high proportion of falls in hospitals involving people with dementia or delirium (8).
Although widely used, research evaluating pressure sensor alarms on beds and chairs has produced disappointing results (6). Despite this lack of evidence, the desire to keep patients safe drives ongoing reliance on such devices (9,10). Wearable sensors may offer advantages over bed and chair pressure alarm systems because they allow for monitoring of multiple risk activities, across multiple locations in multiple patients together with the ability to individualize alarms to patient care needs. Furthermore, there is rapid progress in the field of wearable sensors, moving toward a future where real-time physiological monitoring of patients is integrated with electronic medical records (11).
A new wearable sensor approach called the Ambient Intelligent Geriatric Management (AmbIGeM) system, encompassing patient-worn sensors and movement recognition and location tracking algorithms to trigger alert messages to staff when risk movements occur, was codesigned by our research team and hospital staff with feedback from patients. Preliminary research by our team demonstrated data to support that a wearable sensor system could be accurate and acceptable to patients (12). We hypothesized that such a system would enable staff to intervene before a fall and reduce falls rates and injuries.
The study objective therefore was to investigate the AmbIGeM system in 3 wards across 2 different states while evaluating the effectiveness of the technology in reducing the falls rate, the proportion of fallers, and the injurious falls rate in older people in hospital.
Method
Trial Design and Participants
The implementation research incorporated a concurrent mixed-methods design combining a pragmatic stepped-wedge cluster trial with a survey and qualitative process to gather information from patients and clinical staff on the acceptability and safety of the study. The study protocol has been published but is briefly described here (13).
Participants and Consent
Patients who were 65 years and older and admitted to participating wards were eligible while those receiving palliative care as well as those previously enrolled in the study during the same admission (eg, transfers between wards) were excluded. Supporting inclusiveness of those with dementia, an opt-out consent process was in place in South Australia (SA) while a consent waiver process was in place in Western Australia (WA). Opt-out consent is where participants are provided with information about the research and unless they opt out (decline to participate), it is presumed they will participate. A consent waiver waives the requirement to obtain informed consent. In the study wards, patients and families were informed about the study by placing posters around the ward and giving patients written information about the study. If patients (or person responsible) did not want to participate, they withdrew (WA study site) by telling the clinical or research staff or opted out (SA study site) by signing the form or telling the clinical or research staff within 3 days of entering the ward.
Sample Size
Assuming a falls rate of 7.7 per 1000 participant bed days and the average length of stay of 12.3 days, we calculated that 924 patients would be needed in a patient-level pragmatic trial to achieve 80% power at 5% significance level to detect a relative reduction in falls of 0.53 (ie, a 47% reduction in the falls rate). To account for the clustered nature of the stepped-wedge design, an Intracluster Correlation Coefficient of 0.0027 and an average cluster size of 800 patients over the 100 weeks of the study (excluding the 3 weeks technology testing period prior to the first intervention block) were assumed, resulting in a design effect of 2.6. The study required a total of 2400 patients (1200 in control and 1200 in intervention). The sample size arrived at was guided by pragmatism as well as the study by Dykes et al where the adjusted fall rate in the control unit was 4.75 (3.44–6.54) per 1000 patient days compared to 2.66 (1.87–3.80) per 1000 patient bed days (14).
Allocation and Blinding
The intervention was not blinded and was delivered across 3 clusters (wards) in 2 hospitals in 2 Australian states: SA and WA. The AmbIGeM trial commenced on the 10th of July 2017 and after 103 weeks including 3 weeks of technology testing prior to the first active wedge (commenced 22nd of January 2018), the study completed on the 30th of June 2019.
All wards initially spent 25 weeks in the control period, with 1 ward then changing to an intervention ward each subsequent 25 weeks wedge. The following pragmatic order of transitioning from control to intervention wedge was used to support deployment of the technology from SA: (i) South Ground (28–32 beds) at The Queen Elizabeth Hospital (TQEH), SA, (ii) followed by the 14-bed Geriatric Evaluation and Management Unit (GEMU) at Sir Charles Gairdner Hospital (SCGH) in WA, and (iii) the 32-bed General Medicine (GM) ward at SCGH. While South Ground frequently flexed up from the 28-bed GEMU to a 32-bed ward with the 4 additional beds occupied by general medical patients, this ward is referred to as GEMU in this paper. The GM ward admitted patients of all ages. Summer bed closures affected the WA GEMU (control period) for 16 weeks, from the 22nd of December 2017 to the 16th of April 2018.
Best Practice
Best practice consistent with the Australian falls prevention guidelines for hospitals was in place and continued throughout the study (15). The New South Wales Clinical Excellence Commission Falls Audit Tool-Ward Level was administered during the first week of each wedge to provide a record of best practice falls prevention activity within the wards (16). This best practice framework remained consistent throughout the study.
Intervention
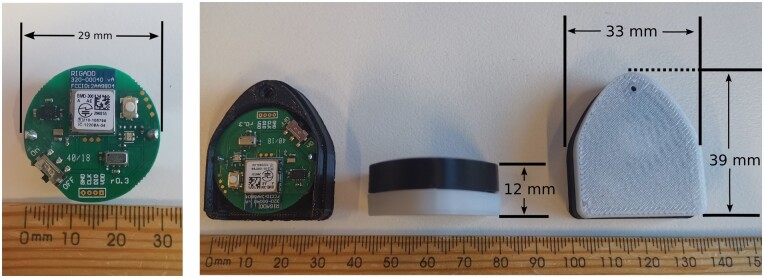
Patients wore a cotton singlet with an encased wearable Bluetooth Low Energy sensor device with integrated triaxial accelerometer and gyroscope sensors. The sensor weighed 15 g (Figure 1) and was positioned in a customized pocket over the sternum. Wearable sensors were cleaned and reused. A protocol with radiology was developed to ensure the wearable sensor was removed prior to Magnetic Resonance Imaging.
Figure 1.
Sensor and casing dimensions.
Wireless signals transmitted from the wearable sensor containing the triaxial accelerometer, gyroscope, and unique sensor ID data were collected by base stations attached to the ceiling of patient rooms. Base stations were positioned above patient beds, toilets and room door exits. Data from the base stations were processed and transmitted over a local area network to a server. Software developed by our team interpreted the data using algorithms. The 3 categories of key software changes made during the study were: (i) changes to improve usability of the software by making modification to the user interface based on staff feedback; (ii) updates to the algorithms used to determine patient activities; and (iii) system upgrades to improve performance and software maintenance. It is possible that the changes to the algorithms and system upgrades could have improved performance, but this was not assessed.
Staff selected patient risk for the day and night periods and it was possible that for some patients, no risk was identified. When patient-specific risk movements were inferred, staff were alerted using vibration or sound or both modes with messages generated by the server software. These were transmitted through the ward Wi-Fi network to the Mobile Apps on Android smartphones to alert staff using vibration or sound or both modes. Unless the staff was adjacent to the patient and had selected to sound, patients would not hear the alerts. The goal was to provide staff an opportunity to intervene and prevent a fall but no record of staff response was recorded. In the intervention phase, the smartphones were provided to all nursing staff in participating wards, and were provided to allied health staff in WA. The AmbIGeM system was allocated a dedicated Wi-Fi network using existing Wi-Fi infrastructure in WA but relied on the health system Wi-Fi network in SA.
The AmbIGeM Mobile App deployed on smartphones allowed staff to define individual patient risk movements for translation by the system to generate a bedside poster and for determining the rules for activation of relevant patient-specific alarms. The risk movement selection facilitated setting individual patient alarm activations by the system for any or all of the following risk movements, and the selections could be updated any time:
• Sitting up after lying on the bed;
• Getting out of from bed or chair;
• Walking out of the room including to the toilet; and
• Walking without an aid where the aid was considered necessary for safety.
The Mobile App allowed staff to deactivate the alert while the alert displayed the patient, time, location, and risk movement. The intention was to provide staff the opportunity to attend to the patient as quickly as possible. Sensors were also attached to participant walking aids to detect movement without an aid where an aid was required for safe mobility.
A Desktop App for falls management was also available at the nurse station to support patient enrollment, bed swaps, and discharge as well as alert staff toward the need for sensor replacements due to an impending depletion of a sensor battery (lasted median 23 days) for individual sensors. Updates on patient activity and alerts were available on the Desktop App and the alarm could also be deactivated from the Desktop App.
Program Fidelity
To ensure program fidelity, protocols were used for staff training, detailing definition and reporting of a fall, use of the Mobile App, putting on the singlet, changing the singlet, enrolling a sensor, use of the Desktop App, and how to respond to the alerts, delivered to the Mobile App. Two weeks prior to the study commencement on each ward, in-service programs were conducted with ward staff to ensure staff understood the definition of a fall including a video designed to assist staff define what constitutes a fall, were familiar with best practice, and were aware how falls should be reported (hospital incident reporting system and patient medical records) (17). In the week before the commencement of the intervention, an in-service program to train ward staff occurred where details of the intervention were provided. Information describing the system was also available in printed form. Once the intervention had commenced, in-service sessions of 15-minute duration on the use of the technology were provided daily for the first week and then weekly in the first month to maintain staff knowledge with regards to the use of the intervention and support troubleshooting.
Technology Adherence
For the purpose of estimating adherence, the percentage of time with valid sensor data was evaluated and defined as (duration with valid sensor data/duration enrolled in study) × 100%. Data were present when the following conditions were met: (i) the participant enrolled into the system and assigned a sensor and (ii) the sample rate of the data (number of unique data packets sent by the assigned sensor and received by the system) exceeded 240 samples per minute. Subsequently, for a given 1-minute period to be counted as a “duration with valid sensor data,” it must satisfy the conditions of: (i) a correctly calibrated sensor; (ii) a sensor worn by a patient (eg, not accidently left on a table); and (iii) a sensor worn in the correct orientation. The admission day was assumed to be from 1200 to 2359 (24-hour clock) while the discharge day was 0000–1200. In this study, daytime was defined as 0701–2059. Based on other falls research, the adherence threshold was set at 63% in this study (18).
Outcomes
The primary outcome measure in this study was falls rate, calculated as the number of falls divided by the number of participant bed days in the participating wards during the control and intervention blocks, and expressed as falls per 1000 participant bed days. A fall in this study was defined as an “event which results in a person coming to rest inadvertently on the ground or floor or other lower level” (19). Research personnel collected falls data (location, injury, time) from 3 sources: (i) health systems computerized incident reports; (ii) daily enquiry of falls from ward team leaders; and (iii) hand searching of patient medical notes or electronic health records in order to maximize accuracy of falls data (20).
The secondary outcome measures in this study were: (i) proportion of participants falling; and (ii) rate of injurious in-patient falls per 1000 participant bed days.
In this study injurious falls refer to those that cause bruising, laceration, fracture, loss of consciousness, or if the patient reports persistent pain (17,21). Moderate injurious falls were recorded when bruising, sprains, cuts, abrasions, seeking medical attention, or a decrease in physical function for a period of 3 days or more were noted (21). Serious injurious falls were when fractures (with radiological confirmation (22)) and sutures were required. As part of hospital policy, clinical staff record any fall in the incident reporting system and medical records and no structured protocol was in place. When it came to recording falls for this research, where research staff were uncertain, the senior investigators (R.V. and K.D.H.) adjudicated.
Other Assessments
Research staff entered patient demographic information (hospital identifier, date of birth, gender, living arrangements pre-hospitalization) within 72 hours of admission. At discharge and predominantly from the discharge summary, the primary reason for admission, post hospital discharge destination, the Charlson Comorbidity Index (23), delirium present during admission or as a primary or complicating diagnosis, if patient was admitted with a fall (within 7 days of admission), if fracture was a primary or complicating diagnosis of this admission, and number of regular medications were recorded. Information relating to recruitment, withdrawal, and adverse events was also recorded.
Data Safety Monitoring
Guided by a charter, a chair and 4 others including representatives from both hospital falls prevention committees formed the independent Data and Safety Monitoring Committee, and reports were provided for their consideration. They met on August 23, 2017, November 10, 2017, August 14, 2018, and March 12, 2019 to ensure the safety of patients over the course of the study.
Ethics
Ethics and governance approval were achieved from TQEH/Lyell McEwin Hospital (LMH)/Modbury Hospital (MH) (HREC/15/TQEH/17) and Curtin University (HRE2017-0449)/SCGH (PRN 2015-110). Funded by the National Health and Medical Research Council of Australia (APP1082197), the trial was registered with the Australian and New Zealand Clinical Trial Registry (ANZCTR): ACTRN 12617000981325.
Statistical Methods
All analyses were conducted using intention-to-treat principles and while blinded to trial phase (intervention or control). The planned analysis of the primary outcome of falls rate was using a Poisson generalized linear regression model including fixed effects for intervention, ward, and time period. Due to overdispersion and nonconvergence of some models, negative binomial models are reported as adjusted rate ratios (ARRs) and 95% CIs. The secondary outcome of the rate of injurious falls was analyzed similarly. The proportion of participants falling was analyzed by binary logistic regression, accounting for ward and time period effects. The Charlson Comorbidity Index was included as a prespecified covariate. Patients recruited to the study during a control period were censored when the ward transitioned to the intervention (or technology testing period for SA), with falls and length of stay data only collected up until the time of transition. A planned subgroup analysis of the effect of the intervention within patients with and without dementia and delirium was conducted, by including the presence of dementia and delirium diagnoses at discharge as an interaction effect. A planned subgroup analysis by time of fall (day 0700–1959/night 2000–0659) was also conducted, but due to nonconvergence of the repeated outcomes model, results are reported for day and night falls rates separately. Significance was determined at the 5% level for all analyses. No imputation of missing outcome data was conducted.
Results
Participant Flow and Recruitment
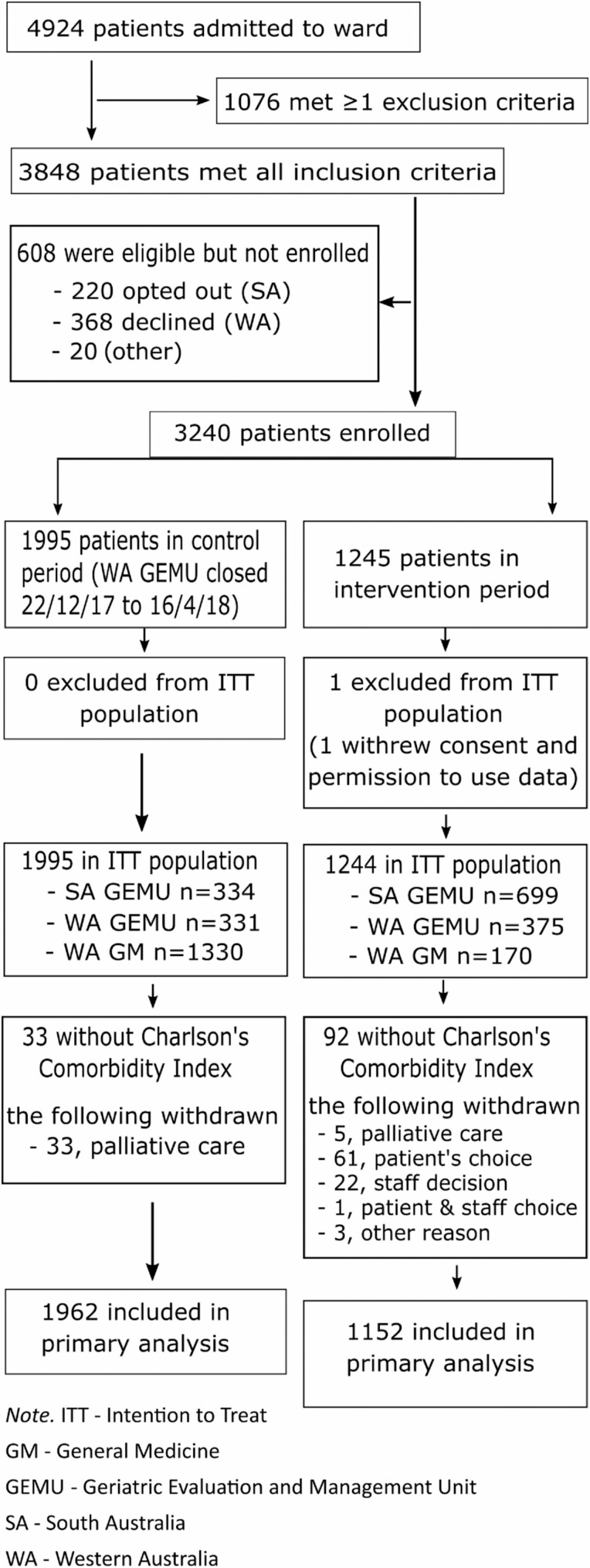
Of the 4924 patients eligible (Figure 2), 3848 met the inclusion criteria and 1076 were excluded. Finally, 3240 patients were enrolled with 1995 control patients and 1244 intervention patients included in the intention-to-treat population.
Figure 2.
Consort flow diagram.
Mean recruitment rates were higher in the control period when compared to the intervention period (SA GEMU 13.4/wk vs 9.3/wk; WA GEMU 8.7/wk vs 7.5/wk; WA GM 17.7/wk vs. 6.8/wk) with the greatest reduction (62%) seen in WA GM.
The higher falls rate observed (10.3/1000 participant bed days) compared to the a priori assumed (7.7/1000 participant bed days), increased the theoretical power to approximately 89% but the lower length of stay observed (11.1 days) compared to assumed (12.3 days) reduced the final theoretical power to 86%.
Baseline Characteristics
There was a larger proportion of GEMU patients (86%) in the intervention group and larger proportion of GM patients (67%) in the control group. The mean age of the study population was 82.7 years (standard deviation [SD] 8.2) with patients in the intervention arm (84.0 [7.9]) being older than those in the control arm (81.9 [8.3]). Patients in the intervention group were more likely to be admitted from the community when compared to the control group (96% vs 89%) and had longer average length of stay (16 vs 11 days). Twenty-two percent of patients were admitted with delirium and 17% with dementia (Table 1, Supplementary Table 1), with a slightly higher proportion in the intervention compared to control (delirium 27% vs 20%; dementia 19% vs 16%). The proportion admitted with a history of falls with or without fracture was higher in the intervention arm at 41% compared to control at 23%. Mortality and discharge to community rates were similar for both groups. There were more patients admitted primarily for infection (35% vs 29%) and more transferred to rehabilitation (inpatient or community; 19% vs 15%) with less discharged home (43% vs 48%) in the intervention period compared to control, when the GM ward was considered on its own.
Table 1.
Patient Characteristics
| Control (N = 1995) | Intervention (N = 1244) | Total (N = 3239) | |
|---|---|---|---|
| Age, years (mean [SD]) | 81.9 (8.3) | 84.0 (7.9) | 82.7 (8.2) |
| Female (n [%]) | 1074 (54%) | 716 (58%) | 1790 (55%) |
| Living in the community pre-hospitalization (n [%]) | 1772 (89%) | 1196 (96%) | 2968 (92%) |
| Charlson Comorbidity Index score (median [IQR])* | 2 [1–4] | 2 [1–4] | 2 [1–4] |
| Proportion with dementia or delirium (n [%])* ,^ | 582 (30%) | 422 (37%) | 1004 (32%) |
| Admitted with falls with or without fractures (n [%])* ,^ | 441 (23%) | 470 (41%) | 911 (29%) |
| Hospital length of stay, days (median [IQR])* | |||
| Total | 11 [7–18] | 16 [11–24] | 13 [8–21] |
| SA GEM | 17 [12–27] | 19 [13–28] | 18 [13–27] |
| WA GEM | 15 [11–22] | 15 [11–21] | 15 [11–21] |
| WA Gen Med | 9 [6–14] | 9 [7–15] | 9 [6–14] |
| Death during admission (n [%])* | 107 (5%) | 18 (2%) | 125 (4%) |
| Discharge destination (n [%])* | |||
| Community | 1013 (52%) | 606 (53%) | 1619 (52%) |
| Residential aged care (permanent) | 193 (10%) | 62 (5%) | 255 (8%) |
| Rehabilitation | 164 (8%) | 104 (9%) | 268 (9%) |
| Transitional care program | 173 (9%) | 98 (9%) | 271 (9%) |
| Died in hospital | 107 (5%) | 18 (2%) | 125 (4%) |
| Others | 312 (16%) | 264 (23%) | 576 (18%) |
Notes: SA GEM = South Australia Geriatric Evaluation and Management; WA GEM = Western Australia Geriatric Evaluation and Management; SD = standard deviation.
*Data were obtained from discharge summary and are not available for participants who withdrew from study during admission and did not grant permission for further data collection. Remaining sample size is 1962 in control and 1152 in intervention.
^Excludes 3 participants in control for whom data were unavailable.
Outcomes and Estimation
There were a total of 371 falls from 273 patients in this study. Injuries were seen in 122 falls with a quarter (n = 103) being classified as moderate or severe and a further 5% (n = 19) as mild injury. Two hundred and fifty-four (69%) of the falls were recorded from the incident reporting system. Twenty-three percent of falls occurred in wet area (e.g. toilet, shower, bathroom) with the majority of falls occurring in the patient rooms (68%).
The overall falls rate increased during the intervention (ARR = 1.41, 95% CI [0.85, 2.34]) period, but was not statistically significant (p = .192) (Table 2). Similarly, the proportion of fallers was nonstatistically significantly increased during the intervention period (odds ratio = 1.54, 95% CI [0.91, 2.61]; p = .105) (Table 2). The injurious falls rate was similar between the 2 periods (ARR = 0.90, 95% CI [0.38, 2.14]; p = .807) (Table 2).
Table 2.
Falls Outcomes
| Intervention (N = 1152) | Control (N = 1962) | Total (N = 3114) | Adjusted Rate Ratio (95% CI), p Value | |
|---|---|---|---|---|
| Falls, rate per 1000 participant bed days (95% CI) | 9.3 (7.0, 12.5) | 6.6 (4.9, 9.0) | 7.9 (6.8, 9.2) | 1.41 (0.85, 2.34), p = .192 |
| N patients with falls | 130 | 128 | 258 | |
| Injurious falls, rate per 1000 participant bed days (95% CI) | 2.5 (1.5, 4.1) | 2.8 (1.7, 4.5) | 2.6 (2.0, 3.3) | 0.90 (0.38, 2.14), p = .807 |
| N patients with injurious falls | 50 | 52 | 102 | |
| N serious falls | 8 | 4 | 12 | |
| N patients with serious falls | 8 | 4 | 12 | |
| Proportion of fallers, having 1 or more falls (95% CI) | 8.9 (6.8, 11.7) | 6.0 (4.5, 8.0) | 7.3 (6.3, 8.4) | OR = 1.54 (0.91, 2.61), p = .105 |
Note: OR = odds ratio.
Rates, proportions, and confidence intervals (CIs) are model-based estimates adjusted for ward, time period, and Charlson Comorbidity Index.
In an exploratory, unplanned subgroup analysis, all outcomes (Table 3) indicated a different effect of treatment in the GEM wards compared to GM (all interaction p < .05), with the intervention being associated with better outcomes than control in the GEM wards, but worse outcomes in GM.
Table 3.
Falls Outcomes by Ward
| Intervention (N = 1152) | Control (N = 1962) | Total (N = 3114) | Adjusted Rate Ratio (95% CI) | p | ||
|---|---|---|---|---|---|---|
| Falls, rate per 1000 participant bed days (95% CI) | GEM | 7.1 (4.9, 10.4) | 11.1 (6.0, 20.5) | 8.9 (7.1, 11.2) | 0.64 (0.27, 1.68) | .002 |
| Gen Med | 14.3 (8.6, 23.7) | 5.7 (4.4, 7.5) | 9.1 (6.8, 12.1) | 2.13 (1.10, 4.10) | ||
| Injurious falls, rate per 1000 participant bed days (95% CI) | GEM | 2.3 (1.3, 4.0) | 6.7 (3.2, 14.1) | 3.9 (2.9, 5.3) | 0.34 (0.11, 1.15) | .018 |
| Gen Med | 3.4 (1.3, 8.8) | 1.1 (0.6, 2.0) | 2.0 (1.1, 3.5) | 2.23 (0.61, 7.90) | ||
| Proportion of fallers, having 1 or more falls (95% CI) | GEM | 8.2 (5.7, 11.6) | 9.6 (5.5, 16.4) | 8.9 (7.2, 11.0) | 0.84 (0.35, 2.19) | .003 |
| Gen Med | 10.0 (6.1, 15.9) | 4.3 (3.3, 5.6) | 6.6 (5.0, 8.7) | 2.21 (1.09, 4.38) |
Notes: GEM = Geriatric Evaluation and Management. p is the p value for the intervention by ward interaction.
Rates, proportions, and confidence intervals (CIs) are model-based estimates adjusted for time period and Charlson Comorbidity Index.
Withdrawal
Overall, there were 33 withdrawals during the control period (Supplementary Table 2a). There were 153 withdrawals during the intervention period (Supplementary Table 2b). The majority of withdrawals during the intervention period were related to the singlet (n = 111; 73%). There were 19 withdrawals relating to the sensor. Three patients pulled apart the sensor and as a result of this staff were advised to not enroll patients (mostly with dementia) who were agitated or fidgety and where already enrolled, to withdraw those patients from the study. To further reduce the risk, the sensor casing was subsequently redesigned without a rivet and enlarged to support a snugger fit in the singlet pocket. A tool was also fabricated to ensure the correct installation of a battery by inserting the battery all the way into the casing and thus ensuring the protections provided by the casing made it difficult for participants to remove the battery.
Adverse Events
There were 24 adverse events recorded during the intervention period with multiple consequences selected for each. The consequences predominantly related to the skin (pressure [n = 2], irritation [n = 10], rash [n = 7], redness [n = 7], and itchiness [n = 8]) and participant (discomfort [n = 1], pulling at sensor [n = 1], and pulling sensor apart [n = 3]).
Technology Adherence
The first intervention wedge was affected by a delay to technology deployment at the start lasting almost 2 weeks and then multiple technical issues relating to the network connectivity between systems at the SA GEMU. Subsequently, there were 3 major technical issues: (i) relating to the Wi-Fi system where the smartphones were unable to connect to the network reliably while roaming; (ii) relating to the introduction of new smartphone models; and (iii) due to the SA GEMU network administrators reconfiguring the local area network and inadvertently disconnecting the server in the process. Two minor events (ie, <1 day) relating to system maintenance contributed to downtime.
Wearable sensor data were available for 1196 (of 1244) participants. The overall median proportion of duration with valid readings was 49% (IQR 25%–67%), similar to that seen at night (median 50%; IQR 25%–71%). In SA GEMU, the median percentage of study time with valid data (Supplementary Table 3) reduced with successive wedges from 53% (IQR 35%–69%) to 40% (IQR 19%–62%) and then 38% (IQR 16%–60%). In WA GEMU, the median percentage of time with valid data remained similar (58%; IQR 44%–72% vs 60%; IQR 38%–72%) across the 2 wedges. The lowest median was seen with WA GM (32%; IQR 14%–59%). Interestingly in WA GM, the falls rate in those with high adherence (defined as ≥63% median percentage time with valid reading) was treble (ie, 33.6 falls/1000 patient bed days vs 11.0 falls/1000 patient bed days) that seen in those with lower adherence.
Bed and chair pressure sensors or pull cord alarm systems were used for a small number of patients throughout the study given staff previous practice and their fear of relying only on the AmbIGeM system for some patients. These were not recorded but an audit in the final intervention wedge revealed that 11 patients in SA wore a pull cord alarm while 25 patients in the WA GEMU and 21 patients in WA GM used a pressure sensor mat.
No differences in rates were seen when daytime was compared to nighttime (Supplementary Table 4) or when those with dementia/delirium were compared to those without (Supplementary Table 5). The effect of the intervention when adherence to the intervention was deemed high was similar to the intention-to-treat analysis.
Discussion
The use of the AmbIGeM technology system in GEMU and GM wards did not significantly reduce falls rate, number of fallers, and injurious falls rate. A post hoc analysis by wards suggested a trend toward reduction in falls and injurious falls rates in the GEMU wards but an increase in those rates in the GM ward. The research is the first time wearable sensor technology involving a large sample of inpatients has been trialed to prevent rather than detect falls and there were few adverse events related to the technology. However, adherence to the technology in terms of the availability of valid data was suboptimal and appeared related to ward size and turnover as well as the duration of time in the intervention as part of research.
Similar to other research when investigated in real-world settings, the intervention did not benefit patient outcomes (9,10). Similar to that noted by Timmons and colleagues who investigated pressure sensor alarm system, we noted that the intervention and the outcome (falls) could not be separated from the broader context of the clinical staff, their established work practices, the ward environment, the organizational culture, and patient or carer expectations. In this research (not published), participants and families viewed the intervention as a useful backup for staff and participants found it acceptable. Staff reported at times that false alerts contributed to ignoring of alerts while delayed alerts did not provide staff with sufficient time to intervene. The intervention was an additional workload for staff that they were willing to undertake if the system was beneficial. While we attempted to overcome potential limitations by codesigning the technology to meet staff and patient needs and gain support for the roll out of the technology, it was difficult to anticipate and respond to all arising requirements in a timely manner given the variability of requirements between staff and also the limited funding and resources available for the conduct of this project. The introduction of new technology into any health care setting introduces new workflow and where there are competing demands, as is the norm, it is more than likely that what is characterized, as research of an unproven intervention is viewed as less of a priority (24). Acceptability of the system from patient, carer, and staff perspectives is planned to be reported separately (13).
While the falls and injurious falls rate increased in the GM ward, the reduced falls and injurious falls rate noted in the GEMU wards provided evidence for further exploration of this technology concept. These findings are also consistent with those reported in a recent Cochrane review that multifactorial interventions appear more effective in reducing falls rates in subacute settings (relative adjusted risk 0.67, 95% CI 0.54 to 0.83) when contrasted to acute or mixed settings (6). Therefore, the quest for an effective strategy for the acute setting continues. A health economic analysis is planned to assess the value of further clinical trials in this area (13).
The recruitment rate was lower in the intervention period compared to the control period. Implementation fatigue is a real risk to lengthy studies and the mean recruitment rate dropped off over the 75 weeks of intervention in the SA GEMU. The largest difference in recruitment rate however was seen with the larger acute GM ward that had a greater patient turnover given shorter median length of stay while caring for patients of all ages. The least change in recruitment rate was noted for the smallest ward providing subacute GEM care (WA) to only older people. Therefore, selection bias possibly contributed to the unusual finding in the GM ward. A limitation of this study was that we did not have approval to investigate the differences between those who enrolled in the trial and those who did not. It has been reported elsewhere that clinical staff sometimes override the random allocation of patients to sensor-related interventions where they subscribe to a view that they know which patients are more likely to fall (25) or are under competing work pressures and it is possible that staff prioritized the intervention to those they identified as being most at risk thus introducing a selection bias. Our previous research investigating the use of health information technology to assess falls risk also demonstrated a lower rate of use (70% vs 61%; p = .08) in the acute medical ward compared to the GEMU (26). The counterargument that the technology intervention somehow contributed to a higher risk of falling in the GM ward is less likely unless the availability of the system to those most at risk resulted in automation complacency (27) reducing staff alertness in this particular busy environment as that association was not seen with the subacute GEM wards.
Reduced adherence to the intervention was recently cited as a major reason why a nurse led multifactorial falls prevention intervention incorporating assessment and care planning in primary care did not lower the rate of a first adjudicated serious fall injury when compared to enhanced usual care (28). Nonadherence to intervention in clinical trials is common and our response to calls for adherence to be reported is a major strength of this study (29). Our interrogation of sensor data on the use of the sensor revealed that the percentage of time with valid data reading was highest in the smaller subacute ward (WA GEMU) but lowest in the larger acute GM ward with 28% deterioration over the 75 weeks of intervention in the SA GEMU. Potentially, and in line with the hypothesis that staff in the GM ward were focused on those with greater risk of falls, higher falls rate (33.6/1000 vs 11.0/1000 participant bed days) were seen in those with high adherence (ie, ≥63%) compared to those with lower adherence with this pattern less noticeable with the GEMU wards.
In this research, a singlet was used to host the sensor but most adverse events and withdrawals related to the use of the singlet. Sensor technology is fast advancing with patch sensors with multiple capabilities more likely to be used in the future (30), removing the need for a singlet. Interest in investigating the effectiveness of wearable patch sensors in improving clinical care is increasing with 1 recent study of intensive care unit patients providing some evidence that wearable sensors guiding patient repositioning including alerting staff to the need, can reduce hospital acquired pressure injuries when compared to usual care (31). Our research adds to the growing body of knowledge and intense interest in this field.
Conclusion
The AmbIGeM system consisting of a movement sensor alarm system was not shown to prevent falls in this adequately powered research. However, our pragmatic trial and comprehensive deployment of a new wearable sensor technology-based intervention in 3 wards across 2 sites in 2 states generated knowledge, novel observations, and new findings. The successful implementation and findings from post hoc analysis pave the way to define future intervention trials investigating novel wearable sensor technologies in hospitals to improve patient outcomes, especially given the rapid advancements in the wearable technology field.
Supplementary Material
Acknowledgments
First and foremost, the authors acknowledge that the research would not have been successful without the significant support and input from the nursing and clinical staff of the GEMU at TQEH (including associate investigator Dr. Pazhvoor Shibu and geriatric medicine advance trainee Dr. Bavand Bikdeli, Dr. David Yu, and Dr. Ayantika Haldar who contributed to data entry) and the GEMU and GM wards of SCGH (including associate investigators Dr. Sean Maher and Mr. Ian Cooper). Also critical were the support received from the ehealth system and casemix staff of both hospitals. The authors also acknowledge the support from the research staff (Mr. Erfan Raygan, Mr. Jaween Nemeshka Ediriweera, Mr. Ian Knight, Mr. Chen Fei, Mr. Hayden Westell, Mr. Ian Linke, Ms. Kathy Bray, Mr. Unyime Jasper, Dr. Lalit Yadav, Ms. F. Waddell). Also acknowledged are the following members of the Independent Data and Safety Monitoring Committee: Dr. Tim Schultz (Chair), Ms. Maureen Tremaine, Ms. Su Kitchen, Associate Professor Peter Hibbert, and Dr. Nick Waldron. This research supported the undergraduate training of Flinders University medical students Ms. Mengqi Zhou and Mr. Nicholas Ng. The authors are also grateful for the support received from SA Health and WA Health for the conduct of this research. The poster incorporated the display of the cognitive identifier symbol of Ballarat Health Service.
Funding
This study was funded by a project grant (1082197) from the National Health and Medical Research Council of Australia. The funding body had no role in the design of the study, collection of the data, analysis, interpretation, or writing of the report. The corresponding author had the final responsibility for the decision to submit for publication and had full access to all the data. Dr. J.D. continued to work on this project after its end date supported by a grant to Professor R.V. from The Hospital Research Foundation. Dr. J.D. is now salaried through a Medical Research Future Fund Grant where she is an associate investigator.
Conflict of Interest
Previously, there was a patent filed (mid-2013) by A/Prof. D.C.R. and Professor R.V. titled system, method, software application, and data signal for determining movement but this has since lapsed. Professor R.V. is the Head of Unit of the Aged & Extended Care Services at The Queen Elizabeth Hospital in South Australia within which the GEM Unit is a service for which, Mr. S.H. is the Nurse Manager. Dr. K.I. is the Falls Lead at Sir Charles Gairdner Hospital in Western Australia.
Author Contributions
R.V. and K.D.H. equally contributed to the study design, selecting participating sites, conducting the research, data collection, data interpretation and drafting of the manuscripts. D.C.R. contributed to the technology design and implementation, conducting the research, data collection, data interpretation and drafting of the manuscript. K.L. designed and conducted the statistical analysis and contributed also to interpretation and drafting of the manuscript. A.W. and J.K. contributed to study design, data interpretation and drafting of manuscript. K.I. and S.H. were clinician leads involved in selecting participating sites, conducting the research, data collection, data interpretation and drafting of the manuscript. J.D., E.B., K.J., and M.C. were research fellows with the study that were involved in the conduct of the study, data collection, data interpretation and drafting the manuscript. C.P. is involved in the analysis and contributed to the drafting of the manuscript.
Data Availability
Requests for data should be directed to the lead author (renuka.visvanathan@adelaide.edu.au) and include a collaboration with at least one of the Chief Investigators (R.V., K.D.H., or D.C.R.). Any request will be assessed for scientific rigor (by a panel consisting of R.V., K.D.H., D.C.R., and K.L.) and given the involvement of hospital patient data, the request must meet ethics request guidelines and be approved by the ethics committees of TQEH/Lyell McEwin Hospital (LMH)/Modbury Hospital (MH), Curtin University, and SCGH. The requestor will be responsible for preparing documentation to the standard required to meet the conditions of the various ethics committees. A data sharing agreement will likely be necessary. Given the multiple analyses planned as well as underway currently, data sharing is at this stage embargoed for a further 2 years.
References
- 1. Beck B, Smith K, Mercier E, et al. Differences in the epidemiology of out-of-hospital and in-hospital trauma deaths. PLoS One. 2019;14:e0217158. doi: 10.1371/journal.pone.0217158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Australian Institute of Health and Welfare. Australia’s Health 2018. Canberra: AIHW; 2018. Accessed June 1, 2021. https://www.aihw.gov.au/reports/australias-health/australias-health-2018/contents/indicators-of-australias-health/falls-resulting-in-patient-harm-in-hospitals. [Google Scholar]
- 3. Morello RT, Barker AL, Watts JJ, et al. The extra resource burden of in-hospital falls: a cost of falls study. Med J Aust. 2015;203:367. doi: 10.5694/mja15.00296 [DOI] [PubMed] [Google Scholar]
- 4. Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806 [DOI] [PubMed] [Google Scholar]
- 5. Cumming RG, Salkeld G, Thomas M, Szonyi G. Prospective study of the impact of fear of falling on activities of daily living, SF-36 scores, and nursing home admission. J Gerontol A Biol Sci Med Sci. 2000;55:M299–M305. doi: 10.1093/gerona/55.5.m299 [DOI] [PubMed] [Google Scholar]
- 6. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi: 10.1002/14651858.CD005465.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cumming RG, Sherrington C, Lord SR, et al. ; Prevention of Older People’s Injury Falls Prevention in Hospitals Research Group . Cluster randomised trial of a targeted multifactorial intervention to prevent falls among older people in hospital. BMJ. 2008;336:758–760. doi: 10.1136/bmj.39499.546030.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hitcho EB, Krauss MJ, Birge S, et al. Characteristics and circumstances of falls in a hospital setting: a prospective analysis. J Gen Intern Med. 2004;19:732–739. doi: 10.1111/j.1525-1497.2004.30387.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahota O, Drummond A, Kendrick D, et al. REFINE (REducing Falls in In-patieNt Elderly) using bed and bedside chair pressure sensors linked to radio-pagers in acute hospital care: a randomised controlled trial. Age Ageing. 2014;43(2):247–253. doi: 10.1093/ageing/aft155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med. 2012;157:692–699. doi: 10.7326/0003-4819-157-10-201211200-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu H, Li P, Yang Z, et al. Construction and application of a medical-grade wireless monitoring system for physiological signals at general wards. J Med Syst. 2020;44:182. doi: 10.1007/s10916-020-01653-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ranasinghe DC, Shinmoto Torres RL, Hill K, Visvanathan R. Low cost and batteryless sensor-enabled radio frequency identification tag based approaches to identify patient bed entry and exit posture transitions. Gait Posture. 2014;39:118–123. doi: 10.1016/j.gaitpost.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 13. Visvanathan R, Ranasinghe DC, Wilson A, et al. Effectiveness of an Ambient Intelligent Geriatric Management system (AmbIGeM) to prevent falls in older people in hospitals: protocol for the AmbIGeM stepped wedge pragmatic trial. Inj Prev. 2019;25:157–165. doi: 10.1136/injuryprev-2017-042507 [DOI] [PubMed] [Google Scholar]
- 14. Dykes PC, Carroll DL, Hurley A, et al. Fall prevention in acute care hospitals: a randomized trial. JAMA. 2010;304:1912–1918. doi: 10.1001/jama.2010.1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Australian Commission on Safety and Quality in Healthcare. Preventing Falls and Harm from Falls in Older People: Best Practice Guidelines for Australian Hospitals. Commonwealth of Australia; 2009. Accessed June 1, 2021. https://www.safetyandquality.gov.au/sites/default/files/migrated/Guidelines-HOSP1.pdf. [Google Scholar]
- 16. The New South Wales Clinical Excellence Commission. CEC Falls Audit Tool-Ward Level. Accessed June 1, 2021. https://docsbay.net/cec-falls-audit-tool-ward-levelaudit-no.
- 17. Haines TP, Massey B, Varghese P, Fleming J, Gray L. Inconsistency in classification and reporting of in-hospital falls. J Am Geriatr Soc. 2009;57:517–523. doi: 10.1111/j.1532-5415.2008.02142.x [DOI] [PubMed] [Google Scholar]
- 18. Morello RT, Barker AL, Ayton DR, et al. Implementation fidelity of a nurse-led falls prevention program in acute hospitals during the 6-PACK trial. BMC Health Serv Res. 2017;17:383. doi: 10.1186/s12913-017-2315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. WHO Global Report on Falls Prevention in Older Age. Geneva: World Health Organization; 2007. Accessed June 1, 2021. https://extranet.who.int/agefriendlyworld/wp-content/uploads/2014/06/WHo-Global-report-on-falls-prevention-in-older-age.pdf. [Google Scholar]
- 20. Hill AM, Hoffmann T, Hill K, et al. Measuring falls events in acute hospitals—a comparison of three reporting methods to identify missing data in the hospital reporting system. J Am Geriatr Soc. 2010;58:1347–1352. doi: 10.1111/j.1532-5415.2010.02856.x [DOI] [PubMed] [Google Scholar]
- 21. Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ. 1997;315:1065–1069. doi: 10.1136/bmj.315.7115.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwenk M, Lauenroth A, Stock C, et al. Definitions and methods of measuring and reporting on injurious falls in randomised controlled fall prevention trials: a systematic review. BMC Med Res Methodol. 2012;12:50. doi: 10.1186/1471-2288-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 24. Timmons S, Vezyridis P, Sahota O. Trialling technologies to reduce hospital in-patient falls: an agential realist analysis. Sociol Health Illn. 2019;41:1104–1119. doi: 10.1111/1467-9566.12889 [DOI] [PubMed] [Google Scholar]
- 25. Meyer G, Köpke S, Haastert B, Mühlhauser I. Comparison of a fall risk assessment tool with nurses’ judgement alone: a cluster-randomised controlled trial. Age Ageing. 2009;38:417–423. doi: 10.1093/ageing/afp049 [DOI] [PubMed] [Google Scholar]
- 26. Teh RC, Visvanathan R, Ranasinghe D, Wilson A. Evaluation and refinement of a handheld health information technology tool to support the timely update of bedside visual cues to prevent falls in hospitals. Int J Evid Based Healthc. 2018;16:90–100. doi: 10.1097/XEB.0000000000000129 [DOI] [PubMed] [Google Scholar]
- 27. Campbell EM, Sittig DF, Guappone KP, Dykstra RH, Ash JS. Overdependence on technology: an unintended adverse consequence of computerized provider order entry. AMIA Annu Symp Proc. 2007;2007:94–98. [PMC free article] [PubMed] [Google Scholar]
- 28. Bhasin S, Gill TM, Reuben DB, et al. A randomized trial of a multifactorial strategy to prevent serious fall injuries. N Engl J Med. 2020;383(2):129–140. doi: 10.1056/NEJMoa2002183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dodd S, White IR, Williamson P. Nonadherence to treatment protocol in published randomised controlled trials: a review. Trials. 2012;13:84. doi: 10.1186/1745-6215-13-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boroojerdi B, Ghaffari R, Mahadevan N, et al. Clinical feasibility of a wearable, conformable sensor patch to monitor motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2019;61:70–76. doi: 10.1016/j.parkreldis.2018.11.024 [DOI] [PubMed] [Google Scholar]
- 31. Pickham D, Berte N, Pihulic M, Valdez A, Mayer B, Desai M. Effect of a wearable patient sensor on care delivery for preventing pressure injuries in acutely ill adults: a pragmatic randomized clinical trial (LS-HAPI study). Int J Nurs Stud. 2018;80:12–19. doi: 10.1016/j.ijnurstu.2017.12.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for data should be directed to the lead author (renuka.visvanathan@adelaide.edu.au) and include a collaboration with at least one of the Chief Investigators (R.V., K.D.H., or D.C.R.). Any request will be assessed for scientific rigor (by a panel consisting of R.V., K.D.H., D.C.R., and K.L.) and given the involvement of hospital patient data, the request must meet ethics request guidelines and be approved by the ethics committees of TQEH/Lyell McEwin Hospital (LMH)/Modbury Hospital (MH), Curtin University, and SCGH. The requestor will be responsible for preparing documentation to the standard required to meet the conditions of the various ethics committees. A data sharing agreement will likely be necessary. Given the multiple analyses planned as well as underway currently, data sharing is at this stage embargoed for a further 2 years.