Abstract
Background
Hospital deaths after sepsis have decreased substantially and most young adult survivors rapidly recover (RAP). However, many older survivors develop chronic critical illness (CCI) with poor long-term outcomes. The etiology of CCI is multifactorial and the relative importance remains unclear. Sepsis is caused by a dysregulated immune response and biomarkers reflecting a persistent inflammation, immunosuppression, and catabolism syndrome (PICS) have been observed in CCI after sepsis. Therefore, the purpose of this study was to compare serial PICS biomarkers in (i) older (vs young) adults and (ii) older CCI (vs older RAP) patients to gain insight into underlying pathobiology of CCI in older adults.
Method
Prospective longitudinal study with young (≤45 years) and older (≥65 years) septic adults, who were characterized by (i) baseline predisposition, (ii) hospital outcomes, (iii) serial Sequential Organ Failure Assessment (SOFA) organ dysfunction scores over 14 days, (iv) Zubrod Performance status at 3-, 6-, and 12-month follow-up, and (v) mortality over 12 months, was conducted. Serial blood samples over 14 days were analyzed for selected biomarkers reflecting PICS.
Results
Compared to the young, more older adults developed CCI (20% vs 42%) and had markedly worse serial SOFA scores, performance status, and mortality over 12 months. Additionally, older (vs young) and older CCI (vs older RAP) patients had more persistent aberrations in biomarkers reflecting inflammation, immunosuppression, stress metabolism, lack of anabolism, and antiangiogenesis over 14 days after sepsis.
Conclusion
Older (vs young) and older CCI (vs older RAP) patient subgroups demonstrate early biomarker evidence of the underlying pathobiology of PICS.
Keywords: Aging, Chronic critical illness, Critical illness, Infection
Sepsis is defined by a dysregulated systemic immune response that causes life-threatening organ dysfunctions and has been called the “quintessential disease of the elderly” (1). It has long been recognized that the incidence of sepsis and in-hospital mortality increase exponentially beyond the age of 65 years (2,3). However, over the past decade with effective implementation of the Surviving Sepsis Campaign (SSC) evidence base guidelines (EBGs), in-hospital mortality has decreased substantially (4,5). While most young survivors rapidly recover (RAP), many older sepsis survivors are, unfortunately, now progressing into clinical trajectory of chronic critical illness (CCI) characterized by prolonged intensive care unit (ICU) stays with low-grade organ dysfunctions and poor posthospital discharge dispositions and dismal long-term outcomes (6,7).
In our recently published prospective study on long-term outcomes after sepsis in surgical ICU patients, 20% of patients were young (≤45 years), 40% were middle-aged (46–64 years), and 40% were older (≥65 years) adults (8). It was observed that significantly more older adults progressed into CCI (22% vs 34% vs 42%) and had notably higher 3-month (9% vs 8% vs 25%), 6-month (11% vs 11% vs 30%), and 12-month (11% vs 14 % vs 32%) mortality. Despite having similar good presepsis functional status, older survivors experienced significantly worse moderate functional disabilities at 3 months after sepsis and did not recover at 12 months compared to middle-aged and young adults. In comparison, the young survivors experienced mild functional restrictions at 3 months and most recovered to baseline by 12 months. The middle-aged adults experienced greater degrees of mild functional restrictions at 3 months but trended toward full recovery by 12 months (8).
The etiology of these poor long-term outcomes after sepsis in older adults is unclear and undoubtedly multifactorial (9). While baseline demographics, comorbidities, and site of infection play a role, the dysregulated systemic immune response that causes sepsis is clearly affected by age and likely plays a dominant role (9,10). It is well documented that older patients have a less robust immune response that renders them more susceptible to infectious challenges and also confounds early diagnosis of sepsis (11). However, recent studies have shown that the innate (proinflammation) and adaptive (immunosuppression) responses within a short time in older patients following sepsis become equally deranged as those seen in younger patients, but older patients have more difficulty returning to immune homeostasis (12,13). This is consistent with our proposed paradigm (based on extensive animal and human studies) that the underlying pathobiology of CCI after sepsis is a persistent inflammation, immunosuppression, and catabolism syndrome (PICS) with impaired angiogenesis that increase the risk of secondary infections, adverse cardiovascular events, poor recovery, and death (7,14–16).
In this manuscript, we compare biomarkers of the host response relevant to PICS over 14 days after sepsis onset in (i) older (vs young) and (ii) older CCI (vs older RAP) study cohorts to gain insight into the early underlying pathobiology of dismal long-term outcomes in older adults after sepsis and hypothesize that the older and older CCI cohorts will have biomarker responses consistent with the PICS paradigm.
Method
Study Design and Population
This was a prospective, observational cohort study over 4 years ending December 31, 2018 that enrolled trauma and surgical ICU patients with new-onset sepsis who were followed for 12 months to document long-term outcomes. This study was conducted by the University of Florida (UF) Sepsis Critical Illness Research Center in collaboration with the UF Institute on Aging (IOA). Patients were recruited from the trauma and surgical ICUs (each with 24 beds) at the UF Health Shands Hospital (Gainesville, FL). Each ICU has a dedicated multidisciplinary ICU team that uses established clinical protocols that ensure consistent implementation of SCC EBGs (17). The study was approved by the UF Institutional Review Board and registered with clinicaltrials.gov (NCT02276417). The patient or legally authorized representative provided informed consent within 96 hours after the patient qualified for study inclusion. If not obtained within 96 hours, all patient data and biological samples were destroyed. Details of the study design with inclusion and exclusion criteria as well as the clinical and laboratory standard operating procedure (SOP) utilized have been published (17). In brief, overall cohort inclusion criteria included (i) age ≥18 years, (ii) clinical diagnosis of sepsis as defined by 2001 consensus guidelines, and (iii) entrance into an electronic medical record evidence-based sepsis SOPs. Exclusion criteria included any of the following: (i) refractory shock (death <24 hours from sepsis protocol initiation) or inability to achieve source control (eg, total bowel ischemic necrosis); (ii) preadmission expected life span <3 months; (iii) patient/proxy not committed to aggressive management; (iv) severe chronic heart failure (New York Heart Association Class IV); (v) Child-Pugh Class C liver disease or pre-liver transplant; (vi) known HIV with CD4+ count <200 cells/mm3; (vii) patients receiving chronic corticosteroids or immunosuppressive agents, including organ transplant recipients; (viii) pregnancy; (ix) institutionalized patients; (x) inability to obtain informed consent within 96 hours of enrollment; (xi) chemotherapy or radiotherapy within 30 days; (xii) severe traumatic brain injury; and (xiii) spinal cord injury resulting in permanent sensory and/or motor deficits. The clinical course of study patients was prospectively documented by experienced research nurses using an established sepsis database. The diagnosis of sepsis, site of infection and initial sepsis severity of each case was adjudicated weekly by a team of bedside clinicians. Predicted mortality was assessed by the acute physiology age chronic health evaluation (Acute Physiology + Age + Chronic Health Evaluation [APACHE]) II and Sequential Organ Failure Assessment (SOFA) scores at 24 hours. Infections were defined using CDC definitions and sepsis was classified as “present on admission” if diagnosed within 48 hours and “hospital acquired” if diagnosed after 48 hours hospital admission. Secondary infections were defined as any probable or microbiologically confirmed bacterial, yeast, fungal, or viral infection requiring treatment and occurring at least 48 hours after sepsis protocol onset during the index hospitalization. Infections within 48 hours of sepsis onset were considered coexisting and therefore excluded. Secondary infections were presented as mean per patient and secondary infections per 100 hospital person days (to adjust for the time at risk). Organ dysfunction progression was assessed by serial SOFA scores. Multiple organ failure (MOF) defined by the Denver MOF score and acute kidney injury (AKI) defined by KDIGO (Kidney Disease: Improving Global Outcomes) score. Patients were classified by 3 in-patient clinical trajectories: (i) early death, (ii) rapid recovery, and (iii) CCI. Early death was defined as death within 14 days of sepsis onset. CCI was defined as an ICU stay greater than or equal to 14 days with evidence of persistent organ dysfunction by SOFA (18). Rapid recovery patients were those not meeting criteria for early death or CCI. Discharge disposition was classified based on known associations with long-term outcomes as either “good” (home with or without health care services, or rehabilitation facility) or “poor” nonhome destinations (Long-Term Acute Care, Skilled Nursing Facility, another acute care hospital, hospice or in-patient death). Performance status was measured by ECOG/WHO/Zubrod Performance Scale that ranges from 0 to 5, with increasing scores reflecting worse performance status: (0) Asymptomatic (fully active), (1) Symptomatic but completely ambulatory (restricted in physically strenuous activity), (2) Symptomatic, <50% in bed during the day (ambulatory and capable of all self-care but unable to perform any work activities), (3) Symptomatic, >50% in bed, but not bedbound (capable of only limited self-care), (4) Bedbound (completely disabled, incapable of any self-care), and (5) Death (17). Baseline (ie, prehospitalization) performance status was based upon patient/proxy reported 4-week recall assessment as soon as possible after sepsis onset. Among survivors, follow-up assessments were performed at 3, 6, and 12 months for mortality (with cross-check validation via the U.S. Social Security Death Index).
Healthy Controls
Age- (median [interquartile range]: 58 [49–64] years), sex- (n = 19, 51% male), and race/ethnicity-matched (n = 31, 84% White; n = 33, 89% non-Hispanic) healthy control subjects (n = 37) were consented, and single blood samples were collected for normal biomarker values. Limited clinical data were collected on these subjects, but any individual with known history of autoimmune disease, taking immunosuppressive medication, active cancer treatment, or active infection was excluded.
Blood Draws and Biomarker Analyses
Blood samples were collected from septic patients at 1, 4, 7, and 14 days after sepsis protocol onset for subjects remaining inpatient. Blood samples were analyzed for biomarkers relevant to the underlying pathobiology of PICS including (i) inflammation (interleukin-6 [IL-6], IL-8, C-reactive protein [CRP], monocyte chemoattractant protein 1 [MCP-1], and absolute neutrophil count [ANC]); (ii) immunosuppression (absolute lymphocyte count [ALC], soluble programmed death ligand 1 [sPDL-1], and IL-10); (iii) stress metabolism (glucagon-like peptide 1 [GLP-1] and albumin); (iv) anabolism (insulin-like growth factor [IGF-1] and insulin-like growth factor-binding protein 3 [IGFBP-3]); and (v) angiogenesis (vascular endothelial growth factor [VEGF], soluble vascular endothelial growth factor receptor 1 [sFlt-1], angiopoietin 2 [Ang2], and interferon gamma-induced protein 10 [IP-10]). The summary of preselected biomarkers’ function, baseline values among sepsis patients, and values determined among healthy controls is reported in Supplementary Table 1. Serum levels of these biomarkers were measured using Luminex multiplex kits (MILLIPLEX multiplex assay, EMD Millipore Corp., Billerica, MA). Complete blood counts with leukocyte differentials, that is, absolute lymphocyte count (ALC) and albumin, were measured by the Clinical and Diagnostic Laboratories at UF Health. For all multiplex analyses, an identical internal control sample was used across all kits to normalize the data.
Study Enrollment and Cohort Selection
In a recently published manuscript, we reported study results describing the epidemiology, functional testing, and long-term outcomes stratified by age based on 328 subjects (8). In this manuscript, we report study results describing the biomarker analyses based on the completed study group of 363 subjects. We have included updated tables (to the previously published tables; see Supplementary Tables 2–4) of the 75 (21%) young (≤45 years), 143 (39%) middle-aged (46–64 years), and 145 (40%) older (≥65 years) adult study cohorts and the CONSORT diagram (Supplementary Figure 1).
In this manuscript, we excluded the middle-aged patients because like their outcomes, their biomarkers fall in between the young and the older cohorts. We chose to compare the young to the older cohort to better demonstrate the difference in biomarkers that reflect host response that are relevant to the PICS paradigm.
Statistical Analysis
Data are presented as frequency and percentage, mean and SD/SE, or median and quartiles. Fisher exact test and the Kruskal–Wallis test were used for comparison of categorical and continuous variables, respectively. SOFA score was imputed for living patients discharged prior to Day 14. For patients with a poor discharge disposition, the last available in-hospital component scores were carried forward. Similarly, for patients with a good disposition, the last available in-hospital component scores were used for hepatic, coagulation, and renal function, while respiratory and CNS component scores were assumed to be 0. Generalized estimating equations using an independent correlation structure were used to compare biomarker and SOFA trajectories between groups. Inverse probability weighting based on concurrent adjudicated Zubrod score was used to account for missing follow-up data, as well as absence due to death. The log-rank test was used to compare Kaplan–Meier product limit estimates of survival between groups. All significance tests were 2-sided, with p value of less than or equal to .05 considered statistically significant. Statistical analyses were performed with SAS (SAS Institute, Cary, NC).
Results
Table 1 summarizes baseline demographics, predisposing factors, characteristics of the inciting septic event, and hospital/postdischarge outcomes between the young and older patients. In comparison to young adults, older adults had lower body mass index, but higher Charlson Comorbidity Index and APACHE II scores. They experienced more septic shock and stayed longer in the ICU on mechanical ventilation. They also experienced more MOF, AKI, and progression into CCI with much higher percentage of poor posthospital discharge disposition. Table 1 also compares older RAP versus older CCI. Compared to older RAP, older CCI patients presented more frequently with hospital-acquired sepsis and septic shock. They had higher APACHE II scores with longer ICU stays on mechanical ventilation. They experienced more MOF, AKI, secondary infections, and a much higher percentage of poor posthospital discharge disposition.
Table 1.
Summary of Baseline Demographics and Characteristics of the Inciting Septic Event and Predisposition by Age Groups
| Age Groups | Young, n = 75 | Older, n = 145 | Older RAP, n = 75 | Older CCI, n = 61 |
|---|---|---|---|---|
| Male, n (%) | 39 (52) | 81 (56) | 39 (52) | 38 (62) |
| Age (y), median (IQR 25th, 75th) | 36 (28, 43)a | 72 (69, 76) | 72 (69, 77) | 72 (69, 76) |
| Body mass index (kg/m2), median (25th, 75th) | 30.3 (25.8, 40.2)a | 28.3 (24.4, 33.9) | 28.3 (24.9, 35.9) | 28.2 (23.4, 32.2) |
| Charlson Comorbidity Index, median (25th, 75th) | 0 (0, 0)a | 4 (4, 6) | 4 (3, 6) | 5 (4, 7) |
| Race, n (%) | ||||
| White | 67 (89) | 133 (91) | 71 (95) | 54 (89) |
| African American | 7 (10) | 10 (7) | 3 (4) | 6 (10) |
| American Indian | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Asian | 0 (0) | 1 (1) | 1 (1) | 0 (0) |
| Unknown | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| Non-Hispanic ethnicity, n (%) | 70 (93)a | 144 (99) | 74 (99) | 61 (100) |
| Sepsis present on admission (≤48 h), n (%) | 51 (68) | 83 (57) | 48 (64)b | 27 (44) |
| Hospital-acquired sepsis (>48 h), n (%) | 24 (32) | 62 (43) | 27 (36)b | 34 (56) |
| Sepsis severity, n (%)a,b | ||||
| Sepsis | 37 (49) | 34 (24) | 25 (33) | 8 (13) |
| Severe sepsis | 30 (40) | 61 (42) | 35 (47) | 24 (39) |
| Septic shock | 8 (11) | 50 (34) | 15 (20) | 29 (48) |
| Site of infection, n (%)a | ||||
| Abdominal | 29 (39) | 71 (49) | 31 (41) | 33 (54) |
| Pulmonary | 12 (16) | 26 (18) | 12 (16) | 14 (23) |
| Skin/soft tissue | 18 (24) | 14 (10) | 10 (13) | 4 (7) |
| Genitourinary | 13 (17) | 19 (13) | 15 (20) | 4 (7) |
| Cardiovascular | 3 (4) | 16 (10) | 7 (10) | 7 (9) |
| APACHE II Score (24 h), median (IQR 25th, 75th) | 12 (7, 17)a | 20 (15, 26) | 17 (14, 23)b | 23 (18, 28) |
| Need for mechanical ventilation, n (%) | 40 (53)a | 111 (77) | 47 (63)b | 56 (92) |
| ICU length of stay, median (IQR 25th, 75th) | 5 (3, 12)a | 9 (4, 17) | 6 (3, 9)b | 20 (15, 25) |
| Secondary infections/patient, mean (SD) | 0.41 (0.84) | 0.57 (0.82) | 0.3 (0.6)b | 1 (1) |
| Secondary infections/100 hospital days, mean (SD) | 2.36 (6.71) | 2.6 (4.45) | 1.5 (3.3)b | 3.4 (3.6) |
| MOF frequency, n (%)—by Denver MOF | 4 (5)a | 31 (21) | 4 (5)b | 25 (41) |
| AKI, n (%)a,b | ||||
| Stage 1 | 16 (21) | 35 (24) | 18 (24)b | 17 (28) |
| Stage 2 | 10 (13) | 29 (20) | 19 (25)b | 6 (10) |
| Stage 3 | 9 (12) | 25 (17) | 5 (7)b | 15 (25) |
| Clinical trajectory, n (%)a,b | ||||
| Early death | 2 (3) | 9 (6) | 0 (0) | 0 (0) |
| Chronic critical illness | 15 (20) | 61 (42) | 0 (0) | 61 (100) |
| Rapid recovery | 58 (77) | 75 (52) | 75 (100) | 0 (0) |
| Poor discharge disposition, n (%)c | 12 (16)a | 91 (63) | 27 (36)b | 55 (90) |
| Good discharge disposition, n (%)c | 63 (84)a | 54 (37) | 48 (64)b | 6 (10) |
Notes: AKI = acute kidney injury; APACHE II = Acute Physiology + Age + Chronic Health Evaluation II; CCI = chronic critical illness; ICU = intensive care unit; IQR = interquartile range; MOF = multiple organ failure; RAP = rapid recovery; SOFA = Sequential Organ Failure Assessment.
Statistical difference was labeled as ayoung vs older adults and bolder RAP vs older CCI, with statistical significance set at p <.05. cPoor discharge disposition is when patients go from hospital to another care facility and good discharge disposition is when patients go home from hospital.
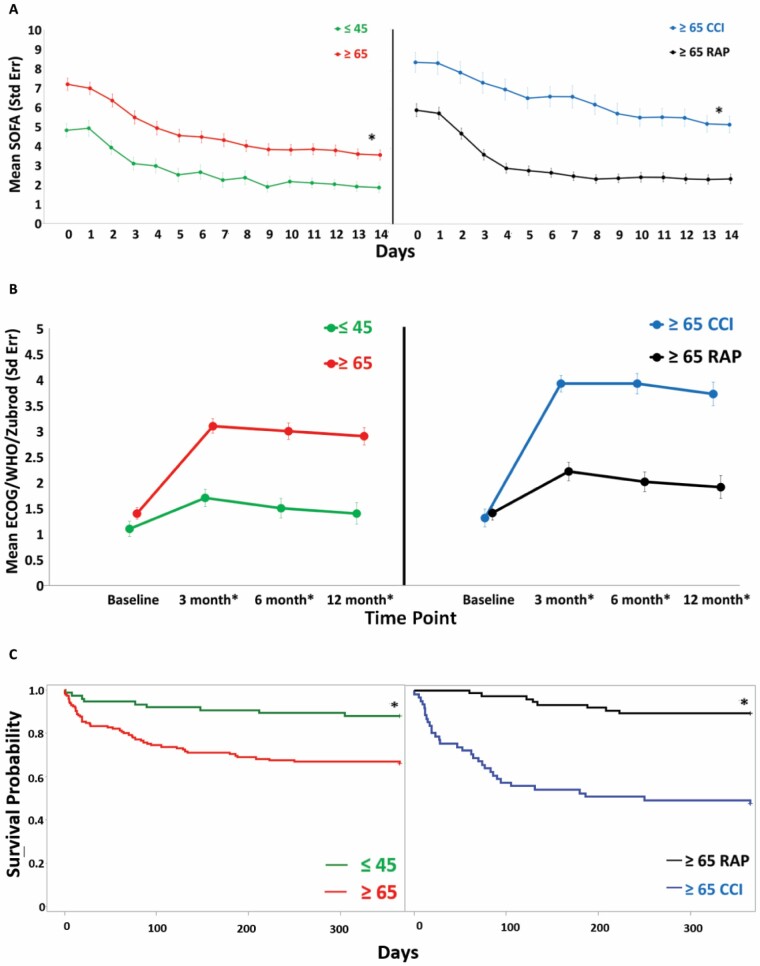
Figure 1A depicts that the older (compared young) and older CCI (compared older RAP) patients had significantly worse serial SOFA scores over 14 days after sepsis onset. Similar comparisons in Figure 1B show that the older and older CCI patients had notably worse Zubrod Performance status over 12 months and in Figure 1C had notably worse 1-year survival curves.
Figure 1.
Clinical outcomes over 12 mo after sepsis onset between the older and young sepsis survivors. (A) A 14-d serial Sequential Organ Failure Assessment (SOFA), (B) 12-mo Zubrod score, and (C) 1-y survival estimates. Data presented as mean ± SE with statistical significance set at p <.05 and asterisk represents statistical significance.
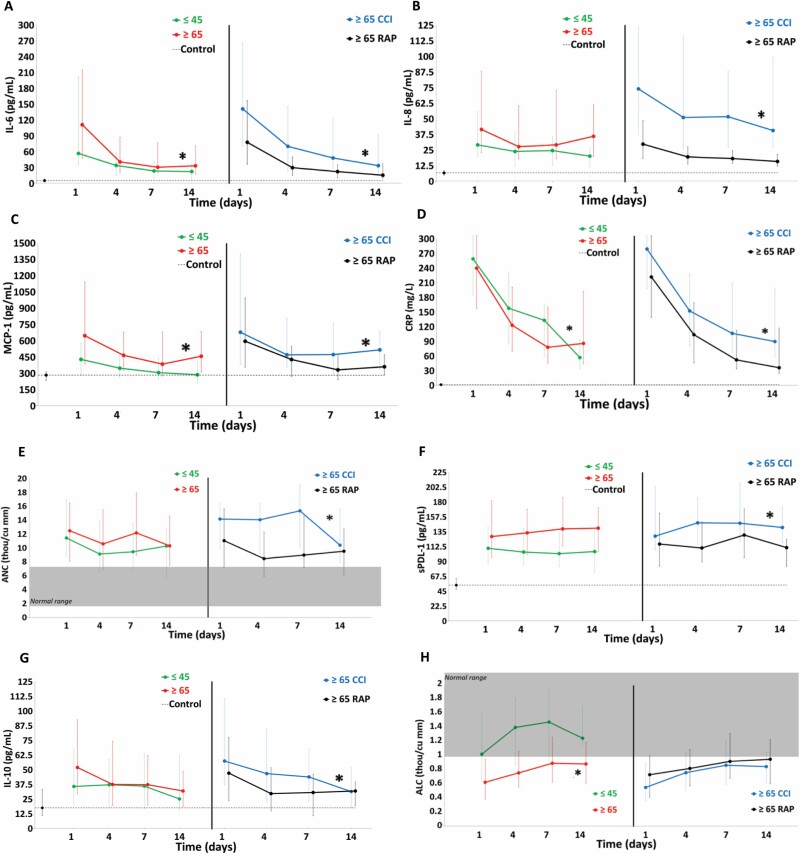
Figure 2 shows circulating proinflammation and immunosuppression biomarkers over 14 days after sepsis onset for study groups and normal controls. For proinflammation (Figure 2A–E), older adults had higher levels of IL-6, MCP-1, and CRP, and IL-8 was not different compared to young patients over 14 days. Among older patients, CCI patients had higher levels of IL-6, IL-8, MCP-1, and CRP compared to RAP. ANC levels were not different in older adults compared to young adults, but older CCI patients (compared to older RAP) had higher ANC levels over 14 days. For immunosuppression (Figure 2F–H), sPDL-1 and IL-10 in older adults were not different from the young, but ALC levels were lower in older patients compared to the young over 14 days. Among older patients, CCI patients (compared to RAP) had higher sPDL-1 and IL-10 levels, but the ALC levels were not different.
Figure 2.
Comparison of biomarkers of proinflammation and immunosuppression between older and young sepsis patients. Proinflammation: (A) interleukin-6 (IL-6), (B) IL-8, (C) monocyte chemoattractant protein 1 (MCP-1), and (D) C-reactive protein (CRP) and (E) absolute neutrophil count (ANC). Immunosuppression: (F) soluble programmed death ligand 1 (sPDL-1), (G) IL-10, and (H) absolute lymphocyte count (ALC) biomarkers. Perforated line represents biomarker levels in matched control subjects. Shaded area represents published normal reference ranges. Data presented as median (25% and 75%) with statistical significance set at p <.05 and asterisk represents statistical significance of the older (vs young) and older chronic critical illness (CCI) (vs older rapidly recover [RAP]) cohorts.
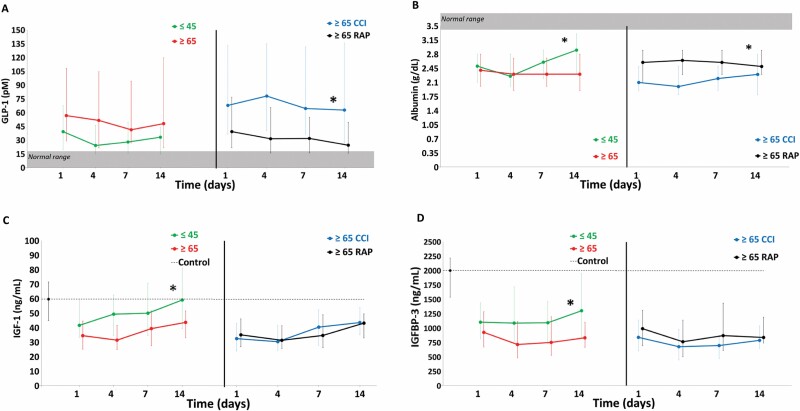
Figure 3 depicts circulating stress metabolism and anabolic biomarkers over 14 days after sepsis onset for study groups and normal controls. For stress metabolism (Figure 3A and B), older patients had higher levels of GLP-1 compared to the young. Among older patients, CCI patients had higher levels of GLP-1 compared to RAP. Albumin levels were higher compared to older patients. Among the older patients, CCI patients had lower albumin levels than RAP. For anabolic biomarkers (Figure 3C and D), older patients had lower levels of IGF-1 and IGFBP-3 than the young. Among older patients, CCI and RAP had similar low levels of IGF-1 and IGFBP-3.
Figure 3.
Comparison of stress and catabolism biomarkers between older and young sepsis patients. Stress metabolism: (A) glucagon-like peptide 1 (GLP-1) and (B) serum albumin level. Anabolism: (C) insulin-like growth factor (IGF-1) and (D) insulin-like growth factor-binding protein 3 (IGFBP-3) biomarkers. Perforated line represents biomarker levels in matched control subjects. Shaded area represents published normal reference ranges. Data presented as median (25% and 75%) with statistical significance set at p <.05 and asterisk represents statistical significance of the older (vs young) and older chronic critical illness (CCI) (vs older rapidly recover [RAP]) cohorts.
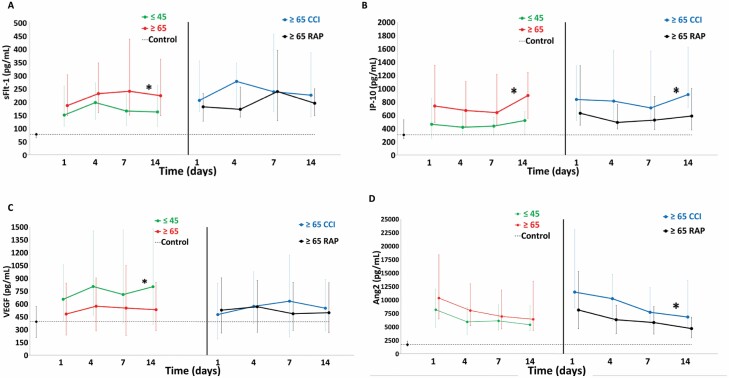
Figure 4 demonstrates circulating angiogenesis biomarkers over 14 days after sepsis onset for study groups and normal controls. For sFlt-1 and IP-10 (Figure 4A and B), older patients had higher levels than the young. Among the older patients, CCI had higher levels of IP-10, but sFlt-1 levels were similar compared to RAP. For VEGF (Figure 4C), young patients had more elevated levels than the older patients. Among the older patients, those with CCI and RAP had similar low elevations. For Ang2 (Figure 4D), older patients had similar levels compared to the young. Among older adults, CCI patients had higher levels compared to RAP.
Figure 4.
Comparison of biomarkers of angiogenesis between older and young sepsis patients. Angiogenesis biomarkers: (A) soluble vascular endothelial growth factor receptor 1 (sFlt-1), (B) interferon gamma-induced protein 10 (IP-10), (C) vascular endothelial growth factor (VEGF), and (D) angiopoietin 2 (Ang2). Perforated line represents biomarker levels in matched control subjects. Data presented as median (25% and 75%) with statistical significance set at p <.05 and asterisk represents statistical significance of the older (vs young) and older chronic critical illness (CCI) (vs older rapidly recover [RAP]) cohorts.
Discussion
The major findings of this study were that older (compared to young) and older CCI (compared to older RAP) adults demonstrate persistent aberrations in host response biomarkers reflecting proinflammation, immunosuppression, stress metabolism, lack of anabolism, and antiangiogenesis over 14 days after the onset of sepsis. Additionally, these finding are consistent with the PICS paradigm, which we hypothesized (based on extensive animal and human data) to represent the underlying pathobiology of CCI after sepsis (7). To our knowledge, this is the first study to prospectively characterize the differences in serial host biomarker levels reflecting PICS in older sepsis patients compared to the young counterparts over 14 days after sepsis onset.
Older sepsis patients demonstrated higher biomarker levels of proinflammation compared to young patients, with the highest levels being observed among older CCI patients. These results are in line with reports showing higher levels of biomarkers of inflammation from short hospital serial measures or long-term posthospital discharge levels in sepsis patients (12,13). However, no studies have reported age comparisons. Ginde et al. reported that middle-aged and older adults had higher levels of proinflammatory biomarkers (including chemokine [CC-motif] ligand-23, CRP, IL-1 receptor antagonist, neutrophil gelatinase-associated lipocalin, peptidoglycan recognition protein, and tumor necrosis factor receptor-1a) over 72 hours in the ICU (13). Older adults have a unique response to infection as compared to their younger counterparts. A significant part of this phenomenon has been attributed to immune senescence, “inflammaging” (chronic low-grade systemic inflammation), which may increase vulnerability to a septic event and to CCI or early death (19,20). Sepsis induces an initial systemic inflammatory “storm,” initiated upon host recognition of pathogen-associated molecular patterns (PAMPs) that leads to bone marrow leukocyte mobilization, host tissue leukocyte infiltration, and organ-specific inflammation. Biomarkers such as IL-6 and IL-8 are among the cytokines that are activated by PAMPs and contribute to suppression of adaptive immunity (21). Yende et al. have shown that CRP levels remain elevated over 12 months after discharge in 26% of mostly older sepsis patients indicating a persistent acute-phase response induced by proinflammatory cytokines (12).
We also showed that older sepsis patients (compared to young) demonstrated higher levels of immunosuppression (lower ALC). Low levels of lymphocytes, known as lymphopenia, are associated with worse outcomes in sepsis patients (22). Low ALC may be even more important in predicting worse outcomes in older sepsis patients, especially that lymphopenia is common in a general older population (23). Higher levels of sPDL-1 and IL-10 were observed only among older CCI compared to older RAP patients (who experienced substantially more secondary infections/100 hospital days than the other subgroups), but not between the young and older adults. IL-10 demonstrates both pro and anti-inflammatory effects in sepsis and may not be indicative of higher immunosuppression in older sepsis patients, but rather be dependent on disease severity (24). This is also consistent with Yende et al. who reported that the levels of PDL-1 were elevated over 12 months in nearly 50% of mostly older septic patients indicating the long-term immunosuppression (12). Notably, the ALC levels between older CCI and older RAP patients were not different. Although the causality is unclear, this is consistent with our previous observations that sepsis induces persistent bone marrow emergency myelopoiesis to promote innate immunity (with production and release of immature neutrophils) with consequent suppression of lymphocyte production (with consequent lymphopenia) and that older patients have a delayed return to immune homeostasis (16,25–27).
We observed that older (compared to young) and older CCI (compared to older RAP) patients had persistent high levels of GLP-1 and low levels of albumin. Others have reported that higher GLP-1 levels are associated with sepsis severity, inflammation, and higher mortality in both ICU and postdischarge (25,28). Proinflammatory IL-6 induces GLP-1 production and AKI (present in over 60% of older CCI patients) impairs renal clearance (29). Elevated GLP-1 levels in older sepsis patients, among other functions, may reflect failure to restore metabolic homeostasis (25). This persistent stress metabolism in part explains the poor nutritional response observed in our CCI patients who received early evidence-based ICU nutritional support (25). In particular, stress metabolism refers to a persistence of the injury stress response where increased counterregulatory hormones (epinephrine, cortisol, and glucagon) combined with inflammatory mediators mobilize endogenous substrates to support ongoing hypermetabolism such as ongoing insulin resistance (with hyperglycemia), protein catabolism (ongoing muscle breakdown), and an acute-phase response (increased CRP and decreased albumin) (30,31). Interestingly, in normal aging, GLP-1 agonists show promise in reducing anabolic resistance and glucose metabolism (32). Furthermore, both older (compared to the young) and older CCI (compared to RAP) patients had lower albumin levels, which is in line with a persistent acute-phase response (induced by IL-6), where reprioritized hepatic protein synthesis results in decreased albumin production by the liver and this is associated with the increased disease severity and mortality (33,34).
Older sepsis survivors demonstrated lower levels of biomarkers of anabolism, compared to young patients. IGF-1 has a role in the maintenance of cell survival and has anti-inflammatory, antioxidative, and antiapoptotic effects, and its binding protein (IGFBP-3) regulates it by decreasing IGF-1 levels (35). Levels of IGF-1 and IGFBP-3 remained lower in older sepsis survivors compared to the young; however, the levels of these biomarkers were not different between the older CCI and RAP sepsis patients. These results are in line with other reports showing lower levels in the acute phase of sepsis and lower survival (35), but also that the anabolic capacity decreases continuously (decrease of IGF-1) after the age 60 (36), which may explain the lack of differences between the older CCI and RAP patients. Additionally, lower IGF-1 and IGFBP-3 were present in the acute respiratory syndrome and predictive of disease severity and 60-day mortality (37).
Disruption of the biomarker levels of angiogenesis during sepsis associates with hematological and renal dysfunction, vascular permeability, and increased mortality (38). Sepsis patients have high prevalence of kidney and endothelial dysfunction, which leads to an antiangiogenic state via a reduction in circulating endothelial progenitor cells due to lower levels of VEGF and higher levels of sFlt-1 and Ang2 (39). We noted substantial deregulation of the angiogenesis biomarkers in older adults compared to the young patients. In line with our results, lower VEGF, and higher sFlt-1 and Ang2 were associated with higher mortality over 24 hours after septic shock onset in mostly older patients (40). Our results also demonstrated that the levels of IP-10, a biomarker of inflammation and antiangiogenesis, were the highest in older CCI patients. In particular, IP-10 contributes to recruiting T lymphocytes and natural killer cells to the sites of infection (41), but it also disrupts angiogenesis by VEGF inhibition and limiting capillary tube formation accompanied by reduced endothelial cell motility, required for the capillary tube formation (42).
Strengths
First, this was a prospective longitudinal study of patients who were managed for new-onset sepsis using established clinical protocols that ensured high compliance with the SSC EBGs. Second, the diagnosis of sepsis, site of infection, and initial sepsis severity of each case was adjudicated weekly by a team of bedside clinicians. Third, the clinical course was prospectively documented by experienced research nurses using an established sepsis database. Fourth, the UF IOA designed and performed the posthospital discharge long-term outcome studies that were relevant to older patient populations.
Limitations
First, this was an observational study, and thus it is difficult to draw causal mechanistic conclusions from the biomarker results. Second, this study was performed at a single tertiary regional medical center and included only surgical and trauma patients admitted to a surgical ICU, which limits the generalizability of the observations. Trauma (type and severity) or type of planned surgery likely contributes to PICS and CCI but it is difficult to ascertain their role because of limited numbers of these patients in our cohort and variability of these presepsis insults. Third, comorbid disease plays an important role in the predisposition and outcomes of sepsis in aging patients. We obtained comorbidity data by concurrent chart review, but a more in-depth interview with the patient/family plus specific biomarkers (such as HbA1C for diabetes) would have allowed quantitation of poor control or severity. Fourth, biomarkers of PICS and organ dysfunction were measured during hospitalization. As a future goal, it would be informative to obtain these biomarkers after hospital discharge to study long-term biomarker aberrations and their resolution to better understand the pathobiology of dismal long-term outcomes.
In conclusion, compared to young adults, older sepsis survivors demonstrate persistent aberrations in PICS biomarkers induced by the dysregulated immune response after sepsis. In older patients, these aberrations are similarly more deranged in those who develop CCI versus those who experience RAP.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the invaluable contributions of the University of Florida Sepsis Critical Illness Research Center (UF SCIRC) staff, including Jennifer Lanz, Ruth Davis, Jillianne Brakenridge, Ashley McCray, Bridget Baisden, Ricky Ungaro, Dina Nacionales, Marvin Dirain, Tabitha Johns, and Ada Malcolm, and the UF IOA staff, including Megan Roberts and Melissa Hinson.
Funding
This work was supported by the National Institute of General Medical Sciences (NIGMS) grants: R01 GM-113945 (P.A.E.), P50 GM-111152 (to R.T.M., S.D.A., G.L.G., B.B., C.L., L.L.M., P.A.E., S.C.B., and F.A.M.), and T32 GM-008721 (D.B.D.). It was also supported by a grant P30 AG028740 (R.T.M., S.D.A., and C.L.). The funding bodies had no role in the design of the study, collection, analysis and interpretation of data, or in writing the manuscript.
Conflict of Interest
None declared.
Author Contributions
F.A.M., S.C.B., S.D.A., A.B., P.A.E., and L.L.M. conceived and designed this clinical study; S.C.B. and D.B.D. adjudicated data from medical records; G.L.G. and B.B. analyzed the data; R.T.M., F.A.M., and S.D.A. drafted the manuscript; S.C.B., C.L., L.L.M., and P.A.E. revised the manuscript; and all coauthors reviewed and approved the manuscript.
References
- 1. Milbrandt EB, Eldadah B, Nayfield S, Hadley E, Angus DC. Toward an integrated research agenda for critical illness in aging. Am J Respir Crit Care Med. 2010;182(8):995–1003. doi: 10.1164/rccm.200904-0630CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angus DC, Linde-Zwirble WT, Lidicker J, et al. . Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 3. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba [DOI] [PubMed] [Google Scholar]
- 4. McKinley BA, Moore LJ, Sucher JF, et al. . Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. J Trauma. 2011;70(5):1153–1166; discussion 1166–1167. doi: 10.1097/TA.0b013e31821598e9 [DOI] [PubMed] [Google Scholar]
- 5. El Solh AA, Akinnusi ME, Alsawalha LN, Pineda LA. Outcome of septic shock in older adults after implementation of the sepsis “bundle”. J Am Geriatr Soc. 2008;56(2):272–278. doi: 10.1111/j.1532-5415.2007.01529.x [DOI] [PubMed] [Google Scholar]
- 6. Brakenridge SC, Efron PA, Cox MC, et al. . Current epidemiology of surgical sepsis: discordance between inpatient mortality and 1-year outcomes. Ann Surg. 2019;270(3):502–510. doi: 10.1097/SLA.0000000000003458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gentile LF, Cuenca AG, Efron PA, et al. . Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi: 10.1097/TA.0b013e318256e000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mankowski RT, Anton SD, Ghita GL, et al. . Older sepsis survivors suffer persistent disability burden and poor long-term survival. J Am Geriatr Soc. 2020;68(9):1962–1969. doi: 10.1111/jgs.16435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. J Am Med Assoc. 2018;319(1):62–75. doi: 10.1001/jama.2017.17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stortz JA, Cox MC, Hawkins RB, et al. . Phenotypic heterogeneity by site of infection in surgical sepsis: a prospective longitudinal study. Crit Care. 2020;24(1):203. doi: 10.1186/s13054-020-02917-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123(3):958–965. doi: 10.1172/JCI64096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yende S, Kellum JA, Talisa VB, et al. . Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2(8):e198686. doi: 10.1001/jamanetworkopen.2019.8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ginde AA, Blatchford PJ, Trzeciak S, et al. . Age-related differences in biomarkers of acute inflammation during hospitalization for sepsis. Shock. 2014;42(2):99–107. doi: 10.1097/SHK.0000000000000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horiguchi H, Loftus TJ, Hawkins RB, et al. . Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. 2018;9:595. doi: 10.3389/fimmu.2018.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mathias B, Delmas AL, Ozrazgat-Baslanti T, et al. . Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg. 2017;265(4):827–834. doi: 10.1097/SLA.0000000000001783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollen MK, Stortz JA, Darden D, et al. . Myeloid-derived suppressor cell function and epigenetic expression evolves over time after surgical sepsis. Crit Care. 2019;23(1):355. doi: 10.1186/s13054-019-2628-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loftus TJ, Mira JC, Ozrazgat-Baslanti T, et al. . Sepsis and Critical Illness Research Center investigators: protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open. 2017;7(7):e015136. doi: 10.1136/bmjopen-2016-015136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stortz JA, Murphy TJ, Raymond SL, et al. . Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock. 2018;49(3):249–258. doi: 10.1097/SHK.0000000000000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salminen A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J Mol Med (Berl). 2021;99(1):1–20. doi: 10.1007/s00109-020-01988-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brummel NE, Bell SP, Girard TD, et al. . Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017;196(1):64–72. doi: 10.1164/rccm.201605-0939OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hotchkiss RS, Moldawer LL, Opal SM, et al. . Sepsis and septic shock. Nat Rev Dis Prime. 2016;2:16045. doi: 10.1038/nrdp.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drewry AM, Samra N, Skrupky LP, et al. . Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383–391. doi: 10.1097/SHK.0000000000000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zidar DA, Al-Kindi SG, Liu Y, et al. . Association of lymphopenia with risk of mortality among adults in the US general population. JAMA Netw Open. 2019;2(12):e1916526. doi: 10.1001/jamanetworkopen.2019.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazer M, Unsinger J, Drewry A, et al. . IL-10 has differential effects on the innate and adaptive immune systems of septic patients. J Immunol. 2019;203(8):2088–2099. doi: 10.4049/jimmunol.1900637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brakenridge SC, Moore FA, Mercier NR, et al. . Persistently elevated glucagon-like peptide-1 levels among critically ill surgical patients after sepsis and development of chronic critical illness and dismal long-term outcomes. J Am Coll Surg. 2019;229(1):58–67.e1. doi: 10.1016/j.jamcollsurg.2019.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vanzant EL, Hilton RE, Lopez CM, et al. . Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care. 2015;19:77. doi: 10.1186/s13054-015-0788-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Metcalf TU, Cubas RA, Ghneim K, et al. . Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell. 2015;14(3):421–432. doi: 10.1111/acel.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perl SH, Bloch O, Zelnic-Yuval D, et al. . Sepsis-induced activation of endogenous GLP-1 system is enhanced in type 2 diabetes. Diabetes Metab Res Rev. 2018;34(4):e2982. doi: 10.1002/dmrr.2982 [DOI] [PubMed] [Google Scholar]
- 29. Lebherz C, Schlieper G, Mollmann J, et al. . GLP-1 levels predict mortality in patients with critical illness as well as end-stage renal disease. Am J Med. 2017;130(7):833–841.e3. doi: 10.1016/j.amjmed.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 30. Williams FN, Jeschke MG, Chinkes DL, et al. . Modulation of the hypermetabolic response to trauma: temperature, nutrition, and drugs. J Am Coll Surg. 2009;208(4):489–502. doi: 10.1016/j.jamcollsurg.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenthal MD, Bala T, Wang Z, Loftus T, Moore F. Chronic critical illness patients fail to respond to current evidence-based intensive care nutrition secondarily to persistent inflammation, immunosuppression, and catabolic syndrome. J Parenter Enteral Nutr. 2020;44:1237–1249. doi: 10.1002/jpen.1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdulla H, Phillips BE, Wilkinson DJ, et al. . Glucagon-like peptide 1 infusions overcome anabolic resistance to feeding in older human muscle. Aging Cell. 2020;19(9):e13202. doi: 10.1111/acel.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magnussen B, Oren Gradel K, Gorm Jensen T, et al. . Association between hypoalbuminaemia and mortality in patients with community-acquired bacteraemia is primarily related to acute disorders. PLoS ONE. 2016;11(9):e0160466. doi: 10.1371/journal.pone.0160466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237(3):319–334. doi: 10.1097/01.SLA.0000055547.93484.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baregamian N, Song J, Jeschke MG, Evers BM, Chung DH. IGF-1 protects intestinal epithelial cells from oxidative stress-induced apoptosis. J Surg Res. 2006;136(1):31–37. doi: 10.1016/j.jss.2006.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zadik Z, Chalew SA, McCarterRJ, Jr., Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60(3):513–516. doi: 10.1210/jcem-60-3-513 [DOI] [PubMed] [Google Scholar]
- 37. White IR. Uses and limitations of randomization-based efficacy estimators. Stat Methods Med Res. 2005;14(4):327–347. doi: 10.1191/0962280205sm406oa [DOI] [PubMed] [Google Scholar]
- 38. Grad S, Ertel W, Keel M, et al. . Strongly enhanced serum levels of vascular endothelial growth factor (VEGF) after polytrauma and burn. Clin Chem Lab Med. 1998;36(6):379–383. doi: 10.1515/CCLM.1998.064 [DOI] [PubMed] [Google Scholar]
- 39. Jesmin S, Zaedi S, Islam AM, et al. . Time-dependent alterations of VEGF and its signaling molecules in acute lung injury in a rat model of sepsis. Inflammation. 2012;35(2):484–500. doi: 10.1007/s10753-011-9337-1 [DOI] [PubMed] [Google Scholar]
- 40. Hou PC, Filbin MR, Wang H, et al. . Endothelial permeability and hemostasis in septic shock: results from the ProCESS trial. Chest. 2017;152(1):22–31. doi: 10.1016/j.chest.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qin S, Rottman JB, Myers P, et al. . The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101(4):746–754. doi: 10.1172/JCI1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98(5):617–625. doi: 10.1161/01.RES.0000209968.66606.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.