Abstract
Background
The randomized DOREMI (Dobutamine Compared to Milrinone) clinical trial evaluated the efficacy and safety of milrinone and dobutamine in patients with cardiogenic shock. Whether the results remain consistent when stratified by acute myocardial infarction remains unknown. In this substudy, we sought to evaluate differences in clinical management and outcomes of acute myocardial infarction complicated by cardiogenic shock (AMICS) versus non‐AMICS.
Methods and Results
Patients in cardiogenic shock (n=192) were randomized 1:1 to dobutamine or milrinone. The primary composite end point in this subgroup analysis was all‐cause in‐hospital mortality, cardiac arrest, non‐fatal myocardial infarction, cerebrovascular accident, the need for mechanical circulatory support, or initiation of renal replacement therapy (RRT) at 30‐days. Outcomes were evaluated in patients with (n=65) and without (n=127) AMICS. The primary composite end point was significantly higher in AMICS versus non‐AMICS (hazard ratio [HR], 2.21; 95% CI, 1.47–3.30; P=0.0001). The primary end point was driven by increased rates of all‐cause mortality, mechanical circulatory support, and RRT. No differences in other secondary outcomes including cardiac arrest or cerebrovascular accident were observed. AMICS remained associated with the primary composite outcome, 30‐day mortality, and RRT after adjustment for age, sex, procedural contrast use, multivessel disease, and inotrope type.
Conclusions
AMI was associated with increased rates of adverse clinical outcomes in cardiogenic shock along with increased rates of mortality and initiation of mechanical circulatory support and RRT. Contrast administration during revascularization likely contributes to increased rates of RRT. Heterogeneity of outcomes in AMICS versus non‐AMICS highlights the need to study interventions in specific subgroups of cardiogenic shock.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03207165.
Keywords: acute myocardial infarction, cardiogenic shock, inotrope, mechanical circulatory support, renal replacement therapy, revascularization
Subject Categories: Myocardial Infarction, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- AMICS
acute myocardial infarction complicated by cardiogenic shock
- CS
cardiogenic shock
- RRT
renal replacement therapy
Clinical Perspective
What Is New?
In patients with cardiogenic shock treated with dobutamine or milrinone, those presenting with acute myocardial infarction had increased 30‐day all‐cause mortality and need for mechanical circulatory support.
Patients with acute myocardial infarction complicated by cardiogenic shock had an increased need for initiation of renal replacement therapy.
Despite concerns about the effect of dobutamine on heart rate and myocardial oxygen consumption, no differences in clinical outcomes were observed between inotropes.
What Are the Clinical Implications?
There is heterogeneity with respect to the etiology of cardiogenic shock, and patients presenting because of acute myocardial infarction are at increased risk of adverse outcomes.
With increased incident renal replacement therapy in patients with cardiogenic shock presenting with myocardial infarction, renal protective strategies such as contrast administration minimization may be important considerations when managing these patients.
Cardiogenic shock (CS) is defined by an inadequate cardiac output state resulting in end‐organ hypoperfusion. 1 Acute myocardial infarction complicated by cardiogenic shock (AMICS) accounts for the majority of cardiogenic shock presentations. 2 , 3 Early revascularization has been shown to improve mortality in CS and remains a cornerstone of management. 4 , 5 More recently, revascularization of culprit‐only disease has become the standard in improving short‐term outcomes in this cohort. 6 , 7 In contrast, CS may also present as a manifestation of chronic cardiac dysfunction and may have a different pathophysiology, response to therapy and prognosis. 2
Management of CS in the cardiac intensive care unit involves hemodynamic stabilization by vasopressors and inotropes. 2 Four major randomized trials including the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK), 4 , 5 Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT‐SHOCK), 7 Tilarginine Acetate Injection in a Randomized International Study in Unstable MI Patients With Cardiogenic Shock (IABP‐SHOCK II), 8 and Intraaortic Balloon Pump in Cardiogenic Shock II 9 have previously evaluated the effect of revascularization and supportive therapies exclusively in AMICS. Although revascularization options have been well‐studied in AMICS, the ubiquitous use of inotropes and vasopressors remains poorly studied. 10 , 11 Furthermore, comparative differences to non‐AMI etiologies of CS have not been evaluated. In CAPITAL‐DOREMI, patients with CS were randomized in a 1:1 ratio to receive either dobutamine or milrinone. 12
Given the lack of robust comparative outcome data in AMICS and non‐AMI etiologies of CS, we sought to evaluate differences between these subgroups of the DOREMI randomized trial.
METHODS
Ethics approval was received from the Ottawa Health Science Network Research Ethics Board and conducted according to the Helsinki Declaration. Written informed consent was obtained from all eligible participants or their substitute decision maker. The trial was registered in ClinicalTrials.gov (NCT03207165). All the data used for this analysis will be made available in the original CAPITAL‐DOREMI trial. 12
Study Design and Oversight
The DOREMI (Dobutamine Compared to Milrinone) trial was a randomized, double‐blind clinical trial of dobutamine versus milrinone in CS. All eligible patients with CS requiring admission to the regional cardiac intensive care unit were randomly assigned (1:1) to dobutamine or milrinone as inotrope therapy. 12 Inclusion criteria includes patients aged ≥18 years who met the Society for Cardiovascular Angiography and Interventions definitions of cardiogenic shock stages B through E 13 and had met an indication for inotrope use: (1) clinical diagnosis of cardiogenic shock and systolic blood pressure <90 mm Hg with signs of end‐organ dysfunction, (2) clinical evidence of systemic and/or pulmonary congestion despite use of vasodilators and/or diuretics, (3) acute coronary syndrome complicated by cardiogenic shock with hemodynamic reduction in cardiac index (<1.8 L/min per m2 and left ventricular end‐diastolic pressure >18 mm Hg), (4) a clinically determined need to augment cardiac output in addition to ongoing vasopressor therapy, or (5) a treating team’s clinical assessment that inotropic therapy is required for developing cardiogenic shock without current evidence of hypoperfusion. For the exclusion criteria, patients were excluded if they had presented with out‐of‐hospital cardiac arrest, were pregnant, initiated an inotrope before randomization, treating physician’s gestalt that the patient was not eligible for the study, patient participating in another interventional trial, or if the patient or substitute decision maker was unable to provide written informed consent for the trial participation. 12
End Points
Patients with both AMI and non‐AMI etiologies of CS were recruited in the DOREMI trial. Patients were categorized as AMICS with a confirmed diagnosis of ST‐segment‒elevation myocardial infarction (STEMI) or non‒ST‐segment‒elevation myocardial infarction (NSTEMI) by the Third Universal Definition of Myocardial Infarction. 14 Non‐infarct related etiologies of CS included decompensated chronic heart failure, valvular heart disease, myocarditis, or low cardiac output state.
The primary end point for this subgroup analysis was the composite of in‐hospital all‐cause mortality, resuscitated cardiac arrest, need for cardiac transplant or mechanical circulatory support, non‐fatal myocardial infarction, transient ischemic attack or stroke, or the initiation of renal replacement therapy (RRT) stratified by AMI and non‐AMI etiologies of CS censored at 30‐days. Secondary end points include components of the primary end point along with median number of days in the cardiac intensive care unit, days of hospital length‐of‐stay, total time on inotropes, and number of patients requiring non‐invasive or invasive mechanical ventilation following randomization. Furthermore, pre‐specified subgroup analyses to explore changes in hemodynamic and biochemical parameters such as heart rate, mean arterial pressure, hourly urine output, vasoactive‐inotropic score, 15 serum creatinine, sodium, potassium, troponin, and creatine kinase levels were performed.
Statistical Analysis
The subgroup analysis according to AMICS was conducted as a post‐hoc analysis. All analyses were performed according to the intention‐to‐treat principle which included all patients according to the group to which they were randomized. Data were summarized as descriptive statistics and presented as proportions (n, %) or mean±SD or median (quartiles, Q1 and Q3). Differences in continuous variables were compared using the Student t‐test or Mann‐Whitney U test and differences in categorical variables were compared by Chi‐Square Test or Fisher Exact Test, as appropriate. Event rates were based on Kaplan‒Meier estimates in the time‐to‐first‐event analysis and compared by log‐rank tests and hazard ratios were calculated by Cox proportional hazards model and presented as hazard ratios (HR) and 95% CI and adjusted for age, sex, volume of contrast used, multivessel disease, and inotrope type. For variables measured more than once throughout the study, a repeated measure mixed model for continuous variables was used to test the statistical significance of the association between AMI and non‐AMI etiologies of CS and outcome. A post‐hoc restricted cubic spline analysis (4 knots) was performed stratifying contrast volume to examine the association between acute kidney injury or renal replacement therapy with contrast volume. All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC, USA) and all figures were generated using GraphPad Prism v8 (GraphPad Software, La Jolla, CA, USA).
Availability of Data and Materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
RESULTS
Baseline and Procedural Characteristics
From September 1, 2017 to May 17, 2020, a total of 319 patients were screened for eligibility in this study. Overall, 192 patients enrolled in the trial and were randomized in a 1:1 fashion to dobutamine or milrinone. In the milrinone group, 31 (32.3%) patients presented with AMICS and 65 (67.7%) presented with non‐AMICS. In the dobutamine group, 34 (35.4%) patients presented with AMICS and 62 (64.6%) presented with non‐AMICS (Figure 1). Amongst the 65 patients presenting with AMICS, 47 (72.3%) patients presented with STEMI and 18 (27.7%) presented with NSTEMI.
Figure 1. Study flow diagram.
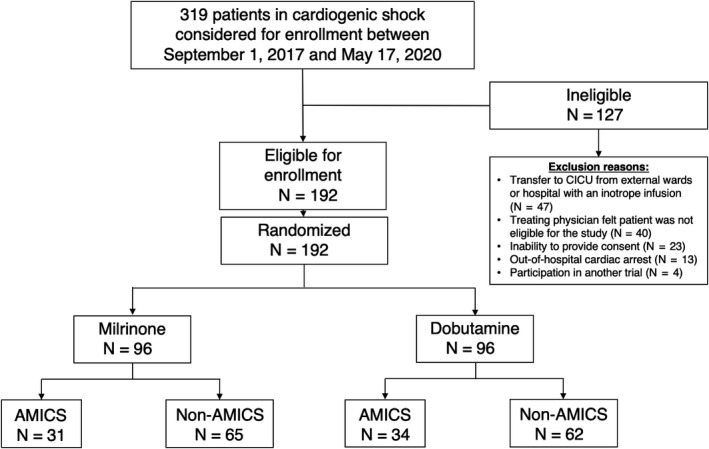
Flow diagram of patient enrollment, showing ineligible patients at screening, randomization 1:1 to inotrope type, and divided by acute myocardial infarction status. AMICS indicates acute myocardial infarction complicated by cardiogenic shock; and CICU, critical intensive care unit.
Baseline characteristics were similar between AMI and non‐AMI etiologies of CS (Table 1). Mean age of patients was 72.1±11.5 years versus 69.6±13.2 years and 21 (32.3%) and 49 (38.6%) were women for AMICS and non‐AMICS, respectively. Patients were evenly distributed in Society for Cardiovascular Angiography and Interventions definition of CS and inotrope randomization. Presence of comorbidities including hypertension, diabetes, dyslipidemia, active smoker, prior coronary artery bypass grafting, myocardial infarction, percutaneous coronary intervention and prior stroke or transient ischemic attack remained similar in both groups. AMICS and non‐AMICS have differences in medications received 24 hours before randomization including aspirin, P2Y12 inhibitor, warfarin, beta‐blocker, diuretic use, and amiodarone administration.
Table 1.
Baseline Characteristics
| Non‐AMI etiology of cardiogenic shock (n=127) | AMI complicated by cardiogenic shock (n=65) | |
|---|---|---|
| Age, y, mean±SD | 69.6±13.2 | 72.1±11.5 |
| Women, n (%) | 49 (38.6) | 21 (32.3) |
| Body mass index, median (IQR) | 26.3 (23.3–31.5) | 26.1 (22.9–30.7) |
| Inotrope, n (%) | ||
| Milrinone | 65 (51.2) | 31 (47.7) |
| Median dose (IQR) | 0.13 (0.00–0.13) | 0.00 (0.00–0.13) |
| Dobutamine | 62 (48.8) | 34 (52.3) |
| Median dose (IQR) | 0.00 (0.00–2.50) | 2.50 (0.00–2.50) |
| Race, n (%) | ||
| White | 112 (88.2) | 53 (81.5) |
| Other races | 15 (11.8) | 12 (18.5) |
| Left ventricular function, n. (%) | ||
| Left ventricular ejection fraction, median (IQR)—% | 25.0 (18.0–36.0) | 27.0 (20.0–45.0) |
| Etiology of ventricular dysfunction | ||
| Ischemic | 64 (50.8) | 64 (98.5) |
| Non‐ischemic | 62 (49.2) | 1 (1.5) |
| Comorbidities, n (%) | ||
| Previous myocardial infarction | 45 (35.4) | 23 (35.4) |
| Previous percutaneous coronary intervention | 30 (23.6) | 19 (29.2) |
| Previous coronary artery bypass grafting | 27 (21.3) | 12 (18.5) |
| Previous stroke/transient ischemic attack | 18 (14.2) | 10 (15.4) |
| Atrial fibrillation | 82 (64.6) | 13 (20.0) |
| Chronic kidney disease | 35 (27.6) | 15 (23.1) |
| Chronic liver disease | 10 (7.9) | 3 (4.6) |
| Chronic obstructive pulmonary disease | 15 (11.8) | 10 (15.4) |
| Hypertension | 84 (66.1) | 42 (64.6) |
| Diabetes | 59 (46.5) | 23 (35.9) |
| Dyslipidemia | 65 (51.2) | 37 (36.9) |
| Active smoker | 19 (15.0) | 9 (13.9) |
| Medications received in 24 h before randomization, n (%) | ||
| Aspirin | 65 (51.2) | 62 (95.4) |
| P2Y12 inhibitor | 37 (27.4) | 62 (95.4) |
| Warfarin | 19 (15.0) | 2 (3.1) |
| Direct oral anticoagulant | 34 (25.2) | 7 (10.8) |
| Statin | 78 (61.4) | 48 (73.9) |
| Beta‐blocker | 71 (55.9) | 22 (33.9) |
| Angiotensin‐converting enzyme inhibitor, angiotensin‐II receptor blocker, or angiotensin receptor neprolysin inhibitor | 62 (45.9) | 23 (35.4) |
| Mineralocorticoid receptor antagonist | 25 (19.7) | 4 (6.2) |
| Nitrates/hydralazine | 19 (14.1) | 7 (10.8) |
| Diuretic | 110 (86.6) | 41 (63.1) |
| Digoxin | 12 (9.5) | 2 (3.1) |
| Amiodarone | 55 (40.7) | 12 (18.5) |
| Society for cardiovascular angiography and interventions cardiogenic shock class, n (%) | ||
| Class B | 7 (5.5) | 4 (6.2) |
| Class C | 106 (83.5) | 49 (75.4) |
| Class D | 11 (8.7) | 11 (16.9) |
| Class E | 3 (2.4) | 1 (1.5) |
| Vasopressor, n (%) | 51 (40.2) | 38 (58.5) |
| Norepinephrine | 51 (40.2) | 38 (58.5) |
| Phenylephrine | 0 (0.0) | 1 (1.5) |
| Vasopressin | 5 (3.9) | 5 (7.7) |
| Epinephrine | 3 (2.4) | 2 (3.1) |
| Dopamine | 1 (0.8) | 3 (4.6) |
| Hemodynamic parameters, median (IQR) | ||
| Heart rate | 94 (75–108) | 88 (72–99) |
| Systolic blood pressure | 108 (93–119) | 108 (98–118) |
| Diastolic blood pressure | 62 (50–72) | 59 (54–70) |
| Mean arterial pressure | 76 (68–84) | 75 (67–84) |
| Intra‐aortic balloon pump, n (%) | 2 (1.6) | 8 (12.3) |
| Ventilation, n (%) | ||
| Non‐invasive | 13 (10.2) | 4 (6.2) |
| Invasive | 16 (12.6) | 24 (36.9) |
AMI indicates acute myocardial infarction; and IQR, interquartile range.
Patients presenting with AMICS were more likely to have multivessel coronary disease (60.0% versus 8.9%; P<0.0001), have contrast exposure (100.0% versus 38.5%; P<0.0001), undergo revascularization (92.3% versus 9.6%; P<0.0001), and longer time to revascularization (10.1 [5.7–21.4] hours versus 4.0 [2.0–7.2] hours; P=0.02; Table 2). Among non‐AMICS, patients who underwent coronary angiography were more likely to have received invasive ventilation (29.0% versus 7.3%; P=0.004; Table S1). We saw no difference in baseline chronic kidney disease (23.1% versus 27.6%; P=0.50) in AMICS versus non‐AMI etiologies of CS.
Table 2.
Biochemical and Procedural Characteristics
| Non‐AMI etiology of cardiogenic shock (n=127) | AMI complicated by cardiogenic shock (n=65) | P value | |
|---|---|---|---|
| Baseline eGFR—mL/min per 1.73 m2, median (IQR) | 63.5 (43.0–94.5) | 63.0 (36.0–89.0) | 0.46 |
| Baseline chronic kidney disease, n (%) (n=172) | 35 (27.6) | 15 (23.1) | 0.50 |
| Catheterization procedural characteristics | (n=52) | (n=65) | |
| Multivessel disease (≥1) | 12 (8.9) | 39 (60.0) | <0.0001 |
| Contrast volume (mL), median (IQR) | 0.0 (0.0–65.0) | 213.0 (147.0–279.0) | <0.0001 |
| Contrast used, n (%) | 52 (38.5) | 65 (100.0) | <0.0001 |
| Coronary intervention, n (%) | 13 (9.6) | 60 (92.3) | <0.0001 |
| GP IIb/IIIa use, n (%) | 0 (0.0) | 1 (1.7) | 0.46 |
| Median time to revascularization—h, median (IQR) | 4.0 (2.0–7.2) | 10.1 (5.7–21.4) | 0.02 |
| Laboratory values at initiation of inotropes | |||
| Hemoglobin, median (IQR), g/L | 118.0 (100.0–135.0) | 116.0 (100.0–134.0) | 0.90 |
| Platelet, median (IQR), g/L | 212.5 (170.0–282.0) | 271.0 (226.0–315.0) | 0.0002 |
| Lactate, median (IQR), mmol/L | 3.0 (2.1–4.5) | 2.8 (1.7–4.3) | 0.43 |
| Serum creatinine, median (IQR), µmol/L | 152.5 (122.0–226.0) | 143.0 (112.0–201.0) | 0.14 |
| Blood urea nitrogen, median (IQR), mmol/L | 15.9 (10.4–24.0) | 12.0 (9.1–16.7) | 0.006 |
| Serum sodium, median (IQR), mmol/L | 135.0 (132.0–138.0) | 137.0 (133.0–139.0) | 0.06 |
| Serum potassium, median (IQR), mmol/L | 4.5 (4.0–4.9) | 4.2 (3.8–4.7) | 0.04 |
| Serum bicarbonate, median (IQR), mmol/L | 22.0 (19.0–26.0) | 20.0 (17.0–23.0) | 0.02 |
| Blood pH, median (IQR) | 7.37 (7.30–7.43) | 7.36 (7.27–7.42) | 0.36 |
| Serum troponin, median (IQR) | 125.5 (43.5–728.0) | 29 540.0 (5578.0–40 000.0) | <0.0001 |
Comparisons were conducted by chi‐squares test or Mann‐Whitney U test. AMI indicates acute myocardial infarction; eGFR, estimated glomerular filtration rate; and IQR, interquartile range.
Clinical Outcomes
As demonstrated in Table 3 and Figure 2, AMICS was associated with increased incidence of the unadjusted primary composite end point at 30‐days (HR, 2.21; 95% CI, 1.47–3.30; P=0.0001; Figure 2A). However, no differences in 30‐day cardiac arrest (HR, 1.68; 95% CI, 0.63–4.51; P=0.30; Figure 2B) nor cerebrovascular accident (HR, 4.63; 95% CI, 0.42–51.15; P=0.17; Figure 2C) was observed between the 2 groups. AMICS was associated with increased rate of 30‐day mortality (HR, 1.62; 95% CI, 1.01–2.59; P=0.04; Figure 2D), mechanical circulatory support (HR, 2.67; 95% CI, 1.21–5.88; P=0.01; Figure 2E) and RRT (HR, 3.14; 95% CI, 1.60–6.14; P=0.001; Figure 2F). The primary composite end point, 30‐day mortality, and initiation of RRT remained associated with AMICS after adjustment of age, sex, contrast use, multivessel disease, and inotrope type (Table 3). When stratified by inotrope type in AMICS, no differences in primary composite end point were observed with dobutamine and milrinone (Figure S1).
Table 3.
Primary and Secondary Outcomes
| Non‐AMI etiology of cardiogenic shock (n=127) | AMI complicated by cardiogenic shock (n=65) | Unadjusted hazard ratio (95% CI) | P value | Adjusted hazard ratio (95% CI)* | P value | |
|---|---|---|---|---|---|---|
| Primary outcome, n (%) | 54 (42.5) | 43 (66.2) | 2.21 (1.47–3.30) | 0.0001 | 2.62 (1.38–4.95) | 0.003 |
| Secondary outcomes, n (%) | ||||||
| All‐cause mortality | 42 (33.1) | 30 (46.2) | 1.62 (1.01–2.59) | 0.04 | 3.11 (1.43–6.76) | 0.004 |
| Resuscitated cardiac arrest | 9 (7.1) | 7 (10.8) | 1.68 (0.63–4.51) | 0.30 | 2.25 (0.45–11.24) | 0.32 |
| Need for mechanical circulatory support or cardiac transplant | 12 (9.5) | 13 (20.0) | 2.67 (1.21–5.88) | 0.01 | 1.93 (0.51–7.29) | 0.33 |
| Intra‐aortic balloon pump | 6 (4.7) | 12 (18.5) | … | … | … | … |
| Impella | 3 (2.4) | 1 (1.5) | … | … | … | … |
| Extracorporeal membrane oxygenation | 1 (0.8) | 0 (0.0) | … | … | … | … |
| Left ventricular assist device | 2 (1.6) | 0 (0.0) | … | … | … | … |
| Non‐fatal myocardial infarction | 1 (0.8) | 0 (0.0) | … | … | … | … |
| Transient ischemic attack or stroke | 1 (0.8) | 2 (3.1) | 4.63 (0.42–51.15) | 0.21 | … | … |
| Initiation of renal replacement therapy | 15 (11.8) | 20 (30.8) | 3.14 (1.60–6.14) | 0.001 | 4.68 (1.69–12.96) | 0.003 |
| Median number of days of cardiac intensive care unit length of stay | 7.0 (4.0–8.0) | 7.0 (6.0–9.0) | … | 0.09 | … | … |
| Median hospital length of stay (d) | 17.0 (9.0–30.0) | 11.0 (4.0–21.0) | … | 0.007 | … | … |
| Median total time on inotropes (h) | 63.0 (28.0–168.0) | 77.0 (32.0–168.0) | … | 0.80 | … | … |
| No. of patients requiring non‐invasive or invasive mechanical ventilation after randomization only | 9 (7.1) | 4 (6.2) | 0.95 (0.65–1.39) | 0.81 | … | … |
Primary composite outcome: composite of all‐cause in‐hospital mortality, resuscitated cardiac arrest, non‐fatal myocardial infarction, transient ischemic attack, or stroke, need for mechanical circulatory support or cardiac transplant, or initiation of renal replacement therapy. Values are reported as n (%) or median (interquartile range). All analyses performed using the intention‐to‐treat principle. P<0.05 is considered statistically significant.
Adjusted for age, sex, contrast use, multivessel disease, and inotrope type.
Figure 2. Kaplan‒Meier estimates in patients with acute myocardial infarction complicated by cardiogenic shock (AMICS) vs non‐AMICS by primary and secondary end points.
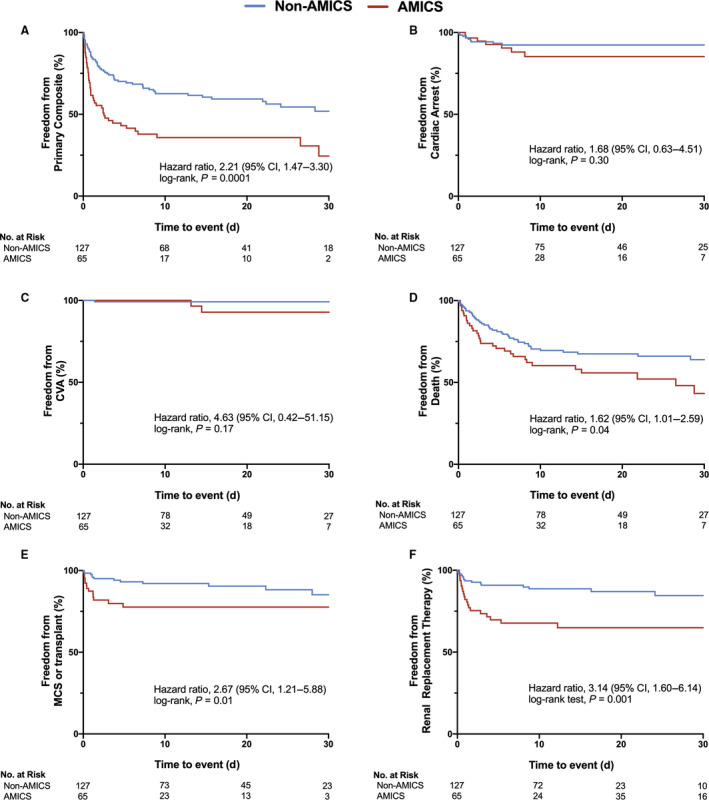
A, AMICS was associated with increased rates of primary composite end point (hazard ratio [HR], 2.21; 95% CI, 1.47–3.30; P=0.0001). B, No differences in rates of cardiac arrest was observed with AMICS vs non‐AMICS (HR, 1.68; 95% CI, 0.63–4.51; P=0.30). C, No differences in rates of CVA was observed with AMICS vs non‐AMICS (HR, 4.63; 95% CI, 0.42–51.15; P=0.21). D, AMICS was associated with increased rates of 30‐day all‐cause mortality (HR, 1.62; 95% CI, 1.01–2.59; P=0.04). E, AMICS was associated with increased rates of need for mechanical circulatory support or cardiac transplant (HR, 2.67; 95% CI, 1.21–5.88; P=0.01). F, AMICS was associated with increased initiation of renal replacement therapy (HR, 3.14; 95% CI, 1.60–6.14; P=0.001). Comparisons were made by log‐rank test and hazard ratios were evaluated using the Cox proportional hazards model. P<0.05 is considered statistically significant. AMICS indicates acute myocardial infarction complicated by cardiogenic shock; CVA, cerebrovascular accident; and MCS, mechanical circulatory support.
Furthermore, no differences in the median number of days in the cardiac intensive care unit (7.0 [6.0–9.0] days versus 7.0 [4.0–8.0] days; P=0.09) or total time on inotropes (77.0 [32.0–168.0] hours versus 63.0 [28.0–168.0] hours; P=0.80) was observed between AMICS and non‐AMI etiologies of CS, respectively (Table 2). Median hospital length‐of‐stay was shorter (11.0 [4.0–21.0] days versus 17.0 [9.0–30.0] days; P=0.01). Elevated level of troponin and creatine kinase was observed in AMICS whereas no differences were seen between AMICS and non‐AMI etiologies of CS in hemodynamic parameters including heart rate, mean arterial pressure, vasoactive‐inotrope score, or lactate levels (Figure 3A through 3F).
Figure 3. Changes in key hemodynamic and biochemical parameters from baseline to 120 hours.
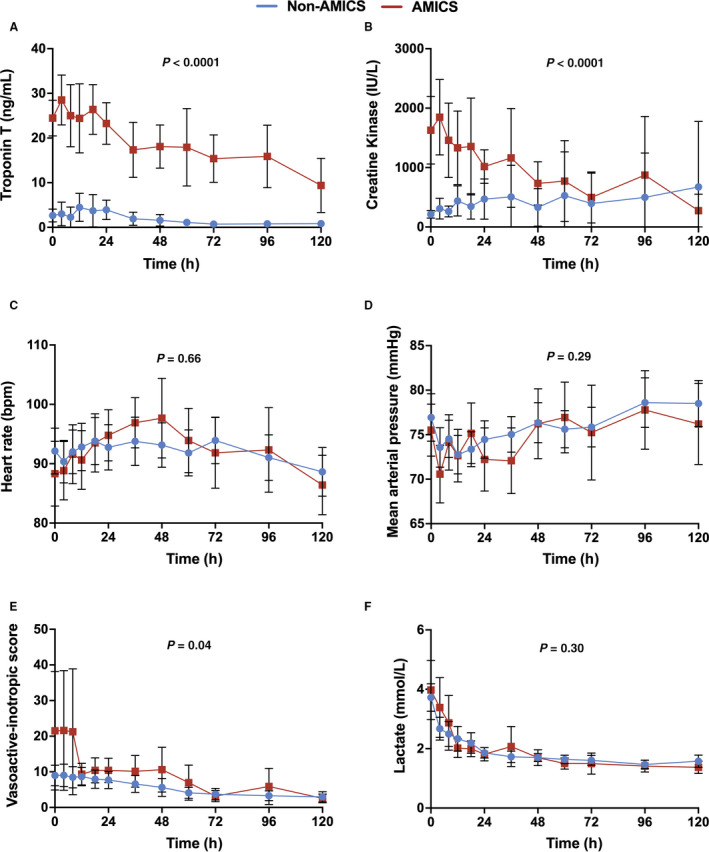
A, Troponin T (ng/mL; P<0.0001). B, Creatine kinase (IU/L; P<0.0001). C, Heart rate (beats per minute [bpm]; P=0.66). D, Mean arterial pressure (mm Hg; P=0.29). E, Vasoactive‐inotropic score (P=0.04). F, Lactate (mmol/L; P=0.30). A repeated measure mixed model was utilized to evaluate differences in the continuous variables between the 2 groups. All panels reveal mean±95% CIs with blue representing non acute myocardial infarction complicated by cardiogenic shock and red representing acute myocardial infarction complicated by cardiogenic shock. AMICS indicates acute myocardial infarction complicated by cardiogenic shock
Renal End Points
When stratified by AMICS versus non‐AMI etiologies of CS, renal function was impaired with a significant decrease in mean urine output levels in AMICS (P=0.0003; Figure 4A) with no significant changes to serum creatinine or potassium levels (Figure 4B and 4C). Procedural contrast volume was higher in AMICS compared with non‐AMI etiologies of CS (213.0 [147.0–279.0] mL versus 0.0 [0.0–65.0] mL; P<0.0001; Figure 4D). A restricted cubic spline analysis reveals a non‐linear relationship between contrast volume used and acute kidney injury (Figure S2). Moreover, the restricted cubic spline analysis shows a non‐linear relationship between contrast volume and renal replacement therapy with an increased risk of renal replacement therapy with contrast volumes ≥225 mL (odds ratio, 1.36; 95% CI, 1.04–1.83; Figure S3).
Figure 4. Renal outcomes.
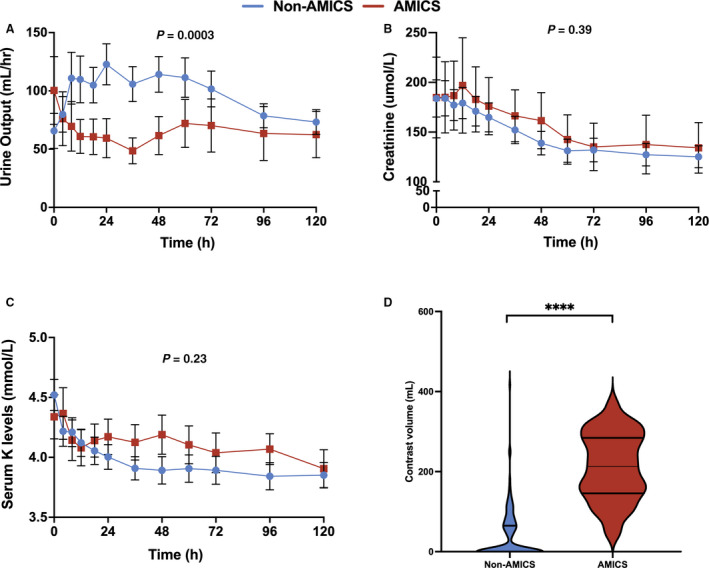
A, Changes in urine output (mL/h) from baseline to 120 hours (P=0.0003). B, Changes in creatinine (µmol/L) from baseline to 120 hours (P=0.39). C, Changes in serum potassium levels (mmol/L) from baseline to 120 hours (P=0.23). D, Contrast volume was elevated in acute myocardial infarction complicated by cardiogenic shock (213.0 [147.0–279.0] mL vs 0.0 [0.0–65.0] mL; P<0.0001). A repeated measure mixed model was used to evaluate differences in the continuous variables between the 2 groups. All panels reveal mean±95% CIs with blue representing nonacute myocardial infarction complicated by cardiogenic shock and red representing patients with acute myocardial infarction complicated by cardiogenic shock. AMICS indicates acute myocardial infarction complicated by cardiogenic shock. **** represents P<0.0001.
DISCUSSION
We sought to evaluate differences in outcomes between AMICS and non‐AMICS in a highly defined population. In this analysis, we identified 2 major findings. First, compared with patients with non‐AMI, individuals presenting with AMI are at higher risk for adverse events. This was largely driven by increased rate of 30‐day all‐cause mortality, need for mechanical circulatory support, and initiation of RRT. Second, urine output was significantly reduced, and contrast administration was higher in patients with AMI likely contributing to increased need for RRT. Importantly, there were no differences in hemodynamic status between AMICS versus non‐AMICS and contrast use may have contributed to differences observed in renal outcomes. Finally, despite concerns about dobutamine increasing heart rate and myocardial oxygen consumption, no differences in clinical outcomes were observed between dobutamine and milrinone. Taken together, these findings suggest important differences in care pathways and outcomes exist between these subgroups of patients with CS.
In this substudy of the randomized DOREMI trial, we evaluated clinical outcomes stratified by AMICS. Previous trials such as the SHOCK trial have demonstrated the benefit of coronary revascularization in AMICS. 4 , 5 Furthermore, CULPRIT‐SHOCK suggested the superiority of culprit‐only revascularization compared with multivessel disease revascularization at the time of presentation on early outcomes including renal dysfunction. 6 , 7 Given their focus on revascularization, these trials were necessarily focused on patients with AMICS and comparative data to non‐AMICS remain limited. Moreover, our results are consistent with a registry study showing a similar rate of in‐hospital mortality in AMICS and non‐AMICS. 3 Despite contemporary supportive treatment with coronary revascularization improving in‐hospital mortality rates, 3 our findings demonstrate high rates of primary composite event in AMICS, highlighting an opportunity to improve outcomes in this patient population.
The use of dobutamine in AMICS raised concerns because of its effect on heart rate and myocardial oxygen consumption. Dobutamine is a synthetic catecholamine with beta‐1 and beta‐2 receptor agonist activity, whereas milrinone is a phosphodiesterase‐3 inhibitor which affects cardiac inotropy, lusitropy, and peripheral vasodilation. Indeed, the effect of different inotropes on mortality in AMICS remains poorly studied with no definitive evidence to date. 16 A substudy of the CardShock trial demonstrated similar outcomes in a combination treatment of norepinephrine with dobutamine or levosimendan. 17 Furthermore, although levosimendan compared with dobutamine was associated with short‐term mortality benefits, it conferred no long‐term mortality benefits in long‐term follow‐up. 16 Our findings complement previous findings in that no differences in the primary composite end point between dobutamine and milrinone in AMICS was observed.
We also observed an increased need for RRT at 30 days in patients with AMICS. No differences in baseline characteristics which may predispose the patient to RRT such as baseline renal function, baseline Society for Cardiovascular Angiography and Interventions class, or chronic kidney disease were observed. Furthermore, both groups had a high rate of acute kidney injury with patients with AMICS more likely to require RRT. In our cohort, patients with AMICS received greater contrast volume during angiography. Iodinated contrast are hypothesized to be toxic to tubular epithelial cells and impede renal blood flow through a combination of arteriolar vasoconstriction and increase in blood osmolality and viscosity—both of which may potentially contribute to contrast‐associated acute kidney injury. 18 , 19 Furthermore, transition to RRT may be partially explained by patients presenting with ST‐segment elevation myocardial infarction who are at high‐risk of contrast‐associated acute kidney injury. 20 Other factors which impact progression to RRT may include athero‐embolic complications. 21 Strategies to restrict contrast use during catheterization and revascularization is paramount to attenuate progression to RRT.
Study Limitations
Certainly, our study is not without limitations. First, it was limited to in‐hospital outcomes as per the design of the clinical trial. Thus, we are not able to comment on long‐term outcomes between the 2 groups and differences may exist. Second, the findings of increased renal injury and RRT in patients with AMI may be unavoidable owing to the need to revascularize patients with an ischemic trigger. However, both CULPRIT‐SHOCK 6 , 7 and recent observational findings support minimizing revascularization and contrast exposure at the time of presentation. 22 Our data echo these studies highlighting worse renal outcomes compared with those without an ischemic trigger. Third, majority of participants in the DOREMI trial presented with Society for Cardiovascular Angiography and Interventions cardiogenic shock stage C. Fourth, we did not account for the competing risk of death with respect to length of stay between the 2 groups in the cohort. Fifth, the evaluation of secondary outcomes such as transient ischemic attack or stroke were limited by small sample size. Finally, only patients with STEMI presenting with AMI underwent a revascularization procedure immediately—a pattern of practice within the randomization center. More aggressive revascularization of patients with NSTEMI may have further magnified differences between the groups—particularly as it pertains to renal outcomes.
CONCLUSIONS
AMICS is associated with increased rates of adverse clinical outcomes compared with non‐AMICS. In particular, all‐cause mortality, RRT, and escalation to mechanical circulatory support were more common in patients with AMICS. Moreover, renal protective strategies such as reducing contrast use and limiting revascularization to culprit‐only lesions maybe beneficial in this cohort.
Sources of Funding
This project was supported by the Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario and Canadian Foundation of Innovation. Jung was funded by the Vanier Canadian Institutes of Health Research Canada Graduate Scholarship.
Disclosures
Dr Hibbert reports funding as a clinical trial investigator from Abbott, Boston Scientific, and Edwards Lifesciences outside of the submitted work. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figures S1–S3
Acknowledgments
The authors would like to thank all the collaborators and CAPITAL Research Group for all their support and guidance for the completion of the project.
Author contributions: Jung, Di Santo, Mathew, and Hibbert contributed to the conception/design, and drafted the work for the study. Abdel‐Razek, Parlow, Simard, Marbach, Gillmore, Mao, Bernick, Theriault‐Lauzier, Fu, Lau, Motazedian, Russo, and Labinaz contributed to the acquisition of the data. Jung, Di Santo, Bernick, and Hibbert contributed to the interpretation of the study. All authors approved the submitted version of the manuscript and agree to be accountable for all aspects of the work to be published.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021570
For Sources of Funding and Disclosures, see page 11.
References
- 1. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 2. Thiele H, Ohman EM, de Waha‐Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40:2671–2683. doi: 10.1093/eurheartj/ehz363 [DOI] [PubMed] [Google Scholar]
- 3. Berg DD, Bohula EA, Van Diepen S, Katz JN, Alviar CL, Baird‐Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward P, Col J, White HD; Investigators S . Early revascularization and long‐term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295:2511–2515. doi: 10.1001/jama.295.21.2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 6. Thiele H, Akin I, Sandri M, de Waha‐Thiele S, Meyer‐Saraei R, Fuernau G, Eitel I, Nordbeck P, Geisler T, Landmesser U, et al. One‐year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379:1699–1710. doi: 10.1056/NEJMoa1808788 [DOI] [PubMed] [Google Scholar]
- 7. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer‐Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 8. Alexander JH, Reynolds HR, Stebbins AL, Dzavik V, Harrington RA, Van de Werf F, Hochman JS. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657–1666. doi: 10.1001/jama.297.15.joc70035 [DOI] [PubMed] [Google Scholar]
- 9. Thiele H, Zeymer U, Neumann F‐J, Ferenc M, Olbrich H‐G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 10. Levy B, Clere‐Jehl R, Legras A, Morichau‐Beauchant T, Leone M, Frederique G, Quenot J‐P, Kimmoun A, Cariou A, Lassus J, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72:173–182. doi: 10.1016/j.jacc.2018.04.051 [DOI] [PubMed] [Google Scholar]
- 11. Fuhrmann JT, Schmeisser A, Schulze MR, Wunderlich C, Schoen SP, Rauwolf T, Weinbrenner C, Strasser RH. Levosimendan is superior to enoximone in refractory cardiogenic shock complicating acute myocardial infarction*. Crit Care Med. 2008;36:2257–2266. doi: 10.1097/CCM.0b013e3181809846 [DOI] [PubMed] [Google Scholar]
- 12. Mathew R, Di Santo P, Jung RG, Marbach JA, Hutson J, Simard T, Ramirez FD, Harnett DT, Merdad A, Almufleh A, et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med. 2021;385:516–525. doi: 10.1056/NEJMoa2026845 [DOI] [PubMed] [Google Scholar]
- 13. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 14. Thygesen K, Alpert Joseph S, Jaffe Allan S, Simoons Maarten L, Chaitman Bernard R, White HD. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 15. Na SJ, Chung CR, Cho YH, Jeon K, Suh GY, Ahn JH, Carriere KC, Park TK, Lee GY, Lee JM, et al. Vasoactive inotropic score as a predictor of mortality in adult patients with cardiogenic shock: medical therapy versus ECMO. Rev Esp Cardiol (Engl Ed). 2019;72:40–47. doi: 10.1016/j.rec.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 16. Schumann J, Henrich EC, Strobl H, Prondzinsky R, Weiche S, Thiele H, Werdan K, Frantz S, Unverzagt S. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;1:CD009669. doi: 10.1002/14651858.CD009669.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tarvasmäki T, Lassus J, Varpula M, Sionis A, Sund R, Køber L, Spinar J, Parissis J, Banaszewski M, Silva Cardoso J, et al. Current real‐life use of vasopressors and inotropes in cardiogenic shock—adrenaline use is associated with excess organ injury and mortality. Crit Care. 2016;20:208. doi: 10.1186/s13054-016-1387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almendarez M, Gurm HS, Mariani J Jr, Montorfano M, Brilakis ES, Mehran R, Azzalini L. Procedural strategies to reduce the incidence of contrast‐induced acute kidney injury during percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1877–1888. doi: 10.1016/j.jcin.2019.04.055 [DOI] [PubMed] [Google Scholar]
- 19. Mehran R, Dangas GD, Weisbord SD. Contrast‐associated acute kidney injury. N Engl J Med. 2019;380:2146–2155. doi: 10.1056/NEJMra1805256 [DOI] [PubMed] [Google Scholar]
- 20. Sgura FA, Bertelli L, Monopoli D, Leuzzi C, Guerri E, Spartà I, Politi L, Aprile A, Amato A, Rossi R, et al. Mehran contrast‐induced nephropathy risk score predicts short‐ and long‐term clinical outcomes in patients with ST‐elevation–myocardial infarction. Circ Cardiovasc Interv. 2010;3:491–498. doi: 10.1161/CIRCINTERVENTIONS.110.955310 [DOI] [PubMed] [Google Scholar]
- 21. Ando G, Costa F, Trio O, Oreto G, Valgimigli M. Impact of vascular access on acute kidney injury after percutaneous coronary intervention. Cardiovasc Revasc Med. 2016;17:333–338. doi: 10.1016/j.carrev.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 22. Khera R, Secemsky EA, Wang Y, Desai NR, Krumholz HM, Maddox TM, Shunk KA, Virani SS, Bhatt DL, Curtis J, et al. Revascularization practices and outcomes in patients with multivessel coronary artery disease who presented with acute myocardial infarction and cardiogenic shock in the US, 2009–2018. JAMA Intern Med. 2020;180:1317–1327. doi: 10.1001/jamainternmed.2020.3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S3
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


