Abstract
Background
Disabilities have implications for health, well‐being, and health care, yet limited information is available on the percentage of adults with congenital heart defects (CHD) living with disabilities. We evaluated the prevalence of disability and associated characteristics among the 2016–2019 CH STRONG (Congenital Heart Survey to Recognize Outcomes, Needs, and Well‐Being) population‐based sample of 19‐ to 38‐year‐olds with CHD from 3 US locations.
Methods and Results
Prevalence of disability types (hearing, vision, cognition, mobility, self‐care, living independently) were compared with similarly aged adults from the general population as estimated by the American Community Survey and standardized to the CH STRONG eligible population to reduce nonresponse bias and confounding. Health‐related quality of life (HRQOL) was measured via Patient‐Reported Outcomes Measurement Information System Global Health Scale T‐scores standardized to US 18‐ to 34‐year‐olds. Separate multivariable regression models assessed associations between disability and HRQOL. Of 1478 participants, 40% reported disabilities, with cognition most prevalent (29%). Of those reporting disability, 45% ever received disability benefits and 46% were unemployed. Prevalence of disability types were 5 to 8 times higher in adults with CHD than the general population. Those with ≥1 disability had greater odds of being female, and of having non‐Hispanic Black maternal race and ethnicity, severe CHD, recent cardiac care, and noncardiac congenital anomalies. On average, adults with CHD and cognition, mobility, and self‐care disabilities had impaired mental HRQOL and those with any disability type had impaired physical HRQOL.
Conclusions
Two of 5 adults with CHD may have disabilities, which are associated with impaired HRQOL. These results may inform healthcare needs and services for this growing population.
Keywords: adult, congenital heart defect, disability, health‐related quality of life
Subject Categories: Congenital Heart Disease, Epidemiology, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- ACS
American Community Survey
- APPROACH‐IS
Assessment of Patterns of Patient‐Reported Outcomes in Adults With Congenital Heart Disease–International Study
- CDC
Centers for Disease Control and Prevention
- CH STRONG
Congenital Heart Survey to Recognize Outcomes, Needs, and Well‐Being
- GMH
Global Mental Health
- GPH
Global Physical Health
- HRQOL
health‐related quality of life
- PROMIS GHS
Patient‐Reported Outcomes Measurement Information System Global Health Scale
Clinical Perspective
What Is New?
Using population‐based data, this study identified that 2 in 5 young adults with congenital heart defects (CHD) have a disability, with cognitive disabilities most common.
Disabilities are 5 to 8 times more common in young adults with CHD than young adults in the general population, even after excluding those with noncardiac congenital anomalies.
Among adults with CHD, disabilities were more common among those who had been born to Black mothers and those with severe CHD.
Among adults with CHD, health‐related quality of life was impaired for those with disabilities, and almost half reported not working in the past 12 months.
What Are the Clinical Implications?
In accordance with the US Surgeon General’s Call to Action in 2005, improving the health and wellness of patients with CHD with disabilities may include provider training and continuing education curricula on the healthcare challenges and best practices in healthcare provision for patients with CHD with disabilities; implementing clinical practices that consider patients’ full range of health concerns, including medical, social, emotional, family, or community needs; and identifying opportunities to improve access to care and services and to offer more inclusive health promotion and wellness services for patients with CHD with disabilities.
Because of advancements in cardiac management, 85% to 90% of individuals with congenital heart defects (CHD) survive to adulthood, with an estimated 1.4 million adults living with CHD in the United States. 1 , 2 Adults with CHD have special healthcare needs and considerations that may be further impacted by the presence of other health conditions like disabilities. 3 While up to 34% of adults with CHD may have cognitive impairment 4 , 5 , 6 and some genetic syndromes are known to result in both heart defects and sensory impairment, 7 , 8 the overall prevalence of disabilities (hearing, vision, cognition, mobility, self‐care, or living independently) among adults with CHD is unknown. A better understanding of the prevalence, characteristics, and outcomes of adults with CHD who also have a disability may help determine healthcare needs and services for this specialized population. Therefore, using data from the 2016–2019 CH STRONG (Congenital Heart Survey to Recognize Outcomes, Needs, and Well‐Being), we assessed prevalence of disability among a population‐based sample of young adults with CHD born in 3 US locations and compared those estimates to that of young adults in the general population. Among young adults with CHD, we further examined associations between disability status and demographic and health characteristics, including health‐related quality of life (HRQOL).
Methods
Requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Centers for Disease Control and Prevention (CDC) at chstrong@cdc.gov.
Study Population
CH STRONG, funded by the CDC, is a cross‐sectional survey on longer‐term outcomes of adults born between 1980 and 1997 and diagnosed with CHD in early childhood (www.chstrong.org). 9 Eligible adults were identified from 3 active, population‐based birth defect registries in Arizona, Arkansas, and the 5 metropolitan counties of Atlanta, Georgia (Clayton, Cobb, DeKalb, Fulton, and Gwinnett). All were recruited between October 2016 and January 2019. Individuals incarcerated or deceased at the time of survey recruitment or unable to complete the survey in English or Spanish were ineligible. If eligible but unable to complete the survey, a proxy (eg, relative or caretaker) could complete the survey on the individual’s behalf. Survey data were linked to registry data to include characteristics at birth and specific type of CHD.
CH STRONG was approved by CDC and University of Arkansas for Medical Sciences’ Institutional Review Boards. The University of Arizona deferred to the CDC Institutional Review Board. A more detailed description of the CH STRONG sampling and design has been published. 9
Data
Congenital Heart Defects
Using the CDC‐modified International Classification of Diseases, Ninth Revision (ICD‐9) with the British Paediatric Association Classification of Diseases diagnosis code extension, CHD was defined as having ≥1 code between 745.000 and 747.430, excluding the minor or unconfirmed CHD codes in Table S1. 9 Data on functional class was unavailable, so CHD severity was determined by diagnosis codes using a previously published algorithm 10 and dichotomized as severe (defects that typically require intervention in the first year of life) or nonsevere (shunt, valve, or other defects that typically do not require early intervention; Table S1 and Figure S1).
Disability
The 6‐item set of Department of Health and Human Services Standard Disability Status Questions was included in the CH STRONG survey. These questions identify individuals who have serious difficulties with hearing; vision (even when wearing glasses); cognition (eg, concentrating, remembering, or making decisions because of a physical, mental, or emotional condition); mobility (eg, walking or climbing stairs); self‐care (eg, dressing or bathing); and living independently (eg, doing errands alone because of a physical, mental, or emotional condition). These are the federal data standard for survey questions on disability, having outperformed other measures in cognitive and field testing. 11
As a comparison group for disability prevalence, we used 1‐year estimates among 18‐ to 35‐year‐olds residing in Arizona, Arkansas, and Metro Atlanta (same 5 counties as CH STRONG) who participated in the 2017 American Community Survey (ACS), an annual federally mandated survey for a random sample of >3.5 million US households. The 2017 survey covered 97.9% of US households and achieved a 93.7% response rate. (https://www.census.gov/programs‐surveys/acs/).
Demographic and Health Characteristics
From registries, we ascertained sex, maternal race and ethnicity, year of birth to calculate age, and diagnosis codes to identify those with any noncardiac congenital anomalies (Table S2), noncardiac birth defects only (a subset of noncardiac congenital anomalies identified by codes outside of 758.000–758.999), chromosomal anomalies only (a subset of noncardiac congenital anomalies, including Down syndrome, identified by codes between 758.000 and 758.999), and Down syndrome only (a subset of chromosomal anomalies identified by codes 758.000–758.090). Time since last visit with a cardiologist was reported via the survey.
Health‐Related Quality of Life
Because of its brevity, ease of administration, and standardization for national comparison, the 10‐item Patient‐Reported Outcomes Measurement Information System Global Health Scale (PROMIS GHS) was selected to assess HRQOL on the CH STRONG survey. PROMIS GHS splits into 2 HRQOL domains: Global Physical Health (GPH) and Global Mental Health (GMH), each summarizing a unique set of 4 items on the 10‐item scale (Table S3). The PROMIS GHS has internal consistency, with a reliability kappa of 0.92, and both the GPH and GMH have high internal consistency with Cronbach’s alpha of 0.81 and 0.86, respectively. 12
GPH and GMH raw scores convert to T‐scores representative of the 2000 US Census. 12 US 18‐ to 34‐year‐olds are estimated to have a mean GPH T‐score of 51.6 (SD=8.4) and mean GMH T‐score of 48.5 (SD=9.7), which we used as reference values to compare with CH STRONG (http://www.healthmeasures.net/score‐and‐interpret/interpret‐scores/promis/reference‐populations). T‐scores above reference values indicate better perceived HRQOL than the general population and scores below indicate worse. Furthermore, individuals with impaired physical and mental HRQOL were defined as those with GPH and GMH T‐scores ½ SD below the reference means (ie, GPH T‐scores <47.4 and GMH T‐scores <43.7), cutoffs representing the minimally important difference between impaired and normal HRQOL. 13 , 14 , 15
The other 2 items not incorporated into the GPH or GMH are:
The general health domain: “In general, would you say your health is … Excellent, Very Good, Good, Fair, or Poor?”
The social functioning domain: “In general, please rate how well you carry out your usual social activities and roles … Excellent, Very Good, Good, Fair, or Poor?”
For both, responses were dichotomized into Excellent/Very Good/Good or Fair/Poor as has been done elsewhere. 16 , 17 General population reference values for the general health and social functioning domains were not available.
Disability Benefits
Among those with CHD and disabilities, we assessed the prevalence of ever receiving disability benefits, of ever being denied disability benefits, and employment status in the past 12 months as reported on the survey.
Statistical Methods
Inclusion Criteria
CH STRONG participants included in the sample responded to all demographic or health characteristics of interest, including all 6 disability questions and all 10 items from the PROMIS GHS. All analyses were conducted using SAS‐callable SUDAAN software version 11.0 (Research Triangle Institute 2011).
Standardization for Comparison With National Estimates
To address potential nonresponse bias and improve comparability with national estimates, 9 we standardized the disability prevalence and HRQOL estimates from the analytic sample to the 9312 eligible individuals in CH STRONG by site, sex, race and ethnicity, birth year, and CHD severity. Furthermore, to address potential confounding when comparing CH STRONG to ACS disability prevalence estimates, we standardized the ACS estimates to the CH STRONG eligible population by the strata they have in common: site (defined as birth state in CH STRONG and current state in ACS) and sex.
Disability Prevalence Estimates
For both CH STRONG and ACS, standardized disability prevalence estimates and 95% CIs were calculated for each disability type and for having ≥1 disability. We calculated Z scores and corresponding P values for the difference in mean proportions between CH STRONG and ACS. Furthermore, standardized prevalence of ≥1 disability among CHD types with >10 cases was calculated.
Demographics, Health, and HRQOL Among Those With CHD by Disability Status and Type
Among the CH STRONG sample, we estimated unstandardized prevalence, adjusted odds ratios (aORs), and 95% CIs of reporting ≥1 disability by demographic and health characteristics. We also estimated the standardized mean GPH and GMH T‐scores and the unstandardized prevalence, aORs, and 95% CIs of reporting poor HRQOL outcomes (fair/poor general health, fair/poor social functioning, impaired physical HRQOL, and impaired mental HRQOL) by presence of disability and disability type. Because nonresponse may not bias associations between variables, but confounding could be a concern, 18 we chose to adjust all models for CHD severity, age, sex, maternal race and ethnicity, and site rather than standardize by these characteristics. Models were additionally adjusted for presence of noncardiac congenital anomalies, except for models examining variables that are components of noncardiac congenital anomalies, such as chromosomal anomalies, noncardiac birth defects, and Down syndrome, or the model examining proxy report since 75% of those who responded via proxy had a non‐congenital cardiac anomaly.
Sensitivity Analyses
We conducted 5 sensitivity analyses excluding CH STRONG participants who had (1) their survey completed by proxy because relatives may report different disabilities and HRQOL than self‐report; (2) noncardiac congenital anomalies, (3) chromosomal anomalies, or (4) Down syndrome because these can be associated with disabilities; and (5) any of the aforementioned criteria.
Results
Sample Characteristics
Of 1656 CH STRONG participants, 11% were excluded for missing data on any of the following: maternal race and ethnicity (n=25), type of survey respondent (ie, self or proxy; n=27), Department of Health and Human Services disability items (n=45), PROMIS GHS items (n=73), or last receipt of cardiac care (n=8). After standardizing the analytic sample (n=1478) to the CH STRONG eligible population, 20% had the survey completed by a proxy, the majority of whom responded that the individual with CHD was mentally unable. The most common primary CHD types in the analytic sample were ventricular septal defects (28%), atrial septal defects (11%), and tetralogy of Fallot (7%). Approximately 65% were non‐Hispanic White individuals, and 35% had co‐occurring noncardiac congenital anomalies.
Prevalence of Disability
Among young adults, disabilities were more common among those with CHD compared with the general population (Figure 1). The standardized prevalence of ≥1 disability and the 6 disability types were 5 to 8 times higher in CH STRONG compared with ACS. Even those with nonsevere CHD were 5 times more likely than the ACS sample to report ≥1 disability. About 40% of the CH STRONG sample reported ≥1 disability compared with 7% in the ACS sample. The most common disability type in both samples was cognition (CH STRONG, 29%; ACS, 5%) followed by independent living (CH STRONG, 22%; ACS, 3%). Reporting ≥1 disability differed among those with severe and nonsevere CHD (45% and 37%, respectively; P=0.006) as did disability in mobility (14% and 10% respectively, P=0.038). Individuals with common atrioventricular canal (81%), interrupted aortic arch (65%), and tricuspid valve atresia (55%) most commonly reported ≥1 disability.
Figure 1. Standardized prevalence of disability among adults with severe and nonsevere CHD in 2016–2019 CH STRONG (Congenital Heart Survey to Recognize Outcomes, Needs, and Well‐Being) compared with 2017 ACS participants.
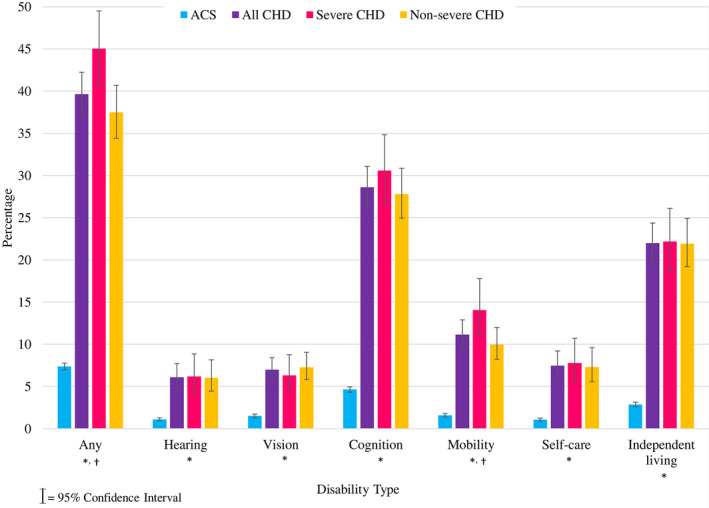
CHD estimates were standardized to the CH STRONG eligible sample (n=9312) by site, sex, race and ethnicity, birth year, and CHD severity. ACS estimates were standardized to the same eligible sample by site and sex. More young adults with CHD reported disabilities compared with ACS participants. *All CHD: ACS P<0.05. †Severe: nonsevere P<0.05. ACS indicates American Community Survey; and CHD, congenital heart defects.
Excluding CH STRONG participants with noncardiac congenital anomalies (n=497), proxy report (n=277), or both (n=567; Figure S2), standardized prevalence of ≥1 disability fell from 40% to 26% to 35%, but was still 5% to 7% higher than the general population. The standardized prevalence for hearing, vision, and self‐care disabilities among those with CHD fell below 5%. However, the standardized prevalence of all disability types, except hearing and vision, in CH STRONG compared with ACS remained elevated (P<0.05). No other results substantially changed in sensitivity analyses.
Multivariable Associations Between Demographic and Health Characteristics and Disability
Among the CH STRONG sample, having ≥1 disability was associated with all examined characteristics, except age (Table 1). The adjusted odds of having severe CHD (aOR, 1.4; 95% CI, 1.1–1.9) and having a recent (≤2 years) visit with a cardiologist (aOR, 1.6; 95% CI, 1.2–2.0) were greater for individuals with ≥1 disability compared with those with no disabilities. Additionally, those with ≥1 disability were 1.6 times (95% CI, 1.3–2.0) as likely to be female and 1.6 (95% CI, 1.1–2.2) times as likely to have maternal non‐Hispanic Black race. They were also more likely to have co‐occurring noncardiac congenital anomalies (aOR, 2.5; 95% CI, 2.0–3.3), including noncardiac birth defects (aOR, 14.0; 95% CI, 8.5–23.1), chromosomal anomalies (aOR, 8.4; 95% CI, 6.1–11.6), and Down syndrome (aOR, 4.0; 95% CI, 3.2–5.1), and to have their survey completed by a proxy (aOR, 16.3; 95% CI, 9.2–29.1). Estimates did not substantially change after excluding individuals with noncardiac congenital anomalies or proxy report.
Table 1.
Demographic and Health Characteristics of Adults with Congenital Heart Defects by Disability Status: CH STRONG, 2016–2019
| Characteristic | ≥1 Disability | No disabilities | Have ≥1 disability: no disabilities | ||||
|---|---|---|---|---|---|---|---|
| n | % |
Standardized* % (95% CI) |
n | % |
Standardized* % (95% CI) |
aOR (95% CI) |
|
| CHD severity † , ‡ | |||||||
| Severe | 216 | 42.5 |
45.0 (40.6–49.5) |
292 | 57.5 |
55.0 (50.5–59.4) |
1.4 (1.1–1.8) |
| Nonsevere | 352 | 36.3 |
37.5 (34.4–40.7) |
618 | 63.7 |
62.5 (59.3–65.6) |
Ref |
| Last visit with a cardiologist † , ‡ | |||||||
| ≤2 y | 312 | 45.0 |
46.4 (42.3–50.5) |
382 | 55.0 |
53.6 (49.5–57.7) |
1.6 (1.2–2.0) |
| >2 y or never | 256 | 32.7 |
35.0 (31.7–38.4) |
528 | 67.3 |
65.0 (61.6–68.3) |
Ref |
| Age at survey completion (y) † , ‡ | |||||||
| 19–24 | 252 | 39.4 |
37.6 (33.8–41.6) |
388 | 60.6 |
62.4 (58.4–66.2) |
Ref |
| 25–30 | 217 | 36.5 |
36.1 (31.5–41.0) |
377 | 63.5 |
63.9 (59–68.5) |
0.9 (0.7–1.1) |
| 31–38 | 99 | 40.6 |
42.6 (36.4–49.0) |
145 | 59.4 |
57.4 (51.0–63.6) |
1.3 (0.9–1.8) |
| Sex † , ‡ | |||||||
| Female | 334 | 41.8 |
42.4 (38.9–46.0) |
466 | 58.3 |
57.6 (54.0–61.1) |
1.6 (1.3–2.0) |
| Male | 234 | 34.5 |
37.0 (33.4–40.8) |
444 | 65.5 |
63.0 (59.2–66.6) |
Ref |
| Maternal race and ethnicity † , ‡ | |||||||
| Hispanic | 48 | 44.0 |
41.2 (29.5–54.0) |
61 | 56.0 |
58.8 (46.0–70.5) |
1.2 (0.7–1.8) |
| NH Black | 88 | 44.9 |
41.3 (36.8–46.0) |
108 | 55.1 |
58.7 (54.0–63.2) |
1.6 (1.1–2.2) |
| NH White | 418 | 37.0 |
36.3 (33.4–39.2) |
713 | 63.0 |
63.7 (60.8–66.6) |
Ref |
| Other ¶ | 14 | 33.3 |
38.8 (34.0–43.8) |
28 | 66.7 |
61.2 (56.2–66.0) |
0.8 (0.4–1.6) |
| Noncardiac congenital anomaly † | |||||||
| Yes | 297 | 59.8 |
60.3 (55.8–64.7) |
200 | 40.2 |
39.7 (35.3–44.2) |
2.5 (2.0–3.3) |
| No | 271 | 27.6 |
27.5 (24.7–30.5) |
710 | 72.4 |
72.5 (69.5–75.3) |
Ref |
| Noncardiac birth defects † , § | |||||||
| Yes | 170 | 48.4 |
49.2 (44.0–54.3) |
181 | 51.6 |
50.8 (45.7–56.0) |
14.0 (8.5–23.1) |
| No | 398 | 35.3 |
36.1 (33.3–39.0) |
729 | 64.7 |
63.9 (61.0–66.7) |
Ref |
| Chromosomal anomalies † | |||||||
| Yes | 127 | 87.0 |
86.3 (81.0–90.3) |
19 | 13.0 |
13.7 (9.7–19.0) |
8.4 (6.1–11.6) |
| No | 441 | 33.1 |
34.6 (32.0–37.2) |
891 | 66.9 |
65.4 (62.8–68.0) |
Ref |
| Down syndrome † | |||||||
| Yes | 111 | 88.8 |
87.2 (81.7–91.3) |
14 | 11.2 |
12.8 (8.7–18.3) |
4.0 (3.2–5.1) |
| No | 457 | 33.8 |
35.4 (32.8–38.0) |
896 | 66.2 |
64.6 (62.0–67.2) |
Ref |
| Survey completed by a proxy † | |||||||
| Yes | 210 | 75.8 |
76.3 (71.2–80.8) |
67 | 24.2 |
23.7 (19.2–28.8) |
16.3 (9.2–29.1) |
| No | 358 | 29.8 |
30.8 (28.3–33.5) |
843 | 70.2 |
69.2 (66.5–71.7) |
Ref |
aOR indicates adjusted odds ratio; CHD, congenital heart defect; CH STRONG, Congenital Heart Survey to Recognize Outcomes, Needs, and Well‐Being; and NH, non‐Hispanic.
Standardized to the CH STRONG eligible sample (n=9312 individuals with CHD in birth defect registries who were not deceased or incarcerated at time of survey) by site, sex, race and ethnicity, birth year, and CHD severity.
Adjusted for CHD severity, age, sex, maternal/race ethnicity, and site.
Model additionally adjusted for noncardiac congenital anomalies.
Model additionally adjusted for chromosomal anomalies.
Other includes non‐Hispanic American Indian or Alaska Native and non‐Hispanic Asian or Pacific Islander.
Standardized HRQOL by Disability Status and Type
In CH STRONG, the standardized mean GPH T‐score (51.9) and GMH T‐score (49.8) among all adults with CHD were comparable to the reference population means (GPH=51.8, GMH=48.5; Figure 2). However, physical and mental HRQOL were lower for those with disabilities; those with ≥1 disability or all of the specific disability types had lower standardized mean GPH or GMH T‐scores compared with those without disabilities (all P<0.001). All mean GPH T‐scores for individuals with disabilities were <47.4, the cutoff for impaired physical HRQOL. Mean GMH T‐scores for those with cognition, mobility, and self‐care disabilities were <43.7, the cutoff for impaired mental HRQOL. Individuals with CHD and mobility disability had the lowest mean scores for both GPH (40.2) and GMH (41.5).
Figure 2. Standardized physical and mental health‐related quality of life of adults with CHD by disability type: CH STRONG (Congenital Heart Survey to Recognize Outcomes, Needs, and Well‐Being), 2016–2019.
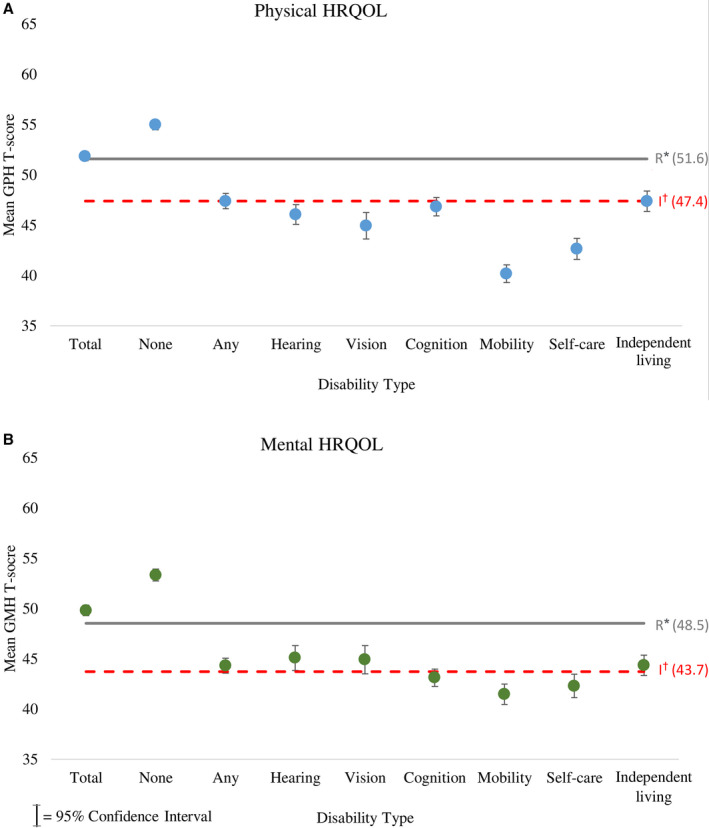
Physical (A) and mental (B) HRQOL T‐scores were standardized to the CH STRONG eligible sample (n=9312) by site, sex, race and ethnicity, birth year, and CHD severity. Mean T‐scores for physical and mental HRQOL among individuals with CHD and disabilities were lower than reference population values, and those with some disabilities fall below the cutoffs for impaired HRQOL. CHD indicates congenital heart defect; GMH, Global Mental Health; GPH, Global Physical Health; and HRQOL, health‐related quality of life. *Reference mean population scores denoted by letter R. †Cutoffs for impaired HRQOL denoted by letter I.
Multivariable Associations With HRQOL by Disability Status and Type
Approximately 14% of the CH STRONG analytic sample reported fair or poor general health and social functioning, 25% reported poor physical HRQOL, and 31% reported poor mental HRQOL (Table 2). Compared with those without disabilities and after adjustment for covariates, individuals with ≥1 disability were more likely to report fair or poor general health (aOR, 5.6; 95% CI, 3.9–7.9), fair or poor social functioning (aOR, 11.4; 95% CI, 7.6–17.0), impaired physical HRQOL (aOR, 7.7; 95% CI, 5.7–10.2), and impaired mental HRQOL (aOR, 7.0; 95% CI, 5.3–9.1). Furthermore, those with each individual disability type had increased odds of fair or poor general health (aORs, 6.1–13.2), fair or poor social functioning (aORs, 9.2–57.0), impaired physical HRQOL (aORs, 7.5–38.8), and impaired mental HRQOL (aORs, 3.5–10.3). While reported associations were strong and statistically significant, in some instances, the 95% confidence intervals were wide (eg, self‐care disability and fair or poor social functioning: aOR=57.0, 95% CI: 26.2‐123.9). After excluding those with noncardiac anomalies or proxy report, the association between impaired mental HRQOL and vision disability as well as the associations between fair or poor social functioning with vision and hearing disabilities were attenuated toward the null. No other associations substantially changed.
Table 2.
Adjusted Odds Ratios of Reduced Health‐Related Quality of Life by Disability Type Among Adults With Congenital Heart Defects: CH STRONG, 2016–2019
| Fair or poor | Fair or poor | Impaired* physical | Impaired † mental | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General health | Social functioning | HRQOL | HRQOL | |||||||||
| Characteristic | n | % |
aOR ‡ (95% CI) |
n | % |
aOR ‡ (95% CI) |
n | % |
aOR ‡ (95% CI) |
n | % |
aOR ‡ (95% CI) |
| Total | 209 | 14.1 | 212 | 14.3 | 367 | 24.8 | 461 | 31.2 | ||||
| Disability type | ||||||||||||
| None | 59 | 6.5 | Ref | 34 | 3.7 | Ref | 101 | 11.1 | Ref | 150 | 16.5 | Ref |
| Any | 150 | 26.4 |
5.6 (3.9–7.9) |
178 | 31.3 |
11.4 (7.6–17.0) |
266 | 46.8 |
7.7 (5.7–10.2) |
311 | 54.8 |
7.0 (5.3–9.1) |
| Hearing | 25 | 31.6 |
9.1 (4.6–17.7) |
23 | 29.1 |
9.2 (4.5–18.7) |
43 | 54.4 |
10.2 (5.8–18.1) |
33 | 41.8 |
3.5 (2.1–6.0) |
| Vision | 26 | 33.3 |
6.6 (3.5–12.4) |
26 | 33.3 |
9.9 (5.1–19.5) |
40 | 51.3 |
7.5 (4.3–13.0) |
34 | 43.6 |
3.7 (2.2–6.2) |
| Cognition | 117 | 28.2 |
6.1 (4.2–8.9) |
142 | 34.2 |
13.8 (9.0–21.0) |
207 | 49.9 |
8.6 (6.3–11.7) |
247 | 59.5 |
8.2 (6.1–11.0) |
| Mobility | 70 | 45.8 |
12.6 (7.9–20.2) |
72 | 47.1 |
21.4 (12.8–35.9) |
127 | 83.0 |
38.8 (23.2–65.0) |
103 | 67.3 |
10.3 (6.8–15.6) |
| Self‐care | 39 | 40.6 |
13.2 (7.0–24.9) |
54 | 56.3 |
57.0 (26.2–123.9) |
76 | 79.2 |
36.7 (19.2–69.9) |
60 | 62.5 |
8.5 (5.1–14.2) |
| Living independently | 78 | 25.2 |
6.3 (4.1–9.8) |
118 | 38.2 |
19.9 (12.3–32.1) |
147 | 47.6 |
9.8 (6.7–14.3) |
170 | 55.0 |
7.8 (5.5–11.0) |
aOR indicates adjusted odds ratio; CH STRONG, Congenital Heart Survey to Recognize Outcomes, Needs, and Well‐Being; and HRQOL, health‐related quality of life.
Defined has having a Global Physical Health T‐score<47.4, representing half a standard deviation below the mean of the general population.
Defined has having a Global Mental Health T‐score<43.7, representing half a SD below the mean of the general population.
Adjusted for CHD severity, age, sex, maternal/race ethnicity, site, and noncardiac congenital anomalies.
Disability Benefits by Work and Defect Type
Among the standardized CH STRONG sample with ≥1 disability, 45% reported ever receiving disability benefits and 21% reported ever being denied disability benefits. Among the disability types, those with self‐care disabilities had both the highest percentage who received disability benefits (63%) and lowest percentage who were ever denied disability benefits (18%). Those with cognitive disabilities had the lowest percentage who ever received disability benefits (46%), and those with mobility disabilities had the highest percentage who were ever denied disability benefits (25%).
Additionally, among the 55% of individuals with single‐ventricle defects who reported ≥1 disability and whose defect may qualify them for disability benefits under Compassionate Allowance, 19 58% reported ever receiving disability benefits, and 30% reported ever being denied disability benefits. About 29% of those with ≥1 disability reported having any full‐time work in the past 12 months (of whom 16% ever received disability benefits); 24% reported part‐time work only (of whom 48% ever received disability benefits); and 46% reported having no work in the past 12 months (of whom 61% ever received disability benefits).
Discussion
In this population‐based survey of young adults with CHD, 40% had ≥1 disability, and disabilities were up to 8 times more prevalent among adults with CHD compared with adults in the general population. The increased prevalence of ≥1 disability was still apparent among young adults with CHD without chromosomal anomalies, ranging from 26% to 35%. Among young adults with CHD reporting disabilities, a little less than half had received disability benefits; 1 in 5 had been denied disability benefits; and a little less than half reported not working in the past 12 months. When stratifying by disability status, those with CHD who also had a disability experienced significantly poorer HRQOL compared with those with CHD without disabilities. We further found that young adults with CHD and cognition, mobility, and self‐care disabilities had impaired mental HRQOL and those with any disability type had impaired physical HRQOL.
Heart trouble has been identified as the third‐most‐common self‐reported cause of disability among adults in the United States, though it is unclear what proportions were caused by acquired cardiovascular conditions other than CHD. 20 Previous studies have more specifically reported that adults with CHD experience cognitive disability more commonly than those without CHD. 4 , 5 One study among 337 adult patients at CHD clinics found that one‐third had significant neurocognitive deficits, similar to 29% reporting cognitive disabilities in CH STRONG. 6 To date, only 3 Dutch cohort studies have investigated mobility limitations among adults with CHD; 1 found adults with CHD had reduced gross motor functioning relative to the general population. 21 , 22 , 23 Even among young children with Down syndrome, those with a co‐occurring CHD were more likely to have greater deficits in motor development than those with Down syndrome without CHD. 24
To our knowledge, little to no information has been published on associations between CHD and difficulty hearing, seeing, or living independently, like those in CH STRONG, even after excluding individuals with known chromosomal anomalies. However, Riehle‐Colarusso et al 25 found that special education service use for vision and hearing impairments was 4 times more common among children with CHD (excluding other birth defects or known syndromes) than children without birth defects.
To our knowledge, this analysis is the first to show an association between disability and non‐Hispanic Black maternal race and ethnicity among adults with CHD. Among individuals with CHD, disparities in survival and other health characteristics by race have been documented. 26 , 27 , 28 , 29 , 30 Specifically, studies have found that non‐Hispanic Black individuals with CHD experience higher mortality than non‐Hispanic Whites, 26 , 27 , 28 , 29 and they are more likely to be hospitalized for infective endocarditis as adults. 30 Authors suggest these differences may be related to access to health care, socioeconomic factors, comorbidities, timely diagnosis, or differences in severity of the lesions. 26 , 27 , 29 While disability is associated with less access to health care in the general population, 31 , 32 , 33 having a disability was associated with receipt of more recent cardiac care among our population‐based sample of young adults with CHD, even after adjusting for CHD severity. Individuals with CHD who also have disabilities may have more interaction with healthcare systems because of more complex health needs or a perception of poorer health. Their perception of health or the referrals from other healthcare providers may prompt these individuals to seek cardiac care more frequently. Supporting this hypothesis, research by Gurvitz et al 34 identified that the most common reasons why cardiac patients do not seek regular cardiac care include feeling well, being unaware that follow‐up was required, and complete absence of medical care. Among young adults with CHD, we also found disability to be associated with female sex and presence of noncardiac congenital anomalies.
In our analysis, those with disabilities had worse HRQOL compared with the US general population, whereas those without disabilities had better physical and mental HRQOL. Only 1 clinic‐based study investigating HRQOL among 74 adolescents and adults with CHD stratified by physical limitations. 35 Similar to our analysis, those with physical limitations had reduced physical and psychological HRQOL compared with those without. Additionally, among >4000 patients with CHD enrolled in the APPROACH‐IS (Assessment of Patterns of Patient‐Reported Outcomes in Adults With Congenital Heart Disease–International Study), poor quality of life was most often observed among those who were job seeking, unemployed, or disabled, though disability was not defined or analyzed apart from employment status, and quality of life is a more broad measure than HRQOL. 36
Study Limitations
For this analysis, self‐report serves as both a strength and a limitation—an individual’s perceptions, beyond objective health measurements, can significantly impact their health outcomes and success of their health care and management. 37 However, self‐report is subjective, and report on disability types and benefits were not clinically or administratively confirmed. Furthermore, data to distinguish between short‐term and permanent disabilities were not available. Classification of CHD severity was limited to diagnosis coding because data on CHD functional class at time of survey were not available. CH STRONG had a 24% survey response rate, which differed by maternal race and ethnicity. 9 To increase representativeness, we standardized our analytic sample to the CH STRONG eligible population (including nonrespondents) by site, sex, birth year, maternal race and ethnicity, and CHD severity when estimating disability prevalence and mean GPH and GMH T‐scores. Additionally, to increase the validity of comparisons to ACS, ACS data among similarly aged participants were standardized to the site and sex distribution of the CH STRONG eligible population. The CH STRONG sample was derived from individuals identified with CHD in early childhood and findings may not be generalizable to young adults whose CHD was identified later in life. Approximately 11% of CH STRONG participants had missing data and were excluded, possibly underestimating the percentage with disabilities by 0.7%.
Conclusions
In a population‐based sample of young adults with CHD in and out of cardiac care, we found 5 to 8 times higher prevalence of all disabilities relative to young adults in the US general population, even when excluding those with other noncardiac anomalies. Among those with CHD, non‐Hispanic Black adults were 60% more likely to have a disability compared with non‐Hispanic White adults. Furthermore, adults with CHD and ≥1 disability had worse physical and mental HRQOL than those without disabilities and almost half reported not working in the past 12 months. To improve the health and wellness of people with disabilities, the US Surgeon General released a Call to Action in 2005 with 4 goals: (1) improve public understanding that persons with disabilities can lead long, healthy, productive lives; (2) improve provider training and capacity to treat the whole person and not just singular needs; (3) promote health and wellness for people with disabilities; and (4) provide access to health care and support services. 38 Implementing policies and practices to recognize and support those with disability within the general CHD community may lead to better connection and usage of resources and, ultimately, improved health and well‐being.
Sources of Funding
None.
Disclosures
None.
Supporting information
Table S1–S3
Figure S1–S2
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This analysis has been replicated by Kristine MaWhinney and Brittany Wright.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022440
For Sources of Funding and Disclosures, see page 11.
References
- 1. Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle‐Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101–109. doi: 10.1161/CIRCULATIONAHA.115.019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122:2264–2272. doi: 10.1161/CIRCULATIONAHA.110.946343 [DOI] [PubMed] [Google Scholar]
- 3. Foster E, Graham TP, Driscoll DJ, Reid GJ, Reiss JG, Russell IA, Sermer M, Siu SC, Uzark K, Williams RG, et al. Task force 2: special health care needs of adults with congenital heart disease. J Am Coll Cardiol. 2001;37:1176–1183. doi: 10.1016/S0735-1097(01)01277-3 [DOI] [PubMed] [Google Scholar]
- 4. Cohen S, Earing MG. Neurocognitive impairment and its long‐term impact on adults with congenital heart disease. Prog Cardiovasc Dis. 2018;61:287–293. doi: 10.1016/j.pcad.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 5. Tyagi M, Austin K, Stygall J, Deanfield J, Cullen S, Newman SP. What do we know about cognitive functioning in adult congenital heart disease? Cardiol Young. 2014;24:13–19. doi: 10.1017/S1047951113000747 [DOI] [PubMed] [Google Scholar]
- 6. Brunmeier A, Reis MP, Earing MG, Umfleet L, Ginde S, Bartz PJ, Cohen S. Identifying self‐reported neurocognitive deficits in the adult with congenital heart disease using a simple screening tool. Congenit Heart Dis. 2018;13:728–733. doi: 10.1111/chd.12646 [DOI] [PubMed] [Google Scholar]
- 7. Belmont JW, Craigen W, Martinez H, Jefferies JL. Genetic disorders with both hearing loss and cardiovascular abnormalities. Adv Otorhinolaryngol. 2011;70:66–74. [DOI] [PubMed] [Google Scholar]
- 8. Mansour AM, Bitar FF, Traboulsi EI, Kassak KM, Obeid MY, Megarbane A, Salti HI. Ocular pathology in congenital heart disease. Eye (Lond). 2005;19:29–34. doi: 10.1038/sj.eye.6701408 [DOI] [PubMed] [Google Scholar]
- 9. Farr SL, Klewer SE, Nembhard WN, Alter C, Downing KF, Andrews JG, Collins RT, Glidewell J, Benavides A, Goudie A, et al. Rationale and design of CH STRONG: Congenital Heart Survey To Recognize Outcomes, Needs, and well‐beinG. Am Heart J. 2020;221:106–113. doi: 10.1016/j.ahj.2019.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glidewell J, Book W, Raskind‐Hood C, Hogue C, Dunn JE, Gurvitz M, Ozonoff A, McGarry C, Van Zutphen A, Lui G, et al. Population‐based surveillance of congenital heart defects among adolescents and adults: surveillance methodology. Birth Defects Res. 2018;110:1395–1403. doi: 10.1002/bdr2.1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Office of Management and Budget . Revisions to the standards for the classification of federal data on race and ethnicity, Federal Register: 62: No. 210, October 30, 1997.
- 12. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the Patient‐Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18:873–880. doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henderson JR II, Kiernan E, McNeer JL, Rodday AM, Spencer K, Henderson TO, Parsons SK. Patient‐reported health‐related quality‐of‐life assessment at the point‐of‐care with adolescents and young adults with cancer. J Adolesc Young Adult Oncol. 2018;7:97–102. doi: 10.1089/jayao.2017.0046 [DOI] [PubMed] [Google Scholar]
- 14. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health‐related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C [DOI] [PubMed] [Google Scholar]
- 15. Sloan J, Symonds T, Vargas‐Chanes D, Fridley B. Practical guidelines for assessing the clinical significance of health‐related quality of life changes within clinical trials. Drug Inf J. 2003;37:23–31. doi: 10.1177/009286150303700105 [DOI] [Google Scholar]
- 16. Jun S, Cowan AE, Bhadra A, Dodd KW, Dwyer JT, Eicher‐Miller HA, Gahche JJ, Guenther PM, Potischman N, Tooze JA, et al. Older adults with obesity have higher risks of some micronutrient inadequacies and lower overall dietary quality compared to peers with a healthy weight, National Health and Nutrition Examination Surveys (NHANES), 2011–2014. Public Health Nutr. 2020;23:2268–2279. doi: 10.1017/S1368980020000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stone CL. A population‐based measure of chronic disease severity for health planning and evaluation in the United States. AIMS Public Health. 2020;7:44–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheung KL, Ten Klooster PM, Smit C, de Vries H, Pieterse ME. The impact of non‐response bias due to sampling in public health studies: a comparison of voluntary versus mandatory recruitment in a Dutch national survey on adolescent health. BMC Public Health. 2017;17:276. doi: 10.1186/s12889-017-4189-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Social Security Administration . Compassionate allowance information ‐ single ventricle. Program Operations Manual System (POMS). 2020.
- 20. Prevalence and most common causes of disability among adults‐‐United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 21. Bol Raap G, Meijboom FJ, Kappetein AP, Galema TW, Yap SC, Bogers AJ. Long‐term follow‐up and quality of life after closure of ventricular septal defect in adults. Eur J Cardiothorac Surg. 2007;32:215–219. doi: 10.1016/j.ejcts.2007.04.023 [DOI] [PubMed] [Google Scholar]
- 22. Kamphuis M, Ottenkamp J, Vliegen HW, Vogels T, Zwinderman KH, Kamphuis RP, Verloove‐Vanhorick SP. Health related quality of life and health status in adult survivors with previously operated complex congenital heart disease. Heart. 2002;87:356–362. doi: 10.1136/heart.87.4.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vandekerckhove KD, Blom NA, Lalezari S, Koolbergen DR, Rijlaarsdam ME, Hazekamp MG. Long‐term follow‐up of arterial switch operation with an emphasis on function and dimensions of left ventricle and aorta. Eur J Cardiothorac Surg. 2009;35:582–588. doi: 10.1016/j.ejcts.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 24. Visootsak J, Mahle WT, Kirshbom PM, Huddleston L, Caron‐Besch M, Ransom A, Sherman SL. Neurodevelopmental outcomes in children with Down syndrome and congenital heart defects. Am J Med Genet A. 2011;155:2688–2691. doi: 10.1002/ajmg.a.34252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riehle‐Colarusso T, Autry A, Razzaghi H, Boyle CA, Mahle WT, Van Naarden BK, Correa A. Congenital heart defects and receipt of special education services. Pediatrics. 2015;136:496–504. doi: 10.1542/peds.2015-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez KN, Morris SA, Sexson Tejtel SK, Espaillat A, Salemi JL. US mortality attributable to congenital heart disease across the lifespan from 1999 through 2017 exposes persistent racial/ethnic disparities. Circulation. 2020;142:1132–1147. doi: 10.1161/CIRCULATIONAHA.120.046822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boneva Roumiana S, Botto Lorenzo D, Moore Cynthia A, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States. Circulation. 2001;103:2376–2381. doi: 10.1161/01.CIR.103.19.2376 [DOI] [PubMed] [Google Scholar]
- 28. Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nembhard WN, Salemi JL, Ethen MK, Fixler DE, DiMaggio A, Canfield MA. Racial/ethnic disparities in risk of early childhood mortality among children with congenital heart defects. Pediatrics. 2011;127:e1128–e1138. doi: 10.1542/peds.2010-2702 [DOI] [PubMed] [Google Scholar]
- 30. Egbe AC, Vallabhajosyula S, Akintoye E, Connolly HM. Trends and outcomes of infective endocarditis in adults with tetralogy of Fallot: a review of the national inpatient sample database. Can J Cardiol. 2019;35:721–726. doi: 10.1016/j.cjca.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 31. Reichard A, Stolzle H, Fox MH. Health disparities among adults with physical disabilities or cognitive limitations compared to individuals with no disabilities in the United States. Disabil Health J. 2011;4:59–67. doi: 10.1016/j.dhjo.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 32. Okoro CA, Hollis ND, Cyrus AC, Griffin‐Blake S. Prevalence of disabilities and health care access by disability status and type among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:882–887. doi: 10.15585/mmwr.mm6732a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matin BK, Williamson HJ, Karyani AK, Rezaei S, Soofi M, Soltani S. Barriers in access to healthcare for women with disabilities: a systematic review in qualitative studies. BMC Womens Health. 2021;21:44. doi: 10.1186/s12905-021-01189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, Ting J, Kuehl K, Earing M, Webb G, et al. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART‐ACHD (the Health, Education, and Access Research Trial). J Am Coll Cardiol. 2013;61:2180–2184. doi: 10.1016/j.jacc.2013.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Teixeira FM, Coelho RM, Proenca C, Silva AM, Vieira D, Vaz C, Moura C, Viana V, Areias JC, Areias ME. Quality of life experienced by adolescents and young adults with congenital heart disease. Pediatr Cardiol. 2011;32:1132–1138. doi: 10.1007/s00246-011-0039-0 [DOI] [PubMed] [Google Scholar]
- 36. Apers S, Kovacs AH, Luyckx K, Thomet C, Budts W, Enomoto J, Sluman MA, Wang J‐K, Jackson JL, Khairy P, et al. Quality of life of adults with congenital heart disease in 15 countries: evaluating country‐specific characteristics. J Am Coll Cardiol. 2016;67:2237–2245. doi: 10.1016/j.jacc.2016.03.477 [DOI] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention . Health‐related quality of life (HRQOL). 2018.
- 38. The surgeon general’s call to action to improve the health and wellness of persons with disabilities. 2005. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3
Figure S1–S2


