Abstract
Background
Atrial fibrillation (AF) commonly occurs in the setting of acute conditions. We aimed to identify the acute conditions associated with secondary AF (AF precipitants) including pneumonia/sepsis, pneumothorax, respiratory failure, myocarditis, pericarditis, alcohol intoxication, thyrotoxicosis, cardiothoracic surgery, other surgery in patients with newly diagnosed AF and determine their association with subsequent oral anticoagulant use.
Methods and Results
We assembled a cohort of patients in the UMass Memorial Healthcare system with a new diagnosis of AF with and without AF precipitants. We used combinations of International Classification of Diseases, Tenth Revision (ICD‐10) codes, Current Procedural Terminology codes, laboratory values, imaging reports, and physician notes including discharge summary texts to identify AF precipitants. We then manually reviewed the individual charts to validate presence of AF precipitants. The study sample consisted of 185 patients with and 172 patients without AF precipitants. Pneumonia/sepsis, myocardial infarction, respiratory failure, and cardiothoracic surgery were the most common precipitants identified. In multivariable analyses adjusting for age, sex, patient comorbidities, left atrial enlargement, left ventricular ejection fraction, and antiplatelet use, patients with AF precipitants were less likely to receive subsequent anticoagulation therapy at 30 days after the initial AF diagnosis (odds ratio, 0.31; 95% CI, 0.19–0.52). The association was persistent after excluding men with CHA2DS2‐VASc score <2 and women with CHA2DS2‐VASc score <3.
Conclusions
Our study highlights lower usage of oral anticoagulant in secondary AF in contemporary clinical practice.
Keywords: anticoagulants, atrial fibrillation, ischemic stroke
Subject Categories: Atrial Fibrillation
Over 5 million Americans have atrial fibrillation (AF), with 12 million projected by 2030. 1 AF is an important ischemic stroke risk factor, increasing the risk 5‐fold and accounting for 1 in 4 ischemic strokes. 2 The presence of AF at the time of ischemic stroke is associated with increased stroke severity, mortality, and risk of recurrence. 3 Nevertheless, in a national outpatient registry of patients with AF, only 60% received any oral anticoagulant (OAC). 4
Approximately one‐third of new AF cases occur in the setting of acute, “reversible” causes 5 (hereafter referred to as secondary AF). Acute conditions associated with secondary AF include pulmonary embolism, sepsis, acute coronary syndrome, myocarditis, pericarditis, thyrotoxicosis, cardiothoracic surgery, and alcohol intoxication (hereafter referred to as AF precipitants). 5 , 6 , 7 AF occurring in the setting of acute conditions was previously thought to be a self‐limited event that is potentially curable with elimination of the underlying cause. 8 However, there is now a considerable body of evidence demonstrating that the AF will likely recur even after elimination of the inciting event. 5 , 7 Importantly, secondary AF is associated with elevated risk of stroke, 5 , 7 and the risk may be similar to the stroke risk in AF without precipitants. 5 In a major departure from previous guidelines, 8 the 2014 US AF guidelines stated that patients with secondary AF require long‐term follow‐up to evaluate for recurrence. The guidelines also highlighted knowledge gaps in long‐term outcomes and the role of OAC in secondary AF. 6 An assessment of current OAC utilization in relation to presence of AF precipitants is a critical, initial step to understanding current prescribing patterns and potential targets for future intervention.
In the current study, we assembled a sample of patients with new‐onset AF with and without AF precipitants using previously developed electronic health record (EHR) algorithm 9 and manual validation. We then measured the association between presence of secondary precipitants and subsequent OAC use.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary file.
Study Sample
We assembled a sample of patients from within the UMass Memorial Healthcare (UMMHC) system with a new diagnosis of AF occurring between April 1, 2018, and December 31, 2019. UMass Memorial Healthcare serves central Massachusetts and has a catchment area of over 1 million lives. We chose the start date of the study period to allow a 6‐month lead‐in period after the Epic Systems EHR went live at UMass Memorial Healthcare. The lead‐in period was necessary to allow providers to enter preexisting diagnoses into the patients’ records. Patients were eligible if we detected the International Classification of Diseases, Tenth Revision (ICD‐10) code I48 for AF or identified AF as a text string (“A. Fib,” “atrial fibrillation,” “afib,” “atrial flutter,” “A. Fib”) in the ECG report, echocardiogram report, or clinician notes (ie, history and physical, progress notes, discharge summaries, emergency room provider notes). We designated the date associated with first AF diagnosis code or text string of AF as the index date. We validated the index date by manual chart review and excluded those who had AF before the index encounter or did not have AF. We also excluded patients with valvular AF (ie, mitral stenosis, rheumatic heart disease of the aortic or mitral valve, and any prosthetic valve), those with incomplete information on demographics or body mass index, those who died within 30 days of AF onset, and those already on anticoagulation therapy at the time of the AF diagnosis. Furthermore, we excluded patients who were diagnosed with definite or possible left ventricular (LV) thrombus or pulmonary embolism at the time of AF diagnosis, as they would have been prescribed an OAC independent of AF. The Figure illustrates patient selection process for the study sample.
Figure 1. Flow diagram of study sample selection.
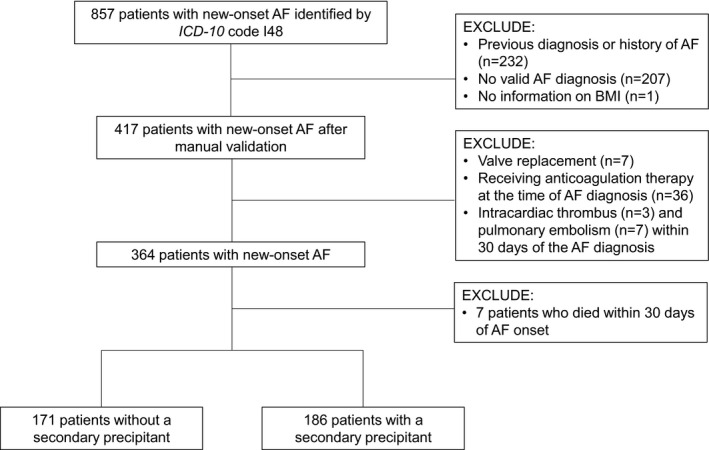
AF indicates atrial fibrillation; BMI, body mass index; and ICD‐10, International Classification of Diseases, 10th Revision.
Outcome
We ascertained OAC use within 30 days after the AF diagnosis by manually reviewing the medication list, physician notes, and discharge summaries.
Independent Variable
We determined presence of an AF precipitant at the time of the AF diagnosis using the EHR algorithms developed by Wang et al. 9 The algorithms use combinations ICD‐10 codes, Current Procedural Terminology codes, laboratory values, imaging reports, and physician notes including discharge summary texts. 9 We searched for presence of the following AF precipitants: pneumothorax, other respiratory disease or failure, sepsis (including pneumonia), myocardial infarction (MI), myocarditis, pericarditis, thyrotoxicosis, cardiothoracic surgery, other surgery, and alcohol intoxication. If any of the algorithms above identified an AF precipitant, we assigned AF as associated with an AF precipitant if it occurred 30 days before, including day of AF diagnosis. We then manually reviewed the patient charts for the entire AF sample to validate presence of an AF precipitant associated with new‐onset AF. We assessed performance of the Wang et al algorithms in our UMass sample.
Covariates: Demographics, Patient Conditions, Echocardiographic Data, and Medication Use
We identified baseline medical comorbidities from the problem list and associated ICD‐10 codes in the EHR for each patient from the encounter associated with the index date. If the information was missing from the index encounter, we used the next closest encounter before the index encounter. We calculated CHA2DS2‐VASc score by adding 1 point for congestive heart failure, hypertension, age ≥65 years, diabetes, vascular disease, and female sex, and 2 points for history of stroke or transient ischemic attack and age ≥75 years. We obtained left atrial (LA) size and LV ejection fraction from echocardiogram reports obtained within 1 year before and 30 days after the first AF diagnosis. We assigned LA size indexed to body surface area (mL/m2) available as structural data to define LA enlargement (>34 mL/m2). 10 When LA size was not available, we searched the summary finding for mention of LA enlargement (ie, “mildly,” “moderately,” “severely” enlarged). We also collected information about LV ejection fraction. When there was a range of ejection fraction reported (eg, 50%–55%), we used the midpoint of the range. (eg, 52.5%). Finally, we determined antiplatelet use within 12 months before the AF diagnosis through electronic capture of the medication list of the most proximal encounter preceding the index date. Selection of the most proximal encounter before AF diagnosis to define antiplatelet use enabled us to distinguish patients starting or discontinuing antiplatelet use during the index encounter from those with prior use. 11
Statistical Analysis
We compared the baseline characteristics between patients with and without an AF precipitant using the t test for continuous variables and the chi‐square test for the categorical variables. We calculated sensitivity, specificity, positive predictive value, and negative predictive value for the AF precipitant algorithms. To determine association between presence of an AF precipitant and initiation of OAC, we constructed 3 multivariable logistic regression models adjusting for (1) age and sex; (2) variables in model 1 plus body mass index, comorbidities (heart failure, diabetes, hypertension, stroke/transient ischemic attack, coronary artery disease, valvular disease, peripheral vascular disease, anemia, and chronic kidney disease); and (3) variables in model 2 plus LV ejection fraction, LA enlargement, and antiplatelet use. In exploratory analyses, we performed subgroup analyses to determine associations between the individual AF precipitants and initiation of an OAC. For these analyses, we permitted patients to contribute to >1 precipitant type. In addition, we performed exploratory analyses excluding men with CHA2DS2‐VASc score <2 and women with CHA2DS2‐VASc score <3 and patients with new diagnosis of AF in the setting of MI.
All analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC). The University of Massachusetts Medical School Institutional Review Board approved the conduct of our study.
RESULTS
Identification of Study Sample and AF Precipitants
Of 857 patients with a new ICD‐10 code for AF, 417 patients had a valid AF diagnosis and complete data on body mass index after manual validation (Figure). Our final sample consisted of 357 patients, 185 (52%) of whom were diagnosed with at least 1 AF precipitant associated with new‐onset AF. Of the 185 patients with a precipitant, 54 (29%) patients experienced ≥2 precipitants. Respiratory failure, sepsis (including pneumonia), MI, and cardiothoracic surgery were the most common precipitants identified (Table S1).
The electronic algorithms by Wang et al 9 to identify at least 1 AF precipitant had the following diagnostic performance in UMass EHR—89% (95% CI, 84–93) positive predictive value, 83% (78–87) negative predictive value, 82% (77–88) sensitivity, and 90% (83–93) specificity. Algorithm performance varied considerably for individual precipitants (Table S1). Because we permitted assignment of >1 precipitant per patient, the resultant sensitivity of each discrete precipitant was less than that for the composite definition of any one precipitant. Concomitantly, the range of specificity was higher for the individual precipitants compared with the composite.
Demographics, Patient Conditions, Echocardiographic Data, and Medication Use
The overall mean age of the sample was 69 years (±14.9), 44% were women, and 93% were White (Table 1). Approximately two‐thirds of patients with new‐onset AF were evaluated by a cardiologist in either an inpatient or outpatient setting within 30 days of the AF diagnosis. Patients with precipitants had greater prevalence of heart failure (31.4% versus 21.5%; P=0.04) and coronary artery disease (33.5% versus 22.1%; P=0.02), and higher mean CHA2DS2‐VASc score (3.8 versus 3.2; P=0.003). Among patients with an echocardiogram available (n=280, 78% of the sample), patients with precipitants were less likely to have normal LV ejection fraction compared with those without (73% versus 84%; P=0.03). There was no significant difference in LA size (n=279) between the 2 groups. Finally, there was no significant difference in antiplatelet use between the 2 patient groups.
Table 1.
Baseline Characteristics of AF Patients With and Without Secondary Precipitants
| Characteristics | Patients with precipitant frequency (% of 185) | Patients without precipitant frequency (% of 172) | P value |
|---|---|---|---|
| Demographics | |||
| Age at AF diagnosis, y | |||
| ≥75 | 77 (41.6) | 60 (34.9) | 0.14 |
| 65–74 | 56 (30.3) | 47 (27.3) | |
| <65 | 52 (28.1) | 65 (37.8) | |
| Mean | 70.7±13.7 | 68.0±16.1 | 0.10 |
| Female sex | 82 (44.3) | 74 (43.0) | 0.80 |
| Non‐White race or Hispanic ethnicity | 11 (6.2) | 13 (7.8) | 0.54 |
| Patient conditions | |||
| Body mass index, kg/m2 | |||
| <18.5 | 2 (1.1) | 2 (1.2) | 0.90 |
| 18.5–24.9 | 40 (21.6) | 32 (18.6) | |
| 25–29.9 | 63 (34.1) | 58 (33.7) | |
| >30 | 80 (43.2) | 80 (46.5) | |
| Congestive heart failure | 59 (31.9) | 37 (21.5) | 0.03 |
| Hypertension | 146 (78.9) | 130 (75.6) | 0.45 |
| Diabetes | 59 (31.9) | 49 (28.5) | 0.48 |
| Stroke/TIA or other systemic embolism | 37 (20.0) | 28 (16.3) | 0.36 |
| Coronary artery disease | 62 (33.5) | 38 (22.1) | 0.02 |
| Valvular disease | 14 (7.6) | 17 (9.9) | 0.44 |
| Peripheral vascular disease | 25 (13.5) | 17 (9.9) | 0.29 |
| Anemia | 21 (11.4) | 13 (7.6) | 0.22 |
| Chronic kidney disease* | |||
| Stages 1–3 | 158 (91.3) | 124 (93.9) | 0.39 |
| Stages 4–5 | 15 (8.7) | 8 (6.1) | |
| CHA2DS2‐VASc score | |||
| Mean | 3.8±2.0 | 3.2±2.0 | 0.002 |
| ≥2 for men and ≥3 for women | 155 (83.8) | 119 (69.2) | 0.001 |
| Smoking history | 122 (66.0) | 104 (60.8) | 0.32 |
| Echographic data (n=280) | |||
| LV ejection fraction ≥50% (n=277) | 115 (73.3) | 101 (84.2) | 0.03 |
| LA enlargement (n=279) | 73 (46.2) | 63 (52.1) | 0.33 |
| Other characteristics | |||
| Antiplatelet use (n=260) | 81 (43.8) | 67 (39.0) | 0.53 |
| Anticoagulant use after AF diagnosis | 75 (40.5) | 105 (61.1) | <0.001 |
| Warfarin | 23 (30.7) | 16 (15.2) | 0.01 |
| DOAC | 52 (69.3) | 89 (84.8) | |
| Cardiology consultation † | 117 (63.2) | 117 (68.0) | 0.34 |
Values are n (%) or mean±SD. AF indicates atrial fibrillation; DOAC, direct oral anticoagulant; LA, left atrial; LV, left ventricular; and TIA, transient ischemic attack.
Stage determined by calculating creatinine clearance using the Cockcroft‐Gault formula.
Cardiology consultation includes patients for whom a cardiologist served as the primary physician or as a consultant.
Initiation of OAC for Patients With New‐Onset AF
Patients with AF precipitants were less likely to receive OACs for new‐onset AF compared with patients without precipitants (40.5% versus 61.1%; P<0.001). In Table 2 we report adjusted association between presence of an AF precipitant and initiation of an OAC among patients with new‐onset AF. After adjusting for age, sex, and patient conditions (model 2), we observed that presence of any AF precipitant was significantly associated with lower odds of OAC use. The association persisted even after adjusting for LV ejection fraction, presence of LA enlargement, and antiplatelet use at the time of AF diagnosis (model 3). Of the individual precipitants, we found that sepsis, respiratory failure, and noncardiothoracic surgery were associated with lower odds of OAC use.
Table 2.
Association Between Presence of a Secondary Precipitant and Initiation of OAC
| Multivariable models | |||
|---|---|---|---|
|
Model 1—age and sex OR (95% CI) |
Model 2—model 1+patient conditions* OR (95% CI) |
Model 3—model 2+other factors † OR (95% CI) |
|
| Secondary precipitant (n=185) | 0.42 (0.28–0.65) | 0.39 (0.25–0.62) | 0.31 (0.19–0.52) |
| Sepsis § (n=64) | 0.51 (0.29–0.89) | 0.45 (0.25–0.82) | 0.46 (0.25–0.85) |
| Myocardial infarction (n=32) | 1.29 (0.62–2.68) | 1.41 (0.65–3.06) | 1.23 (0.53–2.88) |
| Respiratory failure (n=56) | 0.52 (0.29–0.95) | 0.45 (0.24–0.85) | 0.41 (0.21–0.80) |
| Cardiothoracic surgery (n=23) | 0.73 (0.31–1.71) | 0.76 (0.30–1.94) | 0.69 (0.26–1.87) |
| Other surgery (n=25) | 0.44 (0.18–1.04) | 0.39 (0.16–0.98) | 0.37 (0.14–0.96) |
| Other precipitants ‖ (n=40) | 0.71 (0.36–1.38) | 0.74 (0.37–1.49) | 0.67 (0.32–1.39) |
OAC indicates oral anticoagulant; and OR, odds ratio.
Patient conditions include heart failure, hypertension, diabetes, stroke/transient ischemic attack, coronary artery disease, valvular disease, peripheral vascular disease, anemia, and chronic kidney disease.
Other factors include left ventricular ejection fraction, left atrial enlargement, and antiplatelet use.
Includes pneumonia.
We combined myocarditis, pericarditis, alcohol intoxication, pneumothorax, and thyrotoxicosis into a single category due to their small sample sizes. See Table S1 for the sample size for each secondary precipitant.
When we excluded patients in whom the benefit of stroke prophylaxis is less clear (men with CHA2DS2‐VASc score <2 and women with CHA2DS2‐VASc score <3), the association remained the same (n=274 for the sample, 155 with a secondary precipitant; odds ratio, 0.34, 95% CI, 0.19–0.60). In addition, when we excluded new‐onset AF in the setting of MI, in whom the latest US guideline now recommends stroke prophylaxis, 12 there was even lower odds of OAC prescription (n=328 for the sample, 153 with a secondary precipitant; odds ratio, 0.27; 95% CI, 0.15–0.59).
DISCUSSION
Consistent with the prior studies by Lubitz et al 11 and Gundlund et al, 13 we report that patients with new‐onset AF with AF precipitants are less likely to receive OAC compared with those without a precipitant. The relative lack of OAC prescription for patients with AF in the context of AF precipitants persisted after adjustment for multiple factors that predict risk of stroke including CHA2DS2‐VASc score. Of the individual precipitants, noncardiac illnesses including sepsis, respiratory failure, and noncardiothoracic surgery were significantly associated with lower odds of OAC use. Exclusion of men with CHA2DS2‐VASc score <2 and women with CHA2DS2‐VASc score <3 and patients with MI did not change the association. Our work as well as those of others 11 , 13 suggest that AF occurring in the setting of acute precipitants is perceived to be transient by clinicians and therefore does not require long‐term OAC.
However, the 2014 US AF guidelines state that “sparse data support the notion that patients with AF that occurs in the setting of 1 of these potentially ‘reversible’ conditions are, in fact, cured of AF … ” 6 We now have data from both prospective and retrospective observational studies showing that at least 40% of the cases of AF associated with AF precipitants recur during longitudinal follow‐up. 5 , 7 , 9 Given that about one‐third of new AF cases occur in the setting of AF precipitants, 5 the results from the current study highlight the urgent need to determine the optimal stroke prophylaxis strategy in secondary AF through randomized trials.
Our work distinguishes itself from the other studies 11 , 13 in the level of clinical detail and manual validation of AF and presence of AF precipitants. In addition, we adjusted for patient conditions that influence the risk of stroke and bleeding and adjusted for factors that were not available in the prior studies. These included LV ejection fraction and LA size, echocardiographic features that are associated with increased risk of developing AF. 14 The echocardiographic information can help clinicians decide if new‐onset AF with AF precipitants is preexisting or if it will likely to recur even after removal of the precipitant. Given that evidence shows patients with AF treated by cardiologists are more likely to receive guideline‐concordant care for AF, 15 we also adjusted for involvement of a cardiologist in the patients’ care.
We acknowledge several limitations of our findings. Our study is from a single center. Prescription patterns may differ in different health care settings or other parts of the United States. Our sample size was small, and therefore we could not firmly establish the association of individual precipitants with OAC prescription. We also did not have information on duration of the new‐onset AF, which could have been associated with OAC use. Further, we did not have information on echocardiograms and antiplatelet use for everyone. We also did not measure the use of OACs after 30 days of new‐onset AF. In some cases, providers may investigate the burden of AF using ambulatory cardiac rhythm monitors before prescribing OAC. In the current study, we did not investigate clinical outcomes associated with OAC in secondary AF. Finally, as is the case in observational study design, there is the possibility of residual confounding by an unmeasured factor. Nevertheless, the strength of the association demonstrated in our study confirms a significantly lower prescription of OAC in patients with newly diagnosed with AF in the setting of an acute precipitant. Given the risk of AF recurrence during longitudinal follow‐up in secondary AF, 5 future studies on optimal strategy to manage these patients is warranted.
In conclusion, our study highlights feasibility of using EHR algorithms to identify secondary AF and underscores lower usage of OAC in secondary AF in contemporary clinical practice.
Sources of Funding
Dr Ko is supported by American College of Cardiology Foundation/Merck Research Fellowship in Cardiovascular Diseases and Cardiometabolic Disorders. Dr Benjamin is supported by the National Heart, Lung, and Blood Institute (R01HL092577), American Heart Association AF Strategically Focused Research Network (18SFRN34110082), and American Heart Association Tobacco Regulation and Addiction Center (2U54HL120163). Dr Walkey is supported by the National Heart, Lung, and Blood Institute (R01 HL136660 and R01 HL139751). Dr Lubitz is supported by a National Institutes of Health grant (1R01HL139731) and American Heart Association AF Strategically Focused Research Network (18SFRN34250007). Dr McManus is supported by the National Heart, Lung, and Blood Institute (R01HL137794). This work was supported by an investigator‐initiated industry sponsored research award from Boehringer Ingelheim (BIPI QIE2016001). Boehringer Ingelheim Pharmaceuticals, Inc had no role in the design, analysis, or interpretation of the results in this study; Boehringer Ingelheim Pharmaceuticals, Inc was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to Boehringer Ingelheim Pharmaceuticals, Inc substances, as well as intellectual property considerations.
Disclosures
Dr Lubitz receives sponsored research support from Bristol Myers Squibb/Pfizer, Bayer AG, Boehringer Ingelheim, Fitbit, and IBM, and has consulted for Bristol Myers Squibb/Pfizer, Bayer AG, and Blackstone Life Sciences. Dr McManus reports grants and personal fees from Bristol Myers Squibb, grants and personal fees from Pfizer, grants from Boehringer Ingelheim and Philips, nonfinancial support from Apple, personal fees and nonfinancial support from Samsung, grants and personal fees from Flexcon, personal fees from Avania, personal fees from Rose Consulting, grants and personal fees from Heart Rhythm Society, and personal fees and nonfinancial support from Fitbit, outside the submitted work. Dr Alok Kapoor has received research grant support from Pfizer through its independent grants for learning and change the funding mechanism and from Bristol‐Myers Squibb for Independent Medical Education Grants. More recently, he has received research grant support through a competitive process adjudicated and funded by the alliance, which is formed by both Pfizer and Bristol‐Myers Squibb. He has also been awarded a grant by Pfizer to examine conversations between patients and providers. Z. Wang, C. Saleeba, Q. Shi, H. Sadiq, and Dr Crawford have also received research grant support from Bristol Meyers Squibb and Boehringer Ingelheim in the past 3 years (coinvestigator on the grants secured by Drs Kapoor and McManus). The remaining authors have no disclosures to report.
Supporting information
Table S1
For Sources of Funding and Disclosures, see page 6.
REFERENCES
- 1. Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063 [DOI] [PubMed] [Google Scholar]
- 2. O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao‐Melacini P, Zhang X, Pais P, Agapay S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 3. Lin HJ, Wolf PA, Kelly‐Hayes M, Beiser AS, Kase CS, Benjamin EJ, D'Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.STR.27.10.1760 [DOI] [PubMed] [Google Scholar]
- 4. Marzec LN, Wang J, Shah ND, Chan PS, Ting HH, Gosch KL, Hsu JC, Maddox TM. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. doi: 10.1016/j.jacc.2017.03.540 [DOI] [PubMed] [Google Scholar]
- 5. Lubitz SA, Yin X, Rienstra M, Schnabel RB, Walkey AJ, Magnani JW, Rahman F, McManus DD, Tadros TM, Levy D, et al. Long‐term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131:1648–1655. doi: 10.1161/CIRCULATIONAHA.114.014058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long‐term outcomes following development of new‐onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey J‐Y, Lowe JE, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d [DOI] [PubMed] [Google Scholar]
- 9. Wang EY, Hulme OL, Khurshid S, Weng L‐C, Choi SH, Walkey AJ, Ashburner JM, McManus DD, Singer DE, Atlas SJ, et al. Initial precipitants and recurrence of atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e007716. doi: 10.1161/CIRCEP.119.007716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 11. Lubitz SA, Khurshid S, Weng L‐C, Doros G, Keach JW, Gao QI, Gehi AK, Hsu JC, Reynolds MR, Turakhia MP, et al. Predictors of oral anticoagulant non‐prescription in patients with atrial fibrillation and elevated stroke risk. Am Heart J. 2018;200:24–31. doi: 10.1016/j.ahj.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 13. Gundlund A, Kümler T, Bonde AN, Butt JH, Gislason GH, Torp‐Pedersen C, Køber L, Olesen JB, Fosbøl EL. Comparative thromboembolic risk in atrial fibrillation with and without a secondary precipitant‐Danish nationwide cohort study. BMJ Open. 2019;9:e028468. doi: 10.1136/bmjopen-2018-028468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suissa L, Bertora D, Lachaud S, Mahagne MH. Score for the targeting of atrial fibrillation (STAF): a new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009;40:2866–2868. doi: 10.1161/STROKEAHA.109.552679 [DOI] [PubMed] [Google Scholar]
- 15. Barnett AS, Kim S, Fonarow GC, Thomas LE, Reiffel JA, Allen LA, Freeman JV, Naccarelli G, Mahaffey KW, Go AS, et al. Treatment of atrial fibrillation and concordance With the American Heart Association/American College of Cardiology/Heart Rhythm Society Guidelines: findings from ORBIT‐AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation). Circ Arrhythm Electrophysiol. 2017;10:e005051. doi: 10.1161/CIRCEP.117.005051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


