Abstract
Background
The National Patient‐Centered Clinical Research Network Blood Pressure Control Laboratory Surveillance System was established to identify opportunities for blood pressure (BP) control improvement and to provide a mechanism for tracking improvement longitudinally.
Methods and Results
We conducted a serial cross‐sectional study with queries against standardized electronic health record data in the National Patient‐Centered Clinical Research Network (PCORnet) common data model returned by 25 participating US health systems. Queries produced BP control metrics for adults with well‐documented hypertension and a recent encounter at the health system for a series of 1‐year measurement periods for each quarter of available data from January 2017 to March 2020. Aggregate weighted results are presented overall and by race and ethnicity. The most recent measurement period includes data from 1 737 995 patients, and 11 956 509 patient‐years were included in the trend analysis. Overall, 15% were Black, 52% women, and 28% had diabetes. BP control (<140/90 mm Hg) was observed in 62% (range, 44%–74%) but varied by race and ethnicity, with the lowest BP control among Black patients at 57% (odds ratio, 0.79; 95% CI, 0.66–0.94). A new class of antihypertensive medication (medication intensification) was prescribed in just 12% (range, 0.6%–25%) of patient visits where BP was uncontrolled. However, when medication intensification occurred, there was a large decrease in systolic BP (≈15 mm Hg; range, 5–18 mm Hg).
Conclusions
Major opportunities exist for improving BP control and reducing disparities, especially through consistent medication intensification when BP is uncontrolled. These data demonstrate substantial room for improvement and opportunities to close health equity gaps.
Keywords: health equity, high blood pressure, hypertension, quality and outcomes, race and ethnicity
Subject Categories: Health Equity, High Blood Pressure, Hypertension, Quality and Outcomes, Race and Ethnicity
Nonstandard Abbreviations and Acronyms
- NHANES
National Health and Nutrition Examination Survey
- PCORnet
National Patient‐Centered Clinical Research Network
- SBP
systolic blood pressure
Clinical Perspective
What Is New?
Blood pressure (BP) control (<140/90 mm Hg), calculated using real‐world data from 25 health systems across the United States, averaged 62%. BP control was lower in Black patients (57%), and there was substantial variation by health system (range, 44%–74%).
A new class of antihypertensive medication (medication intensification) was prescribed in only 12% (range, 0.6%–25%) of patient visits where BP was uncontrolled.
When a medication intensification event occurred, subsequent systolic BP was 15±20 mm Hg lower on average (range, 5–18 mm Hg).
What Are the Clinical Implications?
Major opportunities exist for improving BP control and reducing disparities.
Hypertension continues to be a leading cause of cardiovascular morbidity and mortality in the United States and worldwide despite its treatability. 1 , 2 A recent study found fluctuating prevalence of hypertension and decreasing rates of blood pressure (BP) control, which places a substantial portion of the US population at increased risk for adverse cardiovascular outcomes. 3
Effective national surveillance of the US BP control rate is essential for monitoring population health and evaluating the impacts of population‐based interventions. Existing national surveillance systems, such as the National Health and Nutrition Examination Survey (NHANES), are often cited as the gold‐standard source for hypertension surveillance. However, data from national surveillance surveys provide no information about healthcare processes relevant to BP management, performance of individual health systems, or variation across health systems, all of which might be useful for health systems interested in improvement. 4
We established the National Patient‐Centered Clinical Research Network (PCORnet) Blood Pressure Control Laboratory to track BP control and process metrics longitudinally in health systems and enable quality improvement at scale using real‐world data from electronic health record (EHR) systems. The BP Control Laboratory is a collaborative partnership including PCORnet entities, the American Medical Association, and the American Heart Association, and features both a surveillance system (BP Track) and pragmatic randomized trials. 5 Herein, we describe our use of BP Track surveillance data to analyze longitudinal trends in BP control performance and process metrics in 25 participating health systems across the United States from January 2017 through March 2020.
Methods
Data‐use agreements with contributing sites prohibit sharing of BP Track data with external investigators. However, the BP Control Laboratory accepts proposals for collaborative analysis and publications. Proposals are subject to review by the BP Control Laboratory Steering Committee for scientific value, avoidance of overlap with previously approved proposals, compliance with our publication policies, and availability of resources for analysis of the data. Interested investigators may contact the corresponding author with inquiries.
The BP Control Laboratory Surveillance System (BP Track) is an ongoing serial cross‐sectional study of BP control across the United States using EHR data. 5 BP Track is built on PCORnet, the National Patient‐Centered Clinical Research Network, 6 , 7 , 8 which supports maintenance and curation of EHR data stored at individual participating health systems in a standardized research‐ready format, the PCORnet common data model. This allows for distribution and application of standardized queries across participating organizations. 9 , 10 BP Track queries are executed against the PCORnet common data model to produce the BP control metrics (Table S1) for a series of 1‐year measurement periods using BP measurements obtained as part of standard clinical care, medication prescribing, and other EHR data linked to encounters at each of the 25 health systems. For this analysis, we used BP Track query results from 25 participating health systems across the United States (Table S2, Figure S1), starting with January 1, 2017 to December 31, 2017, and then every 3 months thereafter for up to 10 measurement periods (most recently April 1, 2019–March 31, 2020) depending on data availability at each health system (see Figures S2 and S3). PCORnet data are refreshed on a quarterly basis, and data quality is evaluated for conformance to the common data model and completeness by the PCORnet Coordinating Center. BP Track results from each health system are compared across measurement periods and across BP Track queries for outliers and discontinuities, then assembled into an analytic data set with health‐system identities masked. BP Track was approved by the lead site's institutional review board as a quality‐improvement research project.
BP Track Study Sample
To be included in analyses (as specified in National Quality Forum 0018: Controlling High Blood Pressure), 11 patients had to (1) be 18 to 85 years of age through the end of a measurement period, (2) have at least one visit during the measurement period at the health system, and (3) have a diagnosis of hypertension according to International Classification of Diseases, Ninth and Tenth Revision (ICD‐9, ICD‐10) codes during the first 6 months of the measurement period or at any time before the measurement period. Patients were excluded if (1) they received hospice services during a measurement period; (2) had a diagnosis or evidence of end‐stage renal disease, dialysis, or renal transplant during or before a measurement period; (3) had a diagnosis of pregnancy during a measurement period; or (4) were receiving care based on an institutional special needs plan or were residing in a long‐term care facility during the measurement period.
BP Control Quality and Process Metrics
BP Track includes BP control quality measures and process measures that track the healthcare processes linked to improving care for patients with hypertension. 12 The technical specifications for all metrics are provided in full detail in Data S1 and in Table S1. Briefly, the 3 quality measures are BP control (<140/<90 mm Hg, National Quality Forum 0018 11 ), BP control to the 2017 Hypertension Clinical Practice Guidelines 13 , 14 goal (<130/<80 mm Hg), and improvement in blood pressure defined as either a reduction of 10 mm Hg in systolic blood pressure (SBP) or achievement of SBP <140 mm Hg among patients with an SBP not previously controlled (Electronic Clinical Quality Improvement Resource Center 15 ). The six BP Track process metrics are based on recommendations in currently available guidance, 13 , 14 and include confirmatory repeated blood pressure measurement, medication intensification (addition of a new class of antihypertensive medication after uncontrolled BP), repeat visit in 4 weeks after uncontrolled BP, average SBP reduction after medication intensification, use of a calcium channel blocker or thiazide or thiazide‐like diuretic among Black patients prescribed at least one medication, and prescription of fixed‐dose combination product among patients prescribed at least 2 classes of medications. Only BP measurements associated with ambulatory visits are included, and as specified in the National Quality Forum 0018, 11 in the event >1 BP measurement is recorded during a visit, the lowest measurement is retained.
Each metric is calculated for each health system, then separately for subgroups defined by age, sex, and race and ethnicity. The SAS code used to generate metric results is available on GitHub (https://github.com/markjpletcher/Blood‐Pressure‐Control‐Laboratory).
Statistical Analysis
We calculated weighted averages for each metric across health systems with weights proportional to the number (N) of observations contributed by each health system (or the number of observations divided by the square of the SD for continuous metrics). The total number of observations for each metric in the most recent measurement period is provided in Table S3. Results are described over time and stratified by 5 racial and ethnic categories: Asian (not Hispanic), Black (not Hispanic), White (not Hispanic), Hispanic (any race), and other/multiple/missing information about race and ethnicity.
For statistical testing, including data from all 25 health systems, we fit generalized linear models clustering by health system, using a binomial distribution for proportion metrics (specifying numerators and denominators) or a Gaussian distribution for continuous metrics (specifying analytic weights=N/SD2). We first compared each metric across race and ethnicity in the most recent measurement period available from each health system. We then conducted a series of hypothesis tests on BP control and BP improvement measures for the 19 health‐system sample with complete data from January 2017 to December 2019 (Figure S3). We described and plotted results over time for each metric, and then tested a linear time trend adjusted for season, a main effect of race and ethnicity, and an interaction between the race and ethnicity group and the linear time trend. For a multivariable adjusted analysis of current disparities, we described each outcome metric across years, then analyzed racial and ethnic differences in the outcome (using calendar year 2019 as the measurement period) with and without adjustment for age and sex, then tested the statistical significance of the race and ethnicity group in an omnibus test. All analyses were conducted using Stata version 16.1 (StataCorp, College Station, TX).
Results
BP control metrics from 25 health systems across the United States passed BP Track quality control review and were included in the analysis. These health systems serve patients in urban, suburban, and rural settings, and most have an academic affiliation (Table S2). A total of 1 737 995 patients with hypertension in the most recent measurement period from each health system are included in our analysis. Ten percent of these patients were <45 years of age, 15% were Black, 9% were Hispanic, 52% were women, 28% had diabetes, 16% had coronary heart disease, and 16% had depression, with variability in patient characteristics by race and ethnicity as shown in Table 1. Baseline medication data (not available stratified by race and ethnicity) showed 70% of patients currently prescribed at least one antihypertensive medication, including angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers (49%), calcium channel blockers (23%), β‐blockers (28%), thiazide or thiazide‐like diuretics (25%), another diuretic (14%), and other antihypertensives (7%). SBP and diastolic BP baseline (from the patients' first visit during the measurement period) were highest among Black patients (136/80±18/12 mm Hg) and lowest in White patients (132/77±17/11 mm Hg). The majority of patients within all racial and ethnic groups had stage 1 or stage 2 hypertension, according to the 2017 Hypertension Clinical Practice Guidelines, 13 at baseline, ranging from 65% in White non‐Hispanic patients to 74% in Black non‐Hispanic patients.
Table 1.
Characteristics of Patients With Hypertension Included in the Most Recent Measurement Period* by Race and Ethnicity (N=1 737 995)
| Characteristics | Asian, not Hispanic, N=43 295, 2.5% | Black, not Hispanic, N=258 018, 14.7% | White, not Hispanic, N=1 227 966, 70.7% | Hispanic, any race, N=153 904, 8.9% | Other/multiple/missing, N=54 812, 3.2% |
|---|---|---|---|---|---|
| Age, y, % | |||||
| 18–44 | 10% | 15% | 8% | 15% | 14% |
| 45–64 | 42% | 52% | 37% | 53% | 46% |
| 65+ | 48% | 33% | 55% | 32% | 40% |
| Sex, % | |||||
| Women | 54% | 62% | 49% | 55% | 49% |
| Men | 46% | 38% | 51% | 45% | 51% |
| Other | 0% | 0% | 0% | 0% | 0% |
| Diabetes, % | 37% | 34% | 25% | 36% | 29% |
| Heart failure, % | 2.9% | 7.8% | 6.6% | 3.6% | 4.5% |
| Coronary artery disease, % | 11% | 10% | 18% | 9% | 13% |
| Depression, % | 8.6% | 12% | 18% | 14% | 13% |
| Baseline systolic blood pressure, mm Hg, mean±SD † | 132±17 | 136±18 | 132±17 | 134±17 | 133±18 |
| Baseline diastolic blood pressure, mm Hg, mean±SD † | 78±11 | 80±12 | 77±11 | 78±11 | 79±11 |
| Baseline blood pressure stage, % † | |||||
| Normal, <120/<80 | 18% | 14% | 18% | 16% | 16% |
| Elevated, 120–129/<80 | 15% | 12% | 16% | 14% | 14% |
| Stage 1, 130–139/80–89 | 34% | 32% | 34% | 32% | 34% |
| Stage 2, 140+/90+ | 32% | 42% | 31% | 37% | 36% |
Measurement periods are 1 year in duration. The most recent measurement period available from each of the 25 participating health systems was used for this analysis (Figure S2).
Baseline blood pressure measurements are taken from the first visit during the 1‐year measurement period. When more than 1 measurement is available during a given visit, the lowest measurement is used. Stage is categorized independently for systolic and diastolic blood pressure, and then the patient is categorized by the higher of the systolic blood pressure stage and the diastolic blood pressure stage. All blood pressure measurements are in units of millimeters mercury.
On average, at the end of the most recent measurement period, 62% of patients with hypertension achieved BP control to <140/<90 mm Hg, with a range of 44% to 74% across health systems, and with substantial variation across racial and ethnic groups (Table 2). Using the 2017 guideline BP goal of <130/<80 mm Hg, substantially fewer patients were in control during the measurement period (30% overall; range, 20%–38%). BP control comparing race and ethnicity was significantly different (P<0.0001), with Black patients having the lowest prevalence of BP control at <140/<90 mm Hg (57%) or <130/<80 mm Hg (25%).
Table 2.
Blood Pressure Control Metrics in the Most Recent Measurement Period* Overall and by Race and Ethnicity
| Blood pressure control metrics | By race/ethnicity, weighted average † | P value § | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Name (range) | Overall, weighted average † (range ‡ ) | Asian, not Hispanic | Black, not Hispanic | White, not Hispanic | Hispanic, any race | Other/multiple/missing | |
| 1 | Blood pressure control, <140/<90 mm Hg, % of patients | 62% (44%–74%) | 66% | 57% | 62% | 62% | 61% | <0.0001 |
| 2 | Blood pressure control to 2017 Hypertension Clinical Practice Guidelines goal, <130/<80 mm Hg, % of patients | 30% (20%–38%) | 33% | 25% | 31% | 30% | 29% | <0.0001 |
| 3 | Improvement in blood pressure, % of patients | 29% (17%–41%) | 30% | 29% | 29% | 29% | 24% | <0.0001 |
| 4 | Confirmatory repeated blood pressure measurement, % of visits | 23% (0%–100%) | 39% | 20% | 22% | 33% | 24% | <0.0001 |
| 5 | Medication intensification after uncontrolled blood pressure, % of visits | 12% (0.6%–25%) | 14% | 13% | 11% | 14% | 14% | <0.0001 |
| 6 | Repeat visit in 4 weeks after uncontrolled blood pressure, % of visits | 35% (15%–47%) | 30% | 37% | 35% | 34% | 32% | <0.0001 |
| 7 | Average SBP reduction after medication intensification, mm Hg±SD | 15±20 (5–18) | 15±19 | 14±20 | 15±20 | 15±19 | 16±20 | 0.005 |
| 8 | Prescription of a CCB or thiazide or thiazide‐like diuretic among Black patients prescribed at least one medication, % of patients | 75% (32%–80%) | N/A | 75% | N/A | 69% | N/A | <0.0001 |
| 9 | Prescription of fixed‐dose combination product among patients prescribed at least 2 classes of medications, % of patients | 25% (0%–90%) | 22% | 26% | 24% | 25% | 27% | 0.082 |
CCB indicates calcium channel blocker; and SBP, systolic blood pressure.
Measurement periods are 1 year in duration. The most recent measurement period available from each of the 25 participating health systems was used for this analysis (Figure S3).
Overall results are calculated as weighted averages of health system–specific results weighted by the total number of observations from each health system meeting eligibility criteria for metric calculation (see definitions in Table S1). The total number of observations for each metric overall and by race and ethnicity are provided in Table S3.
Range represents the lowest and highest metric result from across the 25 participating health systems.
P values represent a hypothesis test of differences across race and ethnicity calculated via generalized linear models (see Methods).
Improvement in BP by the end of the measurement period, defined as a reduction of 10 mm Hg in SBP or SBP <140 mm Hg in previously uncontrolled patients, was achieved by 29% (range, 17%–41%; Table 2). In contrast to BP control metrics, improvement in BP was similar across racial and ethnic groups.
The BP control process metrics varied substantially across health systems, and some racial and ethnic disparities were observed (Table 2). In 23% of visits where uncontrolled BP (>140/90 mm Hg) was noted, a confirmatory repeated BP measurement was documented (health system range, 0%–100%), medication intensification with prescription of a new class of BP medications occurred in 12% (range, 1%–25%), and a repeat visit in 4 weeks occurred in 35% (range, 15%–47%). In patients prescribed at least 2 classes of medications, prescription of a fixed‐dose combination product was documented in 25% (range, 0%–90%). When a medication intensification event occurred, the average SBP reduction at the subsequent visit was large (15 mm Hg; range, 5%–18% mm Hg). Among Black patients, use of a calcium channel blocker or thiazide or thiazide‐like diuretic was common (75%; range, 32%–80%). A small disparity in quality‐of‐care process measures for Black patients was evident for confirmatory repeated BP measurement (20% versus 23%) and average SBP reduction after medication intensification (14±20 mm Hg versus 15±20 mm Hg).
To assess the change in BP control and BP improvement over time, we conducted longitudinal trend analyses including 11 956 509 patient‐years of data from the 19 health‐system samples with complete data from January 2017 to December 2019. Health systems not included in the longitudinal trend analysis were smaller, and had a higher proportion of younger patients and Hispanic patients (Table S4), but were otherwise similar (Table S5). As summarized in Table 3 and Figure 1A, BP control to <140/<90 mm Hg improved in all racial and ethnic groups (Black patients, +2.3%; White patients, +1.5%; Asian patients, +1.4%; Hispanic patients, +1.3%; and other +0.5%; P interaction=0.032 for interaction between time trend and race and ethnicity). Similar trends were observed for BP control to <130/80 mm Hg (Figure 1B, P interaction <0.001) and BP improvement (Figure 1C, P interaction=0.005). However, there was wide variability in BP control to both <140/<90 and <130/<80 mm Hg based on health system (Figure 1, light gray lines).
Table 3.
Relative Likelihood of Blood Pressure Control and Improvement by Race and Ethnicity
| Race and ethnicity | Metric result, % | Odds ratio (95% CI) | |||
|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | Unadjusted* | Adjusted for age and sex | |
| Metric 1, blood pressure control, <140/<90 mm Hg, % of patients | |||||
| Asian, not Hispanic | 64.2% | 65.4% | 65.6% | 1.17 (1.07–1.27) | 1.16 (1.07–1.26) |
| Black, not Hispanic | 54.2% | 55.4% | 56.5% | 0.80 (0.66–0.96) | 0.79 (0.66–0.94) |
| White, not Hispanic | 60.5% | 61.3% | 62.0% | 1 (Ref) | 1 (Ref) |
| Hispanic, any race | 61.5% | 62.6% | 62.8% | 1.03 (0.86–1.25) | 1.02 (0.86–1.22) |
| Other/multiple/missing | 59.6% | 59.3% | 60.1% | 0.93 (0.84–1.02) | 0.92 (0.84–1.01) |
| P value † | <0.0001 | <0.0001 | |||
| Metric 2, blood pressure control to 2017 Hypertension Clinical Practice Guidelines goal, <130/<80 mm Hg, % of patients | |||||
| Asian, not Hispanic | 32.4% | 33.6% | 33.6% | 1.10 (1.04–1.17) | 1.13 (1.06–1.20) |
| Black, not Hispanic | 24.0% | 24.7% | 25.0% | 0.73 (0.66–0.81) | 0.77 (0.69–0.85) |
| White, not Hispanic | 30.4% | 31.1% | 31.4% | 1 (Ref) | 1 (Ref) |
| Hispanic, any race | 29.9% | 30.9% | 31.1% | 0.99 (0.87–1.11) | 1.05 (0.94–1.17) |
| Other/multiple/missing | 29.0% | 28.3% | 28.9% | 0.89 (0.84–0.94) | 0.93 (0.88–0.98) |
| P value * | <0.0001 | <0.0001 | |||
| Metric 3, improvement in blood pressure, % of patients | |||||
| Asian, not Hispanic | 29.8% | 30.1% | 30.9% | 1.04 (0.91–1.19) | 1.03 (0.92–1.15) |
| Black, not Hispanic | 28.6% | 28.9% | 29.4% | 0.97 (0.90–1.04) | 1.00 (0.94–1.07) |
| White, not Hispanic | 29.2% | 29.6% | 30.1% | 1 (Ref) | 1 (Ref) |
| Hispanic, any race | 30.1% | 30.5% | 29.2% | 0.96 (0.85–1.08) | 1.00 (0.91–1.11) |
| Other/multiple/missing | 22.4% | 25.1% | 25.5% | 0.80 (0.72–0.88) | 0.83 (0.75–0.92) |
| P value * | <0.0001 | <0.0001 | |||
Ref indicates reference.
All results account for clustering by health system. Regression results are limited to the final measurement period (calendar year 2019).
P values represent an omnibus test of the contribution of race and ethnicity to the model.
Figure 1. Time trends in blood pressure (BP) control outcomes, 2017 to 2019.
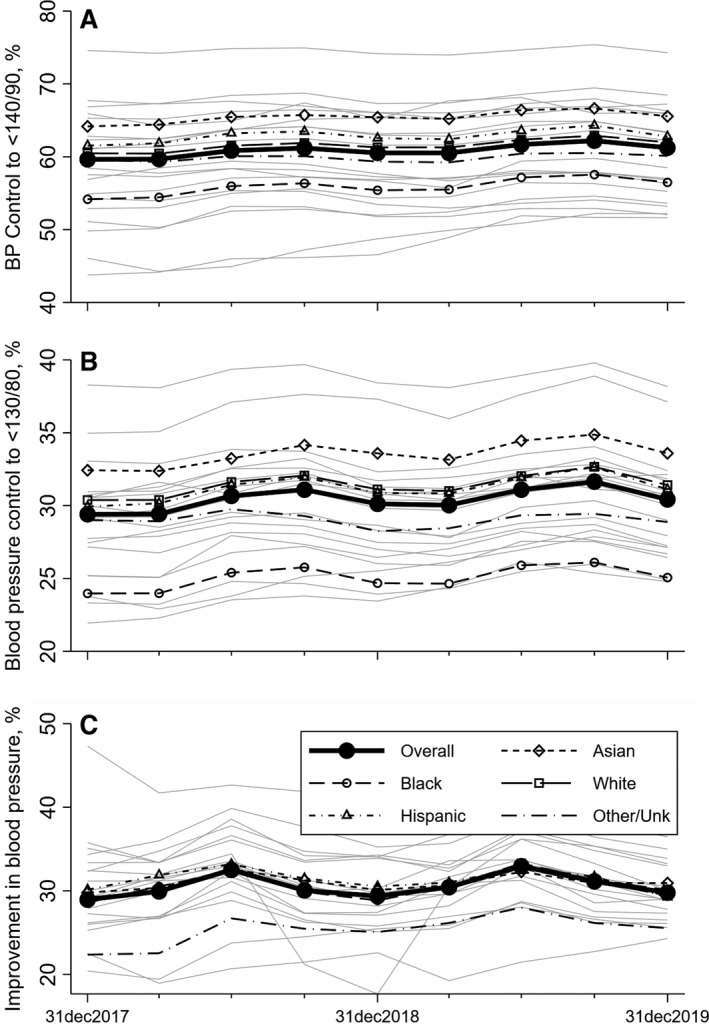
Outcomes were BP control to <140/90 mm Hg (A), BP control to <130/80 mm Hg (B), and improvement in BP (defined as either a reduction of 10 mm Hg in systolic BP or achievement of systolic BP <140 mm Hg in months 10 to 12 of the measurement period among hypertensive patients with a systolic BP not previously controlled) (C). Each data point represents metric results from a 1‐year measurement period. Dates on the x axis represent the ends of those measurement periods (ie, the first measurement period starts on January 1, 2017 and ends December 31, 2017 (Figure S2). Results are weighted averages across participating health systems and are given overall and by race and ethnicity. Light gray lines represent the BP control outcomes for each of the individual health systems included in the time trend analyses, and demonstrate a wide degree of variability based on health system.
Despite differential improvements, BP control by the end of 2019, for both <140/90 and <130/80 thresholds, remained substantially lower in Black patients than in other racial and ethnic groups (Table 3, P<0.0001 for racial and ethnic differences). After adjustment for age and sex, Black patients were 21% less likely to achieve BP control to <140/90 mm Hg compared with White patients (odds ratio [OR], 0.79; 95% CI, 0.66–0.94). A similar pattern is present for BP control to <130/<80 mm Hg. In contrast, Black patients showed identical improvement in BP during calendar year 2019 compared with White patients (OR, 1.00; 95% CI, 0.94–1.07).
Among patients with a BP >140/90 mm Hg, medication intensification with prescription of a new class of antihypertensive occurred at a low overall rate at just 12% (Figure 2A). However, among those patients in whom medication intensification occurred, the subsequent SBP was lower by an average of 15 mm Hg (Figure 2B). These metrics were relatively stable over time on average, although there was variability by health system (Figure 2, light gray lines).
Figure 2. Time trends in medication intensification and systolic blood pressure (SBP) change, 2017 to 2019.
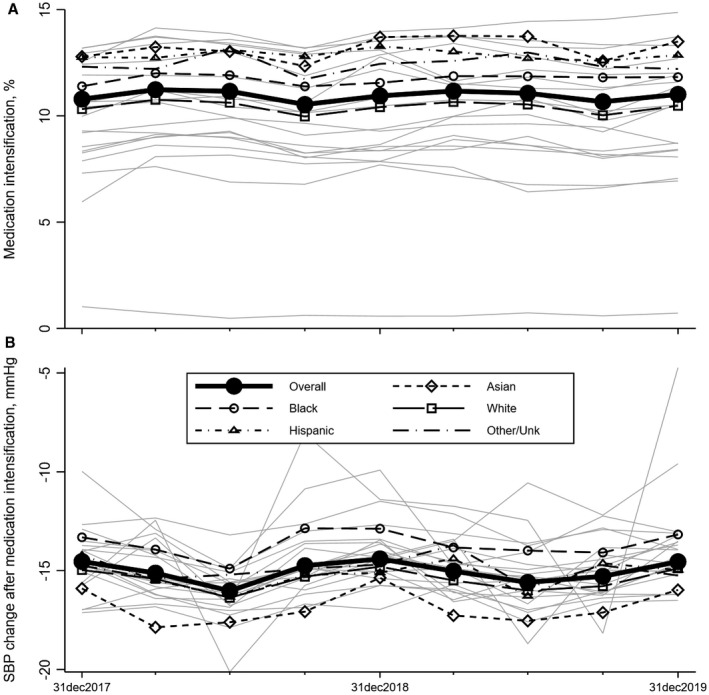
Medication intensification (prescription of a new drug class) (A) occurred in only 12% of patients overall, and occurred less commonly among White participants than other racial and ethnic groups. SBP change (B) among those patients in whom a medication intensification did occur, a drop in SBP of −15 mm Hg was observed on average, and this drop in SBP was consistent across all racial/ethnic groups. Each data point represents metric results from a 1‐year measurement period. Dates on the x axis represent the ends of those measurement periods (ie, the first measurement period starts on January 1, 2017 and ends on December 31, 2017 (Figure S2). Results are weighted averages across participating health systems and are given overall and by race and ethnicity. Light gray lines represent the blood pressure control outcomes for each of the individual health systems included in the time trend analyses, and demonstrate a wide degree of variability based on health system.
Discussion
In this analysis of real‐world healthcare use data from 1.7 million patients with hypertension, we documented major opportunities for improvement in BP control (62% control to <140/<90 mm Hg and 30% control to <130/<80 mm Hg). Racial/ethnic disparities in BP control (especially among Black patients), variability across health systems, and BP control–related healthcare process metrics indicate substantial and specific gaps in quality of care. In particular, prescription of a new class of BP medication for uncontrolled BP (medication intensification), which was associated with large reductions in SBP (15 mm Hg on average), occurred in only 12% of visits for patients with uncontrolled BP. Fixed dose combination medications also appear to be substantially underused (prescribed to only 25% of patients). The current study examines data collected from contemporary patient health visits that occurred since the release of the 2017 Hypertension Clinical Practice Guidelines 13 and shows only a slight improvement from 2017 to 2019.
The results of this study complement a recently published analysis of BP control rates based on NHANES data, which reported BP control (<140/<90 mm Hg) in 43.7% overall and 38.5% among non‐Hispanic Black patients in 2017 to 2018. NHANES data are collected during in‐home interviews or at a mobile examination center. All BP measurements were obtained during the in‐person interviews, and although in 2017 to 2018 it is reported that 93% of NHANES participants had a healthcare visit in the past year, the BP measurements do not reflect data from health systems. 3 Our data, obtained exclusively from EHR data collected from a substantially larger hypertensive cohort during the same period and beyond, indicate that across race and ethnicity, average BP control rates are higher among patients receiving routine medical care at health systems, although with substantial variability across systems (range, 44%–74%). Similarly, recent data from the National Cardiovascular Data Registry PINNACLE (Practice Innovation and Clinical Excellence), which is a registry of ambulatory visits among cardiovascular practice sites, demonstrated BP control in ≈72% of patients with a previous diagnosis of cardiovascular disease, for whom guidelines recommend more intensive BP lowering. 13 , 16
Adherence to the 2017 guidelines recommending BP control to <130/80 mm Hg 13 appears to be low, with only 30% of patients attaining this level of control in the most recent measurement period observed for each health system, a maximum of 38% in our sample, and only a small increasing trend during the time range that we observed. The lag between publication of new guidelines and uptake into clinical practice is known, and barriers to clinician adoption of new guidelines include time constraints, staffing resources, clinician skepticism and/or knowledge of the guidelines, and clinician age. 17 If new treatment guidelines were accompanied by implementation tools designed to overcome these barriers, it might be possible to reduce the lag between guideline release and clinician uptake.
The racial and ethnic disparities in BP control we demonstrated are consistent with recent NHANES‐based estimates 3 of lower BP control in Black patients. BP Track data collected from patients with recent healthcare use indicate that this disparity appears to have slightly narrowed in the last 2 years (2017–2019), yet a 5% absolute difference in BP control remains when comparing White versus Black patients with hypertension. However, we detected only minor healthcare process measure differences related to hypertension management, and no difference in improvement in BP among Black patients with uncontrolled BP compared with patients from other racial/ethnic groups when examined over the course of each 1‐year measurement period. One potential explanation for this discrepancy is that Black patients exhibited higher baseline SBP and diastolic BP levels at the start of each measurement period, so greater reductions in BP are required to attain control. It is also possible that the process measures available in BP Track do not capture critical clinic‐, clinician‐, or patient‐level factors that might contribute to this ongoing disparity. Results from meta‐analyses indicate significant associations between perceived discrimination and hypertension. 18 Perceived discrimination caused by racial trauma induced from everyday discrimination and major life discrimination has also been associated with health behaviors that negatively influence BP (eg, smoking, fewer daily hours of sleep). 19 Race‐ and ethnicity‐related factors, such as discrimination and provider implicit bias, are likely contributors to the racial disparities in hypertension and hypertension control rates observed in the current study.
Medication intensification has a substantial impact on BP trajectories over time and on downstream cardiovascular outcomes such as myocardial infarction and revascularization. 20 There are many ways to classify medication intensification. 21 The approach developed for BP Track counts only prescriptions for a new class of antihypertensive medication, and is limited to visits with SBP ≥140 mm Hg. 22 As expected, we observed substantial reductions in SBP at the visit following medication intensification, but low rates of medication intensification were observed in all racial/ethnic groups (11%–14%). The results in the current study are similar to prior analyses of the National Ambulatory Medical Care Survey, which reported medication intensification (similarly defined as initiation or addition of a new medication) occurring in only 17% of patients with SBP ≥140 mm Hg. 22 Both physician inertia and patient preferences can contribute to missed opportunities for medication intensification; our study could not distinguish them. The long‐term consequences of missed opportunities for medication intensification are substantial for patients including stroke and death. 23 These findings highlight a major opportunity to improve BP control in the United States.
The 2014 Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, 24 and the subsequent 2017 Hypertension Clinical Practice Guidelines 13 recommend that non‐Hispanic Black patients be treated with a thiazide or thiazide‐like diuretic or a calcium channel blocker as initial monotherapy or as part of combination therapy for treatment of uncomplicated hypertension. Recent data from a cohort of Medicare beneficiaries indicated that in 2018, fewer than 20% of Black patients were receiving a thiazide‐type diuretic, whereas almost 40% were receiving a calcium channel blocker as monotherapy. 25 Our BP Track data, collected through 2019, show high levels of compliance with this race‐based quality metric, with 75% of Black patients (albeit younger than the Medicare population) treated with a thiazide‐type diuretic or calcium channel blocker. Whether differential treatment by race is warranted, given that race is a social construct without biological merit, remains controversial. 26 A race‐conscious approach to this issue would urge consideration of all antihypertensive options for Black patients, with the goal of BP control and minimal adverse effects. 27
The 2017 Hypertension Clinical Practice Guidelines 13 recommends that fixed‐dose combination antihypertension products be prescribed whenever feasible based on the associated improved adherence because of decreased pill burden and costs. Our data indicate that although 25% of patients had a fixed‐dose combination product prescribed, the variability was wide (0%–90%), indicating substantial room for improvement. Considering the need for multiple BP‐lowering medications in most patients with hypertension, and that almost every commercially available fixed‐dose combination product is available in a low‐cost generic formulation, there is likely a fixed‐dose combination product suitable for most patients with hypertension.
This analysis is subject to some important limitations. First, the 25 health systems included in BP Track represent a collection of health systems that had already opted into PCORnet, with overrepresentation of academic medical centers. Consequently, these results may not generalize to nonacademic health systems or to all US patients with hypertension. Second, BP Track does not currently collect individual‐level patient or visit data from health systems, so the analysis is limited to reporting prespecified and preprogrammed BP control metrics. No adjustments can be made for individual patient characteristics beyond age, sex, and race/ethnicity. Third, inconsistency and inaccuracy in collection of racial and ethnic data is known to occur in electronic medical records, and our categorizations are crude (eg, the Hispanic ethnicity label masks differences by national origin); these data limitations may influence the associations reported. 28 Fourth, antihypertensive medication dose escalation cannot be accounted for because medication dose is not consistently recorded and captured in the PCORnet common data model. Fifth, there is presumably variability in the techniques used for obtaining usual‐care BP measurements across our 25 health systems, including use of manual versus automated technologies and attended versus unattended measurement protocols. Finally, the average SBP reduction after medication intensification metric likely reflects some regression to the mean along with true SBP‐lowering effects of medication intensification.
Despite these limitations, the results of the current study provide strong evidence of substantial and specific gaps in quality of care for patients diagnosed with hypertension, and a persistent disparity in BP control for Black adults compared with White adults. Discovery of uncontrolled BP during a clinic visit represents a prime opportunity for clinical intervention; this event should trigger confirmatory measurement, medication intensification where appropriate, and timely follow‐up; our data demonstrate how infrequently (and unevenly across health systems) such interventions occur in practice. In particular, medication intensification, the addition of a new class of antihypertensive medication, is clearly effective at lowering BP, but this occurs at rates that remain quite low. BP Track and the BP Control Laboratory infrastructure 5 can be used to identify specific gaps in BP control quality of care at specific health systems, tailor interventions to address those gaps, and track improvements over time.
Sources of Funding
The PCORnet Blood Pressure Control Laboratory is funded by a partnership including the Patient‐Centered Outcomes Research Institute (PCORI contract PaCR‐2017C2‐8153), the American Medical Association (funding and in‐kind support), and the American Heart Association (in‐kind support). The American Medical Association and the American Heart Association are represented on the steering committee. The findings and conclusions are those of the authors and do not necessarily represent the views of Patient‐Centered Outcomes Research Institute, the American Medical Association, or the American Heart Association.
Disclosures
Drs Rakotz and Wozniak are employees of the American Medical Association, and Dr Shay was an employee of the American Heart Association at the time of this work. The funding sources described above partially support salaries (R.M. Cooper‐DeHoff, Dr Fontil, Dr Carton, K.M Shaw, M. Smith, Dr Todd, Dr Chamberlain, Dr O’Brien, M. Faulkner Modrow, Carlos Maeztu, Dr Pletcher) or consulting income (Carlos Maeztu). The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S5
Figures S1–S3
Acknowledgments
The PCORnet Blood Pressure Control Laboratory Team acknowledge the engagement, input, moral support, and other contributions of our patient advisory board (C. Maeztu, K. Sigona, G. Merritt, D. Holmes, P. Williams, and P. Poston) and our clinical nurse stakeholder (J. Sansone).
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022224
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Navar AM, Peterson ED, Wojdyla D, Sanchez RJ, Sniderman AD, D'Agostino RB, Pencina MJ. Temporal changes in the association between modifiable risk factors and coronary heart disease incidence. JAMA. 2016;316:2041–2043. doi: 10.1001/jama.2016.13614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Risk Factors . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foraker RE, Benziger CP, DeBarmore BM, Cené CW, Loustalot F, Khan Y, Anderson CAM, Roger VL; Prevention AHACoEa, Council on Arteriosclerosis TraVB and Health aCoLaC . Achieving optimal population cardiovascular health requires an interdisciplinary team and a learning healthcare system: a scientific statement from the American Heart Association. Circulation. 2021;143:e9–e18. doi: 10.1161/CIR.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pletcher MJ, Fontil V, Carton T, Shaw KM, Smith M, Choi S, Todd J, Chamberlain AM, O’Brien EC, Faulkner M, et al. The PCORnet blood pressure control laboratory: a platform for surveillance and efficient trials. Circ Cardiovasc Qual Outcomes. 2020;13:e006115. doi: 10.1161/CIRCOUTCOMES.119.006115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient‐centered clinical research network. J Am Med Inform Assoc. 2014;21:578–582. doi: 10.1136/amiajnl-2014-002747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selby JV, Krumholz HM, Kuntz RE, Collins FS. Network news: powering clinical research. Sci Transl Med. 2013;5:182fs13. doi: 10.1126/scitranslmed.3006298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forrest CB, McTigue KM, Hernandez AF, Cohen LW, Cruz H, Haynes K, Kaushal R, Kho AN, Marsolo KA, Nair VP, et al. PCORnet® 2020: current state, accomplishments, and future directions. J Clin Epidemiol. 2020;129:60–67. doi: 10.1016/j.jclinepi.2020.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. PCORI . PCORnet: the National Patient‐Centered Clinical Research Network. Available at: https://www.pcori.org/research‐results/pcornet‐national‐patient‐centered‐clinical‐research‐network. Accessed October 30, 2020.
- 10. Qualls LG, Phillips TA, Hammill BG, Topping J, Louzao DM, Brown JS, Curtis LH, Marsolo K. Evaluating foundational data quality in the National Patient‐Centered Clinical Research Network (PCORnet®). EGEMS (Wash DC). 2018;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quality ID #236 (NQF 0018): controlling high blood pressure—national quality strategy domain: effective clinical care. Available at: https://www.acr.org/‐/media/ACR/NOINDEX/Measures/2018_Measure_236_Registry.pdf. Accessed October 30, 2020.
- 12. Egan BM, Sutherland SE, Rakotz M, Yang J, Hanlin RB, Davis RA, Wozniak G. Improving hypertension control in primary care with the measure accurately, act rapidly, and partner with patients protocol. Hypertension. 2018;72:1320–1327. doi: 10.1161/HYPERTENSIONAHA.118.11558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. https://pubmed.ncbi.nlm.nih.gov/30354655/ [DOI] [PubMed] [Google Scholar]
- 14. Casey DE, Thomas RJ, Bhalla V, Commodore‐Mensah Y, Heidenreich PA, Kolte D, Muntner P, Smith SC, Spertus JA, Windle JR, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circ Cardiovasc Qual Outcomes. 2019;12:e000057. doi: 10.1161/HCQ.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. eCQI . Hypertension: improvement in blood pressure. 2018. Available at: https://ecqi.healthit.gov/sites/default/files/ecqm/measures/CMS65v7.html. Accessed October 30 2020.
- 16. Maddox TM, Song Y, Allen J, Chan PS, Khan A, Lee JJ, Mitchell J, Oetgen WJ, Ponirakis A, Segawa C, et al. Trends in U.S. ambulatory cardiovascular care 2013 to 2017: JACC review topic of the week. J Am Coll Cardiol. 2020;75:93–112. doi: 10.1016/j.jacc.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 17. Chan WV, Pearson TA, Bennett GC, Cushman WC, Gaziano TA, Gorman PN, Handler J, Krumholz HM, Kushner RF, MacKenzie TD, et al. ACC/AHA special report: clinical practice guideline implementation strategies: a summary of systematic reviews by the NHLBI Implementation Science Work Group: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e122–e137. doi: 10.1161/CIR.0000000000000481 [DOI] [PubMed] [Google Scholar]
- 18. Dolezsar CM, McGrath JJ, Herzig AJM, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20–34. doi: 10.1037/a0033718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sims M, Diez‐Roux AV, Gebreab SY, Brenner A, Dubbert P, Wyatt S, Bruce M, Hickson D, Payne T, Taylor H. Perceived discrimination is associated with health behaviours among African‐Americans in the Jackson Heart Study. J Epidemiol Community Health. 2016;70:187–194. doi: 10.1136/jech-2015-206390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maddox TM, Ross C, Tavel HM, Lyons EE, Tillquist M, Ho PM, Rumsfeld JS, Margolis KL, O'Connor PJ, Selby JV, et al. Blood pressure trajectories and associations with treatment intensification, medication adherence, and outcomes among newly diagnosed coronary artery disease patients. Circ Cardiovasc Qual Outcomes. 2010;3:347–357. doi: 10.1161/CIRCOUTCOMES.110.957308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rose AJ, Berlowitz DR, Manze M, Orner MB, Kressin NR. Comparing methods of measuring treatment intensification in hypertension care. Circ Cardiovasc Qual Outcomes. 2009;2:385–391. doi: 10.1161/CIRCOUTCOMES.108.838649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:e004188. doi: 10.1161/JAHA.116.004188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim BJ, Cho Y‐J, Hong K‐S, Lee J, Kim J‐T, Choi KH, Park TH, Park S‐S, Park J‐M, Kang K, et al. Treatment intensification for elevated blood pressure and risk of recurrent stroke. J Am Heart Assoc. 2021;10:e019457. doi: 10.1161/JAHA.120.019457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 25. Colvin CL, King JB, Oparil S, Wright JT, Ogedegbe G, Mohanty A, Hardy ST, Huang L, Hess R, Muntner P, et al. Association of race/ethnicity‐specific changes in antihypertensive medication classes initiated among Medicare beneficiaries with the Eighth Joint National Committee Panel Member Report. JAMA Netw Open. 2020;3:e2025127. doi: 10.1001/jamanetworkopen.2020.25127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yudell M, Roberts D, DeSalle R, Tishkoff S. Science and society. Taking race out of human genetics. Science. 2016;351:564–565. doi: 10.1126/science.aac4951 [DOI] [PubMed] [Google Scholar]
- 27. Cerdeña JP, Plaisime MV, Tsai J. From race‐based to race‐conscious medicine: how anti‐racist uprisings call us to act. Lancet. 2020;396:1125–1128. doi: 10.1016/S0140-6736(20)32076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klinger EV, Carlini SV, Gonzalez I, Hubert SS, Linder JA, Rigotti NA, Kontos EZ, Park ER, Marinacci LX, Haas JS. Accuracy of race, ethnicity, and language preference in an electronic health record. J Gen Intern Med. 2015;30:719–723. doi: 10.1007/s11606-014-3102-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
Figures S1–S3


