Abstract
Background
Heart failure (HF) is a known risk factor for ischemic stroke, but data regarding ischemic stroke during hospitalization for acute decompensated HF (ADHF) are limited.
Methods and Results
We analyzed the data from a multicenter registry (Kyoto Congestive Heart Failure [KCHF] Registry) that enrolled 4056 consecutive patients with ADHF in Japan (mean age, 78 years; men, 2238 patients [55%]; acute coronary syndrome [ACS], 239 patients [5.9%]). We investigated the incidence and predictors of ischemic stroke during hospitalization for ADHF. During the hospitalization, 63 patients (1.6%) developed ischemic stroke. The median interval from admission to the onset of ischemic stroke was 7 [interquartile range: 2–14] days, and the most common underlying cause was cardioembolism (64%). Men (OR, 1.87; 95%CI, 1.11–3.24), ACS (OR, 2.31; 95%CI, 1.01–4.93), absence of prior HF hospitalization (OR, 2.21; 95%CI, 1.24–4.21), and high B‐type natriuretic peptide (BNP)/N‐terminal proBNP (NT‐proBNP) levels (above the median) at admission (OR, 3.15; 95%CI, 1.84–5.60) were independently associated with ischemic stroke. In patients without ACS, the independent risk factors for ischemic stroke were fully consistent with those in the main analysis. Higher quartiles of BNP/NT‐proBNP levels were significantly associated with higher incidence of ischemic stroke (P for trend, <0.001). Patients with ischemic stroke showed higher in‐hospital mortality, longer length of hospital stay, and poorer functional status at discharge.
Conclusions
During hospitalization for ADHF, 1.6% of the patients developed ischemic stroke. Men, ACS, absence of prior HF hospitalization, and high BNP/NT‐proBNP levels at admission were independently associated with ischemic stroke.
Keywords: acute heart failure, B‐type natriuretic peptide, ischemic stroke, N‐terminal pro B‐type natriuretic peptide
Subject Categories: Heart Failure, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- ADHF
acute decompensated heart failure
- GUSTO
Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries
- KCHF
Kyoto Congestive Heart Failure
- HFpEF
heart failure with preserved LVEF
- HFmrEF
heart failure with mildly reduced LVEF
- HFrEF
heart failure with reduced LVEF
- TOAST
Trial of Org 10172 Acute Stroke Treatment
Clinical Perspective
What Is New?
During hospitalization for ADHF, 1.6% of patients developed ischemic stroke (median intervals from admission to onset, 7 [interquartile range, 2–14] days), and the most common underlying cause was cardioembolism (64%).
Independent risk factors for ischemic stroke were men, acute coronary syndrome, absence of prior heart failure hospitalization, and high B‐type natriuretic peptide/N‐terminal‐B‐type natriuretic peptide.
Patients with ischemic stroke showed higher in‐hospital mortality and poorer functional status at discharge.
What Are the Clinical Implications?
Ischemic stroke is a serious complication during hospitalization for acute decompensated heart failure, and its incidence is not rare.
We should pay more attention to the prevention of ischemic stroke when treating patients with acute decompensated heart failure, and future studies are warranted to explore the optimal therapy to prevent ischemic stroke.
The presence of heart failure fulfills all of the Virchow’s triad for a hypercoagulable state, increasing the propensity to thrombosis, 1 and is considered to be a risk of ischemic stroke. A previous study demonstrated that the risk of ischemic stroke was 2 to 3‐fold higher in patients with heart failure. 2 Furthermore, it was reported that decompensation further increased the risk of ischemic stroke. A previous population‐based prospective cohort study demonstrated that patients with heart failure had more than 5‐fold increased age‐ and sex‐adjusted risk of ischemic stroke in the first month after diagnosis of heart failure as compared with participants without heart failure, 3 which was also supported by another community‐based study. 4 , 5 The increased risk of ischemic stroke in the acute phase of hospitalization for heart failure was also observed in patients with atrial fibrillation. 6 However, there is a paucity of data on the incidence and risk factors of ischemic stroke during hospitalization for acute decompensated heart failure (ADHF). Therefore, we investigated the incidence and risk factors of ischemic stroke during hospitalization for ADHF using data from a large Japanese registry.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Additional methods can be found in Data S1.
Study Population
The Kyoto Congestive Heart Failure (KCHF) registry is a physician‐initiated, prospective, observational, multicenter cohort study that enrolled consecutive patients who were hospitalized for ADHF for the first time between October 2014 and March 2016 in the 19 participating hospitals in Japan without exclusion (Clinical Trial Registration: NCT02334891 and UMIN000015238). The overall design of the KCHF study has been previously described in detail. 7 We enrolled 4056 consecutive patients with ADHF as defined by the modified Framingham criteria admitted to the participating centers, who received heart failure‐specific treatment involving intravenous drugs within 24 hours after hospital presentation.
Ethics
The investigation conformed with the principles outlined in the Declaration of Helsinki. The study protocol was approved by the ethical committees at Kyoto University Hospital (local identifier: E2311) and each participating hospital. A waiver of written informed consent from each patient was granted by the institutional review boards of Kyoto University and each participating center as the study met the conditions of the Japanese ethical guidelines for medical and health research involving human subjects. 8 , 9 We disclosed the details of the present study to the public as an opt‐out method, and the notice clearly informed patients of their right to refuse enrollment.
Data Collection and Definitions
We collected data on patient demographics, medical history, underlying heart disease, pre‐hospital activities, socioeconomic status, signs, symptoms, medications, laboratory tests at hospital presentation, electrocardiogram, echocardiography, and clinical events during the index hospitalization. Heart failure was classified according to left ventricular ejection fraction (LVEF) as heart failure with preserved LVEF (HFpEF: LVEF ≥50%), heart failure with mildly reduced LVEF (HFmrEF: 40% ≤LVEF <50%), and heart failure with reduced LVEF (HFrEF: LVEF <40%). Atrial fibrillation was defined based on prior history and electrocardiograms at admission. B‐type natriuretic peptide (BNP) or N‐terminal proBNP (NT‐proBNP) was measured at admission at each participating institution using commercially available immunochemical assays. Data values exceeding limits of detection were replaced with the limits of detection. BNP and NT‐proBNP data were available in 3590 patients (88.5%) and 698 patients (17.2%), respectively. Since a conversion formula between BNP and NT‐proBNP has not been established in patients with ADHF, we divided the patients according to the median or quartiles of BNP and NT‐proBNP levels, and NT‐proBNP values were adopted if no BNP values were measured. Antithrombotic therapy was defined as use of intravenous heparin within 24 hours after admission, or prescription of antiplatelet drugs (aspirin, thienopyridines, and cilostazol) and/or oral anticoagulants (vitamin K antagonists and direct oral anticoagulants) at admission.
Ischemic stroke was defined as an episode of sudden onset focal neurologic deficit lasting >24 hours where computed tomography or magnetic resonance imaging showed findings that were consistent with ischemic stroke and relevant to the clinical symptoms. The underlying cause of ischemic stroke was classified into cardioembolism, large‐artery atherosclerosis, small‐vessel occlusion, stroke of other determined cause, and stroke of undetermined cause according to the Trial of Org 10172 Acute Stroke Treatment (TOAST) classification. 10 Intracranial hemorrhage was defined as subarachnoid hemorrhage, hemorrhagic infarction, cerebral bleeding, and subdural hematoma confirmed by computed tomography or magnetic resonance imaging. Major bleeding was defined as moderate or severe bleeding according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) classification. The definitions of the causes of death and in‐hospital adverse events in the KCHF registry were previously reported. 9 The causes of death were adjudicated by a clinical event committee. Death was placed into 1 of 2 categories in the KCHF registry: (1) cardiac death, which includes death related to heart failure and sudden death; and (2) non‐cardiac death, which includes death related to stroke, other vascular death, pulmonary disease, sepsis, other infection, gastrointestinal disease, malignancy, renal failure, and other non‐cardiovascular death. Sudden death was defined as unexplained death in a previously stable patient. Physical activity at discharge was classified by mobility status based on the definition of the Japanese long‐term care insurance system as ambulatory (including those patients using any aid such as stick), use of wheelchair, and bedridden state.
Statistical Analysis
We evaluated the incidence of and the risk factors for ischemic stroke during index hospitalization for ADHF. The categorical variables are presented as numbers and percentages, and were compared using a chi‐square test. The continuous variables are expressed as mean±SD or median with interquartile range (IQR). On the basis of their distributions, the continuous variables were compared using Student’s t test or the Wilcoxon rank sum test. To explore the risk factors of ischemic stroke, we used univariate and multivariable logistic regression models not accounting for the time to events, in order to better visualize the differences in the number of events between groups. Because atrial fibrillation and left ventricular (LV) dysfunction (LVEF <40%) were established risk factors for ischemic stroke in heart failure, we selected these variables and use of intravenous heparin in addition to those variables with significant difference in the univariate analyses as the potential risk factors in the multivariable analysis.
In addition, we sought to investigate the association of BNP/NT‐proBNP levels with the risk of ischemic stroke in detail, because high BNP/NT‐proBNP was reported to be associated with the incidence of ischemic stroke in patients with chronic heart failure. 11 , 12 We performed a multivariable logistic regression analysis using the quartiles of BNP or NT‐proBNP levels. P for trend was calculated by using the quartiles of BNP/NT‐proBNP levels as continuous variable. Because BNP data were available for most of the patients, we investigated the association of BNP level with the incidence of ischemic stroke in 3590 patients with BNP data as a sensitivity analysis. The BNP level was entered into the model as a categorical variable according to the quartile of baseline levels or as a log‐transformed continuous variable. We also conducted multivariable logistic regression analyses in a population excluding patients with acute coronary syndrome as sensitivity analyses, because acute coronary syndrome without heart failure increases the risk for incidence of ischemic stroke in its acute phase. 13
We performed subgroup analyses to investigate the effect of high BNP/NT‐proBNP levels (above the median of each cohort) for ischemic stroke in the subgroups based on the categories used for the multivariable analysis mentioned above (sex, prior heart failure hospitalization, acute coronary syndrome, LV dysfunction, prevalence of atrial fibrillation, and use of intravenous heparin within 24 hours after admission). Odds ratios of high BNP/NT‐proBNP for the incidence of ischemic stroke were estimated in the multivariable logistic regression analysis with adjustment by the 6 variables mentioned above. P values for interaction were calculated by logistic regression analysis.
We also compared the incidence of ischemic stroke and bleeding events (major bleeding and intracranial hemorrhage) in patients with and without antithrombotic therapy using a chi‐square test. In‐hospital death, length of hospital stay, physical activity at discharge, and the rates of the patients who were discharged to home among those who were discharged alive were compared using a chi‐square test.
All statistical analyses were conducted by a physician (M.I.) and a statistician (T.M.) using JMP 12.0. All the reported P values were 2‐tailed, and P values <0.05 were considered statistically significant.
Results
Incidence and Risk Factors of Ischemic Stroke During Hospitalization for ADHF
The mean age of the current study population was 77.9±12.0 years and 44.8% were women (Table 1). A total of 1442 patients (36.2%) had a history of prior hospitalization for heart failure, and 239 patients (5.9%) had acute coronary syndrome at hospital admission. HFpEF, HFmrEF, and HFrEF accounted for 43.2%, 18.5%, and 38.4% of the study population, respectively. Atrial fibrillation was seen in 1898 patients (46.8%), and 662 patients (16.3%) had a history of stroke. The median BNP and NT‐proBNP levels at admission were 721 (IQR: 398–1308) pg/mL and 5880 (IQR: 2721–13 241) pg/mL, respectively. Antiplatelet drugs and oral anticoagulants were prescribed at admission in 1634 patients (40.3%) and 1280 patients (31.6%), respectively, and intravenous heparin was used within 24 hours after admission in 1113 patients (27.4%).
Table 1.
Baseline Characteristics of the Entire Cohort and Patients With and Without Ischemic Stroke
| Variables | Entire cohort (N=4056) | Ischemic stroke (N=63) | No ischemic stroke (N=3993) | P value |
|---|---|---|---|---|
| Age, y | 77.9±12.0 | 76.8±12.3 | 78.0±12.0 | 0.5 |
| Age ≥80 y | 2147 (52.9) | 31 (49.2) | 2116 (53.0) | 0.6 |
| Men | 2238 (55.2) | 44 (69.8) | 2194 (55.0) | 0.02 |
| BMI, kg/m2 | 22.8±4.5 | 21.8±4.2 | 22.8±4.5 | 0.07 |
| BMI ≤22 kg/m2 | 1787 (46.7) | 30 (51.7) | 1757 (46.6) | 0.4 |
| Current smoker | 476 (12.0) | 7 (11.1) | 469 (12.0) | 0.8 |
| Ambulatory | 3149 (78.5) | 48 (76.2) | 3101 (78.5) | 0.7 |
| Prior HF hospitalization | 1442 (36.2) | 13 (21.0) | 1429 (36.5) | 0.009 |
| Ischemic cause | 1327 (32.7) | 30 (47.6) | 1297 (32.5) | 0.01 |
| ACS | 239 (5.9) | 9 (14.3) | 230 (5.8) | 0.01 |
| Non‐ACS | 1088 (26.8) | 21 (33.3) | 1067 (26.7) | 0.3 |
| HFpEF/HFmrEF/HFrEF |
1744/746/1551 (43.2/18.5/38.4) |
28/12/23 (44.4/19.0/36.5) |
1716/734/1528 (43.1/18.5/38.4) |
0.95 |
| Comorbidities | ||||
| Hypertension | 2909 (71.7) | 49 (77.8) | 2860 (71.6) | 0.3 |
| Dyslipidemia | 1549 (38.2) | 23 (36.5) | 1526 (38.2) | 0.8 |
| Diabetes | 1510 (37.2) | 26 (41.3) | 1484 (37.2) | 0.5 |
| Prior myocardial infarction | 908 (22.4) | 12 (19.0) | 896 (22.4) | 0.5 |
| Prior stroke | 662 (16.3) | 14 (22.2) | 648 (16.2) | 0.2 |
| Peripheral artery disease | 343 (8.5) | 5 (7.9) | 338 (8.5) | 0.9 |
| AF | 1898 (46.8) | 29 (46.0) | 1869 (46.8) | 0.9 |
| Chronic kidney disease | 1809 (44.6) | 35 (55.6) | 1774 (44.4) | 0.08 |
| Anemia | 2705 (66.8) | 36 (57.1) | 2669 (67.0) | 0.1 |
| Malignancy | 585 (14.4) | 9 (14.3) | 576 (14.4) | 0.98 |
| Dementia | 770 (19.0) | 15 (23.8) | 755 (18.9) | 0.3 |
| Presentation at emergency room | ||||
| Systolic blood pressure, mm Hg | 147.2±35.3 | 153.2±39.8 | 147.1±35.2 | 0.2 |
| Diastolic blood pressure, mm Hg | 84.5±24.0 | 91.8±27.9 | 84.3±23.9 | 0.04 |
| Pulse rate, bpm | 96.0±27.5 | 104.9±32.1 | 95.8±27.4 | 0.03 |
| Body temperature, °C | 36.5±0.6 | 36.5±0.7 | 36.5±0.6 | 0.8 |
| AF at emergency room | 1457 (35.9) | 23 (36.5) | 1434 (35.9) | 0.9 |
| NYHA III/IV | 1589/1948 (39.4/48.3) | 24/31 (38.1/49.2) | 1565/1917 (39.4/48.3) | 0.8 |
| Biomarkers | ||||
| BNP, pg/mL (N=3590) | 721 [397.75–1307.5] | 1301 [634.5–1731.5] | 719 [394–1294] | <0.001 |
| NT‐proBNP, pg/mL (N=698) | 5880 [2720.75–13 241.5] | 11 929 [4893–31 741.5] | 5809 [2718–12 946.5] | 0.06 |
| High BNP/NT‐proBNP* | 1999 (50.0) | 45 (71.4) | 1954 (49.6) | <0.001 |
| Serum albumin, mg/dL | 3.5±0.5 | 3.3±0.5 | 3.5±0.5 | 0.04 |
| Serum albumin <3 g/dL | 567 (14.4) | 13 (20.6) | 554 (14.3) | 0.2 |
| eGFR, mL/min per 1.73 m2 | 45.7±23.5 | 38.8±18.0 | 45.8±23.5 | 0.003 |
| eGFR <30 mL/min per 1.73 m2 | 1118 (27.6) | 22 (34.9) | 1096 (27.5) | 0.2 |
| Serum Na, mEq/L | 139.0±4.3 | 138.7±3.0 | 139.0±4.3 | 0.4 |
| Serum Na <135 mEq/L | 519 (12.8) | 4 (6.3) | 515 (12.9) | 0.09 |
| Antithrombotic therapy | 2983 (73.5) | 46 (73.0) | 2937 (73.6) | 0.9 |
| Antiplatelet drugs | 1634 (40.3) | 19 (30.2) | 1615 (40.4) | 0.09 |
| Oral anticoagulants | 1280 (31.6) | 18 (28.6) | 1262 (31.6) | 0.6 |
| Warfarin | 872 (21.5) | 12 (19.0) | 860 (21.5) | 0.6 |
| Direct oral anticoagulants | 409 (10.1) | 6 (9.5) | 403 (10.1) | 0.9 |
| Heparin | 1113 (27.4) | 23 (36.5) | 1090 (27.3) | 0.1 |
Continuous variables are expressed as mean±standard deviation or median [interquartile range] according to the distributions. Categorical variables are presented as numbers (percentages). ACS indicates acute coronary syndrome; AF, atrial fibrillation; BMI, body mass index; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; HF with reduced EF; HF, heart failure; HFmrEF, HF with mildly‐reduced EF; HFrEF, HFpEF, HF with preserved ejection fraction (EF); Na, sodium; NT‐proBNP, N‐terminal proBNP; and NYHA, New York Heart Association.
Above the median in the entire cohort.
During hospitalization, 63 patients (1.6%) developed ischemic stroke (Figure 1). The median interval from admission to the onset of ischemic stroke was 7 (IQR: 2–14) days, and 34 patients (54.0%) had ischemic stroke during the first week after hospital admission (Figure 2A). The most common underlying cause of ischemic stroke was cardioembolism (64%), followed by large‐artery atherosclerosis (19%) (Figure 2B).
Figure 1. Study flow chart.
Figure 2. Timing and cause of ischemic stroke during hospitalization for acute decompensated heart failure.
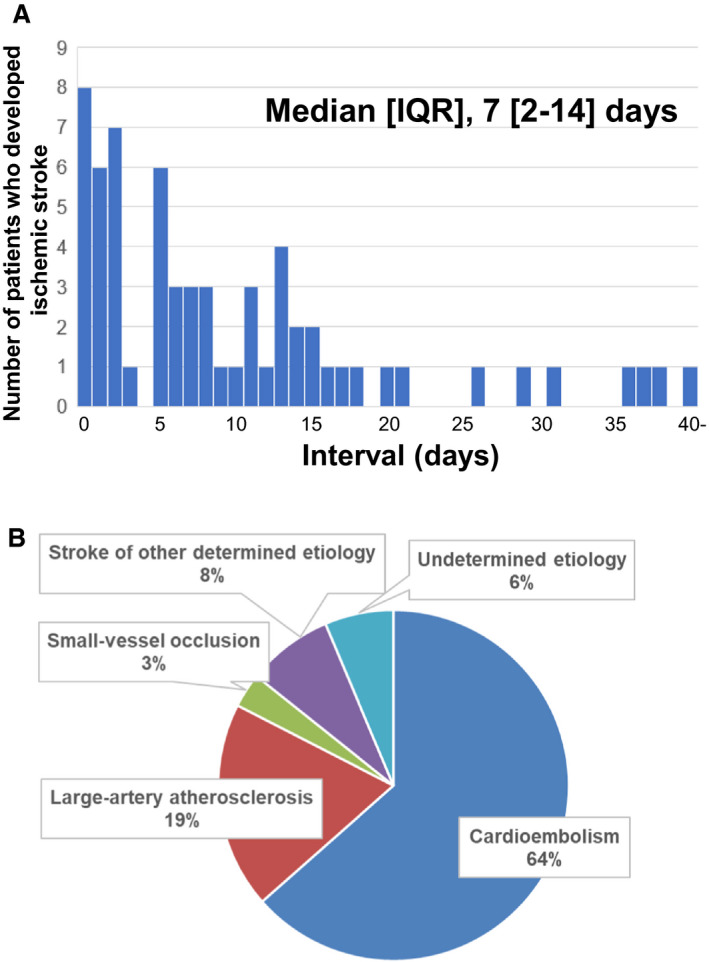
A, Intervals from admission to the onset of ischemic stroke. Intervals were calculated by subtracting the day of admission from the day of onset. B, Underlying cause of incident ischemic stroke. IQR indicates interquartile range.
Table 1 and Table S1 shows the baseline characteristics in patients with and without ischemic stroke. Patients with ischemic stroke as compared with those without had a higher prevalence of men, lower prevalence of prior heart failure hospitalization and higher prevalence of acute coronary syndrome (Table 1). Age was comparable between the two groups. BNP and NT‐proBNP were measured in 3590 and 698 patients, respectively. Patients with ischemic stroke as compared with those without had higher BNP and NT‐proBNP levels. There were no significant between‐group differences in the LVEF category (HFpEF/HFmrEF/HFrEF), presence of atrial fibrillation, history of stroke, or prescription of antithrombotic therapy (Table 1). Prescriptions of medications for heart failure were also comparable between the groups, though prescriptions of loop diuretics and renin angiotensin receptor blockers before admission tended to be lower in patients with ischemic stroke (Table S1).
In the univariate logistic regression analysis, men, acute coronary syndrome, absence of prior hospitalization for heart failure, and high BNP/NT‐proBNP (above the median) were significantly associated with ischemic stroke (Table 2). In the multivariable analysis, men (OR, 1.87; 95%CI, 1.11–3.24), acute coronary syndrome (OR, 2.31; 95%CI, 1.01–4.73), absence of prior heart failure hospitalization (OR, 2.21; 95%CI, 1.24–4.21), and high BNP/NT‐proBNP (OR, 3.15; 95%CI, 1.84–5.60) were independently associated with ischemic stroke (Table 2). In the sensitivity analysis, among 3817 patients—ie, 4056 patients excluding 239 patients (5.9%) with acute coronary syndrome—ischemic stroke occurred in 54 patients (1.4%). The independent risk factors for ischemic stroke in the sensitivity analysis were fully consistent with those in the main analysis (Table S2). When prescription of oral anticoagulants at admission were included in the multivariate analysis, the results were also consistent with those in the main analysis (Table S3).
Table 2.
Univariate and Multivariable Logistic Regression Analysis for the Risk Factors of Ischemic Stroke
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age ≥80 y | 0.86 | 0.52–1.41 | 0.6 | |||
| Men | 1.90 | 1.10–3.26 | 0.02 | 1.87 | 1.11–3.24 | 0.02 |
| BMI ≤22 kg/m2 | 1.23 | 0.73–2.06 | 0.4 | |||
| Current smoker | 0.92 | 0.42–2.03 | 0.8 | |||
| Ambulatory | 0.88 | 0.49–1.57 | 0.7 | |||
| Absence of prior HF hospitalization | 2.16 | 1.17–4.00 | 0.009 | 2.21 | 1.24–4.21 | 0.006 |
| Ischemic cause | 1.89 | 1.15–3.11 | 0.01 | |||
| ACS | 2.73 | 1.33–5.59 | 0.01 | 2.31 | 1.01–4.73 | 0.045 |
| Non‐ACS | 0.73 | 0.43–1.24 | 0.3 | |||
| LV dysfunction (EF<40%) | 0.92 | 0.55–1.55 | 0.8 | 0.64 | 0.37–1.08 | 0.1 |
| Comorbidities | ||||||
| Hypertension | 1.39 | 0.76–2.52 | 0.3 | |||
| Dyslipidemia | 0.93 | 0.55–1.56 | 0.8 | |||
| Diabetes | 1.19 | 0.72–1.97 | 0.5 | |||
| Prior myocardial infarction | 0.81 | 0.43–1.53 | 0.5 | |||
| Prior stroke | 1.47 | 0.81–2.69 | 0.2 | |||
| Peripheral artery disease | 0.93 | 0.37–2.34 | 0.9 | |||
| AF | 0.97 | 0.59–1.60 | 0.9 | 1.53 | 0.92–2.56 | 0.1 |
| Chronic kidney disease | 1.56 | 0.95–2.58 | 0.08 | |||
| Anemia | 0.66 | 0.40–1.09 | 0.1 | |||
| Malignancy | 0.99 | 0.49–2.01 | 0.98 | |||
| Dementia | 1.34 | 0.75–2.41 | 0.3 | |||
| Presentation at emergency room | ||||||
| Systolic blood pressure <100 mm Hg | 0.89 | 0.32–2.47 | 0.8 | |||
| Diastolic blood pressure >90 mm Hg | 1.32 | 0.80–2.19 | 0.3 | |||
| Pulse rate >100 bpm | 1.54 | 0.93–2.55 | 0.1 | |||
| NYHA class IV | 0.96 | 0.59–1.58 | 0.9 | |||
| Body temperature ≥37.5 °C | 0.99 | 0.35–2.74 | 0.98 | |||
| AF at emergency room | 1.03 | 0.61–1.72 | 0.9 | |||
| Biomarkers | ||||||
| High BNP/NT‐proBNP* | 2.54 | 1.47–4.40 | <0.001 | 3.15 | 1.84–5.60 | <0.0001 |
| Serum albumin <3 g/dL | 1.56 | 0.84–2.89 | 0.2 | |||
| eGFR <30 mL/min per 1.73 m2 | 1.41 | 0.84–2.39 | 0.2 | |||
| Serum Na<135 mEq/L | 0.46 | 0.16–1.26 | 0.09 | |||
| Antithrombotic therapy | 0.97 | 0.56–1.70 | 0.9 | |||
| Antiplatelet drugs | 0.64 | 0.37–1.09 | 0.09 | |||
| Oral anticoagulants | 0.87 | 0.50–1.50 | 0.6 | |||
| Heparin | 1.53 | 0.91–2.57 | 0.1 | 1.14 | 0.66–1.93 | 0.6 |
ACS indicates acute coronary syndrome; AF, atrial fibrillation; BNP, B‐type natriuretic peptide; CI, confidence interval; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; LV, left ventricular; Na, sodium; NT‐proBNP, N‐terminal proBNP; NYHA, New York Heart Association; and OR, odds ratio.
Above the median.
Effect of the Levels of High BNP/NT‐proBNP on Ischemic Stroke
The risk for developing ischemic stroke progressively increased according to the increase in BNP/NT‐proBNP levels in both the entire cohort and patients without acute coronary syndrome (Figure 3). Moreover, when we evaluated only those patients in whom BNP values were available, higher BNP levels at admission were also independently associated with higher risk for ischemic stroke both in the entire cohort and in patients without acute coronary syndrome (Figure S1).
Figure 3. Forest plots for the risk for ischemic stroke according to the quartiles of BNP or NT‐proBNP levels.
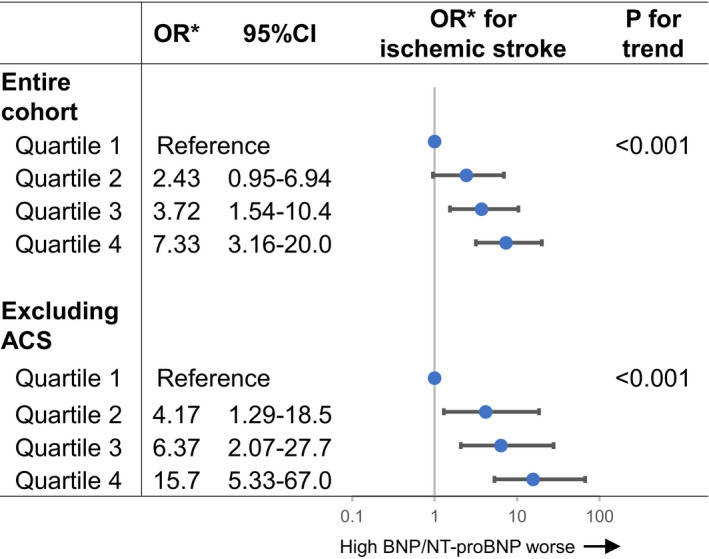
*ORs were adjusted for sex, ACS, prior HF hospitalization, left ventricular dysfunction (ejection fraction <40%), atrial fibrillation, and use of intravenous heparin within 24 hours after admission. ACS indicates acute coronary syndrome; BNP, B‐type natriuretic peptide; CI, confidence interval; NT‐proBNP, N‐terminal proBNP; OR, odds ratio.
In the subgroup analyses based on the variables used in the multivariable analysis, there was no interaction between the subgroup factors and the association of high BNP/NT‐proBNP with ischemic stroke (Figure 4).
Figure 4. Subgroup analysis for the risk for ischemic stroke according to high BNP/NT‐proBNP.
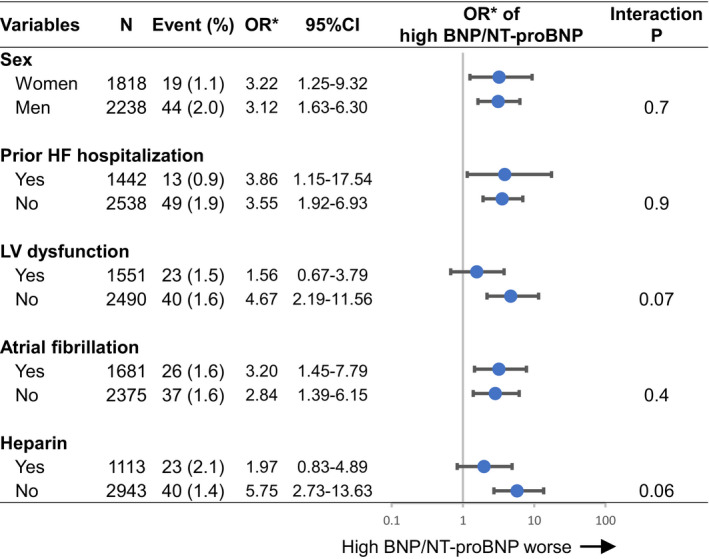
High BNP/NT‐proBNP was defined as above the median in each cohort. *ORs were adjusted for sex, ACS, prior HF hospitalization, LV dysfunction (ejection fraction <40%), atrial fibrillation, and use of intravenous heparin within 24 hours after admission. ACS indicates acute coronary syndrome, BNP, B‐type natriuretic peptide; CI, confidence interval; HF, heart failure; LV, left ventricular; NT‐proBNP, N‐terminal proBNP; and OR, odds ratio.
Incidence of Ischemic Stroke and Bleeding Events in Patients With and Without Antithrombotic Therapy
The incidence of ischemic stroke was not different between patients receiving and those not receiving heparin within 24 hours after hospitalization, while the incidence of major bleeding was significantly higher in patients receiving heparin than in patients not receiving heparin (Table 3). There were no differences in the incidences of ischemic stroke or major bleeding between patients receiving and those not receiving antiplatelet drugs or oral anticoagulants at admission (Table 3).
Table 3.
The Incidence of Ischemic Stroke and Bleeding Events in Patients With and Without Antithrombotic Therapy
| Heparin | (+) (N=1113) | (−) (N=2943) | P value |
|---|---|---|---|
| Ischemic stroke | 23 (2.1) | 40 (1.4) | 0.1 |
| Major bleeding | 44 (4.0) | 48 (1.6) | <0.001 |
| Intracranial hemorrhage | 7 (0.6) | 8 (0.3) | 0.1 |
| Antiplatelet drugs | (+) (N=1634) | (−) (N=2422) | P value |
| Ischemic stroke | 19 (1.2) | 44 (1.8) | 0.09 |
| Major bleeding | 46 (2.8) | 46 (1.9) | 0.06 |
| Intracranial hemorrhage | 8 (0.5) | 7 (0.3) | 0.3 |
| Oral anticoagulants | (+) (N=1280) | (−) (N=2776) | P value |
| Ischemic stroke | 18 (1.4) | 45 (1.6) | 0.6 |
| Major bleeding | 28 (2.2) | 64 (2.3) | 0.8 |
| Intracranial hemorrhage | 4 (0.3) | 11 (0.4) | 0.7 |
Variables are expressed as numbers (percentages).
Clinical Outcomes in Patients With and Without Ischemic Stroke During Hospitalization for ADHF
Patients with ischemic stroke had significantly higher incidence of all‐cause death, cardiac death, and non‐cardiac death than patients without ischemic stroke (Table 4). Among patients who were discharged alive, the length of hospital stay was significantly longer in those with ischemic stroke than in those without, and the proportions of patients who were ambulatory, and patients who were discharged to home, were lower in patients with ischemic stroke than in those without.
Table 4.
In‐Hospital Events and Status at Discharge
| Ischemic stroke (N=63) | No ischemic stroke (N=3993) | P value | |
|---|---|---|---|
| All‐cause death | 19 (30.2) | 252 (6.3) | <0.001 |
| Cardiac death | 9 (14.3) | 181 (4.5) | 0.003 |
| Non‐cardiac death | 10 (15.9) | 71 (1.8) | <0.001 |
| Status at discharge | (N=44) | (N=3741) | |
| Length of hospital stay (d) | 30 [20–44] | 16 [11–24] | <0.001 |
| Ambulatory/wheelchair use/bedridden |
16/16/11 (37.2/37.2/25.6) |
2722/828/127 (74.0/22.5/3.5) |
<0.001 |
| Discharged to home | 18 (41.9) | 3068 (82.6) | <0.001 |
Continuous variables are expressed as median [interquartile range]. Categorical variables are presented as numbers (percentages).
Discussions
The principal findings in the present study were as follows: (1) 1.6% of patients hospitalized for ADHF developed ischemic stroke during hospitalization; (2) men, acute coronary syndrome, absence of prior heart failure hospitalization, and high BNP/NT‐proBNP were independently associated with ischemic stroke; (3) the risk for ischemic stroke progressively increased with increasing BNP/NT‐proBNP levels at admission; (4) patients who developed ischemic stroke had higher mortality rate and poorer functional status at discharge than those who did not develop ischemic stroke.
Ischemic stroke is a serious complication of heart failure. Regarding chronic heart failure, the Framingham study showed that the risk of ischemic stroke was 2‐ to 3‐fold higher in patients with heart failure than in those without. 2 A recent population‐based cohort study also showed that patients with heart failure had 1.5‐ to 2.1‐fold higher risk of ischemic stroke compared to the general population. 5 Decompensation further increases the risk of ischemic stroke through elevated intra‐cardiac and venous pressure, activation of the sympathetic nervous system and the renin‐angiotensin‐aldosterone system, and endothelial dysfunction induced by hypoxia. 14 , 15 , 16 In addition, patients with ADHF are often treated with diuretics after admission, and subsequent dehydration may also cause a hypercoagulable state. 17 Thus, the risk of ischemic stroke is considered to be especially increased in patients with hospitalization for ADHF. However, data on the incidence and predictors of ischemic stroke during hospitalization for ADHF are limited. In our single‐center, retrospective study, 2.6% of the patients with hospitalization for ADHF developed ischemic stroke during hospitalization (interval from admission to the onset of stroke: median 10 days [IQR 5–17 days]). 18 In the present study of 4056 patients hospitalized for ADHF, 63 (1.6%) patients developed ischemic stroke during hospitalization. The incidence rate of 1.6% was not extremely high, but could not be ignored. Moreover, those who developed ischemic stroke showed a higher mortality rate and poorer functional status at discharge. These results suggest that we should pay more attention to the prevention of ischemic stroke when we treat patients with ADHF.
The current study shows that men, acute coronary syndrome, absence of prior HF hospitalization, and high BNP/NT‐proBNP levels at admission were the risks for ischemic stroke. The incidence of ischemic stroke after acute coronary syndrome has been reported in several studies. Acute coronary syndrome causes cardiac injury, leading to cardiac dysfunction and hypokinesis of cardiac chambers, which in turn may predispose to thrombus formation in cardiac chambers and embolism. 13 Catheter‐based coronary reperfusion therapies performed in acute coronary syndrome may also have a risk for ischemic stroke. In addition, ischemic stroke and coronary heart disease share common risk factors and have a similar pathophysiology. 19
High BNP/NT‐proBNP was also a risk for ischemic stroke during hospitalization for ADHF. This is the first study demonstrating an association between BNP/NT‐proBNP levels and the incidence of ischemic stroke during hospitalization for ADHF. Previous studies reported that NT‐proBNP was an independent predictor of ischemic stroke in patients with chronic heart failure without atrial fibrillation, 11 and revealed a significant association of BNP/NT‐proBNP levels with the incidence of ischemic stroke in patients with atrial fibrillation. 20 , 21 An association of BNP/NT‐proBNP levels with the incidence of ischemic stroke was also reported in patients with transient ischemic attack 22 and in asymptomatic patients. 23 BNP/NT‐proBNP are released from the heart in response to pressure and volume overload, and their plasma levels are elevated in patients with heart failure in proportion to disease severity. High BNP/NT‐proBNP levels reflect cardiac congestion, as well as LV systolic and diastolic function. 24 Both severe cardiac congestion and decline in LV function increase thrombogenicity in the heart. Moreover, in patients with these conditions, starting treatment for ADHF is likely to cause greater hemodynamic change, leading to a hypercoagulable state. 25 Thus, elevated BNP/NT‐proBNP levels may become a marker of the propensity for ischemic stroke.
Absence of prior heart failure hospitalization (de novo heart failure hospitalization) was also a risk for ischemic stroke in this study. The clinical characteristics of patients with de novo heart failure hospitalization were previously reported, 26 and included higher blood pressure and a lower rate of prescription of heart failure medications at admission, including renin angiotensin inhibitors, beta blockers, and diuretics. Patients with de novo heart failure might be particularly vulnerable, 27 because initiation of heart failure medications after admission may lead to larger changes in hemodynamics. Actually, decrease in body weight and rise in creatinine levels were lager in patients with ischemic stroke (Table S1), suggesting the association of larger hemodynamic changes with ischemic stroke.
The precise mechanism underlying the sex difference in the incidence of ischemic stroke is unclear. However, it was reported that women had an overall lower age‐adjusted stroke incidence than men, and the difference was suggested to be related to sex steroid hormones, particularly estrogen. 28
Surprisingly, atrial fibrillation was not associated with the incidence of ischemic stroke in this study. Several possible explanations are conceivable. First, as it is well‐known that atrial fibrillation is a major risk for cardioembolic stroke, oral anticoagulants were more often prescribed in this group (Table S4, Table S5), which might reduce the incidence of ischemic stroke. Second, patients without atrial fibrillation showed higher prevalence of ACS, de‐novo heart failure and high BNP/NT‐proBNP (Table S4). These might increase the risks for ischemic stroke in those without atrial fibrillation. Third, the duration of follow‐up was very short in this study, which focused on the incidence of ischemic stroke during hospitalization for ADHF. While atrial fibrillation is the risk of ischemic stroke during long‐term follow‐up, high BNP/NT‐proBNP, a marker of pressure and volume overload, might be more strongly associated with ischemic stroke in the acute phase of heart failure. Importantly, it is noteworthy that, even in patients without atrial fibrillation at admission, 1.6% of the patients developed ischemic stroke during hospitalization.
The optimal therapy to prevent ischemic stroke during hospitalization for ADHF is a topic of debate. 27 Oral anticoagulants are beneficial to prevent ischemic stroke in patients with atrial fibrillation. However, the efficacy of antithrombotic therapy in chronic heart failure patients without atrial fibrillation is controversial, 29 , 30 and there is no data on the efficacy of antithrombotic therapy in ADHF. Consistent with the BRIDGE trial, in which heparin‐bridge therapy failed to prevent incidence of ischemic stroke during the peri‐operative periods of non‐cardiac surgery in patients with atrial fibrillation, who interrupted their course of oral anticoagulants, 31 intravenous heparin was not associated with reduced incidence of ischemic stroke during hospitalization for ADHF in the current study. However, patients receiving heparin had higher prevalence of de‐novo heart failure, high BNP/NT‐proBNP, and ACS, and less prescription of oral anti‐coagulants (Table S4), which might counteract the efficacy of heparin. Direct oral anti‐coagulants have been demonstrated to be beneficial in patient with atrial fibrillation and venous thromboembolisms as alternatives to warfarin and heparin. There is evidence that low dose rivaroxaban started early post‐ADHF discharge reduces thromboembolic events, specifically stroke. 32 , 33 Future studies are warranted to explore the efficacy of heparin or direct oral anticoagulants for preventing ischemic stroke during hospitalization for ADHF.
Limitations
This study has several limitations. First, the results were derived from a prospective observational study; therefore, they only reflect association and not causality. Second, the number of patients with ischemic stroke was limited, which in turn limited the number of covariates, including in the multivariable analysis. Therefore, we cannot rule out the possibility of residual confounding. However, higher BNP/NT‐proBNP levels were consistently associated with ischemic stroke in all the subgroups (Figure 4), which might suggest robustness of the association between BNP/NT‐proBNP levels and ischemic stroke. Third, BNP/NT‐proBNP levels were not analyzed by a unified protocol or kit in a central laboratory, and the difference in natriuretic peptide assays across sites may have introduced some bias. Fourth, the decision regarding the prescription of antithrombotic therapy including intravenous heparin was left to the discretion of the attending physician, which might also have resulted in bias. Fifth, the changes and intensity of antithrombotic therapy, such as APTT, PT‐INR, and dose of direct oral anticoagulants, were not taken into consideration. The quality of anticoagulation therapy might affect the incidence of ischemic stroke. Finally, atrial fibrillation was diagnosed based on previous history and electrocardiograms at admission, and new onset atrial fibrillation after hospitalization was not taken into consideration. Despite these limitations, this study clarified the non‐negligible incidence of ischemic stroke during hospitalization for ADHF from a large‐scale multicenter registry, and could provide new insights on the management of patients with ADHF.
Conclusions
During hospitalization for ADHF, 1.6% of the patients in the present cohort suffered from ischemic stroke. Men, presence of acute coronary syndrome, de novo heart failure hospitalization, and high BNP/NT‐proBNP levels at admission were independently associated with ischemic stroke.
Sources of Funding
This work was supported by the Japan Agency for Medical Research and Development [18059186] (Drs T. Kato, T. Kuwahara, and N. Ozasa).
Disclosures
None.
Supporting information
Data S1
Table S1–S5
Figure S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022525
For Sources of Funding and Disclosures, see page 11.
References
- 1. Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow’s triad revisited. J Am Coll Cardiol. 1999;33:1424–1426. [DOI] [PubMed] [Google Scholar]
- 2. Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011;42:2977–2982. doi: 10.1161/STROKEAHA.111.628479 [DOI] [PubMed] [Google Scholar]
- 3. Alberts VP, Bos MJ, Koudstaal P, Hofman A, Witteman JC, Stricker B, Breteler M. Heart failure and the risk of stroke: the Rotterdam Study. Eur J Epidemiol. 2010;25:807–812. doi: 10.1007/s10654-010-9520-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lip GY, Rasmussen LH, Skjoth F, Overvad K, Larsen TB. Stroke and mortality in patients with incident heart failure: the Diet, Cancer and Health (DCH) cohort study. BMJ Open. 2012;2. doi: 10.1136/bmjopen-2012-000975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adelborg K, Szepligeti S, Sundboll J, Horvath‐Puho E, Henderson VW, Ording A, Pedersen L, Sorensen HT. Risk of stroke in patients with heart failure: a population‐based 30‐year cohort study. Stroke. 2017;48:1161–1168. doi: 10.1161/STROKEAHA.116.016022 [DOI] [PubMed] [Google Scholar]
- 6. Iguchi M, Tezuka Y, Ogawa H, Hamatani Y, Takagi D, An Y, Unoki T, Ishii M, Masunaga N, Esato M, et al. Incidence and risk factors of stroke or systemic embolism in patients with atrial fibrillation and heart failure‐ The Fushimi AF Registry. Circ J. 2018;82:1327–1335. doi: 10.1253/circj.CJ-17-1155 [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto E, Kato T, Ozasa N, Yaku H, Inuzuka Y, Tamaki Y, Kitai T, Morimoto T, Taniguchi R, Iguchi M, et al. Kyoto Congestive Heart Failure (KCHF) study: rationale and design. ESC Heart Failure. 2017;4:216–223. doi: 10.1002/ehf2.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministry of Education C, Sports, Science and Technology. Japan's ethical guidelines for epidemiologic research . Ministry of Health, Labour and Welfare. Accessed June 16, 2020. http://www.lifescience.mext.go.jp/files/pdf/n796_701.pdf
- 9. Yaku H, Ozasa N, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, Yoshikawa Y, Kitai T, Taniguchi R, Iguchi M, et al, on behalf of the KSI. Demographics, management, and in‐hospital outcome of hospitalized acute heart failure syndrome patients in contemporary real clinical practice in Japan ‐ observations from the prospective, multicenter Kyoto Congestive Heart Failure (KCHF) Registry. Circ J. 2018;82:2811–2819. doi: 10.1253/circj.CJ-17-1386 [DOI] [PubMed] [Google Scholar]
- 10. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE, IIIrd . Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 11. Abdul‐Rahim AH, Perez AC, Fulton RL, Jhund PS, Latini R, Tognoni G, Wikstrand J, Kjekshus J, Lip GYH, Maggioni AP, et al. Risk of stroke in chronic heart failure patients without atrial fibrillation: analysis of the Controlled Rosuvastatin in Multinational Trial Heart Failure (CORONA) and the Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca‐Heart Failure (GISSI‐HF) Trials. Circulation. 2015;131:1486–1494. discussion 1494. doi: 10.1161/CIRCULATIONAHA.114.013760 [DOI] [PubMed] [Google Scholar]
- 12. Greenberg B, Peterson ED, Berger JS, Laliberte F, Zhao Q, Germain G, Lejeune D, Wu JW, Lefebvre P, Fonarow GC. Ejection fraction, B‐type natriuretic peptide and risk of stroke and acute myocardial infarction among patients with heart failure. Clin Cardiol. 2019;42:277–284. doi: 10.1002/clc.23140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yaghi S, Pilot M, Song C, Blum CA, Yakhkind A, Silver B, Furie KL, Elkind MS, Sherzai D, Sherzai AZ. Ischemic stroke risk after acute coronary syndrome. J Am Heart Assoc. 2016;5:e002590. doi: 10.1161/JAHA.115.002590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Lorenzo F, Saba N, Kakkar VV. Blood coagulation in patients with chronic heart failure: evidence for hypercoagulable state and potential for pharmacological intervention. Drugs. 2003;63:565–576. doi: 10.2165/00003495-200363060-00004 [DOI] [PubMed] [Google Scholar]
- 15. Sabit R, Thomas P, Shale DJ, Collins P, Linnane SJ. The effects of hypoxia on markers of coagulation and systemic inflammation in patients with COPD. Chest. 2010;138:47–51. doi: 10.1378/chest.09-2764 [DOI] [PubMed] [Google Scholar]
- 16. Bettari L, Fiuzat M, Becker R, Felker GM, Metra M, O'Connor CM. Thromboembolism and antithrombotic therapy in patients with heart failure in sinus rhythm: current status and future directions. Circ Heart Fail. 2011;4:361–368. doi: 10.1161/CIRCHEARTFAILURE.110.959957 [DOI] [PubMed] [Google Scholar]
- 17. Yasaka M, Beppu S. Hypercoagulability in the left atrium: part II: coagulation factors. J Heart Valve Dis. 1993;2:25–34. discussion 35–26. [PubMed] [Google Scholar]
- 18. Hamatani Y, Iguchi M, Nakamura M, Ohtani R, Yamashita Y, Takagi D, Unoki T, Ishii M, Masunaga N, Ogawa H, et al. Incidence and predictors of ischemic stroke during hospitalization for congestive heart failure. Heart Vessels. 2016;31:1154–1161. doi: 10.1007/s00380-015-0719-4 [DOI] [PubMed] [Google Scholar]
- 19. Kajermo U, Ulvenstam A, Modica A, Jernberg T, Mooe T. Incidence, trends, and predictors of ischemic stroke 30 days after an acute myocardial infarction. Stroke. 2014;45:1324–1330. doi: 10.1161/STROKEAHA.113.001963 [DOI] [PubMed] [Google Scholar]
- 20. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, et al. N‐terminal pro‐B‐type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol. 2013;61:2274–2284. doi: 10.1016/j.jacc.2012.11.082 [DOI] [PubMed] [Google Scholar]
- 21. Shimizu H, Murakami YO, Inoue S‐I, Ohta Y, Nakamura KO, Katoh H, Sakne T, Takahashi N, Ohata S, Sugamori T, et al. High plasma brain natriuretic polypeptide level as a marker of risk for thromboembolism in patients with nonvalvular atrial fibrillation. Stroke. 2002;33:1005–1010. doi: 10.1161/hs0402.105657 [DOI] [PubMed] [Google Scholar]
- 22. Rodríguez‐Castro E, Hervella P, López‐Dequidt I, Arias‐Rivas S, Santamaría‐Cadavid M, López‐Loureiro I, da Silva‐Candal A, Pérez‐Mato M, Sobrino T, Campos F, et al. NT‐pro‐BNP: a novel predictor of stroke risk after transient ischemic attack. Int J Cardiol. 2020;298:93–97. doi: 10.1016/j.ijcard.2019.06.056 [DOI] [PubMed] [Google Scholar]
- 23. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994 [DOI] [PubMed] [Google Scholar]
- 24. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 25. Klijn CJ, Kappelle LJ. Haemodynamic stroke: clinical features, prognosis, and management. Lancet Neurol. 2010;9:1008–1017. doi: 10.1016/S1474-4422(10)70185-X [DOI] [PubMed] [Google Scholar]
- 26. Su K, Kato T, Toyofuku M, Morimoto T, Yaku H, Inuzuka Y, Tamaki Y, Ozasa N, Yamamoto E, Yoshikawa Y, et al. Association of previous hospitalization for heart failure with increased mortality in patients hospitalized for acute decompensated heart failure. Circ Rep. 2019;1:517–524. doi: 10.1253/circrep.CR-19-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin AY, Dinatolo E, Metra M, Sbolli M, Dasseni N, Butler J, Greenberg BH. Thromboembolism in heart failure patients in sinus rhythm: epidemiology, pathophysiology, clinical trials, and future direction. JACC. Heart Failure. 2021;9:243–253. doi: 10.1016/j.jchf.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 28. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JGF, Ezekowitz M, Jafri SM, Krol WF, O'Connor CM, Schulman KA, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753 [DOI] [PubMed] [Google Scholar]
- 30. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. doi: 10.1056/NEJMoa1501035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenberg B, Neaton JD, Anker SD, Byra WM, Cleland JGF, Deng H, Fu M, La Police DA, Lam CSP, Mehra MR, et al. Association of rivaroxaban with thromboembolic events in patients with heart failure, coronary disease, and sinus rhythm: a post hoc analysis of the COMMANDER HF trial. JAMA Cardiol. 2019;4:515–523. doi: 10.1001/jamacardio.2019.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mehra MR, Vaduganathan M, Fu M, Ferreira JP, Anker SD, Cleland JGF, Lam CSP, van Veldhuisen DJ, Byra WM, Spiro TE, et al. A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: the COMMANDER HF trial. Eur Heart J. 2019;40:3593–3602. doi: 10.1093/eurheartj/ehz427 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1–S5
Figure S1


