Abstract
Background
Patient‐reported outcomes (PROs) are important measures of treatment response in heart failure. We assessed temporal trends in and factors associated with inclusion of PROs in heart failure randomized controlled trials (RCTs).
Methods and Results
We searched MEDLINE, Embase, and CINAHL for studies published between January 2000 and July 2020 in journals with an impact factor ≥10. We assessed temporal trends using the Jonckheere‐Terpstra test and conducted multivariable logistic regression to explore trial characteristics associated with PRO inclusion. We assessed the quality of PRO reporting using the Consolidated Standards of Reporting Trials (CONSORT) PRO extension. Of 417 RCTs included, PROs were reported in 226 (54.2%; 95% CI, 49.3%–59.1%), with increased reporting between 2000 and 2020 (P<0.001). The odds of PRO inclusion were greater in RCTs that were published in recent years (adjusted odds ratio [aOR] per year, 1.08; 95% CI, 1.04–1.12; P<0.001), multicenter (aOR, 1.89; 95% CI, 1.03–3.46; P=0.040), medium‐sized (aOR, 2.35; 95% CI, 1.26–4.40; P=0.008), coordinated in Central and South America (aOR, 5.93; 95% CI, 1.14–30.97; P=0.035), and tested health service (aOR, 3.12; 95% CI, 1.49–6.55; P=0.003), device/surgical (aOR, 6.66; 95% CI, 3.15–14.05; P<0.001), or exercise (aOR, 4.66; 95% CI, 1.81–12.00; P=0.001) interventions. RCTs reported a median of 4 (interquartile interval , 3–6) of a possible of 11 CONSORT PRO items.
Conclusions
Just over half of all heart failure RCTs published in high impact factor journals between 2000 and 2020 included PROs, with increased inclusion of PROs over time. Trials that were large, tested pharmaceutical interventions, and coordinated in North America / Europe had lower adjusted odds of reporting PROs relative to other trials. The quality of PRO reporting was modest.
Keywords: heart failure, patient‐reported outcomes, randomized controlled trials
Subject Categories: Heart Failure, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- PRO
patient‐reported outcome
Clinical Perspective
What Is New?
Of 417 heart failure randomized controlled trials (RCTs) published in high‐impact factor journals between 2000 and 2020, 54.2% included at least 1 patient‐reported outcome (PRO), with increased inclusion of PROs over the past 20 years.
PROs had greater adjusted odds of inclusion in recently published trials and those that were multicenter; medium‐sized; coordinated in Central and South America; and that tested health service, device / surgery, exercise, and rehabilitation interventions.
A majority (54.4%) of the 226 RCTs reported 4 or less of the 11 Consolidated Standards of Reporting Trials (CONSORT) PRO items, with improved reporting in trials using PRO as a primary outcome or published after the introduction of the CONSORT PRO extension.
What Are the Clinical Implications?
PROs are increasingly collected in heart failure RCTs, but the quality of reporting is modest.
PRO reporting could improve with better adherence to the CONSORT PRO .
Consistent, high‐quality PRO reporting could better inform care in heart failure.
Patient‐reported outcomes (PROs)‐ those reported directly by patients without further interpretation by the clinician or outcome assessor‐ 1 , 2 are important measures of health from the patient's perspective, but are not routinely collected as key outcomes in clinical settings or clinical trials. 3 , 4 , 5 , 6 PROs are particularly important in chronic diseases because they provide information about symptom burden, functional limitations, and social and emotional well‐being. 7 , 8 , 9 Given the importance of PROs, scientific statements from the American Heart Association, 3 the European Society of Cardiology, 4 and regulatory agencies 1 have encouraged the routine collection of PROs in randomized controlled trials (RCTs) involving chronic cardiovascular conditions. PROs remain underused in cardiovascular randomized controlled trials (RCTs), with only 16% of cardiovascular trials published between 2005 to 2008 reporting a PRO. 10
PROs are highly relevant in RCTs of heart failure (HF), given the chronicity, symptom burden, and functional limitations associated with this diagnosis. 3 , 4 PROs can help inform clinical decision making and healthcare policy in HF as long as they are collected and reported without bias. The inclusion of PROs and the quality of PRO reporting are of great importance when evaluating treatments in HF RCTs, but to our knowledge, there have been no studies that have investigated the inclusion and quality of PRO reporting in HF trials.
In this cross‐sectional, systematic bibliometric review, we aimed to evaluate temporal trends and RCT characteristics associated with the inclusion of PROs in impactful HF RCTs, and to assess the quality of PRO reporting over the past 20 years. We analyzed HF RCTs published in high‐impact medical journals and used the Consolidated Standards of Reporting Trials (CONSORT) PRO extension as a guide to quality reporting. 7 We hypothesized that there has been a temporal increase in inclusion of PROs in HF RCTs.
Methods
The study was registered in the International Prospective Register of Systematic Reviews (ID: CRD42020198676). The conduct and the reporting of this study followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. 11 Data may be available upon request as per the Population Health Research Institute Data Sharing Policy. The Population Health Research Institute will approve the use of the data after a committee review. Interested parties may contact the study principal investigator for a copy of the policy.
Data Sources and Searches
We conducted a systematic search of the literature for articles published in MEDLINE, Embase, and CINAHL. The preliminary search strategy for MEDLINE was guided by the senior author (H.V.) and an experienced information specialist. Our search strategy included Medical Subject Headings and keywords such as “heart failure” and “randomized controlled trials.” A full list of included terms for the MEDLINE search strategy is available in Table S1.
Study Selection
We included RCTs that enrolled adult patients (aged ≥18 years) with a primary diagnosis of heart failure and that were published in high impact factor journals in the English language between January 1, 2000 and July 17, 2020. We included primary studies published in journals with an impact factor of ≥10 based on the Web of Science 2019 classification report. 12 For those trials that did not report PROs in the primary publication, we searched for secondary publications with PRO data through until January 6, 2021. We did not apply journal impact‐factor threshold to any secondary publications with PRO data. We excluded studies with methodological designs other than RCTs.
Four reviewers (K.S., M.A., S.W., and Y.E.) independently screened the titles and abstracts to determine eligibility for inclusion. Studies identified as potentially relevant were screened in full text and in duplicate by the same 4 reviewers. We recorded reasons for the exclusion of articles evaluated in full text. Disagreements between reviewers were resolved through discussion, and when required, by consulting the third author. Data extraction was audited by the senior author (H.V.).
Outcomes
The primary outcome was the inclusion of a PRO in the RCT. The secondary outcome was the quality of PRO reporting, as measured by adherence to the CONSORT PRO extension.
Data Extraction
Four reviewers (K.S., M.A., S.W., and Y.E.) independently extracted the following information in duplicate: year of publication, journal impact factor, region of coordinating center, scope of the trial, location of recruitment, trial size, type of consent, type of intervention, level of randomization, number of centers, funding type, and gender of the lead or senior author. Three reviewers (N.L., T.A., and Y.E.) independently extracted, in duplicate, inclusion of and type of PROs, PRO instruments, reporting of PROs as a primary or coprimary end point, PRO results (significant, neutral in the context of the study), reporting of minimal clinically important difference , trial registration, patient and/or public engagement, and CONSORT PRO items. We classified trials that received any industry funding as industry‐funded trials. We classified the gender of the authors using the Web of Science author search engine, institutional websites, and public social media profiles. 13
PRO and Reporting Standards
We included PROs that were reported directly by patients. We used items reported within the CONSORT PRO extension 7 to evaluate the quality of PRO reporting. The 5 primary CONSORT PRO checklist items included: identification of PRO as a primary or secondary outcome in the abstract, reporting of PRO hypotheses in the background, description of the PRO instrument psychometric properties and how the PRO data were collected in the methods, reporting of statistical approaches for dealing with missing PRO data in the methods, and discussion of PRO‐specific limitations and generalizability of the study findings. The 5 subitems included: provision of the rationale for PRO assessment, reporting of the number of participants who completed PROs at each time point, reporting of baseline PRO values, reporting of PRO findings across domains and time points, and interpreting PRO data in relation to clinical outcomes. 7 We added 2 additional items from the CONSORT PRO extension 14 : reporting of the analytic plan for the PRO in the methods and inclusion of the PRO instrument in the supplementary file if not previously published. A table of CONSORT PRO items with descriptors and scoring criteria is available in Table S2. Following calibration exercises, 3 independent authors (N.L., T.A., and Y.E.) evaluated the quality of PRO reporting against the CONSORT PRO recommendations in duplicate.
Statistical Analysis
We summarized data descriptively, including the quality of PRO reporting as measured by adherence to the CONSORT PRO extension. We presented continuous variables as medians and interquartile intervals (IQIs; 25th percentile, 75th percentile) and categorical variables as numbers and percentages, descriptively.
Sample Size
Our sample size was based on the established standards of 10 events per independent variable 15 to achieve a stable logistic regression model. To include 8 independent variables, we required at least 80 HF RCTs with PROs.
Proportions Trend Test
We used the Jonckheere‐Terpstra trend test to assess temporal trends of PRO inclusion in HF RCTs over 5 periods (2000–2003, 2004–2007, 2008–2011, 2012–2015, and 2016–2020).
Multivariable Logistic Regression
We used a multivariable regression model to explore RCT characteristics associated with inclusion of PROs in HF RCTs. The selection of independent variables for the regression analysis was informed by relevance. A list of prespecified hypotheses for the included independent variables was also constructed. We used the following independent variables: year of publication, trial size (n=51–250 and n>250 versus n≤50), region of coordinating center (North America / Europe and Central / South America versus Australia / Asia), type of intervention (health service and device/surgery and exercise/rehabilitation versus pharmaceutical), number of centers (multicenter versus single center), location of recruitment (ambulatory versus inpatient), type of funding (industry versus public), and presence of a woman in the lead or senior author position (yes versus no). Comparator variables were selected based on hypotheses on directionality of associations. We assessed model fit using the Hosmer‐Lemeshow test. We tested model discrimination using area under the receiver operating characteristic curve. We presented adjusted odds ratios (aORs) with 95% confidence intervals (CIs).
Sensitivity Analysis
We described the quality of PRO reporting for RCTs published before and after the introduction of the CONSORT PRO extension in 2013.
All P values were 2‐tailed, with P<0.05 considered statistically significant and reported to the nearest 0.001 decimal places. Data were analyzed using SPSS (version 23; IBM, Armonk, NY).
Results
Our systematic literature search yielded 12 342 unique articles, of which 417 unique RCTs were eligible and included (Figure 1).
Figure 1. The Preferred Reporting Items for Systematic Review and Meta‐Analyses flow diagram of the study selected in this systematic review.
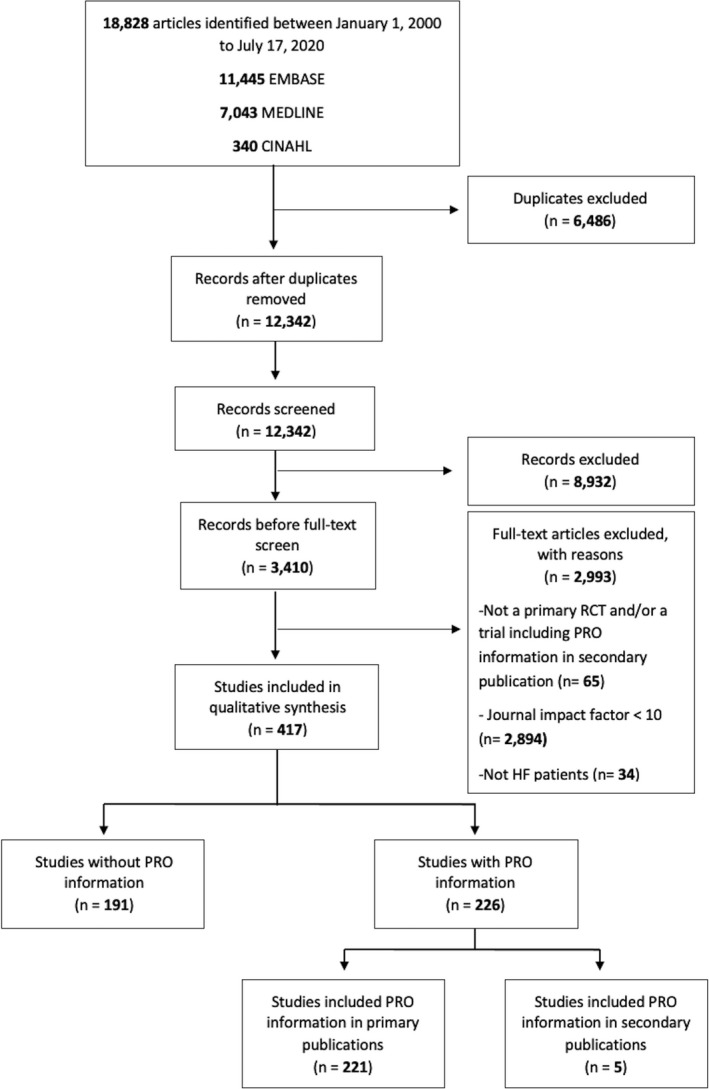
CINAHL indicates Cumulative Index to Nursing and Allied Health Literature; HF, heart failure; PRO, patient‐reported outcome; and RCT, randomized controlled trial.
Baseline Characteristics of Included Studies
The 417 RCTs represented 237 032 participants (median, 120; IQI, 30–406 participants per trial). All RCTs (100.0%) used informed consent. Most of the 417 RCTs were coordinated in Europe or North America (90.4%), recruited patients in ambulatory settings (76.3%), and tested pharmaceutical interventions (65.5%) (Table 1).
Table 1.
Characteristics of Heart Failure RCTs Published in High‐Impact Journals From 2000 to 2020, Stratified According to Inclusion of PROs (N=417)
| Clinical trial characteristic | No. (%) of RCTs with PROs (n=226) | No. (%) of RCTs without PROs (n=191) | No. (%) of total RCTs (N=417) |
|---|---|---|---|
| Trial size | |||
| Small (≤50) | 52 (23.0) | 82 (42.9) | 134 (32.1) |
| Medium (51–250) | 88 (38.9) | 52 (27.2) | 140 (33.6) |
| Large (>250) | 86 (38.1) | 57 (29.8) | 143 (34.3) |
| Primary outcome results | |||
| Positive | 134 (59.3) | 139 (72.8) | 273 (65.5) |
| Neutral | 92 (40.7) | 52 (27.2) | 144 (34.5) |
| Unit of randomization | |||
| Individual | 223 (98.7) | 190 (99.5) | 413 (99.0) |
| Cluster | 3 (1.3) | 1 (0.5) | 4 (1.0) |
| Type of consent | |||
| Informed consent | 226 (100.0) | 191 (100.0) | 417 (100.0) |
| Region of coordinating center | |||
| Europe | 111 (49.1) | 109 (57.0) | 220 (52.8) |
| North America | 94 (41.6) | 63 (33.0) | 157 (37.6) |
| Asia | 4 (1.8) | 13 (6.8) | 17 (4.1) |
| Australia | 6 (2.7) | 3 (1.6) | 9 (2.2) |
| South America | 11 (4.9) | 3 (1.6) | 14 (3.4) |
| Recruitment | |||
| Inpatient | 54 (23.9) | 45 (23.6) | 99 (23.7) |
| Ambulatory | 172 (76.1) | 146 (76.4) | 318 (76.3) |
| Type of intervention | |||
| Health service | 41 (18.1) | 14 (7.3) | 55 (13.2) |
| Exercise/rehabilitation | 19 (8.4) | 10 (5.2) | 29 (7.0) |
| Pharmaceutical | 117 (51.8) | 156 (81.7) | 273 (65.5) |
| Device | 41 (18.1) | 9 (4.7) | 50 (12.0) |
| Surgery/procedure | 8 (3.5) | 2 (1.0) | 10 (2.4) |
| Scope of trial | |||
| National | 154 (68.1) | 143 (74.9) | 297 (71.2) |
| International | 72 (31.9) | 48 (25.1) | 120 (28.8) |
| Type of funding | |||
| Industry* | 123 (54.4) | 99 (51.8) | 222 (53.2) |
| Public | 103 (45.6) | 92 (48.2) | 195 (46.8) |
| No. of centers | |||
| Multicenter | 152 (67.3) | 92 (48.2) | 244 (58.5) |
| Single center | 74 (32.7) | 99 (51.8) | 173 (41.5) |
| Gender* of the lead author | |||
| Man | 189 (83.6) | 162 (84.8) | 351 (84.2) |
| Woman | 37 (16.4) | 29 (15.2) | 66 (15.8) |
| Gender* of the senior author | |||
| Man | 201 (88.9) | 170 (89.0) | 371 (89.0) |
| Woman | 25 (11.1) | 21 (11.0) | 46 (11.0) |
| Year of publication | |||
| 2000–2003 | 46 (20.4) | 77 (40.3) | 123 (29.5) |
| 2004–2007 | 51 (22.6) | 55 (28.8) | 106 (25.4) |
| 2008–2011 | 31 (13.7) | 17 (8.9) | 48 (11.5) |
| 2012–2015 | 40 (17.7) | 13 (6.8) | 53 (12.7) |
| 2016–2020 | 58 (25.7) | 29 (15.2) | 87 (20.9) |
| Trial registration | |||
| Yes | 125 (55.3) | 54 (28.3) | 179 (42.9) |
PRO indicates patient‐reported outcome; and RCT, randomized controlled trial.
We classified trials that received partial or full industry funding as industry‐funded trials. Gender was obtained from online resources.
Among 417 trials included in this study, 5 (1.2%) RCTs engaged patients as research partners.
PRO Types and Temporal Trends
Of the 417 trials, 226 (54.2%; 95% CI, 49.3%–59.1%) included at least 1 PRO, of which 221 (97.8%) included PROs in the primary publications, and 5 (2.2%) included them in secondary publications. Most of the 226 RCTs were coordinated in Europe (49.1%), were multicenter (67.3%), and tested pharmaceutical interventions (51.8%).
Of the 226 RCTs with PROs, 44 (19.5%) reported PROs as the primary or coprimary end point, 137 RCTs (60.6%) reported a single PRO, and 89 RCTs (39.4%) reported ≥2 PROs (Table 2).
Table 2.
PRO Reporting in Heart Failure RCTs (N=226)
| PRO Reporting | |
|---|---|
| No. of PRO per trial, median (IQI) | 1 (1–2) |
| No. of CONSORT PRO items per trial, median (IQI) | 4 (3–6) |
| PRO reported in primary publication, n (%) RCTs | |
| Yes | 221 (97.8) |
| Analysis of PRO, n (%) RCTs | |
| Primary outcome | 17 (7.5) |
| coprimary, composite | 27 (11.9) |
| Secondary outcome | 182 (80.5) |
| No. of PRO instruments used, n (%) RCTs | |
| 1 | 137 (60.6) |
| 2–3 | 76 (33.6) |
| >3 | 13 (5.8) |
| No. of CONSORT PRO items reported, n (%) RCTs | |
| ≤4 | 123 (54.4) |
| 5–7 | 86 (38.1) |
| >7 | 17 (7.5) |
| Report minimal clinically important difference, n (%) RCTs | |
| Yes | 38 (16.8) |
| Type of PRO, n (%) RCTs | |
| HF specific | 98 (43.4) |
| Generic | 64 (28.3) |
| Both | 64 (28.3) |
CONSORT indicates Consolidated Standards of Reporting Trials; HF, heart failure; IQI, interquartile interval; PRO, patient‐reported outcome; and RCT, randomized controlled trial.
Most of the 226 RCTs used HF‐specific instruments including the Minnesota Living With Heart Failure Questionnaire (49.1%) and the Kansas City Cardiomyopathy Questionnaire (23.0%). A minority of studies used generic instruments including the self‐reported dyspnea scale (12.8%) and EuroQol‐5 Dimensions (EQ‐5D, 11.1%) (Table 3).
Table 3.
Types of PRO Instruments Used in Heart Failure RCTs (N=226)
| PRO types | No. (%) of RCTs (N=226) |
|---|---|
| Heart‐failure specific | |
| Minnesota Living With Heart Failure Questionnaire | 111 (49.1) |
| Kansas City Cardiomyopathy Questionnaire | 52 (23.0) |
| Chronic Heart Failure | 7 (3.1) |
| Heart Failure Self‐Care Behaviour Scale | 6 (2.7) |
| Heart Failure Knowledge Score | 5 (2.2) |
| Left Ventricular Dysfunction Questionnaire | 2 (0.9) |
| Medical Psychological Questionnaire for Heart Patients | 1 (0.4) |
| Generic questionnaires | |
| Self‐Reported Dyspnea Scale | 29 (12.8) |
| EuroQol‐5 Dimensions (EQ‐5D) | 25 (11.1) |
| Short Form Survey 36 or 12 | 24 (10.6) |
| Patient Global Assessment | 21 (9.3) |
| Generic Quality of Life | 6 (2.7) |
| Study‐specific scale | 8 (3.5) |
| Other † | 74 (32.7) |
This category included Likert‐based quality of life (QoL) questionnaires, trials using the term QoL without reference to a tool, Iceland QoL Questionnaire, and visual analog scale QoL.
Other included: Visual analog scales (VAS) such as Global Status VAS (7 trials), Sedation VAS (1 trial), Fatigue VAS (4 trials), Thirst VAS (1 trial), Fear of Movement VAS (1 trial), Daily Satisfaction VAS (2 trials), Respiratory Status VAS (2 trials), Solicited Sedation Events Questionnaire (1 trial), McMaster Overall Treatment (3 trials), Ware Satisfaction With Care Scale (1 trial), Guyatt Respiratory Scale (1 trial), Beck Depression Inventory (3 trials), Beck Anxiety Inventory (1 trial), Nausea and Vomiting (postoperative nausea and vomiting) (1 trial), Epworth Sleepiness Scale (6 trials), Duke Activity Status Index (2 trials), International Index of Erectile Function (1 trial), Hospital Anxiety and Depression Scale (4 trials), Zung Self‐Rating Depression Scale (2 trials), MacNew Heart Disease Health‐Related Quality of Life Questionnaire (2 trials), Health Complaints Scale (1 trial), Specific Activity Scale (1 trial), Patient Health Questionnaire (5 trials), Hamilton Rating for Depression (1 trial), Validated National Institutes of Health Patient‐Reported Outcome Measures (1 trial), Seattle Angina Questionnaire (1 trial), Functional Assessment of Chronic Illness Therapy (4 trials), Generalized Anxiety Disorder Questionnaire (2 trials), Decisional Conflict Scale (1 trial), Decision Regret Scale (1 trial), Validated Measures of Control Preferences (1 trial), Peace, Equanimity, and Acceptance (1 trial), Perceived Stress Scale (1 trial), Borg Rating of Perceived Exertion Scale (1 trial), General Symptom Distress Scale (1 trial), Memorial Symptom Assessment Scale (1 trial), Preparedness for Hospital Discharge to Home (1 trial), Care Transitions Measure (1 trial), Measurement System Global Health (1 trial), Measurement System Pain Intensity and Interference (1 trial).
The proportion of RCTs reporting PROs increased significantly from 37.4% (95% CI, 28.8%–46.6%) in 2000 to 2003 to 66.7% (95% CI, 55.8%–76.4%) in 2016 to 2020 (P<0.001) (Figure 2).
Figure 2. Proportion of Heart Failure RCTs with a PRO.
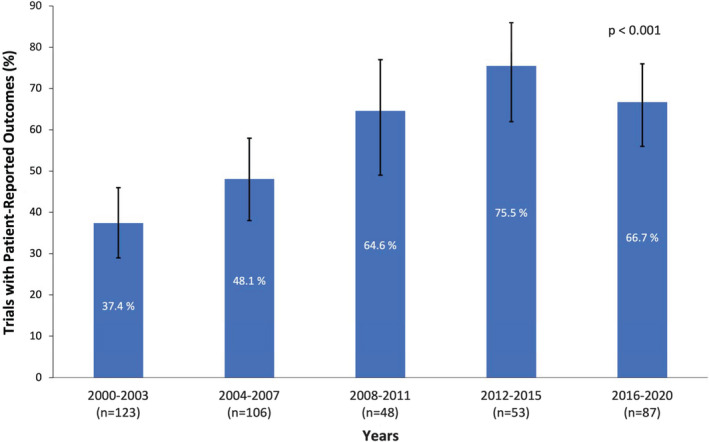
The proportion increased significantly between 2000‐2003 and 2016‐2020. Bars represent 95% CIs. PRO indicates patient‐reported outcome; and RCT, randomized controlled trial.
Multivariable Analysis of Clinical Trial Characteristics Associated With PRO Inclusion
Among 417 RCTs analyzed, the adjusted odds of PRO inclusion were significantly greater in RCTs that were published recently (aOR, 1.08 per year; 95% CI, 1.04–1.12; P<0.001); multicenter (aOR, 1.89; 95% CI, 1.03–3.46; P=0.040) versus single center; medium‐sized (n=51–250) (aOR, 2.35; 95% CI, 1.26–4.40; P=0.008) versus small (n ≤ 50) trials; coordinated in Central / South America (aOR, 5.93; 95% CI, 1.14–30.97; P=0.035) versus Asia / Australia; and that assessed health services (aOR, 3.12; 95% CI, 1.49–6.55; P=0.003), devices, or surgery (aOR, 6.66; 95% CI, 3.15–14.05; P<0.001), or exercise and rehabilitation (aOR, 4.66; 95% CI, 1.81–12.00; P=0.001) versus pharmaceutical interventions.
We did not find a significant association between large (n>250 participants) (aOR, 1.49; 95% CI, 0.69–3.17; P=0.307) versus small trials and PRO inclusion. There were no significant associations between PRO inclusion and other trial characteristics such as ambulatory recruitment (aOR, 1.07; 95% CI, 0.63–1.81; P=0.800) versus inpatient; industry funded (aOR, 1.07; 95% CI, 0.63–1.81; P=0.794) versus publicly funded trials, and trials with a female senior author or led by women (aOR, 0.94; 95% CI, 0.56–1.58; P=0.813) versus RCTs led by men. Our multivariable logistic regression had adequate calibration based on the Hosmer‐Lemeshow test (P=0.585). The area under the receiver operating characteristic curve was 0.77 (95% CI, 0.72–0.81; P<0.001), indicating an acceptable level of discrimination 16 (Figure 3).
Figure 3. Multivariable analysis of trial characteristics associated with inclusion of PROs in HF randomized controlled trials (n=417) published in high impact factor journals 2000–2020.
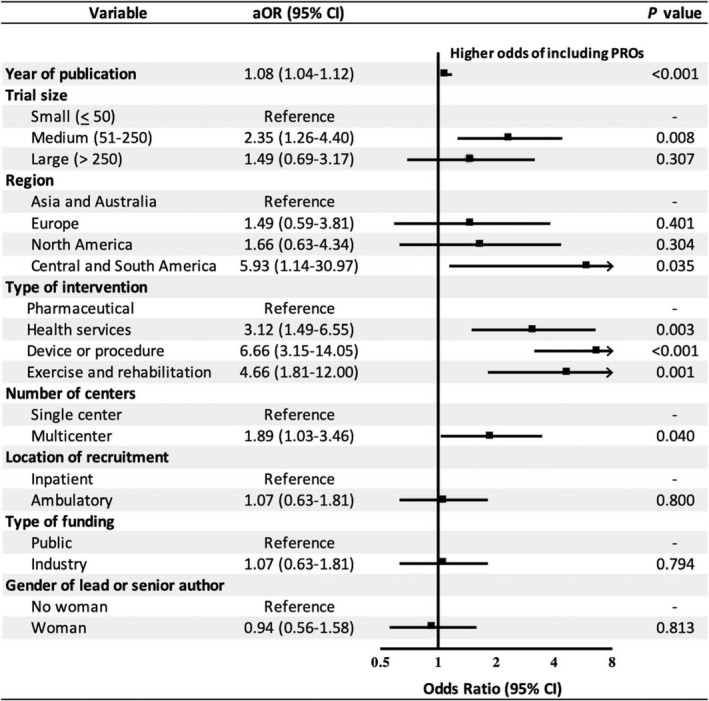
We classified trials that received partial or full industry funding as industry‐funded trials. Gender information of authors was obtained from online resources. aOR indicates adjusted odds ratio; and PRO, patient‐reported outcome.
Quality of PRO Reporting Following the CONSORT PRO Extension Statements
Most of the 226 RCTs reported a statistical analysis plan for PROs (68.6%). Only 8 RCTs used ad hoc, study‐based instruments, of which 3 (37.5%) attached a copy of the tool in the appendix.
Among 226 RCTs with PROs, 123 (54.4%) reported 4 or less of 11 CONSORT PRO items, and no trial reported all items (Table 2). Only a small minority of the 226 RCTs (7.5%) reported >7 CONSORT PRO items. The median number of CONSORT PRO items reported among the 226 RCTs was 4 (IQI, 3–6 items per trial). Of 226 RCTs with PROs, the most commonly reported items were when or how data were collected (70.4%), PRO baseline value (56.6%), instrument validity and reliability (56.2%), and discussion of PRO findings in relation to clinical outcomes (50.9%). PRO hypotheses, discussion of PRO limitations, and statistical approaches for dealing with missing PRO data were reported in a minority of HF RCTs (8.8%, 20.4%, 24.8%, respectively). The median number of CONSORT PRO items reported out of a possible total of 11 was 7 (IQI, 5–8 items per trial) in trials with PRO as a primary end point and 4 (IQI, 3–5 items per trial) in trials with PRO as a secondary end point. Of the 44 RCTs with PRO as a primary end point, the most commonly reported CONSORT PRO items were: when or how data were collected (86.4%), whether the PRO was identified as the primary or secondary outcome in the abstract (84.1%), whether the background and rationale for PRO inclusion was described (75.0%), and whether the PRO findings were interpreted in relation to clinical outcomes (72.7%) (Table 4).
Table 4.
Reporting of PROs According to CONSORT PRO 7 Guidelines (N=226)
| Section | Item | CONSORT PRO Guideline | No. (%) RCTs with PRO as primary or coprimary end point (n=44) | No. (%) RCTs with PRO as secondary end point (n=182) | Total, no. (%) RCTs with PROs (N=226) |
|---|---|---|---|---|---|
| Title and abstract | P1b* | PRO identified in the abstract as primary or secondary outcome | 37 (84.1) | 61 (33.5) | 98 (43.4) |
| Introduction | 2a | Relevant background and rationale for including PRO | 33 (75.0) | 79 (43.4) | 112 (49.6) |
| P2b | PRO and specified PRO domain hypotheses stated | 10 (22.7) | 10 (5.5) | 20 (8.8) | |
| Methods | P6a1 † | PRO instrument psychometric properties cited | 32 (72.7) | 95 (52.2) | 127 (56.2) |
| P6a2 ‡ | When or how PRO data were collected | 38 (86.4) | 121 (66.5) | 159 (70.4) | |
| P12a | Statistical approach for dealing with PRO missing data | 21 (47.7) | 35 (19.2) | 56 (24.8) | |
| 13a § | Study flow diagram describes the number of participants who completed PROs at subsequent trial phases | 6/34 (17.6) | 6/122 (4.9) | 12/156 (7.7) | |
| Results | 15 | Baseline PRO data reported | 31 (70.5) | 97 (53.3) | 128 (56.6) |
| 17a | PRO findings are described with effect size and precision estimate | 26 (59.1) | 85 (46.7) | 111 (49.1) | |
| Discussion | P20/21 | PRO‐specific limitations and implications for the generalizability of study findings and their use in clinical practice are discussed | 20 (45.5) | 26 (14.3) | 46 (20.4) |
| 22 | PROs interpreted in relation to other clinical outcomes | 32 (72.7) | 83 (45.6) | 115 (50.9) | |
| Extension | … | Statistical analysis plan for PRO assessment | 43 (97.7) | 112 (61.5) | 155 (68.6) |
| … | Copy of study‐based PRO included in supplementary file, if not previously published | 1/3 (33.3) | 2/5 (40.0) | 3/8 (37.5) |
CONSORT, indicates Consolidated Standards of Reporting Trials; PRO, patient‐reported outcome; and RCT, randomized controlled trial.
Primary CONSORT PRO items were prefixed with the letter P. Selected items not denoted with the letter P were adapted from the CONSORT 2010 statement based on CONSORT PRO group suggestions.
We scored this item if evidence of at least 1 instrument psychometric property was cited.
We scored this item based on when or how the PRO data were measured. We found 15 trials that collected PRO data via face‐to‐face interview or telephone call.
Of the 70 trials that did not publish their study flowchart, 10 and 60 trials reported PRO as a primary and secondary end point, respectively.
Sensitivity Analysis
RCTs reported a median number of 4 (IQI, 3–5 items per trial) out of 11 possible CONSORT PRO items before the introduction of CONSORT PRO extension and 5 (IQI, 3–7 items per trial) after the introduction of CONSORT PRO extension in 2013.
Discussion
In this cross‐sectional bibliometric review of 417 HF RCTs published in high‐impact medical journals between 2000 and 2020, just over half of the trials included PROs, and the proportion of RCTs reporting PROs increased significantly since 2000. In multivariable analysis, PROs had greater adjusted odds of inclusion in RCTs that were published in more recent years, were multicenter, were medium‐sized, were coordinated in Central and South America, and tested health services, devices, or surgery, and exercise and rehabilitation interventions. Among the 226 RCTs with PROs, the quality of PRO reporting was modest; overall, a majority of trials reported 4 or less of 11 CONSORT PRO items (Figure 4). A greater number of CONSORT PRO items were reported in RCTs that included PROs as the primary outcome or that were published after the introduction of CONSORT PRO extension.
Figure 4. Central illustration.
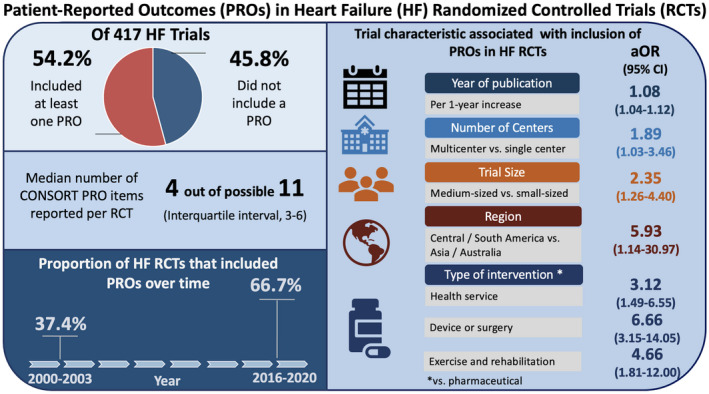
Of 417 Heart Failure RCTs, 226 (54.2%) included at least 1 PRO. The proportion of RCTs with PROs increased significantly between 2000 and 2020 (P<0.001). Year of publication, number of centers, trial size, region of coordinating center, and type of intervention were independently associated with higher adjusted odds of PRO inclusion in HF RCTs.
We found that just over half of the HF RCTs included at least 1 PRO, with an increase in the inclusion of PROs over time. The odds of PRO inclusion were greater in recently published trials after adjusting for other trial factors. The shift toward including PROs in HF RCTs reflects increased recognition of the importance of patient‐perceived health status in a condition associated with significant symptom burden. 3 , 17 , 18 , 19 Over the past 2 decades, multiple HF PRO instruments were also developed to ease the selection of available tools for clinical trials. 20 , 21 , 22 , 23 These efforts followed the recommendations of major cardiovascular societies such as the American Heart Association and European Society of Cardiology that encouraged the collection of PROs in RCTs. 3 , 4 Regulatory agencies such as the US Food and Drug Administration also used PRO data to support approval for medical product labeling claims. 1 The American Heart Association, European Society of Cardiology, and Food and Drug Administration endorsed the inclusion of PROs in early 2009. 1 In addition, funding agencies, such as the Patient‐Centered Outcome Research Institute, support clinical trials with patient‐oriented research methodology. 24 It is likely that these efforts have improved the uptake of PROs as outcome measures in HF RCTs.
The odds of PRO inclusion were higher in medium‐sized trials (n=51–250 participants) and in multicenter trials. The implementation of PROs in clinical trials is logistically complex and requires site personnel training on data collection, management, and follow‐up. 4 , 25 , 26 The inclusion of PROs in medium‐sized trials may be influenced by the manageable participant size and data collection process. The number of centers is also a reflection of the resources used to complete the study. These resources include purchasing copyrighted PRO instruments, 27 hiring an expert statistician for analysis, 28 , 29 and training site staff to maximize PRO data collection. Multicenter trials may provide operational support, include routine monitoring of site operations and providing quality control to standardize data collection, storage, and reporting of PROs. 30 , 31 , 32
We found RCTs that tested health services, device or procedures, and exercise/rehabilitation interventions had higher odds of PRO inclusion. Such trials may use PRO data to better inform routine clinical care on patient perceived health status beyond clinical outcomes such as mortality and hospitalization. 3 , 4 , 18 , 33 This information may facilitate regulatory approval and clinical decision based on patient‐important outcomes that should be included in assessing the net clinical benefit of an intervention. 3 , 4 , 18 , 33 Common efficacy end points, such as survival, can be combined with quality‐of‐life data measured with EQ‐5D to evaluate quality‐adjusted life‐years and health utilities. 4 , 34 PROs can also gauge the trade‐off between clinical efficacy and burden associated with a treatment. 4 For example, patients with advanced HF may consider the benefits of using mechanical circulatory support devices to prolong life, but the device may be burdensome or lead to significant morbidity. 4
PROs had greater odds of inclusion in RCTs coordinated in Central / South America. We found that a majority (78.6%) of the 14 trials in this region included a PRO; among the trials with PROs, 27.3% selected the PRO as the primary or coprimary end point. It is not clear why a greater proportion of trials coordinated in Central or South America included PROs. A majority of these trials were small (57.1% had <50 patients) and may have included a PRO as a primary outcome to detect a meaningful treatment effect; trials require larger sample sizes to achieve adequate statistical power to show a benefit in clinical outcomes. 35 , 36 , 37 Our findings could have been related to chance as well as unmeasured variables that we could not adjust for in our multivariable model.
Only 7.5% of HF RCTs reported >7 CONSORT PRO items in published reports. We found that basic design elements, such as reporting of PRO hypotheses, statistical approaches for dealing with PRO missing data, discussing PRO limitations, and interpreting results in relation to other clinical outcomes, were not commonly reported. These omissions may reduce data quality, research transparency, and compromise PRO interpretability. 37 , 38 , 39 More importantly, low quality of PRO reporting devalues the time and energy that trial participants spend completing PRO questionnaires.
PRO reporting was more thorough when PROs were selected as a primary or coprimary end point, which represented only 10.6% of RCTs included in our study. We found that trials with a secondary PRO publication adequately reported most of the CONSORT PRO items. It is possible that when PROs are reported as secondary end points in the primary manuscript, details around the PRO measures are minimized to adhere to manuscript word limits. Our overall findings of modest‐quality reporting are concordant with previous findings of suboptimal PRO reporting in cancer studies. 40 , 41 Importantly, we found that trials published after the introduction of the CONSORT PRO extension in 2013 reported PRO items with greater quality compared with trials published before 2013, speaking to the importance of guidelines on trial reporting.
This is the first cross‐sectional bibliometric review that identifies gaps in the quality of PRO reporting in HF RCTs published in high‐impact medical journals. Our study is unique in describing the temporal trends and quality of PRO reporting in HF RCTs. A study of 413 cardiovascular trials published between 2005 and 2008 reported that 16% of trials included PROs 10 ; however, it did not assess trial characteristics associated with PRO inclusion or evaluate the quality of PRO reporting. HF trials represented only 13.3% of articles included in this study. Another study of 33 RCTs with preserved ejection fraction found an inadequate quality of reporting based on the standard CONSORT‐2010 checklist. 42 The study did not examine quality of PRO reporting following the CONSORT PRO extension or include trials that tested nonpharmaceutical interventions. Other studies that focused on HF PROs summarized psychometric properties of these instruments to ease the selection of available tools for clinical trials, 20 , 22 , 23 , 43 , 44 , 45 , 46 but did not assess the prevalence or quality of reporting in the HF literature.
Although medical journal editorial policies vary on reporting of PRO data, consistent standards following international reporting guidelines should be encouraged and presented in the instructions for prospective authors. 47 Existing resources, such as the international reporting guidelines published by the CONSORT PRO working group, 7 should be used routinely to guide investigators on best reporting practices for PROs in clinical trials. Other reporting guidelines could be incorporated at the protocol formation stages using the Standard Protocol Items: Recommendations for Interventional Trials PRO extensions. 48 Consistent high‐quality reporting is needed to provide transparent and accurate PRO data to clinicians, healthcare policy makers, and other stakeholders to better inform patient‐centered care in HF.
The strengths of this study include a systematic search strategy and the inclusion of RCTs published over a 2‐decade time span. We incorporated best practices for systematic reviews 11 including protocol registration, independent and duplicate review processes, and data extraction. We used the international CONSORT PRO extension to evaluate quality of PRO reporting. 7
This study has several limitations. We restricted this review to English‐language articles published in medical journals with an impact factor of ≥10. Results may not be generalizable to lower‐impact journals. Recent trials included in our review may have published their PRO data as secondary articles after our last search date. Several studies were published before the development of the CONSORT PRO extension in 2013, and did not have this document to guide reporting. 7 Adherence to other reporting guidelines such as Standard Protocol Items: Recommendations for Interventional Trials PRO extensions 48 was not evaluated in this study. We acknowledge that our multivariable analysis is exploratory and results should be interpreted with caution. Finally, the sample size was selected based on established standards of 10 events per independent variable 15 ; however, the risk of overfitting may appear because of low ratio of events to the degrees of freedom. 49
Conclusions
Among 417 HF RCTs published between 2000 and 2020, 226 (54.2%) trials included at least 1 PRO. Of these 226 RCTs, 44 (19.5%) used PROs as primary or coprimary end points. The proportion of RCTs with PROs increased significantly between 2000 and 2020. PROs had higher adjusted odds of inclusion in RCTs that were published in more recent years, multicenter, medium sized (n=51–250), coordinated in Central /South America, and tested health services, devices, or surgery, or exercise and rehabilitation interventions. Among 226 RCTs with PRO data, 54.4% reported 4 or fewer of 11 CONSORT PRO items, and trials with PRO as a primary or coprimary outcome and published after the introduction of the CONSORT PRO extension in 2013 reported a greater number of CONSORT PRO items. Valuable patient‐reported information is frequently omitted in HF RCTs because of modest reporting. Adequate reporting of PROs is a necessary step in guiding regulatory decisions and clinical care in patients with HF.
Sources of Funding
Dr Van Spall receives research funding from the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada.
Disclosures
None.
Supporting information
Tables S1–S2
Acknowledgments
The authors thank K. Sullivan, M. Alruwayeh, and S. Whitelaw for their assistance with screening and data extraction.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022353
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1.US Department of Health and Human Services Food and Drug Administration Guidance for industry: patient‐reported outcome measures use in medical product development to support labeling claims. Available at: https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/patient‐reported‐outcome‐measures‐use‐medical‐product‐development‐support‐labeling‐claims. Accessed October 21, 2020.
- 2.Canadian Institute of Health Information: Patient‐Reported Outcome Measures (PROMs). Available at: https://www.cihi.ca/en/patient‐reported‐outcome‐measures‐proms. Accessed October 21, 2020.
- 3. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, et al. Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. DOI: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Agewall S, Borggrefe M, Calvert M, Jaime Caro J, Cowie MR, Ford I, Paty JA, Riley JP, Swedberg K, et al. The importance of patient‐reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J. 2014;35:2001–2009. DOI: 10.1093/eurheartj/ehu205. [DOI] [PubMed] [Google Scholar]
- 5. Van Spall HGC, Lee SF, Xie F, Ko DT, Thabane L, Ibrahim Q, Mitoff PR, Heffernan M, Maingi M, Tjandrawidjaja MC, et al. Knowledge to action: rationale and design of the Patient‐Centered Care Transitions in Heart Failure (PACT‐HF) stepped wedge cluster randomized trial. Am Heart J. 2018;199:75–82. DOI: 10.1016/j.ahj.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 6. Van Spall HGC, Lee SF, Xie F, Oz UE, Perez R, Mitoff PR, Maingi M, Tjandrawidjaja MC, Heffernan M, Zia MI, et al. Effect of patient‐centered transitional care services on clinical outcomes in patients hospitalized for heart failure: the PACT‐HF randomized clinical trial. JAMA. 2019;321:753–761. DOI: 10.1001/jama.2019.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD; CONSORT PRO Group . Reporting of patient‐reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309:814–822. DOI: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 8. Blumenthal DM, Strom JB, Valsdottir LR, Howard SE, Wagle NW, Ho KKL, Horn DM, O’Keefe SM, Wasfy JH, Metlay JP, et al. Patient‐reported outcomes in cardiology: comparison of two programs to assess angina burden in coronary artery disease. Circ Cardiovasc Qual Outcomes. 2018;11:e004794. DOI: 10.1161/CIRCOUTCOMES.118.004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weldring T, Smith SM. Patient‐reported outcomes (PROs) and patient‐reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68. DOI: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahimi K, Malhotra A, Banning AP, Jenkinson C. Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: systematic review. BMJ. 2010;341:c5707–c5707. DOI: 10.1136/bmj.c5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. DOI: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Web of Science 2019 Journal Citation Reports (JCR). Available at: https://clarivate.com/webofsciencegroup/article/announcing‐the‐2019‐journal‐citation‐. Accessed July 20, 2020.
- 13. Web of Science Author search. Available at: https://clarivate.libguides.com/woscc/authorid. Accessed July 20, 2020.
- 14. Brundage M, Blazeby J, Revicki D, Bass B, De Vet H, Duffy H, Efficace F, King M, Lam CLK, Moher D, et al. Patient‐reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Qual Life Res. 2013;22:1161–1175. DOI: 10.1007/s11136-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstem AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. DOI: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 16. Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. John Wiley & Sons; 2013. [Google Scholar]
- 17. Jeon YH, Kraus SG, Jowsey T, Glasgow NJ. The experience of living with chronic heart failure: a narrative review of qualitative studies. BMC Health Serv Res. 2010;10:1–9. DOI: 10.1186/1472-6963-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen LA, Hernandez AF, O’Connor CM, Felker GM. End points for clinical trials in acute heart failure syndromes. J Am Coll Cardiol. 2009;53:2248–2258. DOI: 10.1016/j.jacc.2008.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang S, Newton PJ, Inglis S, Luckett T, Krum H, Macdonald P, Davidson PM. Are all outcomes in chronic heart failure rated equally? An argument for a patient‐centred approach to outcome assessment. Heart Fail Rev. 2014;19:153–162. DOI: 10.1007/s10741-012-9369-0. [DOI] [PubMed] [Google Scholar]
- 20. Thompson LE, Bekelman DB, Allen LA, Peterson PN. Patient‐reported outcomes in heart failure: existing measures and future uses. Curr Heart Fail Rep. 2015;12:236–246. DOI: 10.1007/s11897-015-0253-9. [DOI] [PubMed] [Google Scholar]
- 21. Spertus JA, Tooley J, Jones P, Poston C, Mahoney E, Deedwania P, Hurley S, Pitt B, Weintraub WS. Expanding the outcomes in clinical trials of heart failure: the quality of life and economic components of EPHESUS (EPlerenone's neuroHormonal Efficacy and SUrvival Study). Am Heart J. 2002;143:636–642. DOI: 10.1067/mhj.2002.120775. [DOI] [PubMed] [Google Scholar]
- 22. Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L, Valderas JM, Guillemin F, Revicki D, Alonso J. Assessing health‐related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev. 2014;19:359–367. DOI: 10.1007/s10741-013-9394-7. [DOI] [PubMed] [Google Scholar]
- 23. Kelkar AA, Spertus J, Pang P, Pierson RF, Cody RJ, Pina IL, Hernandez A, Butler J. Utility of patient‐reported outcome instruments in heart failure. JACC Heart Fail. 2016;4:165–175. DOI: 10.1016/j.jchf.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Patient‐Centered Outcomes Research Institute (PCORI): what & who we fund. Available at: https://www.pcori.org/funding‐opportunities/what‐who‐we‐fund. Accessed February 4, 2021.
- 25. Mercieca‐Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient‐reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–367. DOI: 10.2147/PROM.S156279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mercieca‐Bebber R, Calvert M, Kyte D, Stockler M, King MT. The administration of patient‐reported outcome questionnaires in cancer trials: interviews with trial coordinators regarding their roles, experiences, challenges and training. Contemp Clin Trials Commun. 2018;9:23–32. DOI: 10.1016/j.conctc.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watkins Bruner D, Bryan CJ, Aaronson N, Blackmore CC, Brundage M, Cella D, Ganz PA, Gotay C, Hinds PS, Kornblith AB, et al. Issues and challenges with integrating patient‐reported outcomes in clinical trials supported by the National Cancer Institute–sponsored clinical trials networks. Am J Clin Oncol. 2007;25:5051. DOI: 10.1200/JCO.2007.11.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Z, Wang X, Barnhart HX, Wang Y. Working with statisticians in clinical research. Stroke. 2018;49:e311–e313. DOI: 10.1161/STROKEAHA.118.022266. [DOI] [PubMed] [Google Scholar]
- 29. Fielding S, Maclennan G, Cook JA, Ramsay CR. A review of RCTs in four medical journals to assess the use of imputation to overcome missing data in quality of life outcomes. Trials. 2008;9:1–6. DOI: 10.1186/1745-6215-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Appel LJ. A primer on the design, conduct, and interpretation of clinical trials. Clin J Am Soc Nephrol. 2006;1:1360–1367. DOI: 10.2215/CJN.02850806. [DOI] [PubMed] [Google Scholar]
- 31. Meinert CL, Tonascia S. Single‐Center Versus Multicenter Trials: Clinical Trials. Oxford University Press; 2009:23–29. [Google Scholar]
- 32. Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single‐center trials. Crit Care Med. 2009;37:3114–3119. DOI: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
- 33. Spertus JA. Evolving applications for patient‐centered health status measures. Circulation. 2008;118:2103–2110. DOI: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 34. Devlin NJ, Brooks R. EQ‐5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy. 2017;15:127–137. DOI: 10.1007/s40258-017-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walters SJ, Jacques RM, Dos Anjos Henriques‐Cadby IB, Candlish J, Totton N, Xian MTS. Sample size estimation for randomised controlled trials with repeated assessment of patient‐reported outcomes: what correlation between baseline and follow‐up outcomes should we assume? Trials. 2019;20:1–6. DOI: 10.1186/s13063-019-3671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sloan JA, Dueck AC, Erickson PA, Guess H, Revicki DA, Santanello NC; Mayo/FDA Patient‐Reported Outcomes Consensus Meeting Group . Analysis and interpretation of results based on patient‐reported outcomes. Value Health. 2007;10:S106–S115. DOI: 10.1111/j.1524-4733.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 37. Revicki DA, Erickson PA, Sloan JA, Dueck A, Guess H, Santanello NC; Mayo/FDA Patient‐Reported Outcomes Consensus Meeting Group . Interpreting and reporting results based on patient‐reported outcomes. Value Health. 2007;10:S116–S124. DOI: 10.1111/j.1524-4733.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 38. Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374:86–89. DOI: 10.1016/S0140-6736(09)60329-9. [DOI] [PubMed] [Google Scholar]
- 39. Kyte D, Ives J, Draper H, Keeley T, Calvert M. Inconsistencies in quality of life data collection in clinical trials: a potential source of bias? Interviews with research nurses and trialists. PLoS One. 2013;8:e76625. DOI: 10.1371/journal.pone.0076625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kyte D, Retzer A, Ahmed K, Keeley T, Armes JO, Brown JM, Calman L, Gavin A, Glaser AW, Greenfield DM, et al. Systematic evaluation of patient‐reported outcome protocol content and reporting in cancer trials. J Natl Cancer Inst. 2019;111:1170–1178. DOI: 10.1093/jnci/djz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Efficace F, Fayers P, Pusic A, Cemal Y, Yanagawa J, Jacobs M, La Sala A, Cafaro V, Whale K, Rees J, et al. Quality of patient‐reported outcome reporting across cancer randomized controlled trials according to the CONSORT patient‐reported outcome extension: a pooled analysis of 557 trials. Cancer. 2015;121:3335–3342. DOI: 10.1002/cncr.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng SL, Chan FT, Maclean E, Jayakumar S, Nabeebaccus AA. Reporting trends of randomised controlled trials in heart failure with preserved ejection fraction: a systematic review. Open Heart. 2016;3:e000449. DOI: 10.1136/openhrt-2016-000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baert A, De Smedt D, De Sutter J, De Bacquer D, Puddu PE, Clays E, Pardaens S. Factors associated with health‐related quality of life in stable ambulatory congestive heart failure patients: systematic review. Eur J Prev Cardiol. 2018;25:472–481. DOI: 10.1177/2047487318755795. [DOI] [PubMed] [Google Scholar]
- 44. Garin O, Ferrer M, Pont À, Rué M, Kotzeva A, Wiklund I, Van Ganse E, Alonso J. Disease‐specific health‐related quality of life questionnaires for heart failure: a systematic review with meta‐analyses. Qual Life Res. 2009;18:71–85. DOI: 10.1007/s11136-008-9416-4. [DOI] [PubMed] [Google Scholar]
- 45. Sedlar N, Socan G, Farkas J, Mårtensson J, Strömberg A, Jaarsma T, Lainscak M. Measuring self‐care in patients with heart failure: a review of the psychometric properties of the European Heart Failure Self‐Care Behaviour Scale (EHFScBS). Patient Educ Couns. 2017;100:1304–1313. DOI: 10.1016/j.pec.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 46. McDonagh J, Martin L, Ferguson C, Jha SR, Macdonald PS, Davidson PM, Newton PJ. Frailty assessment instruments in heart failure: a systematic review. Eur J Cardiovasc Nurs. 2018;17:23–35. DOI: 10.1177/1474515117708888. [DOI] [PubMed] [Google Scholar]
- 47. Cobo E, Cortes J, Ribera JM, Cardellach F, Selva‐O'Callaghan A, Kostov B, Garcia L, Cirugeda L, Altman DG, Gonzalez JA, et al. Effect of using reporting guidelines during peer review on quality of final manuscripts submitted to a biomedical journal: masked randomised trial. BMJ. 2011;343:d6783. DOI: 10.1136/bmj.d6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Calvert M, Kyte D, Mercieca‐Bebber R, Slade A, Chan A‐W, King MT, Hunn A, Bottomley A, Regnault A, Chan A‐W, et al. Guidelines for inclusion of patient‐reported outcomes in clinical trial protocols: the SPIRIT‐PRO extension. JAMA. 2018;319:483–494. DOI: 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 49. Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol. 2016;76:175–182. DOI: 10.1016/j.jclinepi.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


