Abstract
Background
Direct oral anticoagulants (DOACs) are widely used in patients with nonvalvular atrial fibrillation for stroke prevention. However, long‐term adherence to DOACs and clinical outcomes in real‐world clinical practice is not well understood. This study evaluated long‐term medication adherence patterns to DOAC therapy and clinical outcomes in a large US integrated health care system.
Methods and Results
We included adult patients with nonvalvular atrial fibrillation who newly initiated DOACs between 2012 and 2018 in Kaiser Permanente Southern California. Long‐term (3.5 years) adherence trajectories to DOAC were investigated using monthly proportion of days covered and group‐based trajectory models. Factors associated with long‐term adherence trajectories were investigated. Multivariable Poisson regression analyses were used to investigate thromboembolism and major bleeding events associated with long‐term adherence trajectories. Of 18 920 patients newly initiating DOACs, we identified 3 DOAC adherence trajectories: consistently adherent (85.2%), early discontinuation within 6 months (10.6%), and gradually declining adherence (4.2%). Predictors such as lower CHA2DS2‐VASc (0–1 versus ≥5) and previous injurious falls were associated with both early discontinuation and gradually declining adherence trajectories. Early discontinuation of DOAC therapy was associated with a higher risk of thromboembolism (rate ratio, 1.40; 95% CI, 1.05–1.86) especially after 12 months from DOAC initiation but a lower risk of major bleed compared with consistent adherence (rate ratio, 0.48; 95% CI, 0.30–0.75), specifically during the first 12 months following DOAC initiation. A gradual decline in adherence to DOACs was not statistically significantly associated with thromboembolism outcomes compared with consistent adherence.
Conclusions
Although a large proportion of patients with nonvalvular atrial fibrillation were adherent to DOAC therapy over 3.5 years, early discontinuation of DOAC was associated a higher risk of thromboembolic events. Future tailored interventions for early discontinuers may improve clinical outcomes.
Keywords: anticoagulant, atrial fibrillation, medication adherence
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- BIC
Bayesian Information Criterion
- DOACs
direct oral anticoagulants
- KPSC
Kaiser Permanente Southern California
- NVAF
nonvalvular atrial fibrillation
- PDC
proportion of days covered
Clinical Perspective
What Is New?
With a systematic direct oral anticoagulants monitoring program in a US integrated health care system, over 85% patients with nonvalvular atrial fibrillation who were on direct oral anticoagulants were consistently adherent to their therapy for 3.5 years.
Several clinical factors were associated with direct oral anticoagulant adherence trajectories, which can be used for identifying candidates for future adherence interventions.
Early discontinuation of direct oral anticoagulant therapy was associated with a higher risk of thromboembolism compared with consistent adherence.
What Are the Clinical Implications?
Future tailored interventions for early discontinuers may improve clinical outcomes.
Major bleeding events in early treatment periods in those with consistent adherence should be monitored carefully.
Direct oral anticoagulants (DOACs) are widely used for prevention of ischemic stroke in patients with nonvalvular atrial fibrillation (NVAF). 1 , 2 Once patients with NVAF initiate DOAC therapy, most are expected to continue DOAC medications for their lifetime. Compared with warfarin, DOACs are more convenient because they have fewer interactions with food, less need for routine laboratory monitoring, and a lower risk of bleeding. 3 , 4 , 5 However, there is uncertainty whether patients taking DOACs are able to take their medications as directed for a long time without the intensive monitoring and provider follow‐up required for warfarin. 6 Previous studies in the United States have shown that the mean proportion of days covered (PDC) ranges from 0.67 to 0.86 during the first 6 months and between 0.57 to 0.89 during the first 12 months of DOAC therapy 1 , 7 , 8 , 9 , 10 ; in other studies, ≈1 in 3 patients were found to be nonadherent, defined as having PDC <0.80. 4 , 11 , 12 Data on adherence to DOAC therapy beyond 12 months are currently limited. 13
Medication adherence is typically summarized using PDC during a fixed follow‐up time, most frequently 6 or 12 months. 13 More recently, longitudinal adherence patterns have been identified using group‐based trajectory modeling. 14 , 15 , 16 This modeling approach is able to more appropriately categorize complex nonadherence behaviors. Two recent studies identified 4 different adherence patterns of oral anticoagulants using the group‐based trajectory modeling among Medicare beneficiaries such as early discontinuers, late initiators, late discontinuers, and continuous adherers. 17 , 18 However, these studies also focused on adherence during the first 12 months after NVAF diagnosis, and therefore, long‐term adherence trajectories to DOAC therapy are still largely unknown.
In this study, we investigated long‐term medication adherence patterns using group‐based trajectories to DOAC therapy. We further investigated factors associated with long‐term adherence trajectories and clinical outcomes of thromboembolism and major bleeding associated with DOAC medication adherence trajectories.
METHODS
Anonymized data that support the findings of this study may be made available from the investigative team in the following conditions: (1) agreement to collaborate with the study team on all publications, (2) provision of external funding for administrative and investigator time necessary for this collaboration, (3) demonstration that the external investigative team is qualified and has documented evidence of training for human subjects protections, and (4) agreement to abide by the terms outlined in data use agreements between institutions.
Study Cohort
We conducted a retrospective cohort study by identifying new users of DOAC among patients with NVAF. Kaiser Permanente Southern California (KPSC) is a large integrated health care delivery system that provides medical services to its members through its own facilities (15 hospitals, more than 200 outpatient facilities). All clinical care and interactions with the system are captured in comprehensive electronic health records. Over 95% of KPSC members have a pharmacy benefit and, therefore, have an incentive to fill their medication within the system. All dispensed prescriptions and claims outside KPSC are captured in the pharmacy database. This study was approved by the KPSC institutional review board, and patient informed consent was waived.
We included patients ≥18 years of age with a prescription fill for a DOAC (dabigatran, apixaban, edoxaban, or rivaroxaban) between January 2012 and December 2018 in KPSC. The first date of DOAC prescription dispense was defined as the index date. Patients were required to have a diagnosis of atrial fibrillation or atrial flutter (International Classification of Diseases, Ninth Revision [ICD‐9] codes of 427.3, 427.31, 427.32; International Classification of Diseases, Tenth Revision [ICD‐10] codes of I48.x) within 12 months before the index date. Eligible patients also were members of the health plan (allowing a 45‐day gap) and had to have a pharmacy benefit 12 months before the index date. Patients with a mechanical heart valve, mitral valve stenosis, or severe liver disease and those residing in a nursing home or on hospice in the 12 months before the index date were excluded from the analysis. Women who were pregnant before index or during follow‐up were also excluded. Patients were required to have a minimum 30 days follow‐up and 2 prescription dispenses to allow adherence calculations. Therefore, patients with <30 days membership or an outcome event within 30 days of index were also excluded. Two separate cohorts (one for thromboembolic events and the other for major bleeding) were created to evaluate efficacy and safety outcomes separately. The cohort diagram is shown in Figure S1.
Patients were followed from the index date until the outcomes of interest (thromboembolic events or major bleeding), death, disenrollment from the health plan, switching from DOAC therapy to warfarin, or study end date (December 31, 2018), whichever occurred first.
Monthly Medication Adherence
Adherence to any DOAC medication was calculated using pharmacy dispensing data from electronic health records. DOAC adherence was defined using PDC in monthly intervals (denominator of PDC). If a patient was censored before the next monthly interval, the denominator of PDC in that specific period was up to the censoring date instead of 30 days. The refill date was adjusted for patients who refilled their medications earlier than scheduled by shifting the fill date forward to the day after the end of supply of the previous fill. Patients who switched therapy between DOACs (eg, dabigatran to apixaban) were considered as continuing DOAC therapy.
Outcomes
The outcomes of interest were thromboembolism (ischemic stroke and systemic embolism) and major bleeding (intracranial hemorrhage, major gastrointestinal bleeding, and other major bleeding). Hospital discharge diagnoses in the primary position were used to identify these events, which have shown positive predictive values of 77% to 85% (Table S1). 19 , 20
Patient Demographic and Clinical Characteristics
Patient demographic characteristics (age, sex, race and ethnicity) and census block‐level median household income were identified. We included clinically important medical characteristics using 12 months of data before the index date. Clinical characteristics included presence of congestive heart failure, hypertension, diabetes, stroke, transient ischemic attack, peripheral vascular disease, myocardial infarction, bleeding, chronic obstructive pulmonary disease, alcoholism, cancer, injurious falls, and dementia and were defined by diagnosis and procedure codes. Creatinine clearance was calculated using the Cockcroft‐Gault equation recommended by clinical guidelines for DOACs. 21 , 22 CHA2DS2‐VASc and HAS‐BLED scores were calculated to assess stroke and bleeding risk before the index date. 21 Specific medications dispensed in the 12 months before the index date were collected including warfarin, antihypertensive agents, antiarrhythmic agents, and antiplatelet agents. The first DOAC (dabigatran, apixaban, edoxaban, or rivaroxaban) prescribed and the first DOAC prescriber specialty (cardiology, internal medicine/family medicine, others) were collected.
Statistical Analysis
Using the monthly PDC, we applied group‐based trajectory modeling to detect and define groups of patients with similar adherence patterns following initiation of the DOAC medication. 14 Trajectories were modeled using flexible polynomial models based on significant linear, quadratic, and cubic terms. The optimal number of trajectories was selected by comparing Bayesian information criterion and the mean of posterior probabilities in each class. Because PDC captures the number of days covered each month and frequent zero counts were observed, the zero‐inflated Poisson model was used. Long‐term adherence trajectories were investigated until 5% of patients remained in the cohort (3.5 years). Patients were assigned to 1 of the trajectory groups based on the estimated probabilities of membership in each trajectory group. Mean (SD) PDC during follow‐up was compared by adherence trajectory groups.
Descriptive statistics were used to compare patient demographic and clinical characteristics by adherence trajectory groups. To investigate further how these characteristics are associated with each adherence trajectory group, we conducted multinomial logistic regression analyses. Clinically relevant predictors (age, sex, race and ethnicity, CHA2DS2‐VASc score, HAS‐BLED score) were included in the model. Also, other potential predictors were tested as covariates and statistically significant variables from univariate models were included in the final multivariable model. Odds ratios (ORs) with 95% CI were reported.
To evaluate clinical outcomes associated with long‐term adherence trajectories, we calculated thromboembolic and major bleeding events per 100 person‐years during follow‐up. We also evaluated outcomes by timing of events (during the first 12 months, and after 12 months). We conducted multivariable Poisson regression analyses to investigate associations between clinical outcomes and long‐term adherence trajectories. Clinically relevant covariates were tested as covariates and statistically significant variables from univariate models were included in the final multivariable model. Sensitivity analyses were conducted after excluding previous warfarin users and patients with CHA2DS2‐VASc score ≤1. Rate ratios with 95% CIs were reported. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
We included a total of 18 920 patients with NVAF initiating DOAC therapy for the thromboembolic outcomes analysis, and 18 940 patients for bleeding outcomes analysis (Figure S1). Among the 18 920 patients with NVAF, the mean (SD) age was 72.6 (10.6) years, 42.8% were female, and 64.5% were non‐Hispanic White patients, 18.1% Hispanic patients, 8.2% Asian/Pacific Islanders, and 7.2% were non‐Hispanic Black patients (Table 1). Most patients (92.5%) initiated treatment with dabigatran and 5.3% initiated with apixaban. Most patients (82.1%) had CHA2DS2‐VASc score ≥2.
Table 1.
Patient and Clinical Characteristics by Long‐Term Adherence Trajectories
| Characteristics | Total (n=18 920) | Consistent adherence (n=16 111) | Early discontinuation (n=2008) | Gradual decline (n=801) |
|---|---|---|---|---|
| Age, y | 72.6 (10.6) | 73.2 (10.1) | 69.7 (13.3) | 68.9 (12.3) |
| Age category, y | ||||
| <65 | 3889 (20.6%) | 2960 (18.4%) | 650 (32.4%) | 279 (34.8%) |
| 65–74 | 6740 (35.6%) | 5898 (36.6%) | 598 (29.8%) | 244 (30.5%) |
| 75–84 | 6275 (33.2%) | 5519 (34.3%) | 537 (26.7%) | 219 (27.3%) |
| ≥85 | 2016 (10.7%) | 1734 (10.8%) | 223 (11.1%) | 59 (7.4%) |
| Female sex | 8107 (42.8%) | 7056 (43.8%) | 748 (37.3%) | 303 (37.8%) |
| Race or Ethnicity | ||||
| Hispanic | 3421 (18.1%) | 2860 (17.8%) | 415 (20.7%) | 146 (18.2%) |
| Asian/Pacific Islander | 1556 (8.2%) | 1343 (8.3%) | 141 (7.0%) | 72 (9.0%) |
| Non‐Hispanic Black | 1361 (7.2%) | 1117 (6.9%) | 166 (8.3%) | 78 (9.7%) |
| Non‐Hispanic White | 12 206 (64.5%) | 10 481 (65.1%) | 1239 (61.7%) | 486 (60.7%) |
| Multi‐race/Native American/Unknown | 376 (2.0%) | 310 (1.9%) | 47 (2.3%) | 19 (2.4%) |
| Neighborhood household income | ||||
| ≤$25 000 | 184 (1.0%) | 153 (0.9%) | 20 (1.0%) | 11 (1.4%) |
| $25 001–$50 000 | 4143 (21.9%) | 3505 (21.8%) | 465 (23.2%) | 173 (21.6%) |
| $50 001–$80 000 | 7943 (42.0%) | 6810 (42.3%) | 798 (39.7%) | 335 (41.8%) |
| >$80 000 | 6361 (33.6%) | 5409 (33.6%) | 690 (34.4%) | 262 (32.7%) |
| Unknown | 289 (1.5%) | 234 (1.5%) | 35 (1.7%) | 20 (2.5%) |
| Clinical characteristics | ||||
| Congestive heart failure | 3012 (15.9%) | 2614 (16.2%) | 267 (13.3%) | 131 (16.4%) |
| Hypertension | 11 582 (61.2%) | 10 061 (62.4%) | 1053 (52.4%) | 468 (58.4%) |
| Diabetes | 5202 (27.5%) | 4549 (28.2%) | 456 (22.7%) | 197 (24.6%) |
| Stroke/transient ischemic attack | 1332 (7.0%) | 1195 (7.4%) | 96 (4.8%) | 41 (5.1%) |
| Peripheral vascular disease | 408 (2.2%) | 350 (2.2%) | 42 (2.1%) | 16 (2.0%) |
| Intracranial hemorrhage | 66 (0.3%) | 58 (0.4%) | 6 (0.3%) | 2 (0.2%) |
| Gastrointestinal bleed | 1134 (6.0%) | 988 (6.1%) | 103 (5.1%) | 43 (5.4%) |
| Other bleed | 1158 (6.1%) | 999 (6.2%) | 111 (5.5%) | 48 (6.0%) |
| Creatinine clearance (CrCl), mL/min | ||||
| CrCl ≥60 | 13 128 (69.4%) | 11 140 (69.1%) | 1391 (69.3%) | 597 (74.5%) |
| CrCl 30–59 | 4912 (26.0%) | 4264 (26.5%) | 480 (23.9%) | 168 (21.0%) |
| CrCl 15–29 | 308 (1.6%) | 258 (1.6%) | 41 (2.0%) | 9 (1.1%) |
| CrCl <15 | 26 (0.1%) | 21 (0.1%) | 5 (0.2%) | 0 (0%) |
| Missing | 546 (2.9%) | 428 (2.7%) | 91 (4.5%) | 27 (3.4%) |
| Alcoholism | 822 (4.3%) | 706 (4.4%) | 80 (4.0%) | 36 (4.5%) |
| Dementia | 932 (4.9%) | 795 (4.9%) | 101 (5.0%) | 36 (4.5%) |
| Injurious fall | 1620 (8.6%) | 1325 (8.2%) | 181 (9.0%) | 114 (14.2%) |
| Chronic obstructive pulmonary disease | 2331 (12.3%) | 2048 (12.7%) | 204 (10.2%) | 79 (9.9%) |
| Cancer | 1686 (8.9%) | 1467 (9.1%) | 162 (8.1%) | 57 (7.1%) |
| Myocardial infarction | 772 (4.1%) | 655 (4.1%) | 93 (4.6%) | 24 (3.0%) |
| Charlson Comorbidity Index | 2.4 (1.9) | 2.4 (1.9) | 2.1 (1.9) | 2.0 (1.8) |
| CHA2DS2‐VASc score | 2.9 (1.5) | 2.9 (1.4) | 2.4 (1.6) | 2.5 (1.6) |
| CHA2DS2‐VASc score category | ||||
| 0–1 | 3392 (17.9%) | 2518 (15.6%) | 632 (31.5%) | 242 (30.2%) |
| 2 | 4239 (22.4%) | 3676 (22.8%) | 399 (19.9%) | 164 (20.5%) |
| 3 | 4945 (26.1%) | 4320 (26.8%) | 447 (22.3%) | 178 (22.2%) |
| 4 | 4000 (21.1%) | 3535 (21.9%) | 331 (16.5%) | 134 (16.7%) |
| 5 or more | 2344 (12.4%) | 2062 (12.8%) | 199 (9.9%) | 83 (10.4%) |
| HAS‐BLED score | ||||
| 0–1 | 10 073 (53.2%) | 8416 (52.2%) | 1202 (59.9%) | 455 (56.8%) |
| 2 | 6138 (32.4%) | 5337 (33.1%) | 556 (27.7%) | 245 (30.6%) |
| 3 | 2203 (11.6%) | 1914 (11.9%) | 204 (10.2%) | 85 (10.6%) |
| 4 or more | 506 (2.7%) | 444 (2.8%) | 46 (2.3%) | 16 (2.0%) |
| Medication use at baseline | ||||
| Warfarin | 5511 (29.1%) | 4991 (31.0%) | 327 (16.3%) | 193 (24.1%) |
| Antihypertensive medications | 18 067 (86.5%) | 15 567 (87.7%) | 1761 (77.8%) | 739 (85.3%) |
| Antiarrhythmic medications | 1724 (8.3%) | 1403 (7.9%) | 227 (10.0%) | 94 (10.9%) |
| Antiplatelet agents | 5795 (27.8%) | 4945 (27.9%) | 609 (26.9%) | 241 (27.8%) |
| First DOAC | ||||
| Dabigatran | 17 496 (92.5%) | 14 929 (92.7%) | 1838 (91.5%) | 729 (91.0%) |
| Apixaban | 998 (5.3%) | 856 (5.3%) | 99 (4.9%) | 43 (5.4%) |
| Rivaroxaban | 423 (2.2%) | 323 (2.0%) | 71 (3.5%) | 29 (3.6%) |
| Edoxaban | 3 (0%) | 3 (0%) | 0 (0%) | 0 (0%) |
| First DOAC prescriber specialty | ||||
| Cardiology | 5276 (27.9%) | 4607 (28.6%) | 455 (22.7%) | 214 (26.7%) |
| Internal medicine/family medicine | 7964 (42.1%) | 6847 (42.5%) | 820 (40.8%) | 297 (37.1%) |
| Others | 5680 (30.0%) | 4657 (28.9%) | 733 (36.5%) | 290 (36.2%) |
| Mean (SD) follow‐up time in days | 510.0 (415.0) | 481.6 (411.6) | 600.7 (383.0) | 853.8 (372.7) |
The data reported either mean (SD) or N (%). DOAC indicates direct oral anticoagulants.
Long‐Term Adherence Trajectories
Models with up to 6 trajectory groups were considered. Cubic terms were nonsignificant in all models; therefore, the second‐order polynomial model was selected. After model order was determined, Bayesian information criterion selected 3 distinct trajectories, and the mean of posterior probabilities in each class showed good discrimination between groups. Visual inspection of these trajectories supported 3 distinct adherence patterns over 3.5 years of follow‐up. Visual inspection also showed that models allowing up to 6 trajectory groups showed only 3 meaningful trajectory groups, confirming the selection of 3 distinct groups was optimal (consistent adherence [85.2%], early discontinuation [10.6%], and gradual decline [4.2%]) (Figure 1). Among those with 3.5 years of follow‐up, 78.0% were consistently adherent. Mean (SD) PDC for consistently adherent patients was 0.91 (0.17) during the first 6 months and PDCs remained consistently high throughout follow‐up (Figure 1). The PDC of the early discontinuation group was low in the first 6 months (mean [SD] PDC=0.35 [0.20]). The PDC of the gradual decline group was moderately high in the first 6 months (mean [SD]=0.74 [0.27]) and started to decline between 7 and 12 months (mean [SD]=0.36 [0.32]) and stayed below 0.13 after 12 months (Figure 1).
Figure 1. Long‐term adherence trajectories to direct oral anticoagulants.
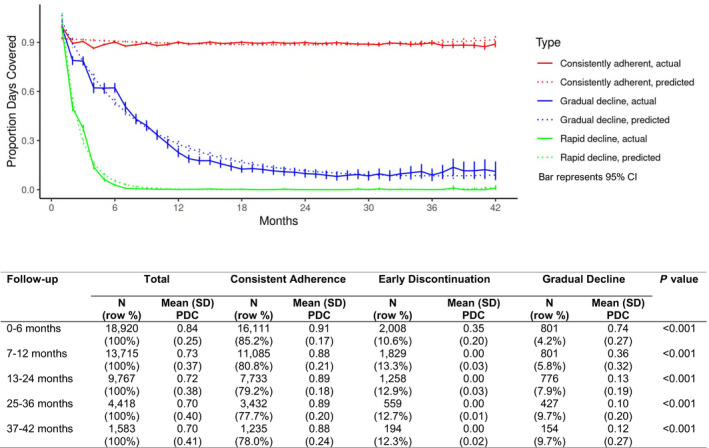
Mean PDC over time by adherence trajectory groups (consistent adherence, early discontinuation, and gradual decline). PDC indicates proportion of days covered.
Patient and Clinical Characteristics Associated With Long‐Term Adherence Trajectory Groups
Patients who were consistently adherent to DOACs were older and a greater proportion were female and non‐Hispanic White patients compared with the early discontinuation and gradual decline groups (Table 1).
Figure 2 shows factors associated with the long‐term adherence trajectory groups. Factors associated with the early discontinuation trajectory include age <65 years or ≥85 years (versus 75–84 years), non‐Hispanic Black or Hispanic (versus non‐Hispanic White), lower CHA2DS2‐VASc scores of 0 or 1 (versus 5 or higher) and lower creatinine clearance (30–59 or <30 mL/min versus ≥60 mL/min), baseline antiarrhythmic agent use, other prescriber specialty (versus cardiology) as a first DOAC prescriber, having dementia, and previous injurious falls (Figure 2A Early Discontinuation). Warfarin users during the baseline period were less likely to have early discontinuation of DOAC therapy. Similar factors were associated with the gradual decline trajectory (Figure 2B Gradual Decline). HAS‐BLED score was not associated with the long‐term adherence trajectories.
Figure 2. Factors associated with long‐term adherence trajectories.
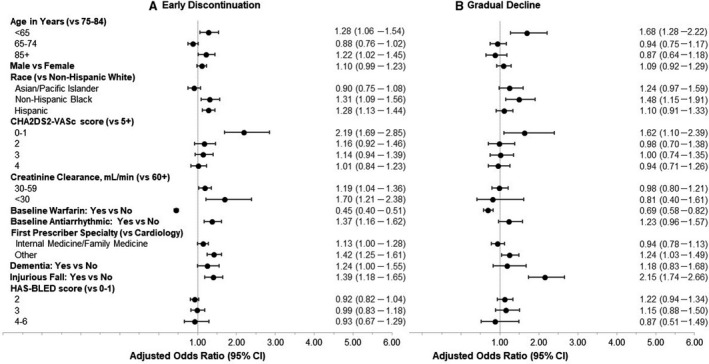
A, Odds ratio (95% CI) of early discontinuation trajectory compared with consistent adherence trajectory. B, Odds ratio (95% CI) of gradually declining adherence trajectory compared with consistent adherence trajectory.
Thromboembolic and Major Bleeding Event by Long‐Term Adherence Trajectories
Thromboembolic and major bleeding events are shown stratified by long‐term adherence trajectory groups as well as follow‐up period (either during the first 12 months or after 12 months from the index date) (Table 2). Thromboembolism event rates were 1.41 per 100 person‐years for the consistent adherence group, 1.78 per 100 person‐years for the early discontinuation group, and 0.96 per 100 person‐years for the gradual decline group. More thromboembolism events occurred during the first 12 months in the consistent adherence group, whereas more thromboembolism events occurred after 12 months from the index date in the early discontinuation and gradual decline groups. Major bleed event rates were 1.46 per 100 person‐years for the consistent adherence group, 0.62 per 100 person‐years for the early discontinuation group, and 0.53 per 100 person‐years for the gradual decline group. More major bleed events occurred during the first 12 months than after 12 months in the consistent adherence group but not in the other 2 groups.
Table 2.
Thromboembolism and Major Bleeding Stratified by Long‐Term Adherence Trajectories
| Consistent adherence | Early discontinuation | Gradual decline | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | First 12 mo | After 12 mo | Total | First 12 mo | After 12 mo | Total | First 12 mo | After 12 mo | |
| Thromboembolic events* | |||||||||
| Total patient, N | 16 111 | 16 111 | 8077 | 2008 | 2008 | 1339 | 801 | 801 | 793 |
| Total person‐years (PY) | 21 259 | 11 943 | 9316 | 3305 | 1809 | 1496 | 1874 | 801 | 1073 |
| Total events, N | 299 | 136 | 83 | 59 | 18 | 25 | 18 | … | 13 |
| Total event rates per 100 PY | 1.41 | 1.14 | 0.89 | 1.78 | 1.00 | 1.67 | 0.96 | … | 1.21 |
| Ischemic stroke, N | 279 | 130 | 77 | 55 | 18 | 21 | 17 | … | 12 |
| Ischemic stroke, rates | 1.31 | 1.10 | 0.83 | 1.66 | 1.00 | 1.40 | 0.91 | … | 1.12 |
| Systemic embolism, N | 24 | 9 | 6 | 4 | … | 4 | 2 | … | 2 |
| Systemic embolism, rates | 0.11 | 0.08 | 0.06 | 0.12 | … | 0.27 | 0.11 | … | 0.19 |
| Major bleed events † | |||||||||
| Total patient, N | 16 167 | 16 167 | 8102 | 1957 | 1957 | 1309 | 816 | 816 | 806 |
| Total PY | 21 323 | 11 983 | 9340 | 3212 | 1764 | 1448 | 1904 | 816 | 1088 |
| Total events, N | 312 | 142 | 80 | 20 | 7 | 9 | 10 | 1 | 7 |
| Total event rates per 100 PY | 1.46 | 1.19 | 0.86 | 0.62 | 0.40 | 0.62 | 0.53 | 0.12 | 0.64 |
| ICH, N | 23 | 11 | 7 | 3 | … | 2 | 2 | 1 | 1 |
| ICH, rates | 0.11 | 0.09 | 0.07 | 0.09 | … | 0.14 | 0.11 | 0.12 | 0.09 |
| GI bleed, N | 271 | 119 | 72 | 16 | 6 | 7 | 8 | … | 6 |
| GI bleed, rates | 1.27 | 0.99 | 0.77 | 0.50 | 0.34 | 0.48 | 0.42 | … | 0.55 |
| Other bleed, N | 29 | 17 | 3 | 1 | 1 | … | … | … | … |
| Other bleed, rates | 0.14 | 0.14 | 0.03 | 0.03 | 0.06 | … | … | … | … |
… no observation. GI indicates gastrointestinal; and ICH, intracranial hemorrhage.
Patients were followed from the index date (first DOAC prescription) until the thromboembolic event, death, disenrollment from the health plan, switching from DOAC therapy to warfarin, or study end date (December 31, 2018), whichever occurred first. The percentages of switch to warfarin were 8.6%, 6.3%, and 3.8% for consistent adherence, early discontinuation, and gradual decline groups, respectively.
Major bleeding event was used as a censoring event instead of thromboembolic event.
Early discontinuation of DOACs was associated with a higher risk of thromboembolism during follow‐up than consistent adherence (rate ratio, 1.40; 95% CI, 1.05–1.86) (Table 3). These differences were mainly from higher event rates after 12 months of follow‐up. Early discontinuation of DOACs was associated with a 2‐fold higher risk of thromboembolism than consistent adherence (rate ratio, 2.22; 95% CI, 1.41–3.50) after 12 months, but there were no differences in thromboembolism during the first 12 months between early discontinuation and consistent adherence groups (rate ratio, 0.86; 95% CI, 0.52–1.42). Early discontinuation of DOACs was also associated with a lower risk of major bleed compared with consistent adherence, mainly driven by higher bleeding event rates during the first 12 months from the consistent adherence group. Gradual decline of adherence to DOACs was not statistically significantly associated with thromboembolism outcomes compared with consistent adherence. Sensitivity analyses for those with CHA2DS2‐VASc score ≥2 and no previous use of warfarin showed consistent results.
Table 3.
Rate Ratios (95% CI) of Thromboembolism and Major Bleeding Associated With Long‐Term Adherence Trajectories
|
Adjusted RR (95% CI) (early discontinuation vs consistent adherence) |
Adjusted RR (95% CI) (gradual decline vs consistent adherence) |
|||||
|---|---|---|---|---|---|---|
| Total | First 12 mo | After 12 mo | Total | First 12 mo | After 12 mo | |
| Total patients | ||||||
| Thromboembolism (N=18 920) | 1.40† (1.05–1.86) | 0.86 (0.52–1.42) | 2.22† (1.41–3.50) | 0.74 (0.46–1.19) | … | 1.51 (0.84–2.73) |
| Major bleed (N=18 940) | 0.48† (0.30–0.75) | 0.36† (0.17–0.77) | 0.82 (0.41–1.64) | 0.40† (0.20–0.73) | 0.11† (0.02–0.82) | 0.78 (0.36–1.70) |
| For patients with CHA2D2‐VASc ≥2 and no previous warfarin use | ||||||
| Thromboembolism (N=10 677) | 1.29 (0.89–1.86) | 0.78 (0.42–1.45) | 2.21† (1.20–4.07) | 0.83 (0.45–1.53) | … | 1.89 (0.89–4.06) |
| Major bleed (N=10 689) | 0.42† (0.23–0.77) | 0.22† (0.07–0.70) | 0.85 (0.36–2.00) | 0.46 (0.20–1.04) | … | 1.26 (0.53–3.00) |
… no observation. For thromboembolism, age, sex, race and ethnicity, CHA2D2‐VASc, prior warfarin use, and creatinine clearance were adjusted in the model. For major bleed, age, sex, race and ethnicity, CHA2D2‐VASc, HAS‐BLED, prior warfarin use, creatinine clearance, dementia, and injurious falls were adjusted. RR indicates rate ratio.
P<0.05.
DISCUSSION
This study identified 3 distinct long‐term adherence trajectories to DOACs. A large proportion of patients with NVAF were adherent to DOAC therapy over 3.5 years. Other identified adherence patterns were early discontinuation within 6 months and gradual decline; patients comprising these 2 groups may be important candidates for future DOAC adherence interventions. To the best of our knowledge, this study is the first to provide information on long‐term adherence trajectories to DOAC therapy and associated outcomes over 3.5 years.
Adherence trajectory modeling has been suggested as a better way to summarize complex longitudinal adherence patterns compared with traditional methods dichotomizing patients as adherent or nonadherent. 14 The trajectory approach may help identifying the timing of interventions as well as the patients most likely to benefit from the intervention by more appropriately categorizing complex nonadherence behaviors. 14 A recent study evaluated an adherence intervention designed to address past adherence trajectories in statin users 23 and was found to be effective. The 3 long‐term adherence trajectories found in our study can be used to identify groups for tailored adherence interventions.
Our study identified early discontinuation as an important subgroup to address because of increased risk of thromboembolism. Patient and clinical factors associated with early discontinuation identified from this study such as race or ethnicity of non‐Hispanic Black or Hispanic, lower creatinine clearance, previous injurious falls, no prior use of warfarin, or having other prescriber specialty would be useful in identifying patients most likely to benefit from an adherence intervention. Interestingly, many predictors associated with early discontinuation were identified as predictors for the gradual decline adherence trajectory. Moreover, some of these predictors such as Black race, use of antiarrhythmic agents, and dementia were identified as predictors associated with decreasing adherence trajectories within 12 months of DOAC initiation in a previous study. 17 However, the current analysis found that the HAS‐BLED score is not associated with early discontinuation adherence trajectories, which is different from other study findings. 17 , 18
Only a few studies from Europe 24 , 25 , 26 and Canada 27 investigated long‐term DOAC adherence over 3 years. These studies reported around 54% to 78% of DOAC initiators were persistent to DOACs after 3 years. Although the methods to define adherence are different, the results from the current analysis show a relatively higher percentage of patients were adherent to DOAC therapy after 3.5 years (85.2% overall, and 78.0% for those with 3.5 years of follow‐up). The mean PDCs during the first 6 months or 7 to 12 months after DOAC initiation was also higher in our study (0.84 and 0.73, respectively) than previous reported ranges. Interestingly, adherence (mean PDC over 12 months) was higher among patients from the Veterans Affairs health care system (0.84–0.89) 7 and relatively lower in a commercially insured population (0.64–0.80) in the United States. 8 The current study results may be more comparable with the results from Veterans Affairs where a DOAC adherence monitoring program has been implemented.
Our study supports the clinical importance of medication adherence. Thromboembolic event rates were similar during the 12 months of DOAC initiation between early discontinuers and consistently adherent patients; however, these rates among early discontinuers during follow‐up after the first 12 months were almost double those of consistent adherers. Consistent with previous studies, 7 , 28 , 29 the current analysis showed that early discontinuation within 6 months from DOAC initiation was associated with a 2‐fold higher risk of thromboembolic events while maintaining a similar risk of bleeds after 12 months of follow‐up. However, major bleeding events in the first 12 months were significantly higher in the consistent adherent group than the early discontinuation group, which suggests careful monitoring is needed in consistent adherers.
Interestingly, patients in our study with a gradually declining adherence pattern had no thromboembolic events during the first 12 months of follow‐up but had a higher rate of thromboembolic events compared with consistently adherent patients after 12 months. The lower event rates during the first 12 months may be because of baseline differences between the 2 groups, where patients in the gradual decline group were younger and had lower CHA2DS2‐VASc scores. No thromboembolic events in the first 12 months for gradual decliners may have affected later medication adherence behaviors; most patients in the gradual decline group were adherent to DOACs in the first 6 months of follow‐up; however, these patients became nonadherent later and had more thromboembolic events later.
This study has several strengths and limitations. We evaluated long‐term adherence trajectories up to 3.5 years in a relatively large population who received DOACs. However, a relatively small number of patients were available for the entire 3.5 years. With an increasing trend of DOAC use, future studies will be able to investigate long‐term adherence in a larger population. Moreover, this study did not evaluate medication adherence by type of DOAC, primarily because most patients received dabigatran. In addition, 18% of the study population had CHA2DS2‐VASc scores of 0 or 1. It is possible that for some patients, DOAC therapy may not be appropriate. Because of the retrospective nature of the study design, we were not able to confirm the appropriateness of DOAC therapy; however when we conducted sensitivity analyses for those with CHA2DS2‐VASc scores ≥2, patients recommended for DOAC therapy, the results were the same. Adherence calculation was based on filled prescription data; therefore, early discontinuation may include both a prescriber’s decision to discontinue the DOAC and patients’ behavior of not filling their DOAC. Also, we required 2 prescription fills for PDC calculation to make sure patients had sufficient data to make a valid estimate of medication adherence. 30 However, this may have inflated DOAC adherence. Although switching from DOAC therapy to warfarin is an appropriate therapeutic option, this study focused on medication adherence to DOACs only. However, a small portion (4%–6%) of early discontinuers and gradual decliners were censored because of the switch to warfarin therapy; therefore, the impact of warfarin switch may be minimal. Lastly, this study was conducted in a single health care system. The study findings may not be generalizable to other settings.
CONCLUSIONS
This study showed that a large portion of patients with NVAF were adherent to DOAC therapy over 3.5 years from a large US integrated health care system. Early discontinuation of DOAC therapy within 6 months was associated with a higher risk of thromboembolic outcomes. Future interventions identifying early discontinuers of DOAC therapy may be warranted to improve medication adherence and ultimately improve clinical outcomes.
Sources of Funding
None.
Disclosures
An reports grants from AstraZeneca, Novartis, and Merck & Co. outside the submitted work. Luong reports grants from Novartis and Vital Strategies outside the submitted work. Reynolds reports grants from Novartis, Vital Strategies, Merck & Co., and Amgen outside the submitted work. Bider is currently an employee of F. Hoffmann—La Roche. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figure S1
Acknowledgments
Authors An and Luong had full access to all the data in the study and take responsibility for its integrity and data analysis.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021601
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Wong SL, Marshall LZ, Lawson KA. Direct oral anticoagulant prescription trends, switching patterns, and adherence in Texas Medicaid. Am J Manag Care. 2018;24:SP309–SP314. [PubMed] [Google Scholar]
- 2. Perreault S, de Denus S, White‐Guay B, Cote R, Schnitzer ME, Dube MP, Dorais M, Tardif JC. Oral anticoagulant prescription trends, profile use, and determinants of adherence in patients with atrial fibrillation. Pharmacotherapy. 2020;40:40–54. doi: 10.1002/phar.2350 [DOI] [PubMed] [Google Scholar]
- 3. Dubois V, Dincq AS, Douxfils J, Ickx B, Samama CM, Dogne JM, Gourdin M, Chatelain B, Mullier F, Lessire S. Perioperative management of patients on direct oral anticoagulants. Thromb J. 2017;15:14. doi: 10.1186/s12959-017-0137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McHorney CA, Crivera C, Laliberte F, Germain G, Wynant W, Lefebvre P. Adherence to rivaroxaban versus apixaban among patients with non‐valvular atrial fibrillation: analysis of overall population and subgroups of prior oral anticoagulant users. PLoS One. 2018;13:e0194099. doi: 10.1371/journal.pone.0194099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Go AS, Singer DE, Toh S, Cheetham TC, Reichman ME, Graham DJ, Southworth MR, Zhang R, Izem R, Goulding MR, et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice: a retrospective cohort study. Ann Intern Med. 2017;167:845–854. doi: 10.7326/M16-1157 [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez RA, Carrier M, Wells PS. Non‐adherence to new oral anticoagulants: a reason for concern during long‐term anticoagulation? J Thromb Haemost. 2013;11:390–394. doi: 10.1111/jth.12086 [DOI] [PubMed] [Google Scholar]
- 7. Borne RT, O'Donnell C, Turakhia MP, Varosy PD, Jackevicius CA, Marzec LN, Masoudi FA, Hess PL, Maddox TM, Ho PM. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the Veterans Health Administration. BMC Cardiovasc Disord. 2017;17:236. doi: 10.1186/s12872-017-0671-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manzoor BS, Lee TA, Sharp LK, Walton SM, Galanter WL, Nutescu EA. Real‐world adherence and persistence with direct oral anticoagulants in adults with atrial fibrillation. Pharmacotherapy. 2017;37:1221–1230. doi: 10.1002/phar.1989 [DOI] [PubMed] [Google Scholar]
- 9. McHorney CA, Ashton V, Laliberte F, Germain G, Wynant W, Crivera C, Schein JR, Lefebvre P, Peterson ED. Adherence to rivaroxaban compared with other oral anticoagulant agents among patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2017;23:980–988. doi: 10.18553/jmcp.2017.23.9.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pham PN, Brown JD. Real‐world adherence for direct oral anticoagulants in a newly diagnosed atrial fibrillation cohort: does the dosing interval matter? BMC Cardiovasc Disord. 2019;19:64. doi: 10.1186/s12872-019-1033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prentice A, Ruiz I, Weeda ER. Medication adherence to rivaroxaban and dabigatran in patients with non‐valvular atrial fibrillation: a meta‐analysis. J Thromb Thrombolysis. 2019;49:360–364. doi: 10.1007/s11239-019-01986-8 [DOI] [PubMed] [Google Scholar]
- 12. Crivera C, Nelson WW, Bookhart B, Martin S, Germain G, Laliberte F, Schein J, Lefebvre P. Pharmacy quality alliance measure: adherence to non‐warfarin oral anticoagulant medications. Curr Med Res Opin. 2015;31:1889–1895. doi: 10.1185/03007995.2015.1077213 [DOI] [PubMed] [Google Scholar]
- 13. Ozaki AF, Choi AS, Le QT, Ko DT, Han JK, Park SS, Jackevicius CA. Real‐world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2020;13:e005969. doi: 10.1161/CIRCOUTCOMES.119.005969 [DOI] [PubMed] [Google Scholar]
- 14. Franklin JM, Shrank WH, Pakes J, Sanfélix‐Gimeno G, Matlin OS, Brennan TA, Choudhry NK. Group‐based trajectory models: a new approach to classifying and predicting long‐term medication adherence. Med Care. 2013;51:789–796. doi: 10.1097/MLR.0b013e3182984c1f [DOI] [PubMed] [Google Scholar]
- 15. Zongo A, Simpson S, Johnson JA, Eurich DT. Change in trajectories of adherence to lipid‐lowering drugs following non‐fatal acute coronary syndrome or stroke. J Am Heart Assoc. 2019;8:e013857. doi: 10.1161/JAHA.119.013857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franklin JM, Krumme AA, Shrank WH, Matlin OS, Brennan TA, Choudhry NK. Predicting adherence trajectory using initial patterns of medication filling. Am J Manag Care. 2015;21:e537–e544. [PubMed] [Google Scholar]
- 17. Chen N, Brooks MM, Hernandez I. Latent classes of adherence to oral anticoagulation therapy among patients with a new diagnosis of atrial fibrillation. JAMA Netw Open. 2020;3:e1921357. doi: 10.1001/jamanetworkopen.2019.21357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernandez I, He M, Chen N, Brooks MM, Saba S, Gellad WF. Trajectories of oral anticoagulation adherence among Medicare beneficiaries newly diagnosed with atrial fibrillation. J Am Heart Assoc. 2019;8:e011427. doi: 10.1161/JAHA.118.011427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 21. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154:1121–1201. doi: 10.1016/j.chest.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 22. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan‐Schilling V, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136 [DOI] [PubMed] [Google Scholar]
- 23. Abughosh SM, Vadhariya A, Johnson ML, Essien EJ, Esse TW, Serna O, Gallardo E, Boklage SH, Choi J, Holstad MM, et al. Enhancing statin adherence using a motivational interviewing intervention and past adherence trajectories in patients with suboptimal adherence. J Manag Care Spec Pharm. 2019;25:1053–1062. doi: 10.18553/jmcp.2019.25.10.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haastrup SB, Hellfritzsch M, Rasmussen L, Pottegard A, Grove EL. Use of non‐vitamin K antagonist oral anticoagulants 2008–2016: a Danish nationwide cohort study. Basic Clin Pharmacol Toxicol. 2018;123:452–463. doi: 10.1111/bcpt.13024 [DOI] [PubMed] [Google Scholar]
- 25. Hellfritzsch M, Husted SE, Grove EL, Rasmussen L, Poulsen BK, Johnsen SP, Hallas J, Pottegard A. Treatment changes among users of non‐vitamin K antagonist oral anticoagulants in atrial fibrillation. Basic Clin Pharmacol Toxicol. 2017;120:187–194. doi: 10.1111/bcpt.12664 [DOI] [PubMed] [Google Scholar]
- 26. Lamberts M, Staerk L, Olesen JB, Fosbol EL, Hansen ML, Harboe L, Lefevre C, Evans D, Gislason GH. Major bleeding complications and persistence with oral anticoagulation in non‐valvular atrial fibrillation: contemporary findings in real‐life Danish patients. J Am Heart Assoc. 2017;6:e004517. doi: 10.1161/JAHA.116.004517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Douros A, Renoux C, Coulombe J, Suissa S. Patterns of long‐term use of non‐vitamin K antagonist oral anticoagulants for non‐valvular atrial fibrillation: Quebec observational study. Pharmacoepidemiol Drug Saf. 2017;26:1546–1554. doi: 10.1002/pds.4333 [DOI] [PubMed] [Google Scholar]
- 28. Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, Gersh BJ, Shah ND, Noseworthy PA. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc. 2016;5:e003074. doi: 10.1161/JAHA.115.003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hernandez I, He M, Brooks MM, Saba S, Gellad WF. Adherence to anticoagulation and risk of stroke among Medicare beneficiaries newly diagnosed with atrial fibrillation. Am J Cardiovasc Drugs. 2019;20:199–207. doi: 10.1007/s40256-019-00371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry‐Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


