Abstract
Background
Among patients with atrial fibrillation and stable coronary artery disease, those with histories of atherothrombotic disease are at high‐risk for future ischemic events. This study investigated the efficacy and safety of rivaroxaban monotherapy in patients with atrial fibrillation, coronary artery disease, and histories of atherothrombotic disease.
Methods and Results
This was a post hoc subanalysis of the AFIRE (Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease) trial. Patients with non‐valvular atrial fibrillation and coronary artery disease were recruited and randomized to receive the rivaroxaban monotherapy or combination therapy with rivaroxaban plus antiplatelet drug. For the purpose of this sub‐study, participants were divided into 2 subgroups, including the atherothrombosis group (those with histories of myocardial infarction, stroke, and/or peripheral artery disease; n=1052, 47.5%) and non‐atherothrombosis group (n=1163, 52.5%). The efficacy end point included cardiovascular events or all‐cause death, while the safety end point was major bleeding. Net adverse events consisted of all‐cause death, myocardial infarction, stroke, or major bleeding. In the atherothrombosis group, rivaroxaban monotherapy was significantly associated with a lower risk of net adverse events when compared with combination therapy (hazard ratio [HR], 0.50; 95% CI, 0.34–0.74; P<0.001), with a decrease in both efficacy (HR, 0.68; 95% CI, 0.47–0.99; P=0.044) and safety (HR, 0.37; 95% CI, 0.19–0.71; P=0.003) end points. By contrast, there were no differences between treatment outcomes for the non‐atherothrombosis group.
Conclusions
Rivaroxaban monotherapy significantly reduced net adverse events as compared with combination therapy for patients with atrial fibrillation, coronary artery disease, and prior atherothrombotic disease.
Registration
URL: https://www.umin.ac.jp/ctr/; Unique identifier: UMIN000016612. URL: https://www.clinicaltrials.gov; Unique identifier: NCT02642419.
Keywords: antiplatelet drug, antithrombotic therapy, atrial fibrillation, coronary artery disease, direct oral anticoagulant
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- AFIRE
Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease
Clinical Perspective
What Is New?
Rivaroxaban monotherapy significantly improved net clinical outcomes in patients with atrial fibrillation and coronary artery disease who had prior atherothrombotic diseases—including myocardial infarction, stroke, and/or peripheral artery disease—compared with the combination therapy with rivaroxaban and single antiplatelet drug, with improvements in both the efficacy and safety end points.
What Are the Clinical Implications?
Because patients with atrial fibrillation and histories of atherothrombotic disease are at high risk not only for thrombotic events but also for bleeding events, antithrombotic therapy should be adjusted to focus on avoiding an increase in bleeding risk rather than eliminating thrombotic activity in these patients.
The randomized AFIRE (Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease) trial was the first study to demonstrate the efficacy and safety of rivaroxaban monotherapy in patients with atrial fibrillation (AF) and stable coronary artery disease (CAD), specifically in comparison with a combination therapy with rivaroxaban plus an antiplatelet agent. 1 In relation to this, the REACH (Reduction of Atherothrombosis for Continued Health) registry 2 found that participants with histories of atherothrombotic events (including stroke, myocardial infarction, and/or peripheral artery disease) were at higher risk for future ischemic events when compared with those with stable atherosclerotic disease. This finding is supported by several other studies, which have also demonstrated that patients with the same types of diseases have poorer prognoses than those with stable CAD alone. 3 , 4 The high rate of recurrent ischemic events in this population underscores the need for intense antithrombotic approaches. Indeed, a post hoc subanalysis performed after the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) trial, which was conducted among patients with prior instances of myocardial infarction, stroke, and/or peripheral artery disease, revealed that dual antiplatelet therapy with clopidogrel plus aspirin provided significant benefits when compared with aspirin monotherapy. 5
While the literature clearly shows that patients with AF and atherothrombotic disease are at high risk for ischemic events, it is also important to note that they are prone to bleeding events. Furthermore, a different risk‐benefit profile for antithrombotic therapy in East Asian patients has been suggested, namely a lower risk of thrombosis and an increased risk of bleeding. 6 , 7 In the context of the AFIRE trial, this poses a crucial question: which strategy is better for use among patients with prior histories of atherothrombotic disease, rivaroxaban monotherapy or rivaroxaban plus a single antiplatelet drug? As a post hoc sub‐study of the AFIRE trial, this study targeted participants with a history of atherothrombotic disease for the purpose of investigating the efficacy and safety of monotherapy with rivaroxaban when compared with the combination therapy with rivaroxaban plus a single antiplatelet drug.
METHODS
Study Design and Patient Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. The AFIRE was a multicenter, randomized, open‐label study in which participants were recruited and enrolled from 294 hospitals in Japan between February 23, 2015 and September 30, 2017. Full details can be found in Yasuda et al (2018) and Yasuda et al (2019). 1 , 8
In brief, eligible patients were diagnosed as having stable CAD and AF with CHADS2 (congestive heart failure, hypertension, age ≥75 years, type 2 diabetes mellitus, previous stroke [doubled]) scores ≥1. Stable CAD was defined based on 1 of the following criteria: (1) history of coronary artery bypass graft or percutaneous coronary intervention (including plain old balloon angioplasty) at least 1 year before enrollment; and (2) evidence of coronary stenosis ≥50% as detected by coronary computerized tomography or coronary angiography. The exclusion criteria included the following: (1) contraindication of rivaroxaban, aspirin, or thienopyridine derivatives (clopidogrel or prasugrel); (2) revascularization within the past year; (3) intended revascularization; (4) history of stent thrombosis; (5) intended invasive surgery; (6) active tumors; (7) poorly controlled hypertension which was defined as ≥160 mm Hg in multiple blood pressure measurements; and (8) being judged as inappropriate by investigators.
The AFIRE trial was conducted in accordance with the Declaration of Helsinki. Its final protocol was approved by the institutional review board of the National Cerebral and Cardiovascular Center, Japan, as well as the review boards of all participating institutions. All patients provided written informed consent before any participation.
Patients were randomized (1:1) to receive either rivaroxaban monotherapy or combination therapy with rivaroxaban plus a single antiplatelet drug (either aspirin or a P2Y12 inhibitor, according to the discretion of the treating physician). Rivaroxaban was prescribed in the standard dose for non‐valvular AF in Japan; that is, 10 mg once daily for patients with creatinine clearances of 15 to 49 mL per minute, or 15 mg once daily for patients with creatinine clearances ≥50 mL per minute. 9 , 10 While a follow‐up was initially planned for a period lasting 24 to 45 months (min/max), the trial was terminated early because of a higher rate of all‐cause death in the combination therapy group. The median treatment and follow‐up durations were 23.1 months (interquartile range, 15.8–31.0) and 24.1 months (interquartile range, 17.3–31.5), respectively.
For the purpose of this study, AFIRE participants were divided into 2 subgroups, including the atherothrombosis group (patients with prior myocardial infarction, stroke, and/or peripheral artery disease) and non‐atherothrombosis group. 2 The risk of stroke was assessed with the CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years [two scores], type 2 diabetes mellitus, previous stroke, transient ischemic attack, or thromboembolism [doubled], vascular disease, age 65–74 years, and sex category) score 11 and the risk of major bleeding was established using the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile international normalized ratio, Elderly, Drugs/Alcohol) score, which assesses the conditions of hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly status (aged >65 years), and concomitant drugs/alcohol usage. 12
Outcomes
Details on the study end points of the AFIRE have been published elsewhere. 1 , 8 Briefly, the primary efficacy end point was a composite of cardiovascular events (ischemic and hemorrhagic stroke, non‐central nervous system embolism, myocardial infarction, and unstable angina requiring revascularization) or all‐cause mortality, while the primary safety end point was major bleeding as defined based on criteria established by the International Society on Thrombosis and Haemostasis. Secondary end points included net adverse clinical events (death from any cause, myocardial infarction, stroke, or major bleeding).
Statistical Analysis
Statistical analyses were conducted using the SAS software, version 9.4 for Windows (SAS Institute). Categorical variables were expressed as frequencies and percentages, then compared using the Chi‐square test or Fisher exact test. Differences in continuous variables were compared using the Wilcoxon rank sum test. The primary efficacy end point, safety end point, and net adverse clinical end point were analyzed using data from the modified intention‐to‐treat population. Kaplan–Meier curves were constructed, with the log‐rank test used to evaluate event‐rate differences between groups. Annual event rates were also compared between groups. Finally, Cox proportional hazard analyses were conducted to test the relative risk between groups, with a 2‐tailed P value of 0.05 set as the significance threshold.
RESULTS
A total of 2240 patients were enrolled in the original AFIRE trial. However, this study excluded 4 for not meeting the eligibility criteria, 2 for a protocol violation, 7 for incomplete registration, and 12 for withdrawal from the trial, thus resulting in 2215 analyzed participants (Figure 1). Of these, 1052 (47.5%) with histories of myocardial infarction, stroke, or peripheral artery disease were categorized into the atherothrombosis group, while the remaining 1163 were placed in the non‐atherothrombosis group. Table 1 includes detailed participant characteristics. Based on HAS‐BLED scores, we also found significantly higher bleeding risks among participants in the atherothrombosis group (ie, HAS‐BLED scores ≥3 for 35.6% versus 17.1% for those in the non‐atherothrombosis group; P<0.001). The CHA2DS2‐VASc score was significantly higher in the atherothrombosis group than in the non‐atherothrombosis group.
Figure 1. Participant flow.
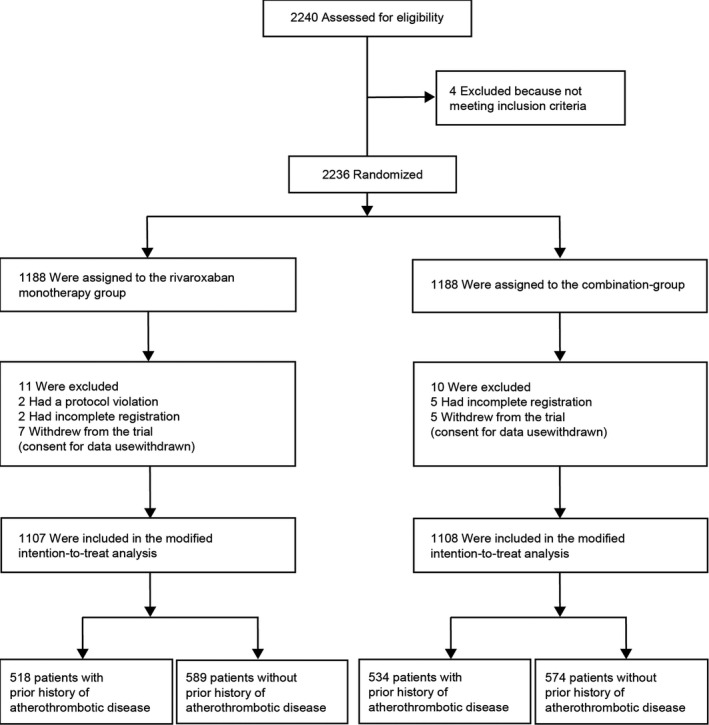
Patients were randomized (1:1) to receive either rivaroxaban monotherapy or combination therapy with rivaroxaban plus antiplatelet drug. For the purpose of this study, AFIRE (Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease) participants were divided into 2 subgroups, including the atherothrombosis group (patients with prior myocardial infarction, stroke, and/or peripheral artery disease) and the non‐atherothrombosis group.
Table 1.
Baseline Characteristics Between Patients With Prior Myocardial Infarction, Stroke, or Peripheral Artery Disease and Those Without
| Non‐Atherothrombosis Group | Atherothrombosis Group | P Value | |
|---|---|---|---|
| n=1163 | n=1052 | ||
| Age, mean (SD), y | 74.4 (7.9) | 74.2 (8.6) | 0.996 |
| <75 y, n (%) | 553 (47.5) | 499 (47.4) | 0.996 |
| ≥75 y, n (%) | 610 (52.5) | 553 (52.6) | |
| Male sex, n (%) | 880 (75.7) | 871 (82.8) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 24.5 (3.6) | 24.5 (3.8) | 0.947 |
| Current smoker, n (%) | 166 (14.3) | 126 (12.0) | 0.116 |
| Diabetes mellitus, n (%) | 446 (38.3) | 481 (45.7) | <0.001 |
| Previous peripheral artery disease, n (%) | 0 (0) | 139 (13.2) | <0.001 |
| Previous stroke, n (%) | 0 (0) | 323 (30.7) | <0.001 |
| Previous myocardial infarction, n (%) | 0 (0) | 777 (73.9) | <0.001 |
| Previous PCI, n (%) | 718 (61.7) | 846 (80.4) | <0.001 |
| Type of stent, n (%) | <0.001 | ||
| Drug eluting | 539/681 (79.1) | 493/763 (64.6) | |
| Bare metal alone | 493/763 (64.6) | 230/763 (30.1) | |
| Unknown | 30/681 (4.4) | 40/763 (5.2) | |
| Previous CABG, n (%) | 92 (7.9) | 160 (15.2) | <0.001 |
| Type of atrial fibrillation, n (%) | 0.041 | ||
| Paroxysmal | 592 (50.9) | 584 (55.5) | |
| Persistent | 196 (16.9) | 143 (13.6) | |
| Permanent | 375 (32.2) | 325 (30.9) | |
| Creatinine clearance | |||
| Mean (SD), mL/min | 63.1 (23.1) | 61.3 (26.7) | 0.002 |
| Distribution, no./total n (%) | 0.004 | ||
| <30 mL/min | 49/1101 (4.5) | 65/991 (6.6) | |
| 30 to <50 mL/min | 289/1101 (26.2) | 304/991 (30.7) | |
| ≥50 mL/min | 763/1101 (69.3) | 622/991 (62.8) | |
| HAS‐BLED score, n (%) | <0.001 | ||
| 0 | 21 (1.8) | 3 (0.3) | |
| 1 | 265 (22.8) | 128 (12.2) | |
| 2 | 640 (55.0) | 505 (48.0) | |
| 3 | 184 (15.8) | 309 (29.4) | |
| 4 | 15 (1.3) | 58 (5.5) | |
| 5 | 0 (0.0) | 7 (0.7) | |
| HAS‐BLED score ≥3, n (%) | 199 (17.1) | 374 (35.6) | <0.001 |
| CHA2DS2‐VASc score, n (%) | <0.001 | ||
| 0 | 1 (0.1) | 0 (0.0) | |
| 1 | 66 (5.7) | 1 (0.1) | |
| 2 | 238 (20.5) | 24 (2.3) | |
| 3 | 381 (32.8) | 154 (14.6) | |
| 4 | 287 (24.7) | 279 (26.5) | |
| 5 | 138 (11.9) | 281 (26.7) | |
| 6 | 46 (4.0) | 203 (19.3) | |
| 7 | 6 (0.5) | 88 (8.4) | |
| 8 | 0 (0.0) | 21 (2.0) | |
| 9 | 0 (0.0) | 1 (0.1) | |
| CHA2DS2‐VASc score ≥4, n (%) | 477 (41.0) | 873 (83.0) | <0.001 |
CABG indicates coronary‐artery bypass grafting; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years (two scores), type 2 diabetes mellitus, previous stroke, transient ischemic attack, or thromboembolism (doubled), vascular disease, age 65–74 years, and sex category; HAS‐BLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly; and PCI, percutaneous coronary intervention.
Clinical Outcomes Between the Atherothrombosis Group and Non‐Atherothrombosis Group
While the event rate of the primary efficacy end point was significantly higher in the atherothrombosis group when compared with the non‐atherothrombosis group (hazard ratio [HR] of 1.33, 95% CI, 1.01–1.74, P=0.041), there were no such differences when looking at the primary safety end point (HR of 1.01, 95% CI 0.67–1.52, P=0.964); see Figure 2A and 2B. Further, net adverse clinical events occured more frequently in the atherothrombosis group when compared with the non‐atherothrombosis group (HR, 1.36; 95% CI, 1.04–1.77; P=0.026); see Figure 2C.
Figure 2. Kaplan‐Meier curves between the non‐atherothrombosis and atherothrombosis groups.
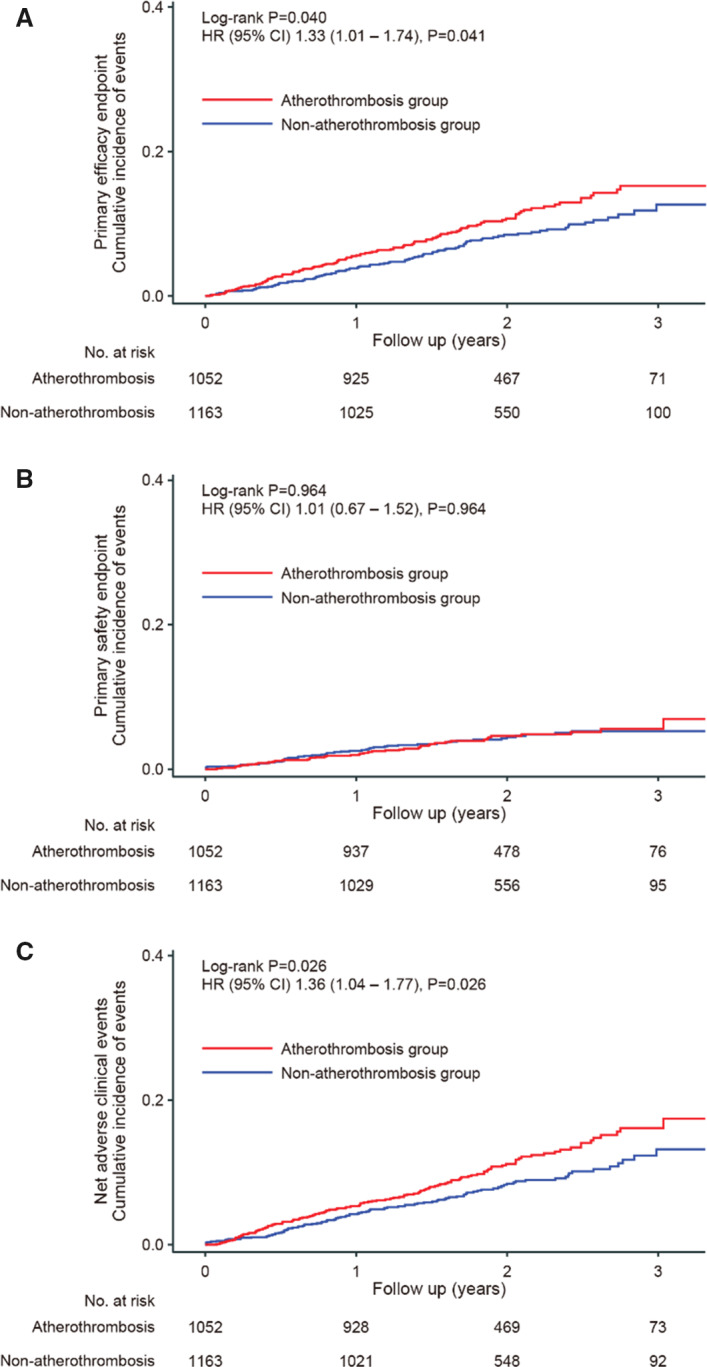
The atherothrombosis group consisted of patients with prior myocardial infarction, stroke, or peripheral artery disease. A, The primary efficacy end point was a composite of cardiovascular events or all‐cause mortality, while (B) the primary safety end point was major bleeding as defined based on criteria established by the International Society on Thrombosis and Haemostasis. C, Net adverse clinical events consisted of death from any cause, myocardial infarction, stroke, and major bleeding. HR indicates hazard ratio.
Intervention Efficacy Between Groups
Baseline characteristics were well‐balanced between the rivaroxaban monotherapy and combination therapy groups, both in the atherothrombosis and non‐atherothrombosis groups (Table 2). Figure 3A shows Kaplan–Meier curves for the primary efficacy end point based on the treatment group and the atherothrombosis/non‐atherothrombosis group. For the atherothrombosis group, the annual event rate was significantly lower in participants treated via rivaroxaban monotherapy when compared with those treated via combination therapy (annual event rate, 4.6% versus 6.8%; log‐rank P=0.043; HR for monotherapy versus combination therapy, 0.68; 95% CI 0.47–0.99; P=0.044); see Figure 3A and Table 3. Meanwhile, no significant differences were found between treatment groups in the non‐atherothrombosis group. Finally, there were no significant interactions between the treatment groups and the atherothrombosis group/non‐atherothrombosis group (P=0.593); see Figure 3A.
Table 2.
Baseline Characteristics
| Atherothrombosis Group | Non‐Atherothrombosis Group | |||
|---|---|---|---|---|
| Rivaroxaban Monotherapy | Combination Therapy | Rivaroxaban Monotherapy | Combination Therapy | |
| n=518 | n=534 | n=589 | n=574 | |
| Age, mean (SD), y | 74.0 (8.7) | 74.4 (8.5) | 74.5 (7.9) | 74.3 (7.9) |
| <75 y, n (%) | 249 (48.1) | 250 (46.8) | 276 (46.9) | 277 (48.3) |
| ≥75 y, n (%) | 269 (51.9) | 284 (53.2) | 313 (53.1) | 297 (51.7) |
| Male sex, n (%) | 420 (82.2) | 441 (83.1) | 449 (76.2) | 431 (75.1) |
| Body mass index, kg/m2 | 24.4(3.9) | 24.6(3.8) | 24.5(3.5) | 24.4(3.7) |
| Current smoker, n (%) | 67 (12.9) | 59 (11.1) | 79 (13.4) | 87 (15.2) |
| Diabetes mellitus, n (%) | 231 (44.6) | 250 (46.8) | 230 (39.0) | 216 (37.6) |
| Previous peripheral artery disease, n (%) | 67 (12.9) | 72 (13.5) | 0 (0) | 0 (0) |
| Previous stroke, n (%) | 148 (28.6) | 175 (32.8) | 0 (0) | 0 (0) |
| Previous myocardial infarction, n (%) | 384 (74.1) | 393 (73.6) | 0 (0) | 0 (0) |
| Previous PCI, n (%) | 422 (81.5) | 424 (79.4) | 359 (61.0) | 359 (62.5) |
| Type of stent, n (%) / total n (%) | ||||
| Drug eluting | 249/383 (65.0) | 244/380 (64.2) | 270/340 (79.4) | 269/341 (78.9) |
| Bare metal alone | 115/383 (30.0) | 115/380 (30.3) | 56/340 (16.5) | 56/341 (16.4) |
| Unknown | 19/383 (5.0) | 21/380 (5.5) | 14/340 (4.1) | 16/341 (4.7) |
| Previous CABG, n (%) | 73 (14.1) | 87 (16.3) | 52 (8.8) | 40 (7.0) |
| Type of atrial fibrillation, n (%) | ||||
| Paroxysmal | 289 (55.8) | 295 (55.2) | 307 (52.1) | 285 (49.7) |
| Persistent | 68 (13.1) | 75 (14.0) | 96 (16.3) | 100 (17.4) |
| Permanent | 161 (31.1) | 164 (30.7) | 186 (31.6) | 189 (32.9) |
| Creatinine clearance | ||||
| Mean (SD), mL/min | 62.5 (28.2) | 60.2 (25.0) | 63.1 (23.4) | 63.2 (22.9) |
| Distribution, no./total n (%) | ||||
| <30 mL/min | 28/493 (5.7) | 37/498 (7.4) | 26/560 (4.6) | 23/541 (4.3) |
| 30 to <50 mL/min | 147/493 (29.8) | 157/498 (31.5) | 153/560 (27.3) | 136/541 (25.1) |
| ≥50 mL/min | 318/493 (64.5) | 304/498 (61.0) | 381/560 (68.0) | 382/541 (70.6) |
| HAS‐BLED score | ||||
| 0 | 2 (0.4) | 1 (0.2) | 15 (2.5) | 6 (1.0) |
| 1 | 70 (13.5) | 58 (10.9) | 137 (23.3) | 128 (22.3) |
| 2 | 253 (48.8) | 252 (47.2) | 309 (52.5) | 331 (57.7) |
| 3 | 146 (28.2) | 163 (30.5) | 98 (16.6) | 86 (15.0) |
| 4 | 28 (5.4) | 30 (5.6) | 8 (1.4) | 7 (1.2) |
| 5 | 3 (0.6) | 4 (0.7) | 0 (0) | 0 (0) |
| HAS‐BLED score ≥3, n (%) | 177 (34.2) | 197 (36.9) | 106 (18.0) | 93 (16.2) |
| CHA2DS2‐VASc score | ||||
| 0 | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| 1 | 0 (0.0) | 1 (0.2) | 31 (5.3) | 35 (6.1) |
| 2 | 7 (1.4) | 17 (3.2) | 115 (19.5) | 123 (21.4) |
| 3 | 76 (14.7) | 78 (14.6) | 199 (33.8) | 182 (31.7) |
| 4 | 160 (30.9) | 119 (22.3) | 149 (25.3) | 138 (24.0) |
| 5 | 131 (25.3) | 150 (28.1) | 67 (11.4) | 71 (12.4) |
| 6 | 96 (18.5) | 107 (20.0) | 21 (3.6) | 25 (4.4) |
| 7 | 39 (7.5) | 49 (9.2) | 6 (1.0) | 0 (0.0) |
| 8 | 8 (1.5) | 13 (2.4) | 0 (0.0) | 0 (0.0) |
| 9 | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CHA2DS2‐VASc score ≥4, n(%) | 435 (84.0) | 438 (82.0) | 243 (41.3) | 234 (40.8) |
There were no significant differences between the 2 groups in the characteristics listed in the atherothrombosis group and non‐atherothrombosis group, respectively. CABG indicates coronary‐artery bypass grafting; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years (two scores), type 2 diabetes mellitus, previous stroke, transient ischemic attack, or thromboembolism (doubled), vascular disease, age 65–74 years, and sex category; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile international normalized ratio, Elderly, Drugs/Alcohol; and PCI, percutaneous coronary intervention.
Figure 3. Kaplan‒Meier curves for prior atherothrombotic disease and treatment group.
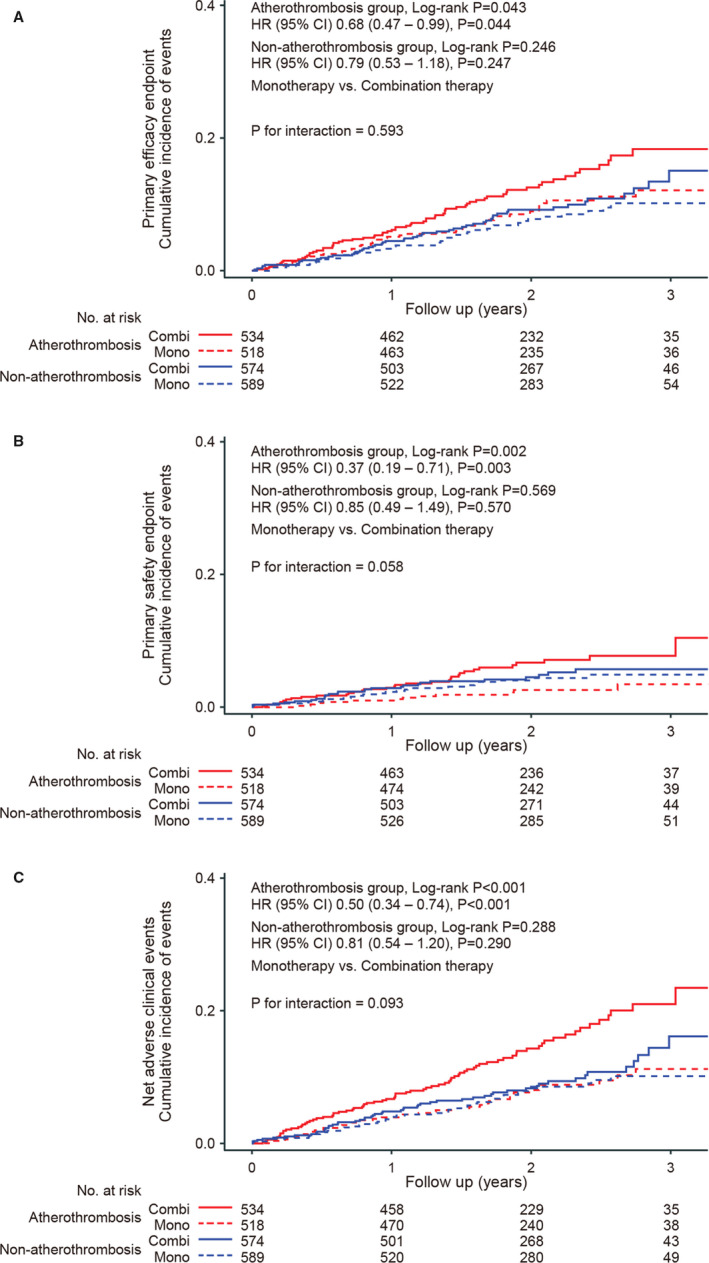
Kaplan–Meier curves for (A) the primary efficacy entpoint, (B) primary safety end point, and (C) net clinical adverse events (4 groups, based on the non‐atherothrombosis/atherothrombosis group and treatment type). Interaction P is for group (non‐atherothrombosis or atherothrombosis)×interventions (monotherapy or combination therapy). Combi indicates combination therapy; HR, hazard ratio; and Mono, monotherapy
Table 3.
Events Details
| Atherothrombosis Group | Non‐Atherothrombosis Group | HR (95% CI) | P Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Rivaroxaban Monotherapy | Combination Therapy | HR (95% CI) | P Value | Overall | Rivaroxaban Monotherapy | Combination Therapy | HR (95% CI) | P Value | |||
| n=1052 | n=518 | n=534 | Monotherapy vs Combination Therapy | n=1163 | n=589 | n=574 | Monotherapy vs Combination Therapy | Atherothrombosis vs Non‐Atherothrombosis | ||||
| Primary efficacy end point | ||||||||||||
| Cardiovascular events or death from any cause | 113 (5.68) | 46 (4.60) | 67 (6.78) | 0.68 (0.47–0.99) | 0.044 | 97 (4.28) | 43 (3.74) | 54 (4.84) | 0.79 (0.53–1.18) | 0.247 | 1.33 (1.01–1.74) | 0.041 |
| Secondary efficacy end points | ||||||||||||
| Ischemic stroke | 24 (1.18) | 10 (0.98) | 14 (1.39) | 0.71 (0.32–1.60) | 0.411 | 25 (1.08) | 11 (0.94) | 14 (1.24) | 0.76 (0.35–1.67) | 0.492 | 1.09 (0.62–1.91) | 0.769 |
| Hemorrhagic stroke | 10 (0.49) | 3 (0.29) | 7 (0.69) | 0.42 (0.11–1.62) | 0.192 | 7 (0.30) | 1 (0.08) | 6 (0.52) | 0.16 (0.02–1.34) | 0.053 | 1.62 (0.62–4.27) | 0.320 |
| Myocardial infraction | 11 (0.54) | 5 (0.49) | 6 (0.60) | 0.84 (0.26–2.76) | 0.778 | 10 (0.43) | 8 (0.68) | 2 (0.18) | 3.87 (0.82–18.23) | 0.065 | 1.23 (0.52–2.91) | 0.631 |
| Unstable angina requiring revascularization | 14 (0.69) | 7 (0.68) | 7 (0.69) | 1.00 (0.35–2.85) | 0.997 | 17 (0.74) | 6 (0.51) | 11 (0.97) | 0.53 (0.20–1.42) | 0.198 | 0.93 (0.46–1.88) | 0.834 |
| Systemic embolism | 0 (0) | 0 (0) | 0 (0) | … | 3 (0.13) | 2 (0.17) | 1 (0.09) | 1.94 (0.18–21.43) | 0.581 | … | 0.100 | |
| Death | 65 (3.17) | 23 (2.23) | 42 (4.13) | 0.54 (0.33–0.90) | 0.016 | 49 (2.10) | 18 (1.52) | 31 (2.70) | 0.59 (0.33–1.05) | 0.069 | 1.53 (1.05–2.21) | 0.024 |
| Cardiovascular | 44 (2.15) | 17 (1.65) | 27 (2.65) | 0.62 (0.34–1.14) | 0.121 | 25 (1.07) | 9 (0.76) | 16 (1.40) | 0.55 (0.24–1.24) | 0.140 | 2.01 (1.23–3.28) | 0.005 |
| Non‐cardiovascular | 21 (1.03) | 6 (0.58) | 15 (1.47) | 0.39 (0.15–1.01) | 0.045 | 24 (1.03) | 9 (0.76) | 15 (1.31) | 0.63 (0.27–1.46) | 0.276 | 1.02 (0.57–1.84) | 0.944 |
| Net adverse clinical events | 117 (5.89) | 40 (3.96) | 77 (7.88) | 0.50 (0.34–0.74) | <0.001 | 98 (4.35) | 44 (3.85) | 54 (4.88) | 0.81 (0.54–1.20) | 0.290 | 1.36 (1.04–1.77) | 0.026 |
| Primary safety end point | ||||||||||||
| Major bleeding | 44 (2.19) | 12 (1.18) | 32 (3.23) | 0.37 (0.19–0.71) | 0.003 | 49 (2.16) | 23 (1.99) | 26 (2.33) | 0.85 (0.49–1.49) | 0.570 | 1.01 (0.67–1.52) | 0.964 |
| Secondary safety end point | ||||||||||||
| Any bleeding | 181 (9.82) | 64 (6.72) | 117 (13.13) | 0.52 (0.38–0.71) | <0.001 | 205 (9.93) | 83 (7.70) | 122 (12.37) | 0.63 (0.48–0.83) | 0.001 | 0.98 (0.80–1.20) | 0.858 |
| Nonmajor bleeding | 151 (8.07) | 55 (5.72) | 96 (10.56) | 0.55 (0.40–0.77) | <0.001 | 170 (8.03) | 67 (6.08) | 103 (10.13) | 0.61 (0.45–0.82) | 0.001 | 1.00 (0.80–1.24) | 0.971 |
Data are shown in total number of events during follow‐up period and annual event rates. HR indicates hazard ratio.
Intervention Safety Between Groups
Figure 3B shows Kaplan–Meier curves for the safety end point according to the treatment group and atherothrombosis/non‐atherothrombosis group. For the atherothrombosis group, participants who were treated via monotherapy had significantly lower annual primary safety event rates when compared with those who were treated via combination therapy (annual event rate, 1.2% versus 3.2%; log‐rank P=0.002; HR for monotherapy versus combination therapy, 0.37; 95% CI, 0.19–0.71; P=0.003); see Figure 3B and Table 3. There were contrasting results for the non‐atherothrombosis group, which showed no differences in annual event rates for the primary safety end point between treatment groups (2.0% in the monotherapy group and 2.3% in the combination therapy group; log rank P=0.569). Further, the interaction between the treatment groups and atherothrombosis/non‐atherothrombosis group reached borderline significance (P=0.058); see Figure 3B.
Net Adverse Clinical Events
Figure 3C shows Kaplan–Meier curves for net adverse clinical events according to the treatment group and atherothrombosis/non‐atherothrombosis group. For the atherothrombosis group, the annual net adverse clinical event rate was significantly lower among patients who were treated via rivaroxaban monotherapy when compared with those who were treated via combination therapy (annual event rate, 4.0% versus 7.9%; log‐rank P<0.001; HR for monotherapy versus combination therapy, 0.50; 95% CI, 0.34–0.74; P<0.001); see Figure 3C and Table 3. The elevated net adverse event risk by the combination therapy in the atherothrombosis group was largely derived from bleeding events. However, there were contrasting results for the non‐atherothrombosis group, in which similar annual net adverse clinical event rates were found between treatment types—3.9% for those who received monotherapy and 4.9% for those who received combination therapy (log‐rank P=0.288); see Figure 3C and Table 3. Nevertheless, the interaction failed to reach significance (P=0.093).
DISCUSSION
In the AFIRE trial, 47.5% of patients had histories of myocardial infarction, stroke, or peripheral artery disease. For the purpose of this study, these participants constituted the atherothrombosis group, while the remaining constituted the non‐atherothrombosis group. In this context, we found higher stroke risk assessed by CHA2DS2‐VASc score and a higher incidence of the primary efficacy end point in the atherothrombosis group, which also contained a greater number of participants who were at increased risk for bleeding. However, the interaction between the intervention type and subgroup (based on prior history of atherothrombotic disease) was not significant. However, for the atherothrombosis group, rivaroxaban monotherapy lasting for 24 months significantly reduced net adverse clinical events when compared with the combination therapy with rivaroxaban plus a single antiplatelet drug. Here, the net clinical benefit of the monotherapy was mainly derived from substantial decreases in bleeding.
These findings indicate that more intense antithrombotic therapy does not provide greater benefits to participants with histories of myocardial infarction, stroke, and/or peripheral artery disease. With that said, it should be noted that too much intensity can produce negative results. In this regard, antithrombotic therapy should be adjusted so as not to increase bleeding risk rather than focusing on the elimination of thrombotic activity, even for patients with histories of atherothrombotic disease.
Is the Treatment Considered Optimal for Antithrombosis?
Previous studies have reported that bleeding presents a substantial risk in subsequent ischemic events. 13 , 14 , 15 Further, a trial known as ADAPT‐DES (the assessment of dual antiplatelet therapy with drug‐eluting stents) 16 revealed that post‐discharge myocardial infarction was associated with a 1.9‐fold greater risk of mortality, whereas the risk associated with post‐discharge bleeding (adjusted HR, 5.03; 95% CI, 3.29–7.66) was much higher. Notably, post‐discharge bleeding was associated with not only all‐cause mortality, but also presented several other risks, including a 4.9‐fold higher risk of cardiovascular deaths, 3.1‐fold higher risk of myocardial infarction, and 3.6‐fold higher risk of stent thrombosis. This suggests that intense antithrombotic strategies may not only increase bleeding events but can also occasionally cause thrombosis and ischemic events.
These results are consistent with previous studies that compared anticoagulant monotherapy with anticoagulant plus antiplatelet therapy. 17 , 18 Specifically, a nationwide observational study in Denmark compared vitamin K antagonist plus antiplatelet therapy with vitamin K antagonist monotherapy, finding that the vitamin K antagonist plus antiplatelet therapy did not provide any benefit over vitamin K antagonist monotherapy for the risk of myocardial infarction or all‐cause mortality. 17
In this study, the patients in the atherothrombosis group had a significantly higher risk of stroke and a higher rate of the cardiovascular events or death from any cause. However, based on this study's findings, adding an antiplatelet medication to rivaroxaban in standard dosage amounts fails to provide additional thromboembolic protection for patients with AF and CAD. In fact, rivaroxaban plus antiplatelet therapy actually increased the risk of bleeding in patients with histories of atherothrombotic disease. As expected from the higher HAS‐BLED scores, the risk of bleeding was markedly increased with more intense antithrombotic treatments, which suggests that the relationship between bleeding and ischemic events may be more conspicuous in the atherothrombosis group. Thus, more intense antithrombotic therapy does not necessarily result in fewer ischemic events; in fact, it may paradoxically increase the risk of such events because of the concomitant risk of elevated bleeding. Figure 4A and 4B depicts the supposed association between the strength of the antithrombotic therapy and the risk of bleeding/ischemic events. Specifically, bleeding activates platelets, which then release a variety of bioactive substances, including CD40 ligand, serotonin, adenosine, adenosine 5’‐diphosphate, von Willebrand factor, platelet membrane‐derived microparticles, and platelet‐derived growth factor. Although there are several possible mechanisms, including biological homeostatic counter‐reactions to antithrombotic therapy, the discontinuation of antithrombotic drugs, and transfusion‐related phenomena (vasoconstriction, the activation of inflammation, apoptosis, and pro‐thrombogenicity), additional research is needed to reach a conclusion. 19
Figure 4. Supposed relationship between antithrombotic therapy strength and risk of ischemic and bleeding events.
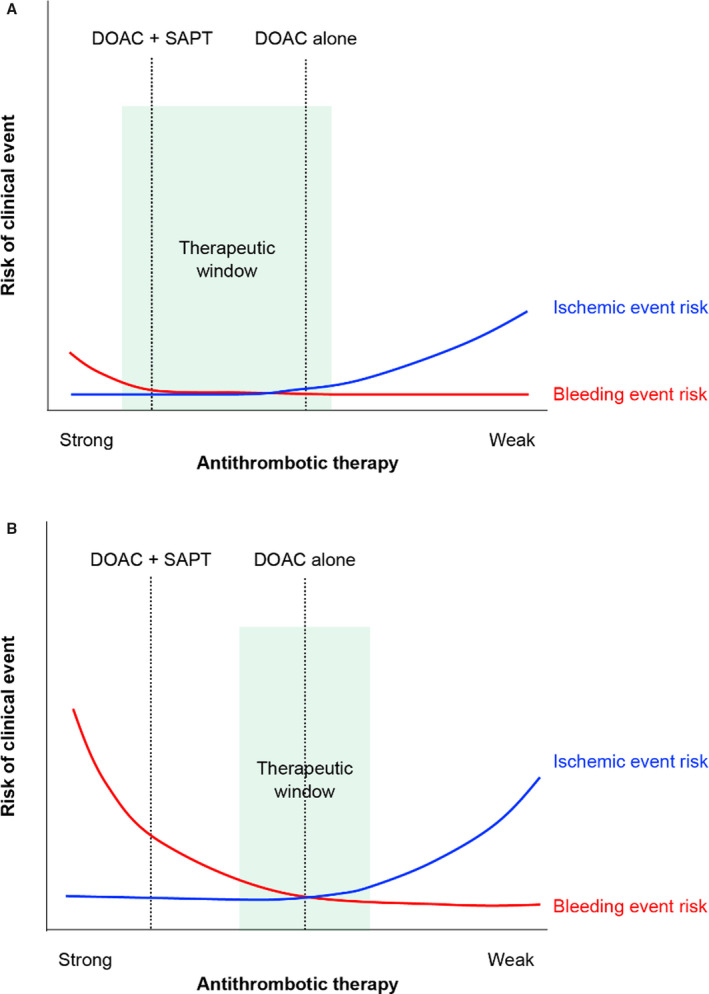
A, Non‐atherothrombosis group; (B) atherothrombosis group. Red lines indicate bleeding event risk, while blue lines indicate ischemic event risk. Bleeding risk was higher in the atherothrombosis group than in the non‐atherothrombosis group, as seen from the right edge of the figures (no antithrombotic therapy). The red line for bleeding risk in the atherothrombosis group rises more steeply than that of the non‐atherothrombosis group. Thus, the optimal “therapeutic window” (green area in the figures) may be narrower and of lesser range in the atherothrombosis group. DOAC indicates direct oral anticoagulant; and SAPT, single antiplatelet therapy.
Previous studies have suggested that patients in East Asian countries are more susceptible to bleeding events, but they have lower risks of thrombotic events when compared with patients in European and American countries; thus, the optimal “therapeutic window” for antithrombotic treatments may be narrower and of lesser range for East Asian patients. 6 , 7 However, these genetic or ethnic differences are still subjects of scholarly discussion. Furthermore, all participants in this study were taking anticoagulant drugs for AF, which indicates an increased bleeding risk by itself. Additionally, a greater number of patients in the atherothrombosis group had high HAS‐BLED scores (≥3) when compared with the non‐atherothrombosis group. This suggests that the optimal “therapeutic window” may have been even narrower and of lesser range for participants in the atherothrombosis group; see Figure 4A and 4B.
Potential of Dual‐Pathway Inhibition Via Rivaroxaban
The coagulation and platelet pathways intersect at several sites. Thrombin is not only an important mediator of the coagulation cascade but also acts as a potent platelet agonist that induces platelet activation and aggregation via activation of protease‐activated receptors on the platelet surface. Because of the synergistic involvement of platelets and coagulation in atherothrombosis, treatment strategies targeting both fibrin generation and platelet activation, termed “dual pathway inhibitions”, are likely to be well‐balanced and effectively prevent atherothrombosis while minimizing bleeding. 20 Recent studies indicate that rivaroxaban produces an inhibitory effect on thrombin‐mediated platelet aggregation via protease‐activated receptors‐1 while directly inhibiting both factor Xa and thrombin generation. 21 Through the unique characteristic of being a potential dual pathway inhibitor, the AFIRE trial demonstrated a well‐balanced outcome: rivaroxaban monotherapy significantly reduced net adverse events in patients with AF, CAD, and histories of atherothrombotic disease.
Limitations
This study also had several limitations. First, its analyses were based on data from the open‐label AFIRE trial, meaning that all reported findings were retrieved post hoc. In the same regard, we did not directly diagnose participants for prior myocardial infarction, stroke, or peripheral artery disease. Second, the AFIRE did not complete its planned follow‐up; as such, we were not able to analyze long‐term data. Third, rivaroxaban was prescribed at the approved dose in Japan (either 10 or 15 mg once daily, according to creatinine clearance) rather than the globally approved once‐daily dose of 20 mg. Fourth, the study population was comprised of a single ethnic group, nevertheless, the study may have direct implications for similar populations and it certainly lays groundwork for further investigations. Fifth, P values for interactions between the treatment strategy and atherothrombotic disease were borderline and were not significant.
Conclusions
When compared with the combination therapy, rivaroxaban monotherapy significantly reduced net adverse events in patients with AF, CAD, and histories of atherothrombotic disease, including myocardial infarction, stroke, and/or peripheral artery disease. While it initially seemed that more intense antithrombotic therapy provided greater benefits to patients with prior atherothrombotic disease, we found that too much intensity could produce negative outcomes. More specifically, these patients were at high risk not only for thrombotic events, but also for bleeding events, in which case bleeding risk may be a more important factor. We therefore suggest that antithrombotic therapy should be adjusted with respect to bleeding risk as well rather than strictly focusing on the elimination of antithrombotic activity. The mechanisms of bleeding and thromboembolism are both multifactorial and complex, which makes it difficult to determine the best antithrombotic therapy strategy for either condition. Further investigations will be necessary to confirm.
Sources of Funding
This work was supported by the Japan Cardiovascular Research Foundation based on a contract with Bayer Yakuhin, Ltd., which had no role in the design of the trial, collection or analysis of the data, interpretation of the trial results, or writing of the article.
Disclosures
Dr Kimura reports grants from the Japan Cardiovascular Research Foundation; grants and personal fees from Bayer Yakuhin, Daiichi Sankyo, Sanofi, MSD, and AstraZeneca; personal fees from Bristol‐Myers Squibb and Nippon Boehringer Ingelheim. Dr Yasuda reports grants from Takeda Pharmaceutical, Abbott, and Boston Scientific; personal fees from Daiichi‐Sankyo and Bristol‐Meyers. Dr Kaikita reports grants from Grants‐in‐Aid for Scientific Research (20K08451) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; grants and personal fees from Bayer Yakuhin and Daiichi Sankyo. Dr Akao reports grants from the Japan Agency for Medical Research and Development; personal fees from Bristol‐Myers Squibb and Nippon Boehringer Ingelheim; grants and personal fees from Bayer Yakuhin and Daiichi Sankyo. Dr Ako reports personal fees from Bayer Yakuhin and Sanofi; grants and personal fees from Daiichi Sankyo. Dr Matoba reports grants from Japan Cardiovascular Research Foundation; personal fees from Nippon Boehringer Ingelheim, Daiichi Sankyo, Astra Zeneca, and Bayer Yakuhin. Dr Nakmaura reports grants and personal fees from Bayer Yakuhin, Daiichi Sankyo, and Sanofi; personal fees from Bristol‐Myers Squibb and Nippon Boehringer Ingelheim. Dr Miyauchi reports personal fees from Amgen Astellas BioPharma, Astellas Pharma, MSD, Bayer Yakuhin, Sanofi, Takeda Pharmaceutical, Daiichi‐Sankyo, Nippon Boehringer Ingelheim, and Bristol‐Myers Squibb. Dr Hagiwara reports grants and personal fees from Bayer Yakuhin; grants from Nippon Boehringer Ingelheim; personal fees from Bristol‐Myers Squibb. Dr Hirayama reports grants and personal fees from Boston Scientific Japan, Otsuka Pharmaceutical, Sanofi, Astellas Pharma, Bristol‐Myers Squibb, Daiichi Sankyo, and Bayer Yakuhin; grants from Fukuda Denshi, Abbott Japan, Japan Lifeline, Takeda Pharmaceutical, Sumitomo Dainippon Pharma; personal fees from Toa Eiyo, Nippon Boehringer Ingelheim, Amgen Astellas BioPharma, and AstraZeneca. Dr Ogawa reports personal fees from Towa Pharmaceutical, Bristol‐Meyers Squibb, Pfizer, Toa Eiyo, Bayer Yakuhin, and Novartis Pharma. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
(J Am Heart Assoc. 2021;10:e020907. DOI: 10.1161/JAHA.121.020907.)
For Sources of Funding and Disclosures, see page 12.
References
- 1. Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103–1113. DOI: 10.1056/NEJMoa1904143. [DOI] [PubMed] [Google Scholar]
- 2. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PWF, Alberts MJ, D’Agostino R, Liau C‐S, et al. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. DOI: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 3. Rosengren A, Wilhelmsen L, Hagman M, Wedel H. Natural history of myocardial infarction and angina pectoris in a general population sample of middle‐aged men: a 16‐year follow‐up of the Primary Prevention Study, Goteborg, Sweden. J Intern Med. 1998;244:495–505. DOI: 10.1111/j.1365-2796.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- 4. Miura T, Soga Y, Doijiri T, Aihara H, Yokoi H, Iwabuchi M, Nobuyoshi M. Prevalence and clinical outcome of polyvascular atherosclerotic disease in patients undergoing coronary intervention. Circ J. 2013;77:89–95. DOI: 10.1253/circj.CJ-12-0535. [DOI] [PubMed] [Google Scholar]
- 5. Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, et al.; Charisma Investigators . Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–1988. DOI: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 6. Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, Taubert K, Smith SC Jr. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014;11:597–606. DOI: 10.1038/nrcardio.2014.104. [DOI] [PubMed] [Google Scholar]
- 7. Kang J, Park K, Palmerini T, Stone G, Lee M, Colombo A, Chieffo A, Feres F, Abizaid A, Bhatt D, et al. Racial differences in ischaemia/bleeding risk trade‐off during anti‐platelet therapy: individual patient level landmark meta‐analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. DOI: 10.1055/s-0038-1676545. [DOI] [PubMed] [Google Scholar]
- 8. Yasuda S, Kaikita K, Ogawa H, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, et al. Atrial fibrillation and ischemic events with rivaroxaban in patients with stable coronary artery disease (AFIRE): protocol for a multicenter, prospective, randomized, open‐label, parallel group study. Int J Cardiol. 2018;265:108–112. DOI: 10.1016/j.ijcard.2018.04.131. [DOI] [PubMed] [Google Scholar]
- 9. Tanigawa T, Kaneko M, Hashizume K, Kajikawa M, Ueda H, Tajiri M, Paolini JF, Mueck W. Model‐based dose selection for phase III rivaroxaban study in Japanese patients with non‐valvular atrial fibrillation. Drug Metab Pharmacokinet. 2013;28:59–70. DOI: 10.2133/dmpk.DMPK-12-RG-034. [DOI] [PubMed] [Google Scholar]
- 10. Kaneko M, Tanigawa T, Hashizume K, Kajikawa M, Tajiri M, Mueck W. Confirmation of model‐based dose selection for a Japanese phase III study of rivaroxaban in non‐valvular atrial fibrillation patients. Drug Metab Pharmacokinet. 2013;28:321–331. DOI: 10.2133/dmpk.DMPK-12-RG-109. [DOI] [PubMed] [Google Scholar]
- 11. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, ESC Scientific Document Group , et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. DOI: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 12. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation the Euro Heart Survey. Chest. 2010;138:1093–1100. DOI: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 13. Held C, Hylek EM, Alexander JH, Hanna M, Lopes RD, Wojdyla DM, Thomas L, Al‐Khalidi H, Alings M, Xavier D, et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: Insights from the ARISTOTLE trial. Eur Heart J. 2015;36:1264–1272. DOI: 10.1093/eurheartj/ehu463. [DOI] [PubMed] [Google Scholar]
- 14. Alberts MJ, Bhatt DL, Smith SC, Rother J, Goto S, Hirsch AT, Steg PG; REACH Registry Investigators . Risk factors and outcomes for patients with vascular disease and serious bleeding events. Heart. 2011;97:1507–1512. DOI: 10.1136/hrt.2010.221788. [DOI] [PubMed] [Google Scholar]
- 15. Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease reply. N Engl J Med. 2019;381:2481. DOI: 10.1056/NEJMoa1904143. [DOI] [PubMed] [Google Scholar]
- 16. Genereux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, et al. Incidence, predictors, and impact of post‐discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66:1036–1045. 10.1016/j.jacc.2015.06.1323. [DOI] [PubMed] [Google Scholar]
- 17. Lamberts M, Gislason GH, Lip GY, Lassen JF, Olesen JB, Mikkelsen AP, Sorensen R, Kober L, Torp‐Pedersen C, Hansen ML. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129:1577–1585. DOI: 10.1161/CIRCULATIONAHA.113.004834. [DOI] [PubMed] [Google Scholar]
- 18. Lee SR, Rhee TM, Kang DY, Choi EK, Oh S, Lip GYH. Meta‐analysis of oral anticoagulant monotherapy as an antithrombotic strategy in patients with stable coronary artery disease and nonvalvular atrial fibrillation. Am J Cardiol. 2019;124:879–885. DOI: 10.1016/j.amjcard.2019.05.072. [DOI] [PubMed] [Google Scholar]
- 19. Goto S, Hasebe T, Takagi S. Platelets: small in size but essential in the regulation of vascular homeostasis—translation from basic science to clinical medicine. Circ J. 2015;79:1871–1881. 10.1253/circj.CJ-14-1434. [DOI] [PubMed] [Google Scholar]
- 20. Olie RH, van der Meijden PEJ, ten Cate H. The coagulation system in atherothrombosis: implications for new therapeutic strategies. Res Pract Thromb Haemost. 2018;2:188–198. DOI: 10.1002/rth2.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perzborn E, Heitmeier S, Laux V. Effects of rivaroxaban on platelet activation and platelet‐coagulation pathway interaction: in vitro and in vivo studies. J Cardiovasc Pharmacol Ther. 2015;20:554–562. DOI: 10.1177/1074248415578172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


