Abstract
Background
Circulating microRNAs are emerging biomarkers for heart failure (HF). Our study aimed to assess the prognostic value of microRNA signature that is differentially expressed in patients with acute HF.
Methods and Results
Our study comprised a screening cohort of 15 patients with AHF and 5 controls, a PCR‐discovery cohort of 50 patients with AHF and 26 controls and a validation cohort of 564 patients with AHF from registered study DRAGON‐HF (Diagnostic, Risk Stratification and Prognostic Value of Novel Biomarkers in Patients With Heart Failure). Through screening by RNA‐sequencing and verification by reverse‐transcription quantitative polymerase chain reaction, 9 differentially expressed microRNAs were verified (miR‐939‐5p, miR‐1908‐5p, miR‐7706, miR‐101‐3p, miR‐144‐3p, miR‐4732‐3p, miR‐3615, miR‐484 and miR‐19b‐3p). Among them, miR‐19b‐3p was identified as the microRNA signature with the highest fold‐change of 8.4 and the strongest prognostic potential (area under curve with 95% CI, 0.791, 0.654–0.927). To further validate its prognostic value, in the validation cohort, the baseline level of miR‐19b‐3p was measured. During a follow‐up period of 19.1 (17.7, 20.7) months, primary end point comprising of all‐cause mortality or readmission due to HF occurred in 48.9% patients, while patients in the highest quartile of miR‐19b‐3p level presented the worst survival (Log‐rank P<0.001). Multivariate Cox model showed that the level of miR‐19b‐3p could independently predict the occurrence of primary end point (adjusted hazard ratio,1.39; 95% CI, 1.18–1.64). In addition, miR‐19b‐3p positively correlated with soluble suppression of tumorigenicity 2 and echocardiographic indexes of left ventricular hypertrophy.
Conclusions
Circulating miR‐19b‐3p could be a valuable prognostic biomarker for AHF. In addition, a high level of circulating miR‐19b‐3p might indicate ventricular hypertrophy in AHF subjects.
Registration
URL: https://www.clinicaltrials.gov. Unique Identifier: NCT03727828.
Keywords: acute heart failure, biomarker, miR‐19b‐3p, prognosis, RNA‐sequencing
Subject Categories: Heart Failure, Biomarkers, Clinical Studies, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- AHF
acute heart failure
- HFpEF
heart failure with preserved ejection fraction
- IVST
interventricular septal thickness
- LVMI
left ventricular mass index
- LVPWT
left ventricular posterior wall thickness
- NYHA
New York Heart Association
- sST2
soluble suppression of tumorigenicity 2
Clinical Perspective
What Is New?
miR‐19b‐3p is identified as a novel prognostic microRNA signature for acute heart failure population.
The baseline level of miR‐19b‐3p was strongly correlated with echocardiographic indexes of left ventricular hypertrophy and the fibrosis‐related biomarker soluble suppression of tumorigenicity 2.
What Are the Clinical Implications?
The expression level of miR‐19b‐3p at the onset of acute heart failure could predict the prognosis and function as a potential biomarker for stratification of patients with acute heart failure.
The association of miR‐19b‐3p with hypertrophic and fibrosis indexes prompts for further investigations to reveal the pathogenesis of acute heart failure.
Acute heart failure (AHF) is a severe cardiac syndrome that carries a high risk of hospital readmission and mortality. Predicting the outcome of patients with AHF has been a challenging task. Although various circulating peptides, such as natriuretic peptides, have been shown to facilitate the diagnosis and guidance of short and long term therapy in patients with AHF their drawbacks limit their potential as prognostic biomarkers. Recently, microRNAs (miRNAs) have been gaining attention due to their promising role as potential biomarkers for stratifying patients with cardiac diseases. 1
miRNAs are small non‐coding RNAs that orchestrate homeostasis through acting on post‐transcription process. Due to their considerable stability, circulating miRNAs have been promoted as ideal biomarkers for heart failure (HF). 2 , 3 Moreover, as miRNAs regulate various biophysiological processes, the presence of certain miRNAs can be harnessed to identify particular types of HF such as ischemic HF, 4 , 5 systolic HF, 6 HF with preserved ejection fraction (HFpEF), 7 and HF due to dilated cardiomyopathy. 8 Hence, miRNAs are promising indicators for the risk stratification and prognosis of HF.
In recent years, over ten miRNAs that play a diagnostic and prognostic role in AHF subjects have been identified, 9 and some of them could indeed uncover the underlying mechanisms contributing to AHF. For instance, an aberrant miR‐22 level could indicate the disturbance of calcium homeostasis and myofilament protein content under stress, 10 and levels of miR‐21 and miR‐30a/b could indicate the severity of acute kidney injury. 11 , 12 Therefore, further investigations of miRNAs are warranted to unravel the mechanisms and optimize the management of HF.
The present study was aimed at exploring circulating miRNAs associated with AHF using RNA‐sequencing and identifying the miRNA signature for prognosis. We then validate its prognostic potential in a prospective cohort incorporating 564 patients with AHF and investigate its clinical relevance with serological and echocardiographical measurements.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Recruitment
The study design is illustrated in Figure 1. Three independent cohorts were built for screening, PCR‐discovery of miRNA candidates, and validation of the miRNA signature. (1) The screening cohort comprised 15 patients with AHF and 5 control subjects; (2) the PCR‐discovery cohort comprised 50 patients with AHF and 26 control subjects; (3) the prospective validation cohort comprised 564 patients with AHF from registered DRAGON‐HF (Diagnostic, Risk Stratification and Prognostic Value of Novel Biomarkers in Patients With Heart Failure) study (ClinicalTrials.gov, NCT03727828). The diagnosis of AHF was in accord with 2016 ESC Guidelines of HF, all patients were consecutively enrolled and received standard‐of‐care according to the guidelines. 1
Figure 1. Flow chart of the study.
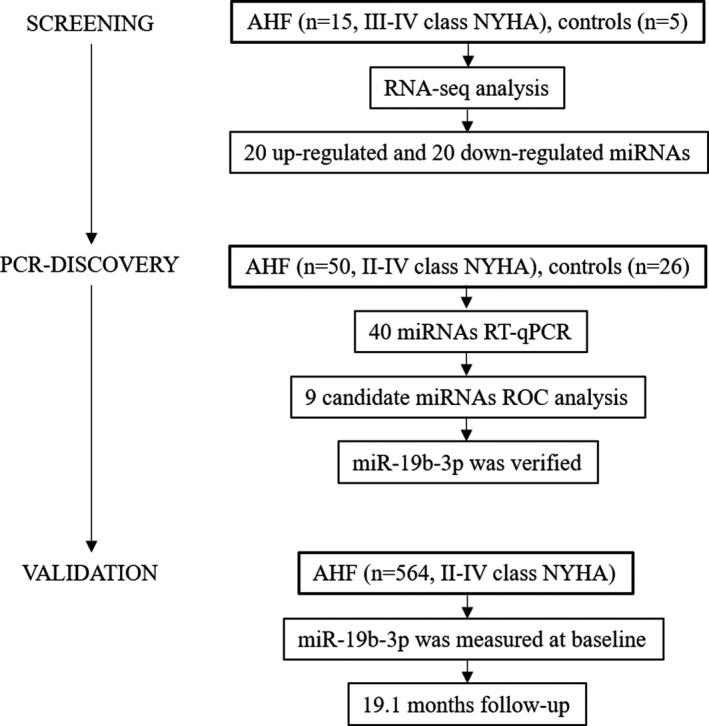
AHF indicates acute heart failure; NYHA, New York Heart Association; ROC, receiver operating characteristics; and RT‐qPCR, Quantitative Real‐time Polymerase Chain Reaction.
Inclusion criteria for the validation cohort were as follow: (1) at least 18 years of age; (2) admission for diagnosed AHF (New York Heart Association [NYHA] classification from II to IV); (3) acquired consent form. Patients were excluded if complicated with one of the following: (1) acute myocardial infarction; (2) acute pulmonary embolism; (3) acute stroke; (4) preexisting organ failure (chronic cirrhosis, uremia, or end‐stage cancer); (5) pregnancy.
This study complies with the Declaration of Helsinki, and has been approved by the local Ethics Committee. All participants have signed informed consent forms.
Data Collection
Demographic characteristics, medical history, and medication were determined at admission. Transthoracic echocardiography was performed by an experienced echocardiologist within 24 hours of admission. Echocardiographic parameters including left ventricular end‐diastolic diameter (LVeDD), left ventricular end‐systolic diameter (LVeSD), interventricular septal thickness (IVST) and left ventricular posterior wall thickness (LVPWT) were measured by two experienced physicians using MyLab 30 CB machine (ESAOTE SPA) according to the recommendations of the American Society of Echocardiography and the European Association of Echocardiography. 13 Left ventricular mass index (LVMI) was calculated by the formula: LVMI (g/m2)=(0.8×1.04×((LVeDD+LVPWT+IVST)3−(LVeDD)3)+0.6)/BSA (body surface area, m2).
Subjects of PCR‐discovery cohort were scheduled to have 1‐year follow‐up. And subjects of validation cohort underwent scheduled follow‐up in the third, sixth, twelfth month, and every year since then. The primary end point was the composite of all‐cause mortality and re‐admission due to HF. At every scheduled follow‐up or readmission, medical history and physical examination were taken by experienced physicians, and electrocardiography and blood samples were collected. Other examinations were conducted as deemed necessary.
Serological Measurements
Baseline blood samples were obtained on admission prior to any treatment. Venous blood samples were collected for detection of soluble suppression of tumorigenicity 2 (sST2), NT‐proBNP (N‐terminal pro brain natriuretic peptide), and other biochemical indicators. NT‐proBNP level was measured by an automated electrochemiluminescence immunoassay (Elecsys proBNP II assay). The remaining blood samples were centrifuged by two‐step method (4 °C at 820×g for 10 minutes, then 4 °C at 16000×g for 10 minutes) 14 and the supernatant was preserved at −80 ℃ with RNase/DNase‐free tubes for further miRNAs and sST2 detection. sST2 levels were detected using Human ST2/IL‐33R Quantikine ELISA Kit (R&D Systems, Minneapolis, MN). 15 The sST2 level of patients were calculated from the mean value of two repeated measurements of blood sample. Total RNAs were extracted from serum samples of screening cohort patients, and RNA‐sequencing was performed to figure out differentially expressed miRNAs. Confirmation and validation of candidate miRNAs were accomplished by using real‐time polymerase chain reaction (PCR) in PCR‐discovery and validation cohort. (The description in details for miRNA profiling, PCR detection, primer sequences and bioinformatic analysis were showed in Data S1 and Table S1 with references [16, 17, 18, 19, 20, 21, 22, 23] cited in the order as in the main manuscript).
Statistical Analysis
Bioinformatics Analysis of RNA‐Sequencing Data
In the screening process, false discovery rate <0.01 and fold change >2 are taken as the screening criteria by default. In total, 20 up‐regulated and 20 down‐regulated miRNAs with the most significant difference (P value <0.05) in expression were selected. Based on GO and KEGG databases, the miRNAs involving in pathophysiological processes of cardiovascular diseases were selected for PCR verification. For miRNA verified in the PCR‐discovery cohort, the diagnostic and prognostic power were assessed by receiver‐operator characteristics (ROC) analysis. To better quantitatively identify the miRNA signature that has the highest diagnostic and prognostic power, the area under curve (AUC) was compared among candidates. Subsequently, the signature was adopted for further validation.
Clinical Data Analysis
Descriptive statistics were obtained for all study variables. Continuous data were expressed as mean±SD or median with interquartile range. Categorical data were expressed as proportions. All continuous variables were compared using the t‐test or the Mann‐Whitney U‐test if appropriate. Categorical variables were analyzed for the study outcome by Fisher exact test or χ2 test. Logarithmic transformation was applied for variables including NT‐proBNP, sST2, and miRNAs in order to conform normal distribution. To validate the prognostic value of the signature and compare with other biomarkers, and further investigate the underlying association, survival analysis was adopted for validation.
The validation cohort was divided into four quartiles based on the baseline level of the miRNA signature. Clinical measurements were compared among four quartiles using analysis of variance if data complied normal distribution with homogeneity of variance or sign rank test if not. The associations of miR‐19b‐3p with clinical measurements was analyzed by Pearson correlation and Spearman rank correlation analysis. The prognosis among four quartiles was assessed by survival analysis using Kaplan‐Meier estimate and P‐value was generated by Log‐Rank test. Besides, univariate and multivariate Cox proportional hazard models were applied to evaluate the prognostic value, and the hazard ratio with corresponding 95% CI was calculated for investigating the risk factors of the primary end point. The multivariate model was adjusted for age, sex, body mass index, systolic blood pressure, NT‐proBNP, atrial fibrillation history, coronary heart disease history, diabetes history, and estimated glomerular filtration rate.
Clinical data were analyzed using SPSS version 25.0 (IBM Corp) and Graphpad Prism 8.0.1 (GraphPad Software, San Diego, CA). For all the statistical analyses, 2‐sided P<0.05 was considered significant.
Results
miRNAs Expression Profile in the Screening Cohort
In the screening phase, demographic characteristics were almost similar between 15 patients with AHF and 5 controls. However, patients with AHF had distinctly higher NT‐proBNP level (2521.0 versus 78.0 ng/L, P<0.001), worse cardiac function (left ventricular ejection fraction, 28.7±7.9% versus 65.4%±2.6%, P<0.001), worse renal function (estimated glomerular filtration rate 76.4±24.4 versus 107.7±22.5 mL/min×1.73 m2, P=0.021), and lower high density lipoprotein levels (1.02±1.17 versus 1.71±0.33 mmol/L, P=0.004) (Table S2).
RNA‐sequence analysis documented that a portion of miRNAs had a strong positive correlation between patients with AHF and controls (Figure S1A). As we displayed overall P‐value (Figure S1B), 218 miRNAs were differentially expressed in patients with AHF. Eventually, 40 miRNA candidates (20 up‐regulated and 20 down‐regulated) with the highest fold‐change were screened for validation (Figure S1C and S1D).
Verification of miRNA Expression and Identification of miRNA Signature
qPCR of the 40 selected miRNAs was undertaken with circulating RNA obtained from the PCR‐discovery cohort composed of 50 patients with AHF and 26 health controls (Table S2). This cohort has an average age of 66.7±11.1 years old, and the proportion of women was 69.3%. Among these, a total of 9 miRNAs (miR‐939‐5p, miR‐1908‐5p, miR‐4732‐3p, miR‐7706, miR‐3615, miR‐484, miR‐19b‐3p, miR‐101‐3p, and miR‐144‐3p) were successfully validated and showed significantly different expression level (Figure 2). miR‐19b‐3p presented the highest level of up‐regulation (fold change=8.4). ROC curve showed that all 9 validated miRNAs could discriminate patients with AHF from controls, and miR‐19b‐3p had the highest AUC value of 0.753 (Figure 3).
Figure 2. Relative expression of 9 verified miRNAs between patients with AHF and control.
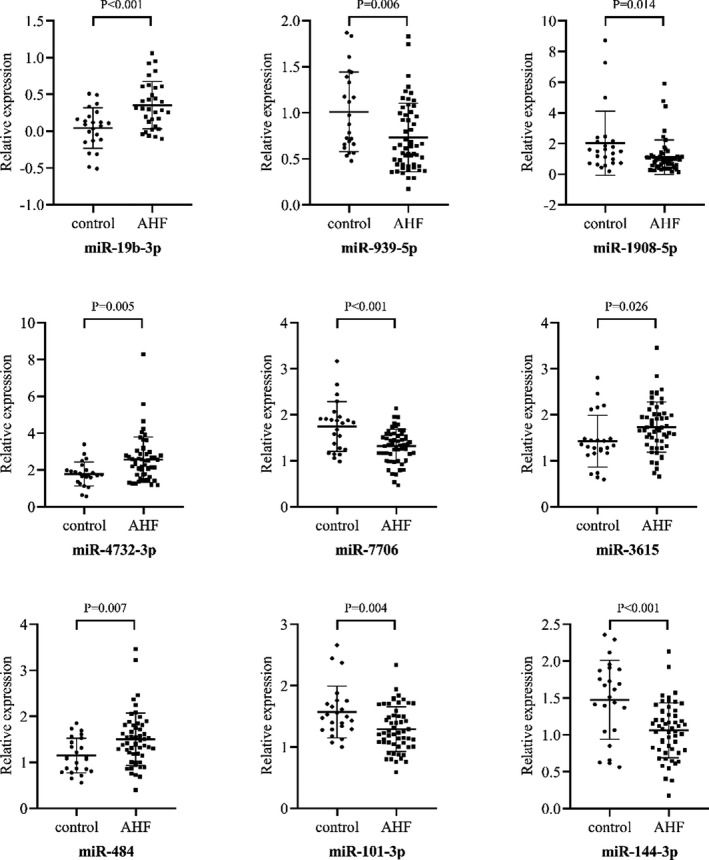
miRNA candidates were verified in 50 AHFs and 26 controls by real‐time quantitative polymerase chain reaction. Out of 40 candidates, 9 were verified with significant differential expression and miR‐19b‐3p presented the highest relative expression fold‐change of 8.4. AHF indicates acute heart failure.
Figure 3. Receiver operating characteristic curves of 9 validated miRNAs for diagnosis of AHF.

Receiver operating characteristic curves were performed to test the diagnostic value of 9 miRNA candidates for AHF in PCR‐discovery cohort. miR‐19b‐3p was found to have the highest discriminative potential for AHF (AUC=0.753). AHF denotes acute heart failure, AUC area under curve. Lower right annotation in each figure showed AUC with 95% CI and P value.
To preliminarily evaluate the prognostic potential of 9 miRNAs candidates, ROC analysis for the occurrence of end point events was performed (Figure 4). Three miRNAs showed significant P‐value (miR‐19b‐3p, miR‐484 and miR‐3615), and miR‐19b‐3p presented the strongest power for discriminating patients with AHF who had occurrence of events from those had not. Therefore, miR‐19b‐3p was identified as the miRNA signature and underwent further validation.
Figure 4. Prognostic power of 9 validated miRNAs by receiver operating characteristic curves.
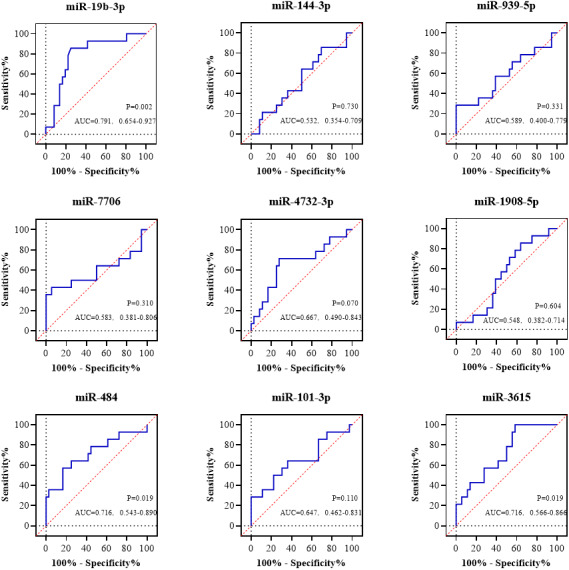
Receiver operating characteristic curves were performed to test the diagnostic value of 9 miRNA candidates for AHF in PCR‐discovery cohort. miR‐19b‐3p was found to have the highest discriminative potential for AHF (AUC=0.753). AHF denotes acute heart failure, AUC area under curve. Lower right annotation in each figure showed AUC with 95% CI and P value.
Validation of miR‐19b‐3p in the Validation Cohort
The clinical significance of miR‐19b‐3p was further evaluated in the validation cohort. A total of 564 patients with AHF were divided into 4 quartiles according to the baseline level of miR‐19b‐3p. As shown in Table, the validation cohort was composed of more men (64.5%), and the median age was 69.0 [62.0, 77.0] years old. Demographic characteristics and medication at discharge (shown in Table S3) were similar among 4 groups.
Table 1.
Clinical Characteristics of 564 Patients With AHF in Quartiles of miR‐19b‐3p Levels
|
Overall (N=564) |
Q1 (N=141) |
Q2 (N=141) |
Q3 (N=141) |
Q4 (N=141) |
P value | |
|---|---|---|---|---|---|---|
| Age, y | 69.0 (62.0, 77.0) | 70.0 (63.0, 82.0) | 68.0 (61.0, 77.0) | 69.0 (62.0, 75.0) | 70.0 (61.0, 78.0) | 0.214 |
| Male/female, n/n | 404/160 | 99/42 | 99/42 | 101/40 | 105/36 | 0.790 |
| BMI, kg/m2 | 24.3±3.6 | 24.3±3.6 | 24.0±3.8 | 24.5±3.6 | 24.5±3.6 | 0.950 |
| Smoking, n (%) | 175 (31.0) | 47 (33.3) | 43 (30.5) | 49 (34.8) | 36 (25.5) | 0.456 |
| NYHA functional class | 2.4±0.8 | 2.4±0.8 | 2.4±0.7 | 2.3±0.8 | 2.5±0.8 | 0.080 |
| HR, bpm | 83.7±18.5 | 83.9±18.7 | 84.1±17.9 | 83.7±18.6 | 82.9±19.0 | 0.977 |
| SBP, mm Hg | 135.5±23.7 | 137.1±23.3 | 133.6±23.4 | 134.5±24.5 | 137.0±23.7 | 0.900 |
| DBP, mm Hg | 79.2±15.8 | 79.6±15.5 | 78.9±15.6 | 78.7±15.5 | 79.4±16.6 | 0.847 |
| Comorbidities | ||||||
| Diabetes, n (%) | 205 (36.4) | 45 (31.9) | 50 (35.5) | 53 (37.6) | 57 (40.4) | 0.463 |
| Hypertension, n (%) | 373 (66.1) | 95 (67.4) | 81 (57.4) | 93 (66.0) | 104 (73.8) | 0.024* |
| CHD, n (%) | 344 (61.0) | 82 (58.2) | 85 (60.3) | 89 (63.1) | 88 (62.4) | 0.657 |
| AF, n (%) | 118 (20.9) | 37 (26.2) | 24 (17.0) | 31 (22.0) | 26 (18.4) | 0.205 |
| ICM, n (%) | 123 (21.8) | 30 (21.3) | 30 (21.3) | 34 (24.1) | 29 (20.6) | 0.893 |
| HCM, n (%) | 8 (1.4) | 2 (1.4) | 1 (0.7) | 2 (1.4) | 3 (2.1) | 0.798 |
| DCM, n (%) | 31 (5.5) | 10 (7.1) | 4 (2.8) | 8 (5.7) | 9 (6.4) | 0.418 |
| Serological measurements | ||||||
| NT‐proBNP, ng/mL | 1918.0 (782.5, 5070.5) | 2170.0 (993.7, 4748.0) | 1960.0 (800.6, 5051.3) | 1986.0 (609.0, 6771.0) | 1636.0 (871.9, 4723.0) | 0.261 |
| vmiR‐19b‐3p (relative expression) | 2.15 (1.63, 3.40) | 1.23 (0.99, 1.43) | 1.89 (1.75, 2.00) | 2.69 (2.36, 2.96) | 5.24 (4.28, 6.73) | <0.001* |
| sST2, ng/mL | 42.3 (36.8, 50.7) | 38.4 (33.0, 42.4) | 40.6 (35.4, 47.2) | 42.6 (48.4, 38.5) | 53.4 (44.9, 67.4) | <0.001* |
| HDL, mmol/L | 0.96 (0.79, 1.20) | 1.02 (0.81, 1.31) | 0.98 (0.81, 1.25) | 0.93 (0.79, 1.13) | 0.94 (0.78, 1.15) | 0.521 |
| LDL, mmol/L | 2.16±0.93 | 2.07±0.91 | 2.15±0.98 | 2.24±0.94 | 2.19±0.88 | 0.627 |
| eGFR, mL/(min*1.73 m2) | 79.2 (56.4, 99.3) | 82.1 (64.2, 101.7) | 81.4 (55.5, 102.9) | 79.2 (57.8, 98.0) | 73.6 (54.9, 95.7) | 0.355 |
| BUN, mmol/L | 8.3±6.0 | 7.7±3.9 | 8.4±4.5 | 8.7±9.3 | 8.5±4.9 | 0.361 |
| Hb1Ac (%) | 6.3 (5.8, 7.4) | 6.4 (5.8, 7.3) | 6.3 (5.8, 7.5) | 6.2 (5.7. 7.0) | 6.3 (5.8, 7.5) | 0.678 |
| Echocardiographic measurements | ||||||
| LVEF (%) | 43.0 (32.0, 55.0) | 44.0 (33.0, 55.0) | 43.0 (30.0, 55.0) | 44.0 (35.8, 56.0) | 40.0 (32.3, 54.8) | 0.675 |
| IVST, mm | 10.0 (9.0, 11.0) | 9.0 (9.0, 10.0) | 9.0 (9.0, 10.0) | 10.0 (9.0, 10.0) | 11.0 (10.0, 13.0) | <0.001* |
| LVPWT, mm | 10.0 (9.0, 10.0) | 10.0 (9.0, 10.0) | 10.0 (9.0, 10.0) | 10.0 (9.0, 10.0) | 10.0 (9.0, 11.0) | 0.001* |
| LVeDD, mm | 50.0 (45.0 57.0) | 49.0 (44.0, 56.0) | 50.0 (44.0, 57.0) | 49.0 (45.0, 56.0) | 51.0 (45.0, 60.0) | 0.151 |
| LVeSD, mm | 35.5 (30.0, 46.0) | 32.0 (29.0, 43.0) | 31.0 (27.0, 45.0) | 34.0 (28.0, 44.0) | 37.0 (30.0, 47.0) | 0.241 |
| LVMI, g/m2 | 113.5±34.8 | 101.0±28.1 | 103.8±25.2 | 110.2±26.8 | 141.1±42.2 | <0.001* |
Continuous variables are presented as means±SD if conform normal distribution or median with interquartile range (IQR) if not. Categorical variables are presented as percentage (%). AF indicates atrial fibrillation; AHF, acute heart failure; BMI, body mass index; BUN, blood urea nitrogen; CHD, coronary heart disease; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate (calculated by MDRD formula); Hb1Ac, hemoglobin A1c; HCM, hypertrophic cardiomyopathy; HDL, high‐density lipoprotein; HR, heart rate; ICM, ischemic cardiomyopathy; IVST, interventricular septum thickness; LDL, low‐density lipoprotein; LVeDD, left ventricular end‐diastolic diameter; LVEF, left‐ventricular ejection fraction; LVeSD, left ventricular end‐systolic diameter; LVMI, left ventricular mass index; LVPWT, left ventricular posterior wall thickness; NT‐proBNP, N‐terminal brain natriuretic peptide precursor; NYHA, New York Heart Association; SBP, systolic blood pressure; and sST2, soluble suppression of tumorigenicity 2.
Significant P value (<0.05).
In further investigating the clinical relevance of miR‐19b‐3p, we found significant positive correlations with serum sST2 (r=0.583) and echocardiographic indexes of left ventricular hypertrophy including IVST (r=0.437), LVPWT (r=0.285) and LVMI (r=0.492). However, no significant correlation was found between miR‐19b‐3p and NT‐proBNP, left ventricular ejection fraction (Figure S2).
To validate the prognostic value of miR‐19b‐3p, occurrence of clinical events were compared among 4 quartiles of the cohort. Figure 5 shows the differences in occurrence of the primary end points (all cause‐mortality or HF readmission) among patients in different quartiles of baseline miR‐19b‐3p levels. Through a follow‐up period of 19.1 [17.7, 20.7] months, the event‐free survival worsened from Q1 to Q4. A count of 83 out of 141 patients with AHF of Q4 reached primary end point, presenting the worst survival among 4 quartiles (Log‐Rank P<0.001).
Figure 5. Survival analysis of patients with AHF divided by baseline level of miR‐19b‐3p.
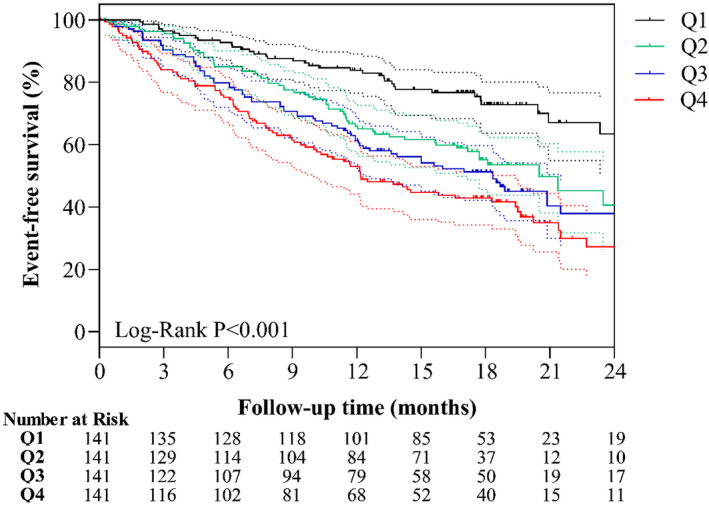
Survival analysis of miR‐19b‐3p for primary end point (all‐cause mortality or readmission due to HF) were performed and survival curves with 95% CI shown with dotted line in corresponding color. Through a follow‐up period of 19.1 [17.7, 20.7] months, survival curves showed group with higher level of miR‐19b‐3p presented worse event‐free survival, with Log‐Rank P value <0.001. Q1, Q2, Q3 and Q4 represent patients with AHF with relative expression of miR‐19b‐3p level lower than 1.63, 1.63‐2.15, 2.15‐3.41, and higher than 3.41, respectively.
Subsequently, multivariate Cox proportional hazard model showed that baseline miR‐19b‐3p levels could predict the occurrence of the primary end points in overall population [hazard ratio, 1.39; 95% CI, 1.18–1.64] (Figure 6).
Figure 6. The Forest plot of multivariate Cox proportional hazard regression model for prognostic evaluation.
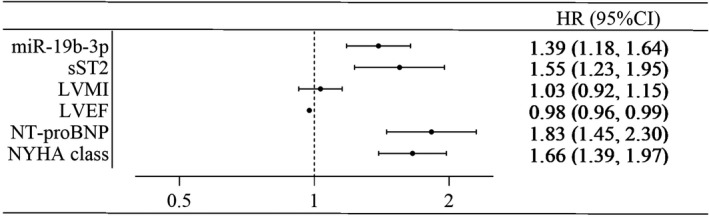
The level of miR‐19b‐3p, sST2, and NT‐proBNP was analyzed after logarithmic transformation.
Discussion
This is a large cohort study investigating the profiling of miRNA among 564 patients with AHF in China. The salient findings from this study include detection of miR‐19b‐3p as the most abundantly expressed miRNA among patients with AHF and the fact that miR‐19b‐3p achieved the highest prognostic value for primary cardiovascular events among patients with AHF. Furthermore, independent positive associations were found between circulating miR‐19b‐3p and serum sST2, LVMI, IVST and LVPWT in the AHF cohort, which implied a positive association between miR‐19b‐3p and ventricular hypertrophy.
In recent decades, multiple miRNAs have been identified as biomarkers for AHF diagnosis or prognosis. 5 up‐regulated miRNAs, including miR‐150‐5p, 24 miR‐1306‐5p, 25 miR‐92b‐5p, 26 miR‐302 family, 27 and miR‐499, 28 and 7 down‐regulated miRNAs, including miR‐7i‐5p, miR‐18a‐5p, miR‐18b‐5p, miR‐223‐3p, miR‐301a‐3p, miR‐423‐5p, and miR‐652‐3p 9 have been previously identified. A growing number of miRNAs were found to play important roles in the pathogenesis of AHF, although not much is known about their association with different clinical features. In the present study, we identified the novel circulating miR‐19b‐3p that not only diagnose AHF, but also revealed its positive association with LV hypertrophy indices and cardiac fibrosis biomarker sST2. This suggests that an elevation of circulating miR‐19b‐3p could indicate fibrogenetic and hypertrophic responses in AHF subjects, which could exacerbate the condition of AHF.
miR‐19b‐3p belongs to the miRNA cluster miR‐17‐92, which is an important modulator in heart diseases. 29 In the in vitro studies, it had been illustrated that miR‐19b‐3p participated in the cardiac fibrosis process through modulating transforming growth factor‐beta pathway and phosphatase and tensin homologue gene expression. Zou et al found that over‐expressed miR‐19a‐3p/19b‐3p in human cardiac fibroblasts could reduce transforming growth factor‐beta signaling by targeting transforming growth factor‐beta receptor II mRNA, and therefore inhibit autophagy‐mediated fibrogenesis. 30 Another study by Zhong et al explored that over‐expressed miR‐19b‐3p in rat cardiac fibroblast could promote proliferation and migration fibroblast by down‐regulating phosphatase and tensin homologue expression. 31 In the in vivo studies, Fang et al have demonstrated in humans that circulating miR‐19b‐3p could indicate diffused cardiac fibrosis ‐ a pivotal pathophysiological alteration of HF, 32 , 33 as confirmed by cardiac magnetic resonance. 34 In the present study, circulating miR‐19b‐3p was proposed to have a significant positive correlation with cardiac fibrosis biomarker sST2. 35 Moreover, we identified that circulating miR‐19b‐3p levels are positive related to LV hypertrophy indices. The enumerated researches on regulating role of miR‐19b‐3p about cardiac fibrosis provided solid evidences to support our finding mentioned above from a molecular biological perspective.
Besides fibrosis, miR‐19b‐3p modulates various pathophysiological processes that influence cardiac metabolism as demonstrated in the settings of diabetic cardiomyopathy, 36 vascular smooth muscle cell proliferation, 37 angiogenesis in endothelial cells of coronary artery, 38 and inhibition of renal fibrosis. 39 Both endothelial dysfunction and RAAS activation by renal remodeling have been demonstrated to deteriorate cardiac failing. Moreover, our clinical evidence also indicates that miR‐19b‐3p predict worsened outcome of patients with AHF, where a higher level of circulating miR‐19b‐3p (divided by median value of 2.15) posed a 1.31‐fold higher risk for mortality and HF readmission of patients with AHF. Integrating all available evidences, we believe that miR‐19b‐3p is a promising prognostic biomarker for patients with AHF, while the mechanism that aggravating cardiac dysfunction remained to be further investigated.
In addition, HF treatment is another crucial factor that would affect the prognosis. While in our prospective cohort, all of the patients were consecutively enrolled and received standard‐of‐care in hospital, and discharged with similar regimen across the quartiles (Table S3). Therefore, in our study, the treatment has little impact on outcome. However from another perspective, studies conducted previously have demonstrated that the level of circulating miRNAs is variable in response to treatment, and the change of miRNAs during admission could also predict adverse outcomes. 9 , 25 Thus, it is an important topic to investigate how specific medication interacts with miRNA in the treatment process of HF. Thus, repeated measurement could also be of clinical importance and further studies are warranted.
Limitations
The present study has several limitations. First, although the other 8 miRNAs failed to outperform miR‐19b‐3p, they could potentially also play a role in diagnosis and prognostication of patients with HF. Combining evaluation with diverse miRNAs, NT‐proBNP, or other valuable markers could be considered in further investigations. Second, we did not repetitively measure the level of miR‐19b‐3p during admission and follow‐up. As miRNAs are dynamic, further studies analyzing repetitive measurement of miR‐19b‐3p are warranted to explore further clinical implication. Third, echocardiography and serum sST2 were not designed to be measured during follow‐up, thus it remained unknown whether miR‐19b‐3p level correlates with follow‐up changes of echocardiographic indices and serum biomarkers. Fourth, we evaluated only ventricular structural parameters by echocardiography, which are not the gold standard for measuring the extent and character of cardiac hypertrophy. Further extensive studies using cardiac magnetic resonance are needed to support the findings. Fifth, in the screening and PCR discovery phase, we performed only ROC analysis without k‐fold cross validation of AUCs. K‐fold analysis is an advantageous approach that could reduce the generalization error and produce a more precise result. However, in our study, the discovery cohort had a limited sample size that was inadequate and independent of a validation cohort, rendering it inappropriate to perform k‐fold analysis. Further studies with a larger sample size are warranted.
Conclusions
Circulating miR‐19b‐3p is independently associated with adverse clinical outcomes in patients with AHF. In addition, a high level of circulating miR‐19b‐3p might indicate adverse cardiac hypertrophy in AHF. Further studies are warranted to reveal the regulatory mechanism underlying the increase in miR‐19b‐3p during AHF.
Sources of Funding
This work has been supported by grants from National Natural Science Foundation of China (No. 81770391 to Dachun Xu) and Clinical Research Plan of Shanghai Hospital Development Center (No. SHDC2020CR3030B to Dachun Xu). Sang‐Bing Ong is supported by a Direct Grant for Research 2020/21 (2020.035), a Project Impact Enhancement Fund (PIEF) (Phase 2‐COVID) (PIEF/Ph2/COVID/08), the Improvement on Competitiveness in Hiring New Faculties Funding Scheme from the Chinese University of Hong Kong (CUHK) and the Lui Che Woo Foundation.
Disclosures
None.
Supporting information
Data S1
Tables S1–S3
Figures S1–S2
Acknowledgments
We gratefully acknowledge the support from the Heart Failure Project Team of the Department of Cardiology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, China.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 2. Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. Micrornas in heart failure: from biomarker to target for therapy. Eur J Heart Fail. 2016;18:457–468. doi: 10.1002/ejhf.495 [DOI] [PubMed] [Google Scholar]
- 3. Gomes CPC, Schroen B, Kuster GM, Robinson EL, Ford K, Squire IB, Heymans S, Martelli F, Emanueli C, Devaux Y, et al. Regulatory RNAs in heart failure. Circulation. 2020;141:313–328. doi: 10.1161/CIRCULATIONAHA.119.042474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vogel B, Keller A, Frese KS, Leidinger P, Sedaghat‐Hamedani F, Kayvanpour E, Kloos W, Backe C, Thanaraj A, Brefort T, et al. Multivariate miRNA signatures as biomarkers for non‐ischaemic systolic heart failure. Eur Heart J. 2013;34:2812–2822. doi: 10.1093/eurheartj/eht256 [DOI] [PubMed] [Google Scholar]
- 5. Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, et al. Preclinical development of a microRNA‐based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol. 2016;68:1557–1571. doi: 10.1016/j.jacc.2016.07.739 [DOI] [PubMed] [Google Scholar]
- 6. Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail. 2012;14:147–154. doi: 10.1093/eurjhf/hfr155 [DOI] [PubMed] [Google Scholar]
- 7. Wong LL, Zou R, Zhou L, Lim JY, Phua DCY, Liu C, Chong JPC, Ng JYX, Liew OW, Chan SP, et al. Combining circulating microRNA and NT‐proBNP to detect and categorize heart failure subtypes. J Am Coll Cardiol. 2019;73:1300–1313. doi: 10.1016/j.jacc.2018.11.060 [DOI] [PubMed] [Google Scholar]
- 8. Zhu X, Wang H, Liu F, Chen L, Luo W, Su P, Li W, Yu L, Yang X, Cai J. Identification of micro‐RNA networks in end‐stage heart failure because of dilated cardiomyopathy. J Cell Mol Med. 2013;17:1173–1187. doi: 10.1111/jcmm.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ovchinnikova ES, Schmitter D, Vegter EL, ter Maaten JM, Valente MAE, Liu LCY, van der Harst P, Pinto YM, de Boer RA, Meyer S, et al. Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail. 2016;18:414–423. doi: 10.1002/ejhf.332 [DOI] [PubMed] [Google Scholar]
- 10. Gurha P, Abreu‐Goodger C, Wang T, Ramirez MO, Drumond AL, van Dongen S, Chen Y, Bartonicek N, Enright AJ, Lee B, et al. Targeted deletion of microRNA‐22 promotes stress‐induced cardiac dilation and contractile dysfunction. Circulation. 2012;125:2751–2761. doi: 10.1161/CIRCULATIONAHA.111.044354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaede L, Liebetrau C, Blumenstein J, Troidl C, Dorr O, Kim WK, Gottfried K, Voss S, Berkowitsch A, Walther T, et al. Plasma microRNA‐21 for the early prediction of acute kidney injury in patients undergoing major cardiac surgery. Nephrol Dial Transplant. 2016;31:760–766. doi: 10.1093/ndt/gfw007 [DOI] [PubMed] [Google Scholar]
- 12. Sun SQ, Zhang T, Ding D, Zhang WF, Wang XL, Sun Z, Hu LH, Qin SY, Shen LH, He B. Circulating microRNA‐188, ‐30a, and ‐30e as early biomarkers for contrast‐induced acute kidney injury. J Am Heart Assoc. 2016;5:e004138. doi: 10.1161/JAHA.116.004138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 14. Xiao J, Gao R, Bei Y, Zhou Q, Zhou Y, Zhang H, Jin M, Wei S, Wang K, Xu X, et al. Circulating miR‐30d predicts survival in patients with acute heart failure. Cell Physiol Biochem. 2017;41:865–874. doi: 10.1159/000459899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, McInnes IB, Dargie HJ, McMurray JJ. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 2010;55:243–250. doi: 10.1016/j.jacc.2009.08.047 [DOI] [PubMed] [Google Scholar]
- 16. Nachamkin I, Panaro NJ, Li M, Ung H, Yuen PK, Kricka LJ, Wilding P. Agilent 2100 bioanalyzer for restriction fragment length polymorphism analysis of the campylobacter jejuni flagellin gene. J Clin Microbiol. 2001;39:754–757. doi: 10.1128/JCM.39.2.754-757.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nair SS, Luu PL, Qu W, Maddugoda M, Huschtscha L, Reddel R, Chenevix‐Trench G, Toso M, Kench JG, Horvath LG, et al. Guidelines for whole genome bisulphite sequencing of intact and FFPET DNA on the illumina HiSEQ X Ten. Epigenetics Chromatin. 2018;11:24. doi: 10.1186/s13072-018-0194-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Ronde MWJ, Ruijter JM, Lanfear D, Bayes‐Genis A, Kok MGM, Creemers EE, Pinto YM, Pinto‐Sietsma SJ. Practical data handling pipeline improves performance of qPCR‐based circulating miRNA measurements. RNA. 2017;23:811–821. doi: 10.1261/rna.059063.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 20. Yusuf NH, Ong WD, Redwan RM, Latip MA, Kumar SV. Discovery of precursor and mature microRNAs and their putative gene targets using high‐throughput sequencing in pineapple (Ananas comosus var. comosus). Gene. 2015;571:71–80. doi: 10.1016/j.gene.2015.06.050 [DOI] [PubMed] [Google Scholar]
- 21. Griffiths‐Jones S, Saini HK, van Dongen S, Enright AJ. Mirbase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human microRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scrutinio D, Conserva F, Passantino A, Iacoviello M, Lagioia R, Gesualdo L. Circulating microRNA‐150‐5p as a novel biomarker for advanced heart failure: a genome‐wide prospective study. J Heart Lung Transplant. 2017;36:616–624. doi: 10.1016/j.healun.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 25. van Boven N, Kardys I, van Vark LC, Akkerhuis KM, de Ronde MWJ, Khan MAF, Merkus D, Liu Z, Voors AA, Asselbergs FW, et al. Serially measured circulating microRNAs and adverse clinical outcomes in patients with acute heart failure. Eur J Heart Fail. 2018;20:89–96. doi: 10.1002/ejhf.950 [DOI] [PubMed] [Google Scholar]
- 26. Wu T, Chen Y, Du Y, Tao J, Li W, Zhou Z, Yang Z. Circulating exosomal miR‐92b‐5p is a promising diagnostic biomarker of heart failure with reduced ejection fraction patients hospitalized for acute heart failure. J Thorac Dis. 2018;10:6211–6220. doi: 10.21037/jtd.2018.10.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li G, Song Y, Li YD, Jie LJ, Wu WY, Li JZ, Zhang Q, Wang Y. Circulating miRNA‐302 family members as potential biomarkers for the diagnosis of acute heart failure. Biomark Med. 2018;12:871–880. doi: 10.2217/bmm-2018-0132 [DOI] [PubMed] [Google Scholar]
- 28. Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, Schroen B. Circulating microRNA‐208b and microRNA‐499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. 2010;3:499–506. doi: 10.1161/CIRCGENETICS.110.957415 [DOI] [PubMed] [Google Scholar]
- 29. Danielson LS, Park DS, Rotllan N, Chamorro‐Jorganes A, Guijarro MV, Fernandez‐Hernando C, Fishman GI, Phoon CK, Hernando E. Cardiovascular dysregulation of miR‐17‐92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013;27:1460–1467. doi: 10.1096/fj.12-221994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zou M, Wang F, Gao R, Wu J, Ou Y, Chen X, Wang T, Zhou X, Zhu W, Li P, et al. Autophagy inhibition of hsa‐miR‐19a‐3p/19b‐3p by targeting TGF‐beta R II during TGF‐beta1‐induced fibrogenesis in human cardiac fibroblasts. Sci Rep. 2016;6:24747. doi: 10.1038/srep24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhong C, Wang K, Liu Y, Lv D, Zheng BO, Zhou Q, Sun QI, Chen P, Ding S, Xu Y, et al. miR‐19b controls cardiac fibroblast proliferation and migration. J Cell Mol Med. 2016;20:1191–1197. doi: 10.1111/jcmm.12858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bayes‐Genis A, de Antonio M, Vila J, Penafiel J, Galan A, Barallat J, Zamora E, Urrutia A, Lupon J. Head‐to‐head comparison of 2 myocardial fibrosis biomarkers for long‐term heart failure risk stratification: ST2 versus galectin‐3. J Am Coll Cardiol. 2014;63:158–166. doi: 10.1016/j.jacc.2013.07.087 [DOI] [PubMed] [Google Scholar]
- 33. Sygitowicz G, Tomaniak M, Filipiak KJ, Koltowski L, Sitkiewicz D. Galectin‐3 in patients with acute heart failure: preliminary report on first polish experience. Adv Clin Exp Med. 2016;25:617–623. doi: 10.17219/acem/60527 [DOI] [PubMed] [Google Scholar]
- 34. Fang L, Ellims AH, Moore XL, White DA, Taylor AJ, Chin‐Dusting J, Dart AM. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J Transl Med. 2015;13:314. doi: 10.1186/s12967-015-0672-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gawor M, Spiewak M, Kubik A, Wrobel A, Lutynska A, Marczak M, Grzybowski J. Circulating biomarkers of hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy assessed by cardiac magnetic resonance. Biomarkers. 2018;23:676–682. doi: 10.1080/1354750X.2018.1474261 [DOI] [PubMed] [Google Scholar]
- 36. Copier CU, Leon L, Fernandez M, Contador D, Calligaris SD. Circulating miR‐19b and miR‐181b are potential biomarkers for diabetic cardiomyopathy. Sci Rep. 2017;7:13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang WB, Li HP, Yan J, Zhuang F, Bao M, Liu JT, Qi YX, Han Y. CTGF regulates cyclic stretch‐induced vascular smooth muscle cell proliferation via microRNA‐19b‐3p. Exp Cell Res. 2019;376:77–85. [DOI] [PubMed] [Google Scholar]
- 38. Mehta JL, Mercanti F, Stone A, Wang X, Ding Z, Romeo F, Khaidakov M. Gene and microRNA transcriptional signatures of angiotensin II in endothelial cells. J Cardiovasc Pharmacol. 2015;65:123–129. doi: 10.1097/FJC.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen W, Zhou ZQ, Ren YQ, Zhang L, Sun LN, Man YL, Wang ZK. Effects of long non‐coding RNA LINC00667 on renal tubular epithelial cell proliferation, apoptosis and renal fibrosis via the miR‐19b‐3p/LINC00667/CTGF signaling pathway in chronic renal failure. Cell Signal. 2019;54:102–114. doi: 10.1016/j.cellsig.2018.10.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1–S2


