Abstract
Background
The Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE‐DAPT) score has been shown to predict out‐of‐hospital major bleeding after myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy (DAPT). However, large validation studies have been scarce and the discriminative ability for patients with a preexisting bleeding risk factor (elderly, underweight, women, anemia, kidney dysfunction, or cancer) in a real‐world setting is unknown.
Methods and Results
Patients undergoing percutaneous coronary intervention for myocardial infarction between 2008 and 2017 were included from the SWEDEHEART (Swedish Web System for Enhancement of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) registry (n=66 295). The predictive value of the PRECISE‐DAPT score for rehospitalization with major bleeding during dual antiplatelet therapy was evaluated using receiver operating characteristic analyses. A high PRECISE‐DAPT score (≥25; n=13 894) was associated with increased risk of major bleeding (3.9% versus 1.8%; hazard ratio [HR], 2.2; 95% CI, 2.0–2.5; P<0.001) compared with a non‐high score (<25; n=52 401). The score demonstrated a c‐statistic of 0.64 (95% CI, 0.63–0.66). The discriminative ability of the score to further stratify bleeding risk in patients with preexisting bleeding risk factors was poor, especially in patients who are elderly (c‐statistic=0.57; 95% CI, 0.55–0.60) or underweight (c‐statistic=0.56; 95% CI, 0.51–0.61), for whom a non‐high PRECISE‐DAPT score was associated with similar bleeding risk as a high PRECISE‐DAPT score in the general myocardial infarction population.
Conclusions
In this nationwide population‐based study, the PRECISE‐DAPT score performed moderately in the general myocardial infarction population and poorly in patients with preexisting bleeding risk factors, where its usefulness seems limited.
Keywords: bleeding, dual antiplatelet therapy, myocardial infarction, PRECISE‐DAPT, SWEDEHEART
Subject Categories: Acute Coronary Syndromes, Myocardial Infarction, Percutaneous Coronary Intervention, Secondary Prevention, Treatment
Nonstandard Abbreviations and Acronyms
- ARD
absolute risk difference
- DAPT
dual antiplatelet therapy
- PLATO
The Study of Platelet Inhibition and Patient Outcomes
- PRECISE‐DAPT
Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy
- SWEDEHEART
Swedish Web System for Enhancement of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies
- TRITON‐TIMI‐38
Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38
Clinical Perspective
What Is New?
In this nationwide population‐based study, the Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE‐DAPT) score showed a moderate ability of predicting rehospitalization with major bleeding in the general population with myocardial infarction and performed poorly in patients with preexisting bleeding risk factors, especially in patients who are elderly or underweight.
In patients with preexisting bleeding risk factors, those with a non‐high PRECISE‐DAPT score (<25) had a risk of major bleeding that was comparable to that of patients with a high PRECISE‐DAPT score (≥25) in the general population with myocardial infarction.
What Are the Clinical Implications?
The usefulness of the PRECISE‐DAPT score seems to be low in patients with myocardial infarction and preexisting risk factors for bleeding.
Dual antiplatelet therapy (DAPT) with aspirin and potent P2Y12 inhibition has improved ischemic outcomes for patients with myocardial infarction (MI) undergoing percutaneous coronary intervention (PCI) with stent implantation. 1 , 2 However, this treatment strategy increases the risk of major bleeding, which has a substantial impact on mortality and is related to the DAPT duration. 3 , 4 An extended course of DAPT beyond the standard 12 months has been shown to further reduce ischemic events at the expense of major bleeding 5 , 6 and is considered an option for patients at high ischemic risk and low bleeding risk. 7 Inversely, in patients with MI at high risk of bleeding, discontinuation of DAPT after 3 or 6 months might be preferable. 8 , 9 , 10 To determine the optimal DAPT duration, a patient‐tailored approach based on ischemic versus bleeding risk is recommended. 7 Existing risk scores to calculate bleeding risk are designed for separate time windows, such as in hospital, 11 , 12 , 13 , 14 within 1 month, 15 or beyond 12 months after the index event. 16 The Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE‐DAPT) score, on the other hand, was developed to predict bleeding rates at 12 months and has demonstrated a potential to identify patients suitable for a short DAPT strategy. 17 The original score consists of 5 variables (age, creatinine clearance, hemoglobin, previous bleeding, and white blood cell count), but a simplified version with 4 variables (excluding white blood cell count) has shown similar qualities. 18 The 5‐item score has been validated in several studies but not the 4‐item version, and neither in a large nationwide population of real‐world patients with MI. 19 , 20 , 21 , 22 Furthermore, we hypothesized that the use of the PRECISE‐DAPT score would provide little or no additional value in patients with preexisting risk factors for bleeding. 23 Therefore, the aim of this study was to investigate the applicability of the 4‐item PRECISE‐DAPT score in a large national registry of patients with MI undergoing PCI with following DAPT treatment as well as in patients with preexisting risk factors for bleeding (advanced age, underweight, women, anemia, kidney dysfunction, or cancer).
Methods
Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data were obtained from the SWEDEHEART (Swedish Web System for Enhancement of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) registry on patients (≥18 years) undergoing PCI for ST‐segment–elevation MI or non‐ST‐segment–elevation MI between January 2008 and December 2017 at 1 of the 30 PCI centers in Sweden (n=93 613; Figure 1). 24 We linked the SWEDEHEART registry to the Swedish National Patient Registry and the Swedish Prescribed Drugs Registry, to retrieve data on International Classification of Diseases, Tenth Revision (ICD‐10), codes from electronic healthcare records and on prescribed drugs dispensed from any pharmacy in Sweden. 25 , 26 Patients who were on treatment with oral anticoagulants at any time during the past 6 months before or 12 months after the index event were excluded (n=11 016). Furthermore, we excluded patients who were not prescribed DAPT (n=3108), consisting of aspirin in addition to a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) post‐PCI, or who had missing values for DAPT prescription status (n=3991). Patients with missing values for any of the components of the 4‐item PRECISE‐DAPT score (age, creatinine clearance, hemoglobin, and previous bleeding) were also excluded (n=4481). Data on prescribed DAPT at discharge were collected from SWEDEHEART, but only patients with a recorded dispensation in the Swedish Prescribed Drugs Registry within 3 months after the index event were included in further analyses (n=66 295), to take patient adherence into account. Furthermore, as data on drug dispensations were available for predefined time intervals (the first 3 months, between 3 and 6 months, and between 6 and 12 months), patients were considered to have continued DAPT for a total of 3, 6, or 12 months or longer. It was assumed that patients who received dispensations of aspirin and P2Y12 inhibitors at pharmacy were compliant to prescribed medications. The ethical committee of Lund University approved the study (2015/297).
Figure 1. Study design.
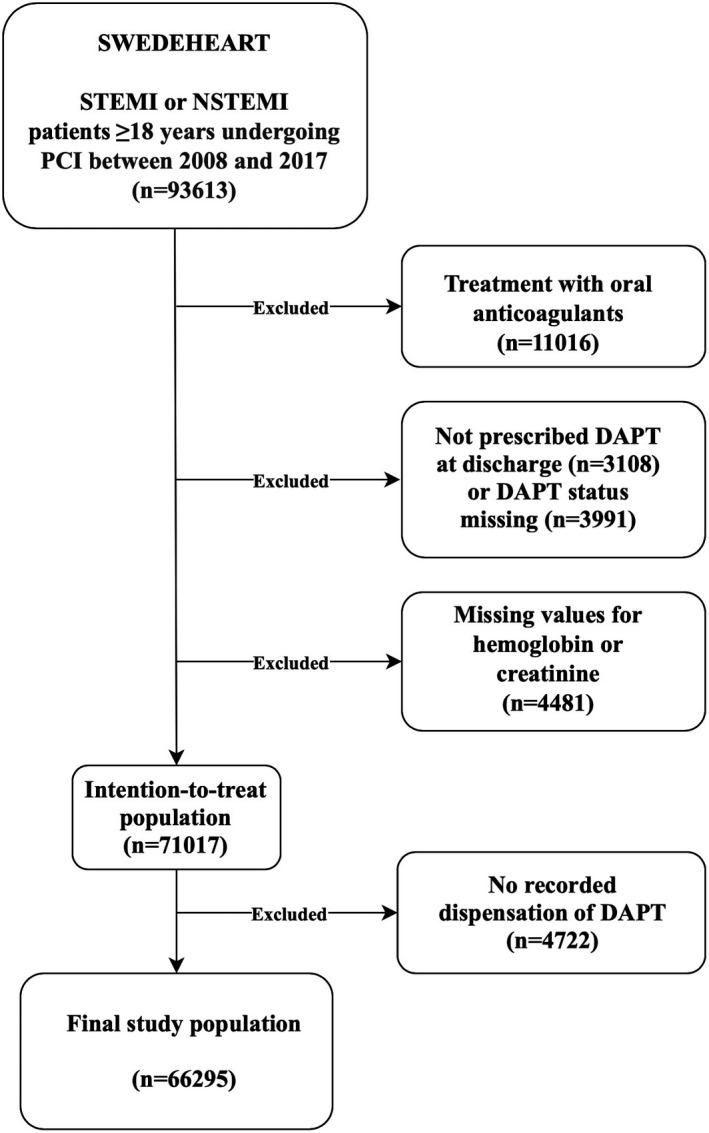
DAPT indicates dual antiplatelet therapy; NSTEMI, non‐ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and SWEDEHEART, Swedish Web System for Enhancement of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies.
Bleeding Definition
Major bleeding events were defined as bleeding requiring hospitalization with an ICD‐10, code of cerebral, gastrointestinal, genitourinary, airway, eye, or ear bleeding, anemia after acute bleeding or iron deficiency anemia secondary to blood loss, or bleeding related to surgical or medical intervention (Table S1). To maximize the sensitivity of our analysis, bleeding events were included regardless of whether they were recorded as the main diagnosis or a secondary diagnosis during rehospitalization. Maximum follow‐up was 12 months after the index event. Bleeding events happening before day 7 after the PCI procedure were censored, as early bleedings are likely to be a result of the invasive procedure rather than DAPT. 17 We also censored bleedings occurring after discontinuation of DAPT.
Statistical Analysis
Patient characteristics were compared using the chi‐square test or the independent samples t test. To compute the 4‐item PRECISE‐DAPT score, hazard ratios (HR) for its variables, as reported in the appendix of the PRECISE‐DAPT derivation study, were used to calculate the corresponding beta coefficients. 17 The sums of truncated predictor variables times their respective beta coefficient were scaled and rounded to an integer between 0 and 100. 17 The variable for creatinine clearance, used to determine the PRECISE‐DAPT score, was computed using the Chronic Kidney Disease Epidemiology Collaboration formula. 27 Kaplan‐Meier event rates for major bleeding were estimated for patients with high (≥25) or non‐high PRECISE‐DAPT score (<25) and compared with the log‐rank test as well as with univariable Cox regression. The performance of the PRECISE‐DAPT score to predict major bleeding was tested in receiver operating characteristic analyses with 95% CI for the c‐statistic. As a sensitivity analysis, patients with missing values for creatinine clearance and hemoglobin were included in the study population and multiple imputation was performed for missing values using 10 imputations and 10 iterations for every imputation. The score was further analyzed among several traditional high bleeding risk subgroups: elderly (≥75 years), underweight (<60 kg), women, patients with anemia (hemoglobin <120 g/L for women, <130 g/L for men), patients with kidney dysfunction (creatinine clearance <60 mL/min per 1.73 m2), and patients with cancer (within 3 years). Furthermore, univariable and multivariable Cox regression was used to identify baseline patient characteristics that were associated with major bleeding or stent thrombosis. The risk of stent thrombosis was assessed during the first year after DAPT discontinuation using DAPT discontinuation as time point zero. Variables with a P<0.2 in the univariable analyses were included in the multivariable models. Analyses were done using IBM SPSS (version 25) or Stata (version 15.1). A 2‐tailed P<0.05 was considered to indicate statistical significance.
Results
Patient Characteristics
In the 66 295 patients included in the study, the median PRECISE‐DAPT score was 14 (interquartile range 7–23; Figure 2A). Patients with a high PRECISE‐DAPT score (≥25; n=13 894) were considerably older (mean 78 versus 63 years), had lower body weight, were more often women, and had higher frequencies of relevant comorbidities, compared with patients with non‐high PRECISE‐DAPT score (<25; n=52 401; all P<0.001; Table 1). Patients with a high PRECISE‐DAPT score were more often treated with cardiovascular medications on admission and were less frequently prescribed ticagrelor or prasugrel as well as other evidence‐based secondary preventive medications on discharge, compared with patients with a non‐high PRECISE‐DAPT score (Table 1). Furthermore, radial artery access and drug‐eluting stents were less commonly used in patients with a high PRECISE‐DAPT score.
Figure 2. The distribution of the PRECISE‐DAPT score (A) in the whole study population (n=66 295) of patients with myocardial infarction treated with percutaneous coronary intervention and subsequent dual antiplatelet therapy, divided into score risk categories with green denoting very low risk, blue low risk, purple moderate risk, and red high risk. Kaplan‐Meier failure functions for major bleeding at 12 months stratified into 4 (B) or 2 risk categories (D). Receiver operating characteristic curve for the PRECISE‐DAPT score displaying the c‐statistic with 95% CI (C).
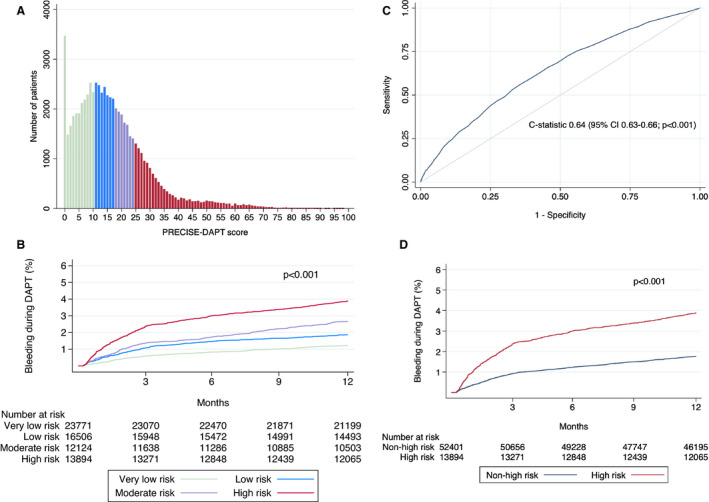
PRECISE‐DAPT indicates Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy.
Table 1.
Patient Characteristics Stratified by Non‐High (<25) or High (≥25) PRECISE‐DAPT Score in 66 295 Patients With Myocardial Infarction Treated With PCI and Subsequent Dual Antiplatelet Therapy
|
Non‐high PRECISE‐DAPT (<25) n=52 401 |
High PRECISE‐DAPT (≥25) n=13 894 |
P value | Missing or unknown n (%) | |
|---|---|---|---|---|
| Age, y, mean±SD | 63±10 | 78±9 | <0.001 | 0 (0.0) |
| Age ≥75 y n (%) | 7150 (13.6) | 10 304 (74.2) | <0.001 | 0 (0.0) |
| Body mass index, mean±SD | 27.5±4.4 | 26.4±4.4 | <0.001 | 11 705 (17.7) |
| Body weight <60 kg, n (%) | 1921 (4.2) | 1378 (11.3) | <0.001 | 8647 (13.0) |
| Women, n (%) | 12 397 (23.7) | 6116 (44.0) | <0.001 | 0 (0.0) |
| ST‐segment–elevation myocardial infarction, n (%) | 24 528 (46.8) | 6315 (45.5) | 0.006 | 0 (0.0) |
| Hypertension, n (%) | 22 782 (43.5) | 9235 (66.5) | <0.001 | 909 (1.4) |
| Hyperlipidemia, n (%) | 15 010 (28.6) | 5574 (40.1) | <0.001 | 1217 (1.8) |
| Current smoking, n (%) | 15 487 (29.6) | 1645 (11.8) | <0.001 | 3410 (5.1) |
| Diabetes, n (%) | 7532 (14.4) | 3164 (22.8) | <0.001 | 373 (0.6) |
| Diabetes with insulin therapy, n (%) | 3024 (5.8) | 1643 (11.8) | <0.001 | 451 (0.7) |
| Peripheral artery disease, n (%) | 976 (1.9) | 963 (6.9) | <0.001 | 0 (0.0) |
| Heart failure, n (%) | 878 (1.7) | 1329 (9.6) | <0.001 | 0 (0.0) |
| Chronic obstructive pulmonary disease, n (%) | 2243 (4.3) | 1145 (8.2) | <0.001 | 0 (0.0) |
| Cancer within 3 y, n (%) | 694 (1.3) | 636 (4.6) | <0.001 | 0 (0.0) |
| Dementia, n (%) | 93 (0.2) | 98 (0.7) | <0.001 | 0 (0.0) |
| Previous myocardial infarction, n (%) | 6050 (11.5) | 3268 (23.5) | <0.001 | 1086 (1.6) |
| Previous PCI, n (%) | 4650 (8.9) | 1964 (14.1) | <0.001 | 21 (0.0) |
| Previous coronary artery bypass graft, n (%) | 2000 (3.8) | 1152 (8.3) | <0.001 | 17 (0.0) |
| Previous stroke, n (%) | 1361 (2.6) | 1755 (12.6) | <0.001 | 0 (0.0) |
| Previous bleeding, n (%) | 0 (0.0) | 2468 (17.8) | <0.001 | 0 (0.0) |
| Hemoglobin, mean±SD | 144.2±13.4 | 129.5±18.0 | <0.001 | 0 (0.0) |
| Anemia, n (%)* | 4579 (8.7) | 5268 (37.9) | <0.001 | 0 (0.0) |
| Creatinine clearance, mean±SD | 86.3±14.6 | 56.9±19.4 | <0.001 | 0 (0.0) |
| Kidney dysfunction, n (%) † | 2354 (4.5) | 8245 (59.3) | <0.001 | 0 (0.0) |
| Radial access, n (%) | 38 468 (73.4) | 9242 (66.5) | <0.001 | 0 (0.0) |
| Bifurcation lesion, n (%) | 6030 (11.5) | 1483 (10.7) | 0.006 | 0 (0.0) |
| Complex lesion, n (%) | 9203 (17.6) | 2852 (20.5) | <0.001 | 0 (0.0) |
| Drug‐eluting stent | 33 383 (69.0) | 7850 (63.8) | <0.001 | 5624 (8.5) |
| Stent length (mm), mean±SD | 19.9±7.0 | 19.6±7.0 | <0.001 | 5624 (8.5) |
| Admission medications, n (%) | ||||
| Aspirin | 10 645 (20.3) | 5956 (42.9) | <0.001 | 695 (1.0) |
| P2Y12 inhibitors | <0.001 | 679 (1.0) | ||
| Clopidogrel | 1231 (2.4) | 822 (5.9) | ||
| Ticagrelor | 237 (0.5) | 125 (0.9) | ||
| Prasugrel | 12 (0.0) | 7 (0.0) | ||
| ACEi/ARB | 13 718 (26.2) | 5727 (41.3) | <0.001 | 887 (1.3) |
| β‐blockers | 11 113 (21.2) | 5751 (41.4) | <0.001 | 913 (1.4) |
| Statins | 10 654 (20.4) | 4238 (30.5) | <0.001 | 707 (1.1) |
| Periprocedural medications, n (%) | ||||
| Aspirin | 51 726 (98.7) | 13 669 (98.4) | 0.002 | 3 (0.0) |
| Clopidogrel | 25 494 (48.7) | 7262 (52.3) | <0.001 | 3 (0.0) |
| Ticagrelor | 25 784 (49.2) | 6088 (43.8) | <0.001 | 0 (0.0) |
| Prasugrel | 2178 (4.2) | 431 (3.1) | <0.001 | 0 (0.0) |
| Glycoprotein IIb/IIIa inhibitor | 8493 (16.2) | 1548 (11.1) | <0.001 | 0 (0.0) |
| Discharge medications, n (%) | ||||
| Aspirin | 52 401 (100.0) | 13 894 (100.0) | 0 (0.0) | |
| Clopidogrel | 24 735 (47.2) | 7972 (57.4) | <0.001 | 0 (0.0) |
| Ticagrelor | 26 324 (50.2) | 5762 (41.5) | <0.001 | 0 (0.0) |
| Prasugrel | 1210 (2.3) | 132 (1.0) | <0.001 | 0 (0.0) |
| ACEi/ARB | 44 170 (84.3) | 11 258 (81.0) | <0.001 | 30 (0.0) |
| β‐blockers | 47 893 (91.4) | 12 451 (89.6) | <0.001 | 7 (0.0) |
| Statins | 51 378 (98.0) | 12 818 (92.3) | <0.001 | 10 (0.0) |
ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker; PCI, percutaneous coronary intervention; and PRECISE‐DAPT indicates Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy.
Anemia is hemoglobin <120 g/L for women and <130 g/L for men.
Kidney dysfunction is creatinine clearance <60 mL/min per 1.73 m2.
Score Performance and Outcomes in the Whole Study Population
The median follow‐up was 365 days and the median time to major bleeding was 77 days (interquartile range 34–188). Patients with a high PRECISE‐DAPT score (≥25; n=13 894) were at higher absolute risk of major bleeding during follow‐up (3.9% versus 1.8%, absolute risk difference [ARD]=2.1 percentage points; HR, 2.2; 95% CI, 2.0–2.5; P<0.001) compared with patients with a non‐high PRECISE‐DAPT score (<25; n=52 401; Table 2; Figure 2D). Patients were consistently separated according to their absolute bleeding risk also when stratified into 4 risk categories: very low (1.2%), low (1.9%), moderate (2.7%), or high (3.9%) risk (P<0.001; Figure 2B). The comparison between high and non‐high PRECISE‐DAPT score showed coherent results when examining different bleeding sites, including cerebral (0.3% versus 0.1%), gastrointestinal (2.0% versus 0.8%), genitourinary (0.8% versus 0.4%), or other (1.2% versus 0.6%; all P<0.001). Furthermore, a receiver operating characteristic analysis yielded a c‐statistic of 0.64 (95% CI, 0.63–0.66; Figure 2C). Results were unchanged when major bleeding events before day 7 after the PCI procedure also were taken into account (c‐statistic=0.64; 95% CI, 0.62–0.65), as well as when analyzing multiply imputed data (Table S2). Using a cutoff at 25 score points to categorize the PRECISE‐DAPT score into a high (≥25) or non‐high (<25) score represents a positive predictive value for major bleeding of 3.8% and a negative predictive value of 98.3%. Furthermore, the positive likelihood ratio at this cutoff was 1.8, and the negative likelihood ratio 0.8. Applying this to the pretest probability of major bleeding (2.1%; probability of major bleeding in the whole study population), produces a positive (PRECISE‐DAPT ≥25) posttest probability of 3.8% and a negative (PRECISE‐DAPT <25) posttest probability of 1.7%.
Table 2.
Major Bleeding Outcomes at 12 Months Stratified by Non‐High (<25) or High (≥25) PRECISE‐DAPT Score and Receiver Operating Characteristic Analyses Assessing the Performance of the Score
| Kaplan‐Meier event rate, n/total n (%) | HR (95% CI) | P value | C‐statistic (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| Non‐high PRECISE‐DAPT score (<25) | High PRECISE‐DAPT score (≥25) | |||||
| All patients, n=66 295 | 891/52 401 (1.8) | 526/13 894 (3.9) | 2.2 (2.0–2.5) | <0.001 | 0.64 (0.63–0.66) | <0.001 |
| Age ≥75 y, n=17 454 | 207/7150 (3.0) | 383/10 304 (3.8) | 1.3 (1.1–1.5) | 0.003 | 0.57 (0.55–0.60) | <0.001 |
| Body weight <60 kg, n=3299 | 62/1921 (3.3) | 55/1378 (4.1) | 1.2 (0.9–1.8) | 0.236 | 0.56 (0.51–0.61) | 0.033 |
| Women, n=18 513 | 217/12 397 (1.8) | 198/6116 (3.3) | 1.9 (1.5–2.3) | <0.001 | 0.62 (0.60–0.65) | <0.001 |
| Anemia, n=9847* | 143/4579 (3.2) | 270/5268 (5.3) | 1.7 (1.4–2.0) | <0.001 | 0.60 (0.58–0.63) | <0.001 |
| Kidney dysfunction, n=10 599 † | 55/2354 (2.4) | 290/8245 (3.6) | 1.5 (1.1–2.0) | 0.005 | 0.61 (0.58–0.64) | <0.001 |
| Cancer within 3 y, n=1330 | 29/694 (4.3) | 42/636 (6.8) | 1.6 (1.0–2.6) | 0.050 | 0.59 (0.53–0.66) | 0.009 |
Results are shown for the whole study population of patients with myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy as well as for subgroups. PRECISE‐DAPT indicates Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy. HR indicates hazard ratio.
Anemia is hemoglobin <120 g/L for women and <130 g/L for men.
Kidney dysfunction is creatinine clearance <60 mL/min per 1.73 m2.
High Bleeding Risk Subgroups
A high PRECISE‐DAPT score was associated with a statistically significant higher absolute risk of major bleeding for elderly patients (3.8% versus 3.0%, ARD, 0.8 percentage points; P=0.003), women (3.3% versus 1.8%, ARD, 1.5 percentage points; P<0.001), patients with anemia (5.3% versus 3.2%, ARD, 2.1 percentage points; P<0.001), patients with kidney dysfunction (3.6% versus 2.4%, ARD, 1.2 percentage points; P=0.005), and patients with cancer (6.8% versus 4.3%; ARD, 2.5 percentage points; P=0.050), but not for patients who were underweight (4.1% versus 3.3%, ARD, 0.8 percentage points; P=0.236), compared with a non‐high PRECISE‐DAPT score (Table 2). The PRECISE‐DAPT score was able to predict major bleeding for all high bleeding risk subgroups, but with only poor to moderate discriminative capability, including elderly (c‐statistic=0.57; 95% CI, 0.55–0.60), underweight (c‐statistic=0.56; 95% CI, 0.51–0.61), women (c‐statistic=0.62; 95% CI, 0.60–0.65), patients with anemia (c‐statistic=0.60; 95% CI, 0.58–0.63), patients with kidney dysfunction (c‐statistic=0.61; 95% CI, 0.58–0.64), and patients with cancer (c‐statistic 0.59; 95% CI, 0.53–0.66; Table 2; Figure 3).
Figure 3. Receiver operating characteristic curves for the PRECISE‐DAPT score in several subgroups of patients with myocardial infarction treated with percutaneous coronary intervention and subsequent dual antiplatelet therapy.
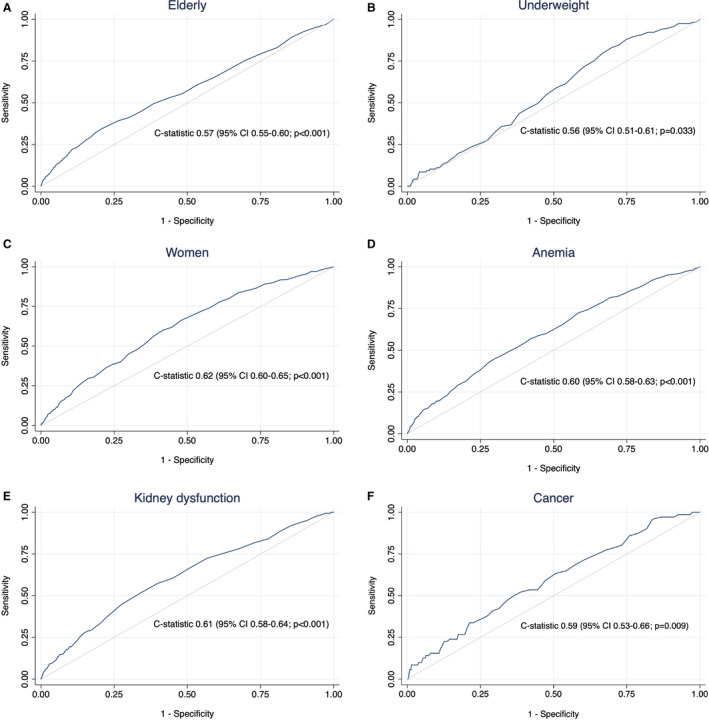
PRECISE‐DAPT indicates Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy. Elderly (≥75 years; n=17 454) (A), underweight (<60 kg; n=3299) (B), women (n=18 513) (C), patients with anemia (hemoglobin <120 g/L for women, <130 g/L for men; n=9847) (D), patients with kidney dysfunction (creatinine clearance <60 mL/min per 1.73 m2; n=10 599) (E), and patients with cancer (n=1330) (F).
Risk Factors for Major Bleeding and Stent Thrombosis
The strongest associations with major bleeding during DAPT in univariable analysis among the 66 295 patients included in the present study were found for advanced age (≥75 years), peripheral artery disease, heart failure, cancer, dementia, previous bleeding, and baseline anemia (Table 3). This was consistent in multivariable analysis (Table 4). For patients who ended their DAPT at 12 months or earlier (n=60 227), risk factors for subsequent stent thrombosis during the first year after DAPT discontinuation were found to be hyperlipidemia; peripheral artery disease; previous MI, PCI, or coronary artery bypass grafting before index hospitalization; and the presence of complex lesions in the univariable analysis (Table 5). In the multivariable model, previous PCI and complex lesions remained as independent risk factors for stent thrombosis (Table 6). The use of drug‐eluting stents as compared with bare‐metal stents was found to be a strong protecting factor against stent thrombosis.
Table 3.
Univariable Cox Regression Analysis for Rehospitalization With Major Bleeding in 66 295 Patients With Myocardial Infarction Treated With PCI and Subsequent Dual Antiplatelet Therapy
| HR (95% CI) | P value | |
|---|---|---|
| Age (for each 10 y increase)* | 1.5 (1.4–1.5) | <0.001 |
| Age ≥75 y | 2.0 (1.8–2.2) | <0.001 |
| Body weight <60 kg | 1.8 (1.5–2.1) | <0.001 |
| Women | 1.1 (1.0–1.2) | 0.239 |
| ST‐segment–elevation myocardial infarction | 1.1 (1.0–1.2) | 0.048 |
| Hypertension | 1.3 (1.2–1.4) | <0.001 |
| Hyperlipidemia | 1.0 (0.9–1.1) | 0.619 |
| Current smoking | 1.0 (0.9–1.2) | 0.545 |
| Diabetes | 1.2 (1.1–1.4) | 0.002 |
| Peripheral artery disease | 2.1 (1.7–2.6) | <0.001 |
| Heart failure | 2.0 (1.6–2.5) | <0.001 |
| Chronic obstructive pulmonary disease | 1.7 (1.4–2.1) | <0.001 |
| Cancer within 3 y | 2.6 (2.1–3.3) | <0.001 |
| Dementia | 2.5 (1.4–4.7) | 0.003 |
| Previous myocardial infarction | 1.1 (0.9–1.2) | 0.385 |
| Previous PCI | 1.0 (0.8–1.2) | 0.943 |
| Previous coronary artery bypass graft | 1.0 (0.8–1.3) | 0.935 |
| Previous stroke | 1.5 (1.2–1.8) | <0.001 |
| Previous bleeding | 2.6 (2.2–3.1) | <0.001 |
| Hemoglobin (for each 10 g/L increase) † | 0.5 (0.4–0.5) | <0.001 |
| Anemia ‡ | 2.4 (2.1–2.7) | <0.001 |
| Creatinine clearance (for each 10 mL/min per 1.73 m2 increase) § | 0.9 (0.8–0.9) | <0.001 |
| Kidney dysfunction ‖ | 1.7 (1.5–1.9) | <0.001 |
| Drug‐eluting stent | 1.3 (1.1–1.5) | <0.001 |
| Radial access | 1.0 (0.9–1.1) | 0.970 |
| Discharge medications | ||
| Ticagrelor ¶ | 1.4 (1.2–1.5) | <0.001 |
| Prasugrel ¶ | 1.1 (0.7–1.6) | 0.722 |
| Angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker | 0.9 (0.8–1.0) | 0.146 |
| β‐blockers | 0.8 (0.7–1.0) | 0.053 |
| Statins | 0.8 (0.6–1.0) | 0.064 |
HR indicates hazard ratio; and PCI, percutaneous coronary intervention.
Age was truncated below 50 years.
Hemoglobin was truncated above 120 g/L and below 100 g/L.
Anemia is hemoglobin <120 g/L for women and <130 g/L for men.
Creatinine clearance was truncated above 100 mL/min per 1.73 m2.
Kidney dysfunction is creatinine clearance <60 mL/min per 1.73 m2.
Clopidogrel was used as reference.
Table 4.
Multivariable Cox Regression Analysis for Rehospitalization With Major Bleeding in 51 713 Patients With Myocardial Infarction Treated With PCI and Subsequent Dual Antiplatelet Therapy
| HR (95% CI) | P value | |
|---|---|---|
| Age (for each 10 y increase)* | 1.3 (1.2–1.4) | <0.001 |
| Body weight <60 kg | 1.3 (1.1–1.6) | 0.013 |
| ST‐segment–elevation myocardial infarction | 1.1 (1.0–1.2) | 0.140 |
| Hypertension | 0.9 (0.8–1.1) | 0.237 |
| Diabetes | 1.0 (0.9–1.2) | 0.744 |
| Peripheral artery disease | 1.4 (1.1–1.8) | 0.019 |
| Heart failure | 1.3 (1.0–1.7) | 0.024 |
| Chronic obstructive pulmonary disease | 1.3 (1.1–1.7) | 0.010 |
| Cancer within 3 y | 1.6 (1.2–2.1) | 0.004 |
| Dementia | 2.1 (1.1–4.1) | 0.028 |
| Previous stroke | 0.8 (0.6–1.1) | 0.175 |
| Previous bleeding | 2.1 (1.7–2.6) | <0.001 |
| Hemoglobin (for each 10 g/L increase) † | 0.6 (0.5–0.7) | <0.001 |
| Creatinine clearance (for each 10 mL/min per 1.73 m2 increase) ‡ | 1.0 (0.9–1.0) | 0.036 |
| Drug‐eluting stent | 1.1 (1.0–1.3) | 0.099 |
| Discharge medications | ||
| Ticagrelor § | 1.4 (1.2–1.6) | <0.001 |
| Angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker | 0.9 (0.8–1.1) | 0.429 |
| β‐blockers | 0.9 (0.7–1.0) | 0.094 |
| Statins | 1.2 (0.8–1.6) | 0.373 |
HR indicates hazard ratio; and PCI, percutaneous coronary intervention.
Age was truncated below 50 years.
Hemoglobin was truncated above 120 g/L and below 100 g/L.
Creatinine clearance was truncated above 100 mL/min per 1.73 m2.
Clopidogrel was used as reference.
Table 5.
Univariable Cox Regression Analysis for Stent Thrombosis Within 12 Months After Discontinuation of Dual Antiplatelet Therapy in 60 227 Patients With Myocardial Infarction Treated With PCI Who Did Not Continue With Dual Antiplatelet Therapy Beyond 12 Months From Their Index Myocardial Infarction
| HR (95% CI) | P value | |
|---|---|---|
| Age (for each 10 y increase)* | 0.9 (0.8–1.1) | 0.274 |
| Age ≥75 y | 0.8 (0.5–1.3) | 0.315 |
| Body weight <60 kg | 0.5 (0.2–1.7) | 0.298 |
| Women | 0.7 (0.4–1.1) | 0.111 |
| ST‐segment–elevation myocardial infarction | 1.1 (0.7–1.6) | 0.722 |
| Hypertension | 0.9 (0.6–1.3) | 0.485 |
| Hyperlipidemia | 2.0 (1.4–3.0) | <0.001 |
| Current smoking | 1.1 (0.7–1.8) | 0.574 |
| Diabetes | 1.1 (0.6–1.8) | 0.754 |
| Diabetes with insulin therapy | 1.2 (0.6–2.5) | 0.634 |
| Peripheral artery disease | 2.6 (1.2–5.7) | 0.013 |
| Heart failure | 1.3 (0.5–3.5) | 0.638 |
| Chronic obstructive pulmonary disease | 0.6 (0.2–1.8) | 0.577 |
| Cancer within 3 y | 0.5 (0.1–3.5) | 0.471 |
| Previous myocardial infarction | 2.1 (1.4–3.4) | 0.001 |
| Previous PCI | 2.9 (1.8–4.6) | <0.001 |
| Previous coronary artery bypass graft | 3.5 (2.0–6.2) | <0.001 |
| Previous stroke | 1.6 (0.7–3.5) | 0.228 |
| Previous bleeding | 0.8 (0.3–2.6) | 0.723 |
| Hemoglobin (for each 10 g/L increase) † | 0.9 (0.5–1.5) | 0.578 |
| Anemia ‡ | 1.2 (0.7–2.0) | 0.544 |
| Creatinine clearance (for each 10 mL/min per 1.73 m2 increase) § | 1.0 (0.9–1.1) | 0.986 |
| Kidney dysfunction ‖ | 1.5 (0.9–2.4) | 0.101 |
| Bifurcation lesion | 0.8 (0.4–1.5) | 0.455 |
| Complex lesion | 2.0 (1.3–3.1) | 0.001 |
| Drug–eluting stent | 0.4 (0.3–0.6) | <0.001 |
| Stent length (for each mm increase) | 1.0 (1.0–1.0) | 0.297 |
HR indicates hazard ratio; and PCI, percutaneous coronary intervention.
Age was truncated below 50 years.
Hemoglobin was truncated above 120 g/L and below 100 g/L.
Anemia is hemoglobin <120 g/L for women and <130 g/L for men.
Creatinine clearance was truncated above 100 mL/min per 1.73 m2.
Kidney dysfunction is creatinine clearance <60 mL/min per 1.73 m2.
Table 6.
Multivariable Cox Regression Analysis for Stent Thrombosis Within 12 Months After Discontinuation of Dual Antiplatelet Therapy in 53 513 Patients With Myocardial Infarction Treated With PCI Who Did Not Continue With Dual Antiplatelet Therapy Beyond 12 Months From Their Index Myocardial Infarction
| HR (95% CI) | P value | |
|---|---|---|
| Women | 0.7 (0.4–1.2) | 0.232 |
| Hyperlipidemia | 1.4 (0.9–2.4) | 0.162 |
| Peripheral artery disease | 1.2 (0.4–3.4) | 0.717 |
| Previous myocardial infarction | 1.1 (0.5–2.1) | 0.855 |
| Previous PCI | 2.1 (1.1–4.2) | 0.035 |
| Previous coronary artery bypass graft | 1.9 (0.9–4.0) | 0.074 |
| Kidney dysfuction* | 1.1 (0.6–1.9) | 0.817 |
| Complex lesion | 2.1 (1.3–3.3) | 0.002 |
| Drug‐eluting stent | 0.4 (0.2–0.6) | <0.001 |
HR indicates hazard ratio; and PCI, percutaneous coronary intervention.
Kidney dysfunction is creatinine clearance <60 mL/min per 1.73 m2.
Discussion
The key findings of this large nationwide population‐based validation study of the PRECISE‐DAPT score for real‐world patients with MI treated with PCI and subsequent DAPT were as follows. The PRECISE‐DAPT score identified a subset of patients with a higher comorbidity burden and a more than doubled absolute risk for major bleeding. The score demonstrated a moderate discriminative capability for major bleeding during DAPT within 12 months. In patients with preexisting risk factors for bleeding, its ability to predict major bleeding was poor, especially for patients with advanced age, low body weight, anemia, or cancer. In these patient subgroups, those with a non‐high PRECISE‐DAPT score (<25) had a similar risk of major bleeding as patients with a high PRECISE‐DAPT score (≥25) in the general MI population.
Score Validation
The PRECISE‐DAPT score was initially validated for Thrombolysis in Myocardial Infarction major or minor bleeding in PLATO (The Study of Platelet Inhibition and Patient Outcomes) (c‐statistic=0.70) and the Bern PCI registry (c‐statistic=0.63). 17 Successively, the PRECISE‐DAPT score has been evaluated for different bleeding definitions and compared with other bleeding risk scores, demonstrating moderate to good predictive capability. 19 , 20 , 21 International guidelines have suggested using the score as a way of identifying patients at high risk of bleeding who might benefit from a shorter DAPT period. 7 , 10 , 28 In consistency with most previous validation studies, 17 , 20 , 21 this large nationwide registry study showed that the PRECISE‐DAPT score had a discriminative ability for major bleeding that was moderate but, on the other hand, was better than chance and comparable to other widely used risk scores within the field of cardiology. 29 However, although the negative predictive value of the PRECISE‐DAPT score was high (98.3%), a non‐high PRECISE‐DAPT score represents a mere drop in risk of major bleeding from 2.1% to 1.7%. Furthermore, although a non‐high PRECISE‐DAPT score was associated with better bleeding outcomes than a high PRECISE‐DAPT score, the vast majority of patients with a high score do not experience a major bleeding during DAPT (96.2%).
The Optimal DAPT Duration
The PRECISE‐DAPT score derivation study used data from randomized trials comparing different DAPT durations and showed that patients with a high PRECISE‐DAPT score (≥25) experienced a decreased bleeding risk, without an increased risk of ischemic events, when DAPT was discontinued after 3 to 6 months. 17 , 18 On the contrary, patients with a non‐high PRECISE‐DAPT score (<25) did not benefit from a shorter DAPT course regarding bleeding, whereas the risk of ischemic complications was higher. 17 , 22 Based on these findings, it would be reasonable to use the score to guide clinicians in deciding the optimal DAPT duration that balances ischemic protection with the risk of bleeding. 7 However, in our study, the median time to major bleeding was 77 days, which is comparable with results from the PLATO and TRITON‐TIMI‐38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38) studies but lower than the 158 days in the PRECISE‐DAPT derivation study. 1 , 2 , 17 Taking into account these findings that a majority of patients experienced bleeding within 6 months, or within 3 months in our study, a shortened DAPT duration of 3 or 6 months would not help these early bleeders, unless one would use even shorter treatment regimens. 30 Importantly, however, the use of the PRECISE‐DAPT score to shorten the DAPT duration to 3 or 6 months would be valuable for patients with later bleeding events.
High Bleeding Risk Subgroups
A small previous study evaluated the PRECISE‐DAPT score in elderly patients and found that the majority of patients had a score ≥25 and would therefore be considered at high bleeding risk. 23 We hypothesized that the score would have little or no extra value in patients with preexisting risk factors for bleeding, including advanced age, 31 underweight, 1 women, 32 patients with anemia, 33 kidney dysfunction, 34 or cancer. 35 In the present study, we found a poor discriminative ability of the score for both patients who are elderly or underweight. In these patient subgroups, the absolute risk of major bleeding in patients considered to be at non‐high risk according to the score (PRECISE‐DAPT <25) was comparable to that of patients in the general population with MI considered at high bleeding risk (PRECISE‐DAPT ≥25). Furthermore, patients with anemia or cancer with a non‐high PRECISE‐DAPT score had an absolute risk of major bleeding comparable to, or higher, than that of patients with high PRECISE‐DAPT scores in the general MI population, indicating that these comorbidities are high‐risk features regardless of PRECISE‐DAPT score status. Our results suggest that the use of the PRECISE‐DAPT score adds limited value in patients with preexisting risk factors for bleeding, who should be deemed a priori at high risk of bleeding.
Prediction of Bleeding and Stent Thrombosis
In addition to the 4 variables included in the PRECISE‐DAPT score (age, creatinine clearance, hemoglobin, and previous bleeding), we found independent associations with the occurrence of major bleeding for low body weight, peripheral artery disease, heart failure, chronic obstructive pulmonary disease, cancer, dementia, and DAPT with ticagrelor as compared with clopidogrel. Perhaps these additional risk factors could help to improve the discriminatory power of the PRECISE‐DAPT score. Furthermore, we found that complex lesions and a history of PCI before index hospitalization were associated with a 2‐fold increased risk of stent thrombosis after DAPT cessation and that the use of drug‐eluting stents was protective against stent thrombosis. In many risk scores like HAS‐BLED and CHA2DS2VASC, there are multiple risk factors that are similar for both bleeding and ischemic outcomes. 29 However, our findings show interestingly that the 3 strongest risk factors for stent thrombosis are not associated with increased risk of bleeding. This opens several possibilities for patient‐tailored medicine where patients with high stent thrombosis risk (complex lesions, a history of previous PCI before index hospitalization, and use of bare‐metal stents) should be treated with long‐term DAPT to a higher degree, taking concomitant bleeding risk into account.
Strengths and Limitations
This large observational study was limited by the lack of data on some important variables. We did not have information on intended or randomized DAPT duration and could therefore not test the ability of the score to select patients suitable for a shortened treatment regimen. We did, however, have data on DAPT dispensations from the pharmacy and were able to censor patients when their treatment was terminated in an attempt to exclude bleeding events unrelated to DAPT. Furthermore, the SWEDEHEART registry does not hold data on white blood cell count. It was therefore not possible for us to validate the 5‐item score, although the 4‐item version has demonstrated similar qualities. 18 Additionally, the 4‐item score might be more practical, as white blood cell count is not routinely measured in many hospitals. Lastly, we did not have access to any standardized bleeding definition, as was used in the derivation study. 17 A strength of the present study is that it relies on the use of a nationwide and validated registry with data on all‐comer patients undergoing PCI in Sweden. 24
Conclusions
In this large population‐based study of patients with MI treated with PCI and DAPT, the PRECISE‐DAPT score was moderate at predicting risk of major bleeding. Furthermore, the score performed poorly in patients with a preexisting risk factor for bleeding, especially in patients with advanced age, low body weight, anemia, or cancer for whom a non‐high PRECISE‐DAPT score (<25) was associated with a similar or higher absolute risk of major bleeding compared with that of patients with a high PRECISE‐DAPT score (≥25) in the general population with MI. For these patients, who should per se be considered at high risk of bleeding, the score seems to be of limited use.
Sources of Funding
This work was supported by the Swedish Heart and Lung Foundation, the Swedish Scientific Research Council, the Swedish Foundation for Strategic Research (as part of the TOTAL‐AMI [Tailoring of Treatment in All Comers with Acute Myocardial Infarction] project), and Thorsten Westerström's Research Foundation. The sponsors had no role in the design and conduct of this study; in the collection, analysis, and interpretation of the data; or in the preparation, review, and approval of the article.
Disclosures
Dr Koul reports personal fees from BMS and personal fees from Pfizer outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
For Sources of Funding and Disclosures, see page 11.
References
- 1. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F‐J, Ardissino D, De Servi S, Murphy SA, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482 [DOI] [PubMed] [Google Scholar]
- 2. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 3. Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, Armstrong PW, White HD, Held C, Aylward PE, et al. Trade‐off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the thrombin receptor antagonist for clinical event reduction in acute coronary syndrome (TRACER) randomized trial. Eur Heart J. 2017;38:804–810. doi: 10.1093/eurheartj/ehw525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navarese EP, Andreotti F, Schulze V, Kolodziejczak M, Buffon A, Brouwer M, Costa F, Kowalewski M, Parati G, Lip GYH, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta‐analysis of randomised controlled trials. BMJ. 2015;350:h1618. doi: 10.1136/bmj.h1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857 [DOI] [PubMed] [Google Scholar]
- 6. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, Normand S‐L, Braunwald E, Wiviott SD, Cohen DJ, et al. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valgimigli M, Bueno H, Byrne RA, Collet J‐P, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 8. Palmerini T, Della Riva D, Benedetto U, Bacchi Reggiani L, Feres F, Abizaid A, Gilard M, Morice MC, Valgimigli M, Hong MK, et al. Three, six, or twelve months of dual antiplatelet therapy after des implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta‐analysis of six randomized trials and 11 473 patients. Eur Heart J. 2017;38:1034–1043. doi: 10.1093/eurheartj/ehw627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, et al. Ticagrelor with or without aspirin in high‐risk patients after PCI. N Engl J Med. 2019;381:2032–2042. doi: 10.1056/NEJMoa1908419 [DOI] [PubMed] [Google Scholar]
- 10. Collet J‐P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2020;42:1289–1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 11. Simonsson M, Winell H, Olsson H, Szummer K, Alfredsson J, Hall M, Dondo TB, Gale CP, Jernberg T. Development and validation of a novel risk score for in‐hospital major bleeding in acute myocardial infarction: the SWEDEHEART score. J Am Heart Assoc. 2019;8:e012157. doi: 10.1161/JAHA.119.012157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moscucci M, Fox KA, Cannon CP, Klein W, Lopez‐Sendon J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24:1815–1823. doi: 10.1016/S0195-668X(03)00485-8 [DOI] [PubMed] [Google Scholar]
- 13. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, et al. Baseline risk of major bleeding in non‐ST‐segment‐elevation myocardial infarction: the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines) bleeding score. Circulation. 2009;119:1873–1882. doi: 10.1161/CIRCULATIONAHA.108.828541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP. In‐hospital major bleeding during ST‐elevation and non‐ST‐elevation myocardial infarction care: derivation and validation of a model from the ACTION registry‐GWTG. Am J Cardiol. 2011;107:1136–1143. doi: 10.1016/j.amjcard.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 15. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–2566. doi: 10.1016/j.jacc.2009.09.076 [DOI] [PubMed] [Google Scholar]
- 16. Yeh RW, Secemsky EA, Kereiakes DJ, Normand S‐L, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–1749. doi: 10.1001/jama.2016.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong M‐K, Kim H‐S, Colombo A, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE‐DAPT) score: a pooled analysis of individual‐patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5 [DOI] [PubMed] [Google Scholar]
- 18. Costa F, van Klaveren D, Colombo A, Feres F, Räber L, Pilgrim T, Hong M‐K, Kim H‐S, Windecker S, Steyerberg EW, et al. A 4‐item PRECISE‐DAPT score for dual antiplatelet therapy duration decision‐making. Am Heart J. 2020;223:44–47. doi: 10.1016/j.ahj.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 19. Choi SY, Kim MH, Cho YR, Sung Park J, Min Lee K, Park TH, Yun SC. Performance of PRECISE‐DAPT score for predicting bleeding complication during dual antiplatelet therapy. Circ Cardiovasc Interv. 2018;11:e006837. doi: 10.1161/CIRCINTERVENTIONS.118.006837 [DOI] [PubMed] [Google Scholar]
- 20. Abu‐Assi E, Raposeiras‐Roubin S, Cobas‐Paz R, Caneiro‐Queija B, Martinez‐Reglero C, Rodriguez‐Rodriguez JM, Baz A, Iniguez‐Romo A. Assessing the performance of the PRECISE‐DAPT and PARIS risk scores for predicting one‐year out‐of‐hospital bleeding in acute coronary syndrome patients. EuroIntervention. 2018;13:1914–1922. doi: 10.4244/EIJ-D-17-00550 [DOI] [PubMed] [Google Scholar]
- 21. Bianco M, D'ascenzo F, Raposeiras Roubin S, Kinnaird T, Peyracchia M, Ariza‐Solé A, Cerrato E, Manzano‐Fernández S, Gravinese C, Templin C, et al. Comparative external validation of the PRECISE‐DAPT and PARIS risk scores in 4424 acute coronary syndrome patients treated with prasugrel or ticagrelor. Int J Cardiol. 2020;301:200–206. doi: 10.1016/j.ijcard.2019.11.132 [DOI] [PubMed] [Google Scholar]
- 22. Choi KH, Song YB, Lee JM, Park TK, Yang JH, Choi JH, Choi SH, Oh JH, Cho DK, Lee JB, et al. Clinical usefulness of PRECISE‐DAPT score for predicting bleeding events in patients with acute coronary syndrome undergoing percutaneous coronary intervention: an analysis from the SMART‐DATE randomized trial. Circ Cardiovasc Interv. 2020;13:e008530. doi: 10.1161/CIRCINTERVENTIONS.119.0085 [DOI] [PubMed] [Google Scholar]
- 23. Guerrero C, Ariza‐Sole A, Formiga F, Martinez‐Selles M, Vidan MT, Aboal J. Applicability of the PRECISE‐DAPT score in elderly patients with myocardial infarction. J Geriatr Cardiol. 2018;15:713–717. doi: 10.11909/j.issn.1671-5411.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804 [DOI] [PubMed] [Google Scholar]
- 25. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundstrom A, Westerholm B, Rosen M. The new Swedish prescribed drug register‐opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735. doi: 10.1002/pds.1294 [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 29. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488 [DOI] [PubMed] [Google Scholar]
- 30. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, et al. Effect of 1‐month dual antiplatelet therapy followed by clopidogrel vs 12‐month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT‐2 randomized clinical trial. JAMA. 2019;321:2414–2427. doi: 10.1001/jama.2019.8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wester A, Attar R, Mohammad MA, Isma N, James S, Omerovic E, Erlinge D, Koul S. Bivalirudin versus heparin monotherapy in elderly patients with myocardial infarction: a prespecified subgroup analysis of the VALIDATE‐SWEDEHEART trial. Circ Cardiovasc Interv. 2020;13:e008671. doi: 10.1161/CIRCINTERVENTIONS.119.008671 [DOI] [PubMed] [Google Scholar]
- 32. Yu J, Mehran R, Grinfeld L, Xu KE, Nikolsky E, Brodie BR, Witzenbichler B, Kornowski R, Dangas GD, Lansky AJ, et al. Sex‐based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: three year results from the HORIZONS‐AMI trial. Catheter Cardiovasc Interv. 2015;85:359–368. doi: 10.1002/ccd.25630 [DOI] [PubMed] [Google Scholar]
- 33. Wester A, Attar R, Mohammad MA, Andell P, Hofmann R, Jensen J, Szummer K, Erlinge D, Koul S. Impact of baseline anemia in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a prespecified analysis from the VALIDATE‐SWEDEHEART trial. J Am Heart Assoc. 2019;8:e012741. doi: 10.1161/JAHA.119.012741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saltzman AJ, Stone GW, Claessen BE, Narula A, Leon‐Reyes S, Weisz G, Brodie B, Witzenbichler B, Guagliumi G, Kornowski R, et al. Long‐term impact of chronic kidney disease in patients with ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention: the HORIZONS‐AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. JACC Cardiovasc Interv. 2011;4:1011–1019. doi: 10.1016/j.jcin.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 35. Bharadwaj A, Potts J, Mohamed MO, Parwani P, Swamy P, Lopez‐Mattei JC, Rashid M, Kwok CS, Fischman DL, Vassiliou VS, et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. 2020;41:2183–2193. doi: 10.1093/eurheartj/ehz851 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


