Abstract
Background
White matter hyperintensity (WMH), characterized by hyperintensities on T2‐weighted fluid‐attenuated inversion recovery brain magnetic resonance imaging, has been linked to an increased risk of ischemic stroke (IS). Endothelial dysfunction is an indicator of vascular dysfunction, predicting the risk of IS. This study aimed to investigate the association between endothelial dysfunction and regional WMH, and its impact on future risk of IS.
Methods and Results
We enrolled 219 patients (mean age, 53.1±14.1 years; 34.7% men) who underwent peripheral endothelial function assessment using reactive hyperemia peripheral arterial tonometry and brain magnetic resonance imaging without any history of IS. Volumetric WMH segmentation was automatically extrapolated using a validated automated digital tool. Total and juxtacortical WMH volume/intracranial volume (%) increased with aging and became more prominent in patients aged >50 years (n=131) than those aged ≤50 years (n=88) (total WMH: ≤50 years, Pearson r=0.24, P=0.03; >50 years, Pearson r=0.62, P<0.0001; juxtacortical WMH: ≤50 years, Pearson r=0.09, P=0.40; >50 years, Pearson r=0.55, P<0.0001). Reactive hyperemia peripheral arterial tonometry index was negatively associated with total and juxtacortical WMH volume/intracranial volume (%) in patients aged >50 years after adjustment for other covariates (reactive hyperemia peripheral arterial tonometry index, standardized β coefficient −0.17, P=0.04). Juxtacortical WMH volume/intracranial volume (%) was associated with an increased risk of IS during median follow‐up of 6.5 years (hazard ratio, 1.47; 95% CI, 1.05–1.92; P=0.03).
Conclusions
Peripheral endothelial dysfunction is associated with an increased volume of juxtacortical WMH in patients aged >50 years, which is a potential marker to predict future risk of IS.
Keywords: endothelial dysfunction, ischemic stroke, reactive hyperemia peripheral arterial tonometry, white matter disease
Subject Categories: Vascular Biology, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- ICV
intracranial volume
- RH‐PAT
reactive hyperemia peripheral arterial tonometry
- WMH
white matter hyperintensity
Clinical Perspective
What Is New?
Aging, especially for patients aged >50 years, is associated with cerebral parenchymal changes characterized by white matter hyperintensity.
Peripheral microvascular endothelial dysfunction is associated with an increased volume of juxtacortical white matter hyperintensity in patients aged >50 years.
Increased volume of juxtacortical white matter hyperintensity is associated with an increased risk of ischemic stroke.
What Are the Clinical Implications?
In patients aged >50 years, peripheral microvascular endothelial dysfunction was associated with an increased volume of juxtacortical white matter hyperintensity, which could be a potential marker to predict future risk of ischemic stroke.
Impact of microvascular endothelial dysfunction on future risk of ischemic stroke could be potentially through regional loss of cerebrovascular reserve.
Peripheral microvascular endothelial dysfunction is associated with an increased risk of ischemic stroke and its incremental prognostic value in predicting ischemic stroke over conventional stroke risk scores, such as CHA2DS2‐VASc score and revised Framingham Stroke Risk Score, suggesting a potential link in underlying disease process between peripheral microvascular endothelial dysfunction and ischemic stroke. 1 Previous studies also suggested that the cerebral microvascular endothelium might be the primary site of injury caused by multiple risk factors, leading to increased permeability of the blood–brain barrier in cerebral small vessels and subsequent glial/neuronal damage. 2 Serum markers of endothelial dysfunction, such as intercellular adhesion molecule‐1, vascular cell adhesion molecule‐1, and vascular endothelial growth factor, are associated with the progression of cerebral small vessel disease, including white matter hyperintensity (WMH) and lacunes in patients with a history of ischemic stroke. 3 However, no studies have directly explored the association between cerebral small vessel disease and systemic microvascular endothelial dysfunction, using a measure of microvascular vasomotor response.
WMH is a common finding of brain magnetic resonance imaging (MRI) in the elderly, characterized by bilateral, mostly symmetrical hyperintensities on T2‐weighted fluid‐attenuated inversion recovery images without cavitation. 4 WMH has been linked to cognitive decline, gait disturbance, and 3.5‐times increased risk of ischemic stroke. 5 , 6 The underlying pathophysiology of WMH has been attributed, at least in part, to vascular dysfunction 7 , 8 ; however, the role of microvascular endothelial dysfunction on the progression of WMH is not clear. Furthermore, although some investigators indicated the potential regional difference of WMH in pathogenesis and clinical consequences, most published literature refers only to total WMH in the analyses. 4 , 9
We hypothesized that the link between WMH and ischemic stroke could be mediated by endothelial dysfunction. Therefore, this study aimed to investigate the impact of peripheral microvascular endothelial dysfunction on WMH, with further categorization based on the regions of WMH. Additionally, we assessed the predictive value of WMH in different regions for future risk of ischemic stroke.
Methods
The data that supported the findings of this study are available from the corresponding author upon reasonable request.
Study Population
In this observational cohort study, we enrolled patients who underwent peripheral microvascular endothelial function testing using the EndoPAT 2000 device (Itamar Medical, Caesarea, Israel) at Mayo Clinic between January 2006 and February 2014 and brain MRI during follow‐up. The decision to perform reactive hyperemia peripheral arterial tonometry (RH‐PAT) testing was at the clinical discretion of the evaluating physicians for assessment of chest pain and/or cardiovascular risk. Similarly, the decision to perform brain MRI was at the clinical discretion of the evaluating physician for assessment of headache, syncope, dementia, and/or motor/sensory neurological symptoms. Patients with a documented history or current diagnosis of ischemic stroke or hemorrhagic stroke were excluded from our analysis. The study was conducted in accordance with the guidelines of the Declaration of Helsinki. The Mayo Clinic Institutional Review Board approved the study protocol (19‐011946). All patients provided written informed consent for participation in the current study. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Clinical Assessment
Clinical history, laboratory data, and current medications were collected from a detailed chart review by an investigator blinded to RH‐PAT and volumetric WMH data. Data were collected on the following parameters: (1) sex, age, and smoking status; (2) dyslipidemia, defined by a documented history of dyslipidemia or treatment with lipid‐lowering therapy; (3) type 2 diabetes mellitus, defined as a documented history of or treatment for type 2 diabetes mellitus; (4) hypertension, defined as a documented history of or treatment for hypertension; (5) coronary artery disease, diagnosed by coronary angiography or computed tomography coronary angiography; (6) a documented history of atrial fibrillation; and (7) a documented history of transient ischemic attack. Ten‐year atherosclerotic cardiovascular disease risk was estimated with the Atherosclerotic Cardiovascular Disease Risk Estimator Plus tool. 10 Patients were followed‐up from the date of RH‐PAT testing for ischemic stroke over follow‐up. All ischemic stroke events were identified in accordance with the American Heart Association/American Stroke Association definition 11 and classified into cardioembolic, lacunar, and large artery disease, using the modified TOAST (Trial of Org10172 in Acute Stroke Treatment) criteria. 12 All diagnoses of ischemic stroke were made by experienced neurologists at the Mayo Clinic.
Assessment of Microvascular Endothelial Function
RH‐PAT was measured using EndoPAT (Itamar Medical), which is a Food and Drug Administration–approved fingertip device recording distal fingertip signal to evaluate peripheral microvascular endothelial function, as previously described. 13 Briefly, the study protocol included a 5‐minute baseline measurement, followed by a 5‐minute inflation of a blood pressure cuff around the patient's test arm with a pressure of 60 mm Hg above baseline systolic blood pressure up to 200 mm Hg, followed by a 6‐minute period of peripheral arterial tonometry measurement after deflation of the cuff. Blood pressure cuff occlusion was not applied to the control arm (contralateral arm). RH‐PAT ratio was determined as the average pulse wave amplitude for a 1‐minute period beginning 1 minute after pressure cuff deflation divided by the average pulse wave amplitude during the 3.5‐minute baseline period before pressure cuff inflation. The RH‐PAT index was computed automatically by normalizing baseline signal and indexing the RH‐PAT ratio on the test arm to that of the control arm. Per clinical protocol, patients were instructed to stop all vasoactive medications, including calcium channel blockers, β‐blockers, and long‐acting nitrates, for at least 24 hours, and fast for at least 4 hours and abstain from coffee and tobacco use on the day of the RH‐PAT testing.
Assessment of Brain MRI
MRI images were acquired on 1.5T/3T MRI scanners (GE Healthcare, Waukesha, WI). The presence of significant acute or chronic territorial infarction, brain tumors, and inflammatory/infective diseases was ruled out in all patients by experienced neuroradiologists and/or neurologists at Mayo Clinic. The brain volumetric data were automatically extrapolated from a structured 3‐dimensional T1‐weighed image using the volBrain online tool. 14 Similarly, volumetric WMH segmentation data were automatically extrapolated from both a structured 3‐dimensional T1‐weighed image and fluid‐attenuated inversion recovery image using the volBrain online tool. 15 The absolute count of WMH lesions, absolute total volume of WMH (cubic centimeters), total volume of WMH/intracranial volume (ICV) (%), and total volume of WMH/white matter volume (%) were calculated, with further categorization based on the regions of WMH (periventricular, juxtacortical, and deep white matter). 16 , 17
Statistical Analysis
Continuous variables distributed normally were expressed as the mean±standard deviation, and those with a skewed distribution were expressed as the median with interquartile range. Categorical variables were expressed as frequency (percent). Enrolled patients were divided into 2 groups; those aged ≤50 years and those aged >50 years based on the fact that WMH starts to increase in middle age. 9 For between‐group comparisons, an unpaired t test was used for normally distributed continuous variables, Mann‐Whitney U test for nonnormally distributed variables, and χ2 test (and Fisher exact test) for categorical variables. Correlations between 2 variables were assessed using the Pearson correlation test for parametric variables. Nonparametric variables were log‐transformed natural log (LN) to fit normal distribution. Multiple linear regression analyses were performed to estimate the independent effect of peripheral microvascular endothelial function on the progression of WMH, with additional stratification by age. Adjustments were made for age, cardiovascular risk factors (hypertension, dyslipidemia, and diabetes mellitus), coronary artery disease, atrial fibrillation, transient ischemic attack history, and duration from peripheral microvascular endothelial function assessment to brain MRI. These covariates were chosen for clinical relevance. Finally, univariate and multivariate Cox proportional hazard ratio (HR) analyses were performed to estimate the effect of WMH on the risk for ischemic stroke from the date of RH‐PAT testing. For all tests, a P value <0.05 was considered statistically significant. All statistical analyses were performed using JMP Pro software (SAS Institute, Cary, NC) and R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographic Characteristics
Between January 2006 and February 2014, 687 patients presented to the Mayo Clinic and underwent RH‐PAT testing using the EndoPAT 2000 device for assessment of chest pain and/or cardiovascular risk. Brain MRI images were available in 235 patients without a documented history or current diagnosis of ischemic or hemorrhagic stroke at the time of brain MRI. We excluded 16 patients because of artifacts or lack of T1‐weighed or fluid‐attenuated inversion recovery images, leaving a total of 219 patients in the final analyses. Baseline demographic data are summarized in Table 1. Patients were followed up for a median of 6.5 years. The median duration between peripheral microvascular endothelial function testing and brain MRI was 0 years (interquartile range, −1 to 2 years). Patients aged >50 years (n=131) were significantly more likely to have traditional cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia, and chronic kidney disease) than patients aged ≤50 years (n=88). The prevalence of preexisting coronary artery disease, atrial fibrillation, and a history of transient ischemic attack was also significantly higher in patients aged >50 years than patients aged ≤50 years (Table 1).
Table 1.
Baseline Demographic Data
| All | ≤50 years | >50 years | P Value | |
|---|---|---|---|---|
| N=219 | n=88 | n=131 | ||
| Age, y | 53.1±14.1 | 39.7±9.0 | 62.1±8.7 | <0.0001 |
| Men, n (%) | 76 (35) | 29 (33) | 47 (36) | 0.66 |
| Hypertension, n (%) | 108 (49) | 25 (28) | 83 (63) | <0.0001 |
| Diabetes mellitus, n (%) | 25 (11) | 5 (6) | 20 (15) | 0.03 |
| Dyslipidemia, n (%) | 142 (65) | 43 (49) | 99 (76) | <0.0001 |
| Smoking history, n (%) | 72 (33) | 30 (34) | 42 (32) | 0.75 |
| Chronic kidney disease, n (%) | 38 (19) | 7 (9) | 31 (25) | 0.003 |
| Coronary artery disease, n (%) | 47 (22) | 7 (8) | 40 (31) | <0.0001 |
| Transient ischemic attack, n (%) | 24 (11) | 4 (5) | 20 (15) | 0.01 |
| Atrial fibrillation, n (%) | 11 (5) | 1 (1) | 10 (8) | 0.03 |
| 10‐year Cardiovascular risk score | 3.4 (1.3–9.5) | 1.0 (0.5–2.1) | 5.6 (2.6–12.7) | <0.0001 |
| RH‐PAT index | 2.1 (1.8–2.5) | 2.1 (1.8–2.6) | 2.1 (1.7–2.5) | 0.48 |
| Systolic BP, mm Hg | 122±17 | 116±14 | 126±18 | <0.0001 |
| Diastolic BP, mm Hg | 74±12 | 74±14 | 74±10 | 0.98 |
| LDL, mg/dL | 105±41 | 111±41 | 101±40 | 0.10 |
| HDL, mg/dL | 58±18 | 55±16 | 60±19 | 0.08 |
| Triglyceride, mg/dL | 112 (79–150) | 114 (75–179) | 112 (80–144) | 0.66 |
| Fasting plasma glucose, mg/dL | 102±25 | 97±24 | 105±26 | 0.04 |
| HbA1c, % | 5.6 (5.2–6.0) | 5.2 (5.0–5.5) | 5.8 (5.6–6.7) | <0.0001 |
| Creatinine, mg/dL | 0.93±0.25 | 0.88±0.17 | 0.97±0.29 | 0.01 |
| eGFR, mL/min per 1.73 m2 | 74.4±20.9 | 81.8±20.3 | 69.5±19.8 | <0.0001 |
| Aspirin, n (%) | 111 (51) | 29 (33) | 82 (63) | <0.0001 |
| Statins, n (%) | 92 (42) | 20 (23) | 72 (55) | <0.0001 |
| Antihypertensive, n (%) | 118 (54) | 37 (42) | 81 (62) | 0.004 |
| Antidiabetic, n (%) | 21 (10) | 3 (4) | 18 (14) | 0.01 |
BP indicates blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; and RH‐PAT, reactive hyperemia peripheral arterial tonometry.
Brain Volumetric Analysis
Volumetric data of the brain segments and WMH are summarized in Table 2. The brain tissue volume, especially for white matter tissue, was significantly smaller in patients aged >50 years than those aged ≤50 years, whereas their cerebrospinal fluid volume was significantly larger. Total gray matter volume was not different between the 2 groups. Correlation between age and brain morphology was further subdivided by its anatomical structure and is summarized in Figure S1.
Table 2.
Brain Volumetric Segmentation Analysis
| All, | ≤50 years | >50 years | P Value | |
|---|---|---|---|---|
| N=219 | n=88 | n=131 | ||
| Tissue brain, cm3 | 1166±147 | 1197±180 | 1145±115 | 0.02 |
| Tissue brain/ICV, % | 84.9±5.5 | 85.8±6.3 | 84.2±4.9 | 0.06 |
| Tissue WM, cm3 | 451±91 | 479±99 | 432±79 | 0.0003 |
| Tissue WM/ICV, % | 32.8±5.4 | 34.3±5.1 | 31.8±5.3 | 0.001 |
| Tissue GM, cm3 | 715±118 | 718±137 | 713±104 | 0.79 |
| Tissue GM/ICV, % | 52.0±6.8 | 51.4±7.6 | 52.4±6.2 | 0.33 |
| CSF, cm3 | 208±80 | 196±83 | 216±77 | 0.08 |
| CSF/ICV, % | 15.1±5.5 | 14.2±6.3 | 15.8±4.9 | 0.06 |
| WMH no. | 12 (6–19) | 7 (3–12) | 15 (9–22) | <0.0001 |
| WMH, cm3 | 0.8 (0.2–3.0) | 0.3 (0.1–0.8) | 1.6 (0.6–5.4) | <0.0001 |
| WMH/ICV, % | 0.06 (0.02–0.23) | 0.02 (0.00–0.06) | 0.12 (0.05–0.39) | <0.0001 |
| WMH/WM, % | 0.14 (0.04–0.54) | 0.05 (0.01–0.13) | 0.32 (0.10–1.06) | <0.0001 |
CSF indicates cerebrospinal fluid; GM, grey matter; ICV, intracranial volume; WM, white matter; and WMH, white matter hyperintensity.
Impact of Aging on WMH
WMH volume was significantly larger in patients aged >50 years than those aged ≤50 years (Table 2). Scatter plot showed that total volume of WMH/ICV (%) increased with aging, and it became more prominent in patients aged >50 years (Figure 1A). Regional difference in WMH volume with aging is summarized in Figure S2. Correlation between age and WMH volume, with further categorization based on age (>50 years versus ≤50 years) and cerebral regions, is summarized in Figure 1B. Total WMH volume/ICV (%) increased with aging and became more prominent in patients aged >50 years (≤50 years, r=0.24; P=0.03; >50 years, r=0.62; P<0.0001, P for interaction <0.0001). Interestingly, the impact of aging on WMH volume was different among the cerebral regions. Correlation between age and periventricular WMH volume/ICV (%) was similar between patients aged >50 years and those aged ≤50 years (≤50 years, r=0.26; P=0.02; >50 years, r=0.32; P=0.001, P for interaction <0.0001). Correlation between age and juxtacortical WMH was not significant in patients aged ≤50 years, whereas a moderate significant positive correlation was observed in those aged >50 years (≤50 years, r=0.09; P=0.40; >50 years, r=0.55; P<0.0001, P for interaction <0.0001). Correlation between age and deep WMH was not significant in patients aged ≤50 years, whereas mild positive correlation was observed in those aged >50 years (≤50 years, r=0.19; P=0.08; >50 years, r=0.31; P=0.001, P for interaction=0.16).
Figure 1. Correlation between age and WMH.
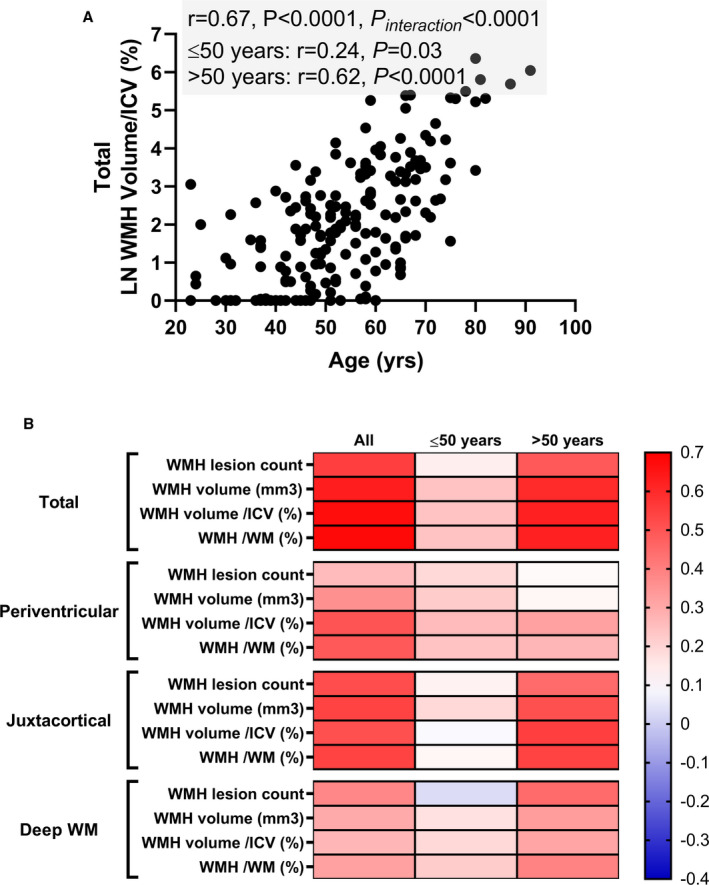
A, Scatter plot showing the association between age and total WMH volume/ICV (%). WMH volume/ICV (%) increases with aging, but becomes more prominent after age 50 years. B, Heat map showing the Pearson correlation (r) between age and WMH lesion count and volume. WMH volume is shown in LN absolute volume (mm3), normalized by intracranial volume (%) and by white matter volume (%). ICV indicates intracranial volume; LN, natural log; WM, white matter; and WMH, white matter hyperintensity.
Impact of Peripheral Microvascular Endothelial Function on WMH
To investigate the independent effect of peripheral microvascular endothelial function on WMH volume, multiple regression analysis was performed. RH‐PAT index was not significantly associated with LN total WMH volume/ICV (%); however, lower LN RH‐PAT index was significantly associated with higher LN juxtacortical WMH volume/ICV (%) in patients aged >50 years even after adjustment for age, other risk factors (hypertension, dyslipidemia, diabetes mellitus), coronary artery disease, atrial fibrillation, transient ischemic attack history, and duration between RH‐PAT testing and brain MRI (LN RH‐PAT index, unstandardized β coefficient −0.77, standardized β coefficient −0.17; P=0.04, P for interaction with age=0.15) (Figure 2A through 2D). Hypertension was marginally associated with higher LN total WMH volume/ICV (%) and LN juxtacortical WMH volume/ICV (%) in patients aged >50 years after adjustment for the same covariates (standardized β coefficient 0.16, P=0.05; standardized β coefficient 0.17, P=0.12). In patients aged >50 years, only atherosclerotic cardiovascular disease risk score and LN RH‐PAT index was significantly associated with LN juxtacortical WMH volume/total WMH volume (%), whereas individual cardiometabolic risk factors were not (Table 3).
Figure 2. Impact of RH‐PAT index on WMH volume/ICV (%).
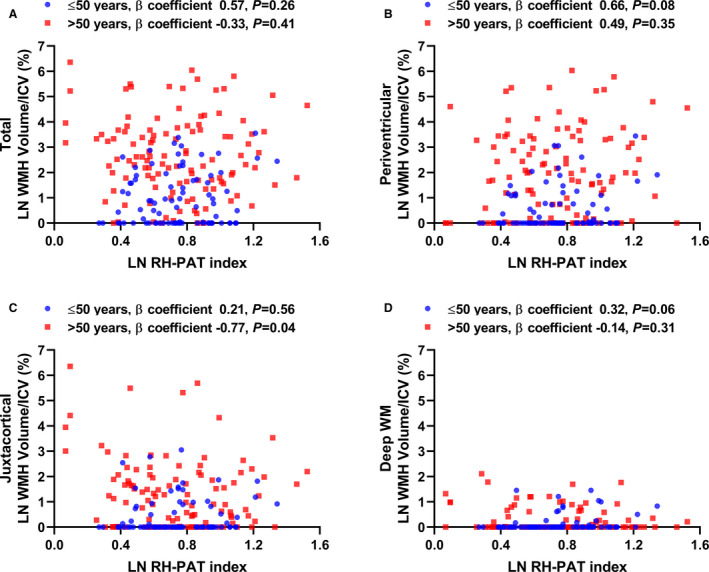
Scatter plot showing the association between RH‐PAT index and (A) total, (B) periventricular, (C) juxtacortical, and (D) deep WM WMH volume/ICV (%). The values of β coefficients from linear regression models adjusted for age, hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, transient ischemic attack history, coronary artery disease, and duration between RH‐PAT testing and brain magnetic resonance imaging are shown in the figures. Lower LN RH‐PAT index is significantly associated with higher juxtacortical WMH volume/ICV (%) in patients aged >50 years. ICV indicates intracranial volume; LN, natural log; RH‐PAT, reactive hyperemia peripheral arterial tonometry; WM, white matter; and WMH, white matter hyperintensity.
Table 3.
Effects of Cardiometabolic Risk Factors on LN Juxtacortical WMH Volume/Total WMH Volume (%) (>50 years)
| Unstandardized Coefficient | Standardized Coefficient | t | P Value | ||
|---|---|---|---|---|---|
| β | Standard Error | β | |||
| Hypertension | 0.92 | 2.13 | 0.03 | 0.43 | 0.66 |
| Systolic blood pressure, mm Hg | −0.004 | 0.12 | −0.002 | −0.03 | 0.97 |
| Diabetes mellitus | 3.59 | 3.44 | 0.07 | 1.04 | 0.30 |
| HbA1c, % | −0.29 | 4.07 | −0.01 | −0.07 | 0.94 |
| Fasting plasma glucose, mg/dL | 0.11 | 0.09 | 0.09 | 1.22 | 0.22 |
| Dyslipidemia | −1.78 | 2.23 | −0.06 | −0.80 | 0.43 |
| LDL‐C, mg/dL | −0.01 | 0.05 | −0.01 | −0.11 | 0.91 |
| Triglyceride, mg/dL | 0.02 | 0.02 | 0.07 | 0.93 | 0.36 |
| Smoking history | 0.59 | 2.27 | 0.02 | 0.26 | 0.80 |
| 10‐year ASCVD risk score | 0.61 | 0.27 | 0.19 | 2.25 | 0.03 |
| LN RH‐PAT index | −18.23 | 7.70 | −0.17 | −2.37 | 0.02 |
ASCVD indicates atherosclerotic cardiovascular disease; HbA1c, hemoglobin A1c; LDL‐C, low‐density lipoprotein cholesterol; LN, natural log; RH‐PAT, reactive hyperemia peripheral arterial tonometry; and WMH, white matter hyperintensity.
Impact of WMH on Future Risk of Ischemic Stroke
We identified 13 patients who developed ischemic stroke (1 cardioembolic and 12 lacunar strokes) during median (interquartile range) follow‐up of 6.5 years (1.3–8.8 years) (1.0% per person‐year). To elucidate the impact of WMH on the future risk of ischemic stroke, Cox proportional HR analysis was performed. Total WMH volume/ICV (%) was significantly associated with an increased risk of ischemic stroke, with an HR of 1.48 (95% CI, 1.08–2.04; P=0.02). Interestingly, only juxtacortical WMH volume was significantly associated with an increased risk of ischemic stroke, with an HR of 1.47 (95% CI, 1.05–1.92; P=0.03) (Figure 3). These associations remained significant even after adjustment for diabetes mellitus (total WMH volume/ICV [%]: adjusted HR, 1.45; 95% CI, 1.05–2.00; P=0.03; juxtacortical WMH volume/ICV [%]: adjusted HR, 1.46; 95% CI, 1.04–1.94; P=0.03). Increased volume of total and juxtacortical WMH tended to be associated with an increased risk of ischemic stroke after adjustment for hypertension (total WMH volume/ICV [%]: adjusted HR, 1.33; 95% CI, 0.95–1.90; P=0.10; juxtacortical WMH volume/ICV [%]: adjusted HR, 1.33; 95% CI, 0.93–1.79; P=0.08) or dyslipidemia (total WMH volume/ICV [%]: adjusted HR, 1.37; 95% CI, 0.98–1.90; P=0.10; juxtacortical WMH volume/ICV [%]: adjusted HR, 1.37; 95% CI, 0.97–1.81; P=0.07), whereas these association became less relevant after adjustment for age (total WMH volume/ICV [%]: adjusted HR, 1.22; 95% CI, 0.78–1.92; P=0.38; juxtacortical WMH volume/ICV [%]: adjusted HR, 1.19; 95% CI, 0.78–1.76; P=0.39). There was a borderline significant association between peripheral microvascular endothelial dysfunction defined as RH‐PAT index ≤2.0 1 , 13 and an increased risk of ischemic stroke with a HR of 2.84 (95% CI, 0.92–10.47; P=0.07).
Figure 3. Regional WMH volume and future risk of ischemic stroke.
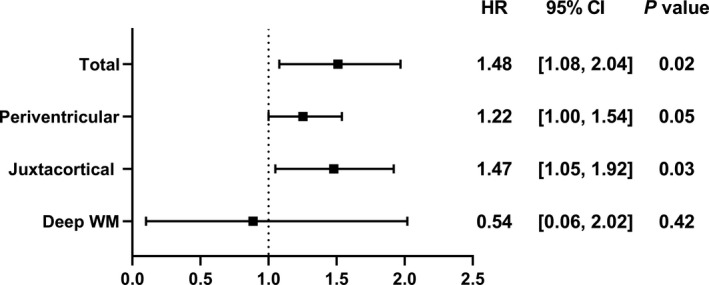
Forest plot showing the association between WMH volume/ICV (%) and future risk of ischemic stroke. Cox proportional HR analysis shows that total WMH volume/ICV (%) and juxtacortical WMH volume/ICV (%) is significantly associated with an increased risk of ischemic stroke during follow‐up. ICV, intracranial volume; HR, hazard ratio; WM, white matter; and WMH, white matter hyperintensity.
Discussion
This study demonstrated that WMH increased with aging, and became particularly prominent after age 50 years. Despite similar cerebral parenchymal changes in brain MRI, this study also highlighted the regional difference of WMH volume with aging, potentially reflecting a different underlying pathophysiology of WMH among cerebral regions. Interestingly, in patients aged >50 years, peripheral microvascular endothelial dysfunction was associated with an increased volume of WMH in the juxtacortical white matter, which in turn was significantly associated with an increased risk of ischemic stroke. This study suggests the impact of microvascular endothelial dysfunction on future risk of ischemic stroke potentially through regional loss of cerebrovascular reserve.
Different Pathophysiology of WMH in Different Regions
WMH is considered to be a sign of small vessel disease related to chronic hypoperfusion and alterations in the blood–brain barrier. 18 Age and hypertension are established risk factors for the progression of WMH. However, other conventional cardiovascular risk factors, such as dyslipidemia and diabetes mellitus, are not consistently associated with WMH, 19 which disfavors vascular dysfunction as the origin of all WMH. Previous postmortem studies suggest that multiple histopathological findings can correspond to WMH on brain MRI, such as nonspecific demyelination, axonal loss, or higher levels of microglial activation. 20 , 21 , 22 Furthermore, the juxtacortical WMH is more relevant to cognitive decline as compared with deep and periventricular WMH, suggesting regional differences in WMH in terms of the underlying pathophysiology and clinical implications. 17 Another observation also suggested that juxtacortical lesions can be related to a wide variety of neurologic symptoms, including headache, insomnia, anxiety, and depression. 23 In this study, we observed a significant association between peripheral microvascular endothelial dysfunction and increase in juxtacortical WMH volume. Interestingly, the periventricular and deep white matter has been considered to be more vulnerable to ischemic insult than juxtacortical white matter, because the former 2 regions are supplied by nonoverlapping terminal arterioles with limited collateral flow compared with juxtacortical WMH. 24 , 25 One possible explanation to this discrepancy could be the difference between macrovascular versus microvascular function, given that RH‐PAT index is an indicator of microvascular rather than macrovascular vasomotor response. However, our observation should be validated in other larger populations.
Endothelial Dysfunction and WMH
Endothelial dysfunction may theoretically contribute to histopathological cerebral parenchymal alterations observed in WMH, given the crucial role of the endothelium in the cerebral circulation: (1) endothelial cells are the site of blood–brain barrier, controlling the movement of ions, molecules, and cells; (2) endothelial cells affect resting cerebral blood flow as well as vasomotor responses to shear stress, neurotransmitters, and metabolic factors; and (3) endothelial cells affect the function of neurons, microglia, and oligodendrocytes. 26 We previously showed that peripheral microvascular endothelial dysfunction per se is associated with an increased risk of ischemic stroke. 1 However, there is a lack of evidence to show its mechanistic link to ischemic stroke. In this study, we demonstrated the association between microvascular endothelial dysfunction and juxtacortical WMH, where WMH volume is significantly associated with future risk of ischemic stroke. The proportion of juxtacortical WMH out of total WMH was significantly associated with overall cardiovascular risk and RH‐PAT index, consistent with the significant association between higher cardiovascular risk burden and WMH. 27 These results may indicate the systemic nature of endothelial dysfunction reflecting summative contribution of cardiovascular risk factors, and may partly explain the mechanistic link between peripheral microvascular endothelial dysfunction and ischemic stroke. We previously demonstrated that peripheral microvascular endothelial dysfunction could identify high‐risk patients for ischemic stroke and other cardiovascular diseases in individuals with chest pain and minimal cardiovascular risk 1 , 28 ; however, it is not elucidated if assessment of microvascular endothelial function could provide further prognostic information above and beyond conventional risk factors in the general population, requiring future studies.
Limitations
This study has several limitations. First, because of its observational cohort design, it is challenging to derive causal associations from the current study. The evaluation of RH‐PAT and brain MRI was performed at the discretion of the evaluating physician. Some selection bias cannot be excluded, thus potentially affecting the generalizability of current observation, requiring future validation studies. Also, there was some time lag between RH‐PAT testing and brain MRI (median [interquartile range], 0 [−1 to 2] years); however, even after adjustment for this lag, peripheral microvascular endothelial dysfunction was independently associated with an increased volume of juxtacortical WMH in patients aged >50 years. The lack of data on cognitive function limited our ability to meaningfully show the effects of aging and microvascular endothelial dysfunction on the association between WMH and cognitive decline/dementia. Second, we measured WMH lesion count and volume using the automated digital tool. Although validated in previous studies, 14 , 15 misclassification of the lesions might have occurred; however, to minimize this risk, we excluded patients with a documented history or a current diagnosis of stroke. The presence of central nervous system tumors and inflammatory/infective diseases was also ruled out in all patients by experienced neuroradiologists and/or neurologists at Mayo Clinic. Exclusion of patients with a documented history or current diagnosis of stroke might have weakened the association between peripheral endothelial dysfunction and WMH in periventricular/deep white matter regions. However, previous observation showing that the serum levels of apoB (apolipoprotein B) (contributing to atherosclerosis 29 ) and homocysteine (contributing to endothelial dysfunction 30 ) are associated with juxtacortical WMH may support our current observation. 23 Finally, because of the small number of events during the follow‐up, the hazard risk of ischemic stroke was not adjusted for other possible covariates, requiring further studies to validate our current observation. Also, previously observed association between peripheral microvascular endothelial dysfunction and ischemic stroke 1 was marginal in this study; thus, mediation analysis should be performed in a larger cohort to show the link between peripheral microvascular endothelial dysfunction and ischemic stroke through juxtacortical WMH.
Conclusions
Aging, especially for patients aged >50 years, is associated with cerebral parenchymal changes characterized by WMH. Peripheral endothelial dysfunction is associated with loss of juxtacortical white matter, where WMH could act as a potential marker to predict future risk of ischemic stroke. This study suggests the regional difference in pathophysiology and clinical consequences of WMH, requiring further validation. Whether improvement in endothelial dysfunction translates into a reduction of regional volume of WMH and incidence of ischemic stroke remains to be determined in future studies.
Sources of Funding
This study was partly supported by the National Institutes of Health (grant no. DK120292 and DK122734) and the Mayo Foundation.
Disclosures
Dr Lerman declares consulting for Itamar Medical. The remaining authors have no disclosures to report.
Supporting information
Figure S1–S2
For Sources of Funding and Disclosures, see page 9.
References
- 1. Toya T, Sara JD, Ahmad A, Nardi V, Taher R, Lerman LO, Lerman A. Incremental prognostic impact of peripheral microvascular endothelial dysfunction on the development of ischemic stroke. J Am Heart Assoc. 2020;9:e015703. DOI: 10.1161/JAHA.119.015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry. 2005;76:617–619. DOI: 10.1136/jnnp.2004.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arba F, Giannini A, Piccardi B, Biagini S, Palumbo V, Giusti B, Nencini P, Maria Gori A, Nesi M, Pracucci G, et al. Small vessel disease and biomarkers of endothelial dysfunction after ischaemic stroke. Eur Stroke J. 2019;4:119–126. DOI: 10.1177/2396987318805905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. DOI: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inzitari D, Pracucci G, Poggesi A, Carlucci G, Barkhof F, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow‐up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:b2477. DOI: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. DOI: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wardlaw JM, Allerhand M, Doubal FN, Valdes Hernandez M, Morris Z, Gow AJ, Bastin M, Starr JM, Dennis MS, Deary IJ. Vascular risk factors, large‐artery atheroma, and brain white matter hyperintensities. Neurology. 2014;82:1331–1338. DOI: 10.1212/WNL.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan U, Porteous L, Hassan A, Markus HS. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry. 2007;78:702–706. DOI: 10.1136/jnnp.2006.103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum Brain Mapp. 2009;30:11671155–11671167. DOI: 10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lloyd‐Jones DM, Braun LT, Ndumele CE, Smith SC, Sperling LS, Virani SS, Blumenthal RS. Use of risk assessment tools to guide decision‐making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Circulation. 2019;139:e1162–e1177. DOI: 10.1161/CIR.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 11. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ(, Culebras A, Elkind MSV, George MG, Hamdan AD, Higashida RT, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. DOI: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perera KS, Sharma M, Connolly SJ, Wang J, Gold MR, Hohnloser SH, Lau CP, Van Gelder IC, Morillo C, Capucci A, et al. Stroke type and severity in patients with subclinical atrial fibrillation: an analysis from the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT). Am Heart J. 2018;201:160–163. DOI: 10.1016/j.ahj.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 13. Toya T, Sara JD, Corban MT, Taher R, Godo S, Herrmann J, Lerman LO, Lerman A. Assessment of peripheral endothelial function predicts future risk of solid‐tumor cancer. Eur J Prev Cardiol. 2020;27:608–618. DOI: 10.1177/2047487319884246. [DOI] [PubMed] [Google Scholar]
- 14. Manjón JV, Coupé P. volBrain: an online MRI brain volumetry system. Front Neuroinform. 2016;10:30. DOI: 10.3389/fninf.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Porcu M, Garofalo P, Craboledda D, Suri JS, Suri HS, Montisci R, Sanfilippo R, Saba L. Carotid artery stenosis and brain connectivity: the role of white matter hyperintensities. Neuroradiology. 2020;62:377–387. DOI: 10.1007/s00234-019-02327-5. [DOI] [PubMed] [Google Scholar]
- 16. Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiat. 2008;64:273–280. DOI: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dadar M, Maranzano J, Ducharme S, Collins DL. White matter in different regions evolves differently during progression to dementia. Neurobiol Aging. 2019;76:71–79. DOI: 10.1016/j.neurobiolaging.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 18. McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH, Hortobagyi T, Ince P, Jellinger K, Gao J, et al. Post‐mortem assessment in vascular dementia: advances and aspirations. BMC Med. 2016;14:129. DOI: 10.1186/s12916-016-0676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scharf EL, Graff‐Radford J, Przybelski SA, Lesnick TG, Mielke MM, Knopman DS, Preboske GM, Schwarz CG, Senjem ML, Gunter JL, et al. Cardiometabolic health and longitudinal progression of white matter hyperintensity: the mayo clinic study of aging. Stroke. 2019;50:3037–3044. DOI: 10.1161/STROKEAHA.119.025822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jorgensen DR, Shaaban CE, Wiley CA, Gianaros PJ, Mettenburg J, Rosano C. A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am J Physiol Heart Circ Physiol. 2018;314:H1117–H1136. DOI: 10.1152/ajpheart.00535.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gouw AA, Seewann A, van der Flier WM , Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. DOI: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 22. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–165. DOI: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 23. Shan Y, Tan S, Wang Y, Li K, Zhang L, Liao S, Zhou LI, Deng Z, Hu X, Li H, et al. Risk factors and clinical manifestations of juxtacortical small lesions: a neuroimaging study. Front Neurol. 2017;8:497. DOI: 10.3389/fneur.2017.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsushita K, Kuriyama Y, Nagatsuka K, Nakamura M, Sawada T, Omae T. Periventricular white matter lucency and cerebral blood flow autoregulation in hypertensive patients. Hypertension. 1979;1994:565–568. DOI: 10.1161/01.HYP.23.5.565. [DOI] [PubMed] [Google Scholar]
- 25. Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 26. Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. 2017;120:449–471. DOI: 10.1161/CIRCRESAHA.116.308427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song R, Xu H, Dintica CS, Pan K‐Y, Qi X, Buchman AS, Bennett DA, Xu W. Associations between cardiovascular risk, structural brain changes, and cognitive decline. J Am Coll Cardiol. 2020;75:2525–2534. DOI: 10.1016/j.jacc.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. DOI: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 29. Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Jungner I, Walldius G. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment. Lancet. 2003;361:777–780. DOI: 10.1016/S0140-6736(03)12663-3. [DOI] [PubMed] [Google Scholar]
- 30. Toya T, Sara JD, Lerman B, Ahmad A, Taher R, Godo S, Corban MT, Lerman LO, Lerman A. Elevated plasma homocysteine levels are associated with impaired peripheral microvascular vasomotor response. Int J Cardiol Heart Vasculature. 2020;28:100515. DOI: 10.1016/j.ijcha.2020.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1–S2


