Abstract
Background
We examined the association of long‐term exposure to air pollution and road traffic noise with incident heart failure (HF).
Methods And Results
Using data on female nurses from the Danish Nurse Cohort (aged >44 years), we investigated associations between 3‐year mean exposures to air pollution and road traffic noise and incident HF using Cox regression models, adjusting for relevant confounders. Incidence of HF was defined as the first hospital contact (inpatient, outpatient, or emergency) between cohort baseline (1993 or 1999) and December 31, 2014, based on the Danish National Patient Register. Annual mean levels of particulate matter with a diameter <2.5 µm since 1990 and NO2 and road traffic noise since 1970 were estimated at participants' residences. Of the 22 189 nurses, 484 developed HF. We detected associations with all 3 pollutants, with hazard ratios (HRs) of 1.17 (95% CI, 1.01–1.36), 1.10 (95% CI, 0.99–1.22), and 1.12 (95% CI, 0.99–1.26) per increase of 5.1 µg/m3 in particulate matter with a diameter <2.5 µm, 8.6 µg/m3 in NO2, and 9.3 dB in road traffic noise, respectively. We observed an enhanced risk of HF incidence for those exposed to high levels of the 3 pollutants; however, the effect modification of coexposure was not statistically significant. Former smokers and nurses with hypertension showed the strongest associations with particulate matter with a diameter <2.5 µm (P effect modification<0.05).
Conclusions
We found that long‐term exposures to air pollution and road traffic noise were independently associated with HF.
Keywords: air pollution, cohort study, heart failure, morbidity, road traffic noise
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors
Nonstandard Abbreviations and Acronyms
- DNC
Danish Nurse Cohort
- IPW
inverse probability weight
- L den
overall weighted 24‐hour noise level
- PM2.5
particulate matter with a diameter <2.5 µm
Clinical Perspective
What Is New?
A large prospective cohort study including 22 189 Danish nurses with detailed information on historical exposures investigated environmental risk factors of heart failure.
Associations with incident heart failure were strongest and most robust with particulate matter with a diameter <2.5 µm and suggestive with road traffic noise.
Former smokers and patients who were hypertensive were most susceptible to the adverse effects of particulate matter with a diameter <2.5 µm on heart failure.
What Are the Clinical Implications?
The study suggests that air polluiton and road traffic noise can increase the risk of heart failure, and that former smokers or those with hypertension may be most susceptible to the adervse effects of air pollution.
Clinicians should inform their patients regarding air pollution and road traffic noise related adverse effects on the heart.
Heart failure (HF) is characterized by the reduced ability of the heart to pump or fill with blood, which affects 26 million people worldwide. 1 HF is associated with high mortality, frequent hospitalization, poor quality of life, or multiple comorbidities, 2 and risk factors of HF include smoking, alcohol consumption, and obesity as well as preexisting hypertension, coronary artery disease, diabetes, and myocardial infarction. Air pollution has been recognized as a risk factor for ischemic and coronary cardiovascular disease, 3 , 4 whereas evidence on HF is more limited and novel. Air pollution may induce oxidative stress, inflammation, imbalance of the autonomic nervous system, and endothelial dysfunction, all associated with the cardiovascular disease progression related to HF. 5 , 6 A number of studies have shown that short‐term exposure to high levels of particulate matter with a diameter <2.5 µm (PM2.5) and NO2, over several hours or days, can lead to HF onset, leading to HF hospitalization or deaths. 7 Evidence on whether long‐term exposure to air pollution over many years can lead to the development of HF is more limited, with 10 studies on HF incidence 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 and 1 on mortality, 18 with somewhat mixed results.
Road traffic noise is an increasingly recognized environmental stressor that can lead to inflammatory responses and oxidative stress as well as cause annoyance and sleep disturbance. 19 Road traffic noise has been linked to ischemic heart disease, 19 whereas only 4 studies considered HF, 10 , 14 , 20 , 21 2 suggesting associations. 14 , 20 As air pollutants and road traffic noise share a major source— traffic—it is important to consider the independent or interactive effects of the 2 exposures on health. Only 3 studies on air pollution and HF had data on road traffic noise and suggested that NO2 and road traffic noise independently influence HF incidence or mortality. 10 , 14 , 20 Moreover, no studies considered possible effect modification of coexposure on the association of PM2.5 and noise with HF, as high exposure to 1 pollutant may make the body more susceptible to the hazardous effects of another pollutant. 14
Here we examined the associations of long‐term exposure to air pollution (PM2.5 and NO2) and road traffic noise, independently and jointly, with incident HF, and considered possible effect modification by lifestyle and comorbidity.
Methods
Scripts for statistical analyses are available from the University of Copenhagen upon request. Data requests should be directed to the University of Copenhagen and are subject to their data access policies.
Participants
The Danish Nurse Cohort (DNC) was designed to examine the effects of hormone replacement therapy in the Danish population 22 and was initiated by sending questionnaires to members of the Danish Nurse Organization in 1993, and again, with the recruitment of new nurses, in 1999. Among 33 704 eligible female nurses aged >44 years who either worked or were retired in 1993 or 1999, 28 731 (85.2%) were enrolled in the DNC. As 4 participants had missing information on vital status during the study period, the remaining 28 727 participants were considered in this study (Figure S1). Upon enrollment, participants answered a comprehensive questionnaire on body mass index (BMI), lifestyle factors (smoking, alcohol consumption, physical activity, and dietary habits), preexisting diseases, reproductive health, and working conditions. All participants were linked to the Danish Civil Registration System 23 to extract data on death or emigration date until December 31, 2014. Participants’ residential addresses with complete moving history and moving dates since 1970 and until 2014 were obtained from the Danish Central Person Register.
The investigation conforms with the principles outlined in the Declaration of Helsinki. The DNC was approved by the Scientific and Ethical Committee of Copenhagen and Frederiksberg Municipalities (approval number [KF] 01‐103/93) and the Danish Data Protection Agency (J. number 1993‐1110‐1151), and the nurses who were included in the original DNC provided informed written consent.
Outcome Definitions
Information on HF was extracted by linking the cohort participants to the Danish National Patient Register, which includes records on all contacts in Danish hospitals since 1977. 24 We defined incidence of HF as a first hospital contact (inpatient, outpatient, or emergency room) between cohort baseline in 1993 or 1999 and December 31, 2014, which resulted in a primary discharge diagnosis of HF (International Classification of Diseases, Eighth Revision [ICD‐8] codes before 1994: 427.0 or 427.1; International Classification of Diseases, Tenth Revision [ICD‐10] codes after 1994: I50, I11.0, I42.0, or I42.9). The HF discharge diagnoses from the Danish National Patient Register have been previously validated by comparison to the hospital records at a Danish University Hospital cardiac care unit in 2005 to 2007, showing high validity with positive predictive values of 84.0% for overall HF and 77.9% for incident HF. 25
Air Pollution and Road Traffic Noise
We estimated annual mean concentrations of PM2.5 (since 1990) and NO2 (since 1970) based on the participants’ residential addresses using the Danish air pollution modeling system, DEHM/UBM/AirGIS (www.au.dk/AirGIS). 26 Road traffic noise levels were estimated using the validated model system, Nord2000, 27 which included noise contributions from roads within a 3 km radius from participants’ residential addresses. Road traffic noise was estimated as the overall weighted 24‐h noise level adding a penalty of 5 dB to the evening hours and 10 dB to the night hours (overall weighted 24‐hour noise level [L den]). For the year that participants changed the address, we calculated annual PM2.5, NO2, and L den levels at the year of an address change as weighted means of the 2 addresses' exposure levels (the old address and the new one). We calculated 1‐year, 3‐year, and 23‐year running means of PM2.5, NO2, and L den and assigned the multiyear residential exposures in a yearly time interval between the baseline year and last year of follow‐up. The 3‐year mean exposure to all pollutants was considered as the main exposure as this was the longest available exposure window for all 3 pollutants (data for PM2.5 available since 1990).
Covariates
We used information on individual characteristics collected at the cohort baseline in 1993 or 1999, including smoking status (never, former, or current), alcohol consumption (never; moderate, 1–14 drinks per week; or heavy, ≥15 drinks per week), leisure time physical activity (low, medium, or high), marital status (married, separated, divorced, single, or widowed), parity (none or at least 1 child), employment status (working, homemaker, retired, unemployed/rehabilitation, or other), use of oral contraceptives (never or ever), and hormone replacement therapy (never, previous, or current). We categorized participants into 3 job strain categories (low, high, or unemployed) by combining the following 3 items: workload, busyness, and control of work. Based on the municipality of the residential address, the level of urbanization was defined: urban (cities, ie, densely populated areas [at least 50% of the population lives in urban centers]), suburban (town and suburbs, ie, intermediate density areas [<50% of the population lives in rural grid cells and <50% of the population lives in urban centers]), and rural (thinly populated areas [≥50% of the population lives in rural grid cells]). Average municipality income in Danish Kroner in 1993 was assigned to each participant.
Statistical Analysis
We examined the association between air pollutants, noise, and HF using time‐varying Cox regression models with age (years) as the underlying time scale. In model 1, we examined associations between 3‐year running means of PM2.5, NO2, and L den and incident HF, separately for each pollutant, after adjusting for age as a time scale and the year of cohort entry (1993 or 1999). In model 2, we additionally controlled for the individual‐level (BMI, smoking status, alcohol consumption, leisure time physical activity, marital status, parity, employment status, use of oral contraceptives, hormone replacement therapy, and job strain) and area‐level (the level of urbanization and average municipality income) confounders. We then fitted 2‐pollutant and 3‐pollutant models controlling for PM2.5, NO2, and L den mutually to examine the independent effects of air pollutants and road traffic noise on incident HF. Akaike information criterion was used to evaluate the model fits. 28 The interquartile range (IQR) of the exposure was calculated as the difference between the 25th and 75th percentiles of the exposure levels. An association between exposure to a pollutant and incident HF was expressed as a hazard ratio (HR) with 95% CIs per IQR increase in the pollutant. We visualized the shape of an association between long‐term exposure to PM2.5, NO2, and L den and incident HF by using spline function with 3 degrees of freedom and tested for a deviation from linearity using a likelihood ratio test.
We investigated the effect modification of coexposures on the association between PM2.5 and HF on a multiplicative scale. 29 We first categorized both NO2 and L den into 2 levels—low and high (≤75th and >75th percentiles of exposure range)—and then estimated the association between PM2.5 and HF in a single coexposure modifier (ie, NO2, low or high; L den, low or high) or 2 coexposure modifiers (ie, a combination of NO2 and L den, low‐low, low‐high, high‐low, or high‐high). Effect modification on the multiplicative scale was presented as HRs of HF per IQR increase in PM2.5. In addition to the formal framework to investigate effect modification, we explored the risk of HF for those exposed to high levels of the 3 pollutants compared with low levels by stratifying the data into the following 3 groups: low levels of all 3 pollutants (PM2.5, NO2, and L den), high levels of 1 or 2 pollutants, and high levels of all 3 pollutants. We then estimated HRs for each group compared with the reference group (low exposures to all 3 pollutants). In a sensitivity analysis, we estimated the risk of HF associated with the multiple exposures based on different cutoffs of the pollutants (eg, 25th and 50th percentiles).
Potential effect modifiers of the association between PM2.5 and incident HF were identified from the literature 8 , 9 , 11 , 14 , 16 and included age (<65 years or ≥65 years), obesity (BMI <30 kg/m2 or ≥30 kg/m2), smoking (never, former, or current), alcohol consumption (never, moderate, or heavy), physical activity (low, medium, or high), shift work (day, evening, night, or rotating), history of hypertension and diabetes (no or yes), and urbanization (urban, suburban, or rural). The effect modification was evaluated in model 2 by testing the analysis of variance for the 2 models with and without the product term of a pollutant and an effect modifier. All tests for effect modification for which P<0.05 were considered statistically significant.
We conducted several sensitivity analyses. First, we explored potential calendar effects caused by a linear association between the time‐varying primary exposure and calendar time 30 by comparing the associations with and without adjustment for calendar year using a spline function with 3 degrees of freedom. Second, as 23% of the original DNC participants were excluded because of missing information on covariates and exposure, we estimated inverse probability weight (IPW)–adjusted HRs to reduce selection bias as a result of missing data 31 (N=22 189 for included and N=6509 for excluded). We regressed a binary variable (1=included and 0=excluded participants) on the following covariates: age, baseline year, marital status, parity, and use of oral contraceptives (N=28 277). We selected the covariates when they were most available and significantly associated with the binary variable (inclusion versus exclusion). From the logistic regression, we estimated the probability of inclusion, took the inverse of probability (ie, IPW), and assigned the IPW to each participant. All analyses were conducted using R (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Of 28 727 participants, 29 participants with HF hospital contacts before baseline, 3422 with missing information on exposure during the follow‐up, and 3087 with missing information on covariates at baseline were excluded (Figure S1). The excluded participants were different at cohort baseline from those included in the analyses; they were older and less likely to be married; had normal weight; were never and former smokers, physically active, moderate and heavy drinkers, and actively working; were more likely to have ever used hormone therapy and never used oral contraceptives; were more likely to have hypertension and diabetes; and developed HF during the follow‐up (Table S1).
Of the remaining 22 189 participants, a total of 484 developed HF (mean age, 60.4 years) during 401 930 person‐years (mean, 18.1 person‐years) of follow‐up. Those who developed HF were more likely to be older and have a history of hypertension or diabetes and less likely to use oral contraceptives at baseline compared with those who never had HF during the follow‐up (Table 1).
Table 1.
Descriptive Statistics for Participants From the Danish Nurse Cohort at the Year of Cohort Entry in 1993 or 1999 by the Incident Heart Failure Status at the End of Follow‐Up
| Total, N=22 189 | No heart failure, N=21 705 | Heart failure, N=484 | |
|---|---|---|---|
| Age at baseline, y, mean±SD | 52.6±7.7 | 52.4±7.6 | 60.4±9.4 |
| Marital status, n (%) | |||
| Married | 15 656 (70.6) | 15 379 (70.9) | 277 (57.2) |
| Separated | 384 (1.7) | 378 (1.7) | 6 (1.2) |
| Divorced | 2593 (11.7) | 2530 (11.7) | 63 (13.0) |
| Single | 2224 (10.0) | 2155 (9.9) | 69 (14.3) |
| Widowed | 1332 (6.0) | 1263 (5.8) | 69 (14.3) |
| Body mass index, kg/m2, mean±SD | 23.7±3.5 | 23.7±3.5 | 24.6±4.2 |
| Body mass index, n (%) | |||
| Underweight, <18.5 kg/m2 | 542 (2.4) | 522 (2.4) | 20 (4.1) |
| Normal weight, 18.5–25 kg/m2 | 15 366 (69.3) | 15 094 (69.5) | 272 (56.2) |
| Overweight, 25–30 kg/m2 | 5032 (22.7) | 4891 (22.5) | 141 (29.1) |
| Obese, ≥30 kg/m2 | 1249 (5.6) | 1198 (5.5) | 51 (10.5) |
| Smoking status, n (%) | |||
| Never | 7724 (34.8) | 7583 (34.9) | 141 (29.1) |
| Former | 6735 (30.4) | 6600 (30.4) | 135 (27.9) |
| Current | 7730 (34.8) | 7522 (34.7) | 208 (43.0) |
| Alcohol consumption, n (%) | |||
| None, 0 drinks/wk | 3345 (15.1) | 3236 (14.9) | 109 (22.5) |
| Moderate, 1–14 drinks/wk | 13 726 (61.9) | 13 454 (62.0) | 272 (56.2) |
| Heavy, ≥15 drinks/wk | 5118 (23.1) | 5015 (23.1) | 103 (21.3) |
| Physical activity, n (%) | |||
| Low | 1445 (6.5) | 1392 (6.4) | 53 (11.0) |
| Medium | 14 802 (66.7) | 14 452 (66.6) | 350 (72.3) |
| High | 5942 (26.8) | 5861 (27.0) | 81 (16.7) |
| Diagnosis—hypertension, n (%) | |||
| No | 19 412 (87.5) | 19 073 (87.9) | 339 (70.0) |
| Yes | 2750 (12.4) | 2605 (12.0) | 145 (30.0) |
| Diagnosis—diabetes, n (%) | |||
| No | 21 767 (98.1) | 21 309 (98.2) | 458 (94.6) |
| Yes | 257 (1.2) | 233 (1.1) | 24 (5.0) |
| Hormone therapy use, n (%) | |||
| Never | 16 270 (73.3) | 15 960 (73.5) | 310 (64.0) |
| Past | 2136 (9.6) | 2048 (9.4) | 88 (18.2) |
| Current | 3783 (17.0) | 3697 (17.0) | 86 (17.8) |
| Oral contraceptive use, n (%) | |||
| Never | 8669 (39.1) | 8373 (38.6) | 296 (61.2) |
| Ever | 13 520 (60.9) | 13 332 (61.4) | 188 (38.8) |
| Parity | |||
| None | 3172 (14.3) | 3069 (14.1) | 103 (21.3) |
| ≥1 child | 19 017 (85.7) | 18 636 (85.9) | 381 (78.7) |
| Employment status, n (%) | |||
| Actively working | 17 901 (80.7) | 17 667 (81.4) | 234 (48.3) |
| Homemaker | 379 (1.7) | 367 (1.7) | 12 (2.5) |
| Retired | 3582 (16.1) | 3349 (15.4) | 233 (48.1) |
| Unemployed/rehabilitation | 142 (0.6) | 140 (0.6) | 2 (0.4) |
| Other | 185 (0.8) | 182 (0.8) | 3 (0.6) |
| Job strain, n (%) | |||
| Low | 17 147 (77.3) | 16 927 (78.0) | 220 (45.5) |
| High | 803 (3.6) | 788 (3.6) | 15 (3.1) |
| Not working | 4239 (19.1) | 3990 (18.4) | 249 (51.4) |
| Urbanization level, n (%) | |||
| Urban | 6827 (30.8) | 6649 (30.6) | 178 (36.8) |
| Suburban | 5094 (23.0) | 4994 (23.0) | 100 (20.7) |
| Rural | 10 268 (46.3) | 10 062 (46.4) | 206 (42.6) |
| Average municipality income, 1000 Danish Kroner, mean±SD | 158.8±22.0 | 158.8±22.0 | 160.6±24.0 |
The 3‐year running means of PM2.5, NO2, and L den at baseline were 21.0 µg/m3 (IQR, 5.1 µg/m3), 13.5 µg/m3 (IQR, 8.6 µg/m3), and 52.6 dB (IQR, 9.3 dB), respectively. The 3‐year running mean of NO2 was moderately correlated with PM2.5 (Spearman’s rank correlation coefficient [ρ]=0.58) and L den (ρ=0.62), whereas a correlation between PM2.5 and L den was low (ρ=0.38; Table 2). Similar patterns were observed with 1‐year and 23‐year mean exposures (Table S2). Those who developed HF lived in areas with higher levels of PM2.5, NO2, and L den at cohort baseline compared with those who remained free of HF at the end of follow‐up (Table S3).
Table 2.
Distribution of 3‐Year Running Means of Air Pollutants and Road Traffic Noise at the Cohort Baseline in 1993 or 1999 and Spearman’s Rank Correlation Coefficients Between the Exposure Levels
| Exposure | Mean±SD | Interquartile range | Percentile | Spearman’s rank correlation coefficient | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | PM2.5 | NO2 | |||
| PM2.5, µg/m3 | 21.0±3.5 | 5.1 | 15.3 | 18.5 | 20.8 | 23.6 | 26.4 | ||
| NO2, µg/m3 | 13.5±8.1 | 8.6 | 5.6 | 8.2 | 11.1 | 16.8 | 28.4 | 0.58 | |
| L den , dB | 52.6±8.0 | 9.3 | 37.3 | 48.6 | 53.0 | 57.8 | 64.7 | 0.38 | 0.62 |
L den indicates overall weighted 24‐hour noise level; and PM2.5, particulate matter with a diameter <2.5 μm.
We observed linear relationships between exposures to air pollution (PM2.5 and NO2) and L den and incident HF (Figure 1). The likelihood ratio tests suggested no deviations from linearity in the association between 3‐year running means of exposures and HF (P value ranged between 0.3 and 0.5).
Figure 1. Relationship between exposure to 3‐year means of (A) PM2.5, (B) NO2, and (C) road traffic noise and incident heart failure in the Danish nurse cohort (N=22 189).
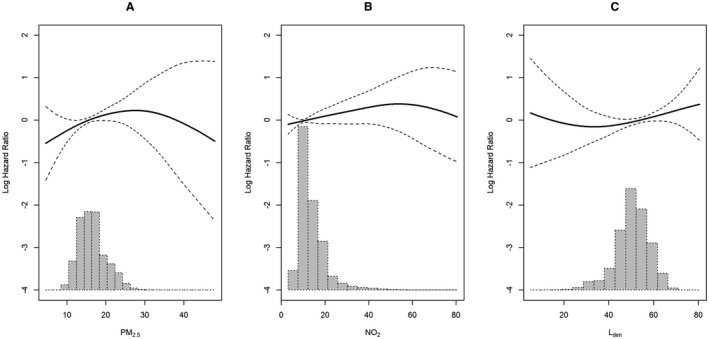
Associations between 3‐year exposures to (A) PM2.5, (B) NO2, and (C) road traffic noise and incident heart failure in the Danish nurse cohort were expressed in a solid spline line with 95% CIs (dashed spline lines). Histograms of distribution of 3‐year exposures are drawn in light gray. Models adjusting for age (underlying time), a strata term of year of cohort entry (1993/1999), and individual‐level and area‐level covariates. L den indicates overall weighted 24‐hour noise level; and PM2.5, particulate matter with a diameter <2.5 μm.
In the crude model, we observed a significant association between PM2.5 and incident HF with an HR of 1.36 (95% CI, 1.20–1.55) per 5.1 µg/m3 increase in PM2.5. With additional adjustment for the individual‐level and area‐level covariates in model 2, we observed an attenuated association (HR, 1.17 [95% CI, 1.01–1.36] per IQR increase in PM2.5; Table 3). Figure S2 shows a lower Akaike information criterion value in model 2 than in model 1 and models with mutual adjustment. Associations between PM2.5 and HF were suggestive in the 2‐pollutant and 3‐pollutant models, although statistical significance was attenuated (Table 3). Additional adjustment for the calendar year in the model increased the estimates of HRs (Table S4). IPW‐adjusted HRs were similar to the main results (Table S5).
Table 3.
Hazard Ratios (95% CI) of Incident Heart Failure Associated With an Interquartile Range Increase in 3‐Year Mean Exposures to PM2.5, NO2, and L den in the Danish Nurse Cohort (N=22 189)
| Exposure | Crude model | Fully adjusted model | Model of 2 pollutants | Model of 3 pollutants | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2+PM2.5 | Model 2+NO2 | Model 2+L den | Model 2+PM2.5, NO2, and L den | |
| PM2.5 | 1.36 (1.20–1.55)* | 1.17 (1.01–1.36)* | — | 1.13 (0.93–1.37) | 1.14 (0.97–1.33) | 1.14 (0.94–1.38) |
| NO2 | 1.18 (1.08–1.29)* | 1.10 (0.99–1.22) † | 1.04 (0.91–1.19) | — | 1.06 (0.93–1.20) | 1.00 (0.85–1.17) |
| L den | 1.18 (1.06–1.32)* | 1.12 (0.99–1.6) † | 1.09 (0.96–1.23) | 1.08 (0.93–1.24) | — | 1.09 (0.94–1.26) |
Model 1 adjusts for age (underlying time) and a strata term of year of cohort entry (1993/1999). Model 2 adjusts for individual‐level and area‐level covariates in addition to the covariates in model 1. HR indicates hazard ratio; L den, overall weighted 24‐hour noise level (interquartile range, 9.3 dB); NO2, nitrogen dioxide (interquartile range, 8.6 µg/m3); and PM2.5, particulate matter with a diameter <2.5 μm (interquartile range, 5.1 µg/m3).
P<0.05.
P<0.05.
Associations between NO2 and incident HF were significant in the crude model with an HR of 1.18 (95% CI, 1.08–1.29) per 8.6 µg/m3 increase in NO2, and attenuated after adjustment for the individual‐level and area‐level covariates to 1.10 (95% CI, 0.99–1.22). Additional adjustment for PM2.5 and L den resulted in a reduced HR for NO2 to unity (1.00; 95% CI, 0.85–1.17; Table 3).
Similar to air pollutants, associations between L den and incident HF were significant in the crude model with HRs of 1.18 (95% CI, 1.06–1.32) per 9.3 dB in L den, and attenuated after adjustment for the individual‐level and area‐level covariates to 1.12 (95% CI, 0.99–1.26). The 3‐year mean of L den showed a suggestive association with incident HF after mutual adjustment for coexposures (PM2.5 and NO2; HR, 1.09; 95% CI, 0.94–1.26; Table 3). Similar suggestive associations were also observed with 1‐year and 23‐year means of L den (Table S6).
We observed no significant effect modification of coexposures (NO2 and L den) on the association with PM2.5 (Table S7). However, the exploratory analysis showed a higher risk of HF for those exposed to high levels (≤75th percentile) of the 3 pollutants (PM2.5, NO2, and L den): HRs of 1.15 (95% CI, 0.94–1.41) for high exposures to 1 or 2 pollutants and 1.43 (95% CI, 1.02–1.99) for high exposures to all 3 pollutants compared with those with low exposures to all 3 pollutants. Among the 25%, 50%, and 75% cutoff levels, those exposed to multiple pollutants ≥75% were most at risk (Table S8).
We observed significant effect modification by smoking status and hypertension at baseline on the association between a 3‐year running mean of PM2.5 and incident HF (Figure 2 and Table S9), showing the strongest associations in former smokers with an HR of 1.72 (95% CI, 1.25–2.36) and those with hypertension with an HR of 1.41 (95% CI, 1.02–1.93). Those with obesity and diabetes also showed greater HRs, but the differences in HRs were not statistically significant.
Figure 2. Hazard ratios of incident heart failure associated with 3‐year exposure to PM2.5 by subgroups in the Danish Nurse Cohort study (N=22 189).
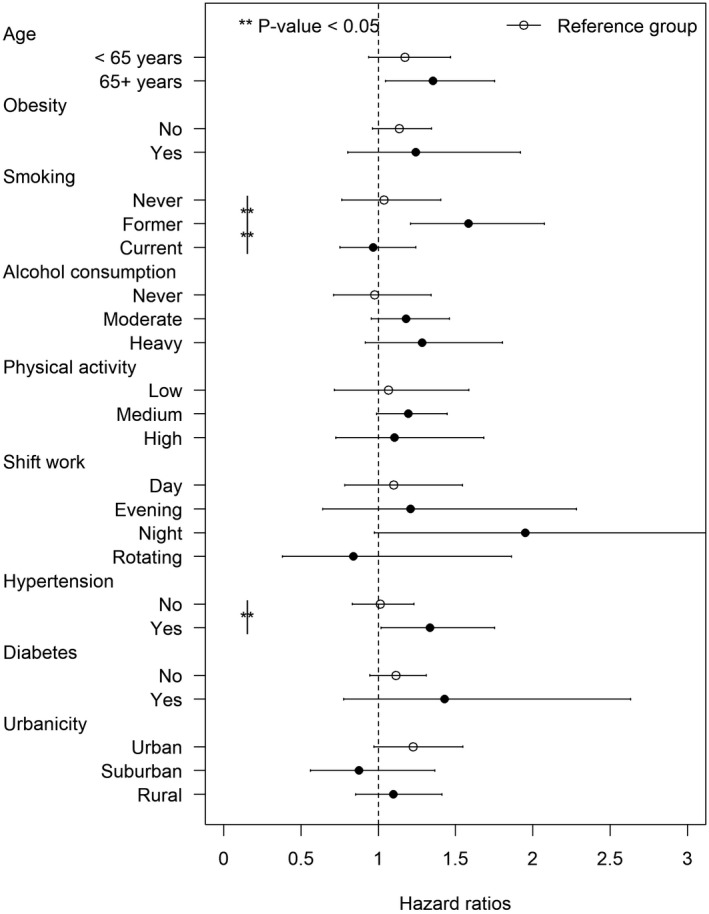
Solid circles indicate hazard ratios of incident heart failure per interquartile range (5.1 µg/m3) increase in PM2.5 among the subgroups compared with the reference groups (empty circles). Horizontal lines indicate 95% CIs. Models adjusting for age (underlying time), a strata term of year of cohort entry (1993/1999), and individual‐level and area‐level covariates. PM2.5 indicates particulate matter with a diameter <2.5 μm.
Discussion
In a nationwide cohort of Danish female nurses, we found that long‐term exposures to PM2.5 and road traffic noise were associated with HF incidence. The associations remained suggestive even after mutual adjustment for copollutants. We observed an enhanced risk of HF incidence for those exposed to high levels of 3 pollutants; however, the effect modification of coexposure was weak. Former smokers and nurses with hypertension seemed to be most susceptible to air pollution exposure with respect to the risk of HF.
As this is the first study to consider the associations between PM2.5, NO2, and road traffic noise simultaneously with incident HF, we cannot compare our results on effect modification of coexposure to these 3 pollutants directly to previous findings. However, the results from single pollutant models were generally in line with previous studies, suggesting an association between long‐term exposure to PM2.5 and incident HF. 8 , 9 , 11 , 16 , 17 Among the previous studies, Carey et al reported the greatest risk of incident HF associated with long‐term exposure to PM2.5 based in Greater London, United Kingdom, with 209 215 cohort participants with an HR of 2.04 (95% CI, 1.09–3.81) per 5.1 µg/m3 increase in PM2.5, 11 followed by the Netherlands Cohort Study on Diet and Cancer (N=120 852) with an HR of 1.66 (95% CI, 1.18–2.33), 18 and the Primary Prevention Study (N=5850) from Sweden with an HR of 1.50 (95% CI, 1.06–2.13). 15 North American studies also demonstrated positive associations between PM2.5 and HF, with HRs ranging between 1.07 and 1.30. 9 , 16 , 17 In contrast, a Dutch cohort study, the European Prospective Investigation Into Cancer and Nutrition (N=33 831), did not find an association between annual PM2.5 concentrations and incident HF, with an HR of 0.43 (95% CI, 0.16–1.20 12 ; Table S10).
We found that the association of NO2 with incident HF was sensitive to adjustment for PM2.5 or L den, after which it attenuated to null. Evidence to date on NO2 and HF suggested positive associations. However, few studies provided 2‐pollutant models for NO2. In contrast to our results, Bai et al reported that the association with NO2 remained robust even after adjustment for PM2.5. 10 On the other hand, 4 other studies detecting associations between NO2 and HF did not additionally adjust for PM2.5 or noise. 8 , 9 , 12 , 13 In another Danish cohort based on the 2 largest cities (Copenhagen and Aarhus), Sørensen et al reported a robust association of NO2 with incident HF even after adjustment for L den, 14 but lacked data on PM2.5. The discrepancy between Sørensen et al’s study and the present study could be explained by different follow‐up periods (mean follow‐up: 13.4 years in Sørensen et al study versus 18.1 years in the present study), age distribution at baseline (mean age at baseline 56.2 versus 52.6 years), sex (52.9% women versus 100% women), urbanicity (2 largest metropolitan areas versus all Denmark), NO2 levels at baseline (15.7 µg/m3 versus 13.5 µg/m3), and incidence rate of HF in 2 studies (5.0% versus 2.2%).
Of the 4 studies on road traffic noise and HF, 2 studies found no associations, 10 , 21 whereas 2 others detected associations, robust to adjustment for NO2. 14 , 20 These mixed results may be explained by different noise‐modeling approaches. In the city of Toronto, Canada (1996–2012), poor resolution of noise estimated at the postal code level may explain the null association with HF in the study, 10 whereas the finer resolution of road traffic noise exposure (10 m×10 m) in the same population resulted in a positive association with HF (HR, 1.07; 95% CI, 1.06–1.09). 20 A total of 2 European studies with road traffic noise at the address level also found significant positive associations between residential road traffic noise and HF. 14 , 21 We observed strong associations between residential road traffic noise and incident HF in the crude model, but the estimates attenuated after adjustment for NO2 or PM2.5. Although previous studies on road traffic noise and HF have considered a confounding effect of NO2, 10 , 14 , 20 this is the first study to examine whether PM2.5 confounded the association between road traffic noise and HF. However, as correlations among the 3 pollutants were high or moderate, caution is warranted when interpreting the results.
We explored calendar effects as Griffin et al reported that estimated effect size was deviated from the true estimation in the model with adjustment for calendar time that was correlated with time‐varying exposure. 30 In the present study, we observed that PM2.5 levels decreased over time; however, NO2 and L den levels were not linearly related to calendar time. Accordingly, we observed that the estimated HR associated with PM2.5 was sensitive (ie, increased) to adjustment for calendar year, but estimated HRs with NO2 and L den were robust to the adjustment. This is in line with the authors’ previous studies 32 , 33 showing that the estimated effect size was higher in the model with adjustment for calendar year compared with the model without adjustment for calendar year. Although this study ascertained that the higher estimated effect size of PM2.5 may come from the negative linear relationship between PM2.5 and calendar year, the impact of calendar year should be explored in other cohort studies.
We identified populations that are vulnerable to exposure to PM2.5. Former smokers and those with preexisting hypertension at baseline showed enhanced associations between long‐term exposure to PM2.5 and incident HF, as compared with never smokers and those without hypertension, respectively. However, previous studies have reported mixed results: a Canadian study found greater estimates among smokers compared with nonsmokers, 16 whereas other studies found either no significant differences of estimates by smoking status or stronger associations among never smokers. 8 , 11 , 18 Smoking is a well‐known risk factor for HF, with markedly increased risk among current smokers and more moderately increased risk among former smokers. 34 One potential explanation of our finding of the increased risk associated with PM2.5 exposure among former smokers might be that they are already at an increased risk as a result of earlier smoking priming for the effect of ambient air pollution. In contrast, the effect of current smoking is so strong that the effects of air pollution are difficult to detect. Another explanation is that those who stopped smoking might have done so, as they have already noticed some bad influence on their health, whereas those who continued were potentially a more resistant subpopulation.
We did not observe multiplicative effect modification of coexposure on the association between PM2.5 and HF. However, the exploratory analysis showed that those exposed to high levels of the 3 pollutants had a higher risk of HF compared with those exposed to low levels. The result suggested a susceptible population who live in high levels of air pollutants and road traffic noise, and they may be targeted for the greatest potential to prevent HF. However, given these findings, the effect modification of coexposure should be explored in other cohorts.
Our finding of greater associations between PM2.5 and HF in individuals with hypertension and diabetes is in line with Sørensen et al, who detected a stronger association between NO2 exposure incident HF among participants who were hypertensive and diabetic compared with participants who were neither hypertensive nor diabetic, respectively. 14 Shin et al suggested a strong link between road traffic noise and hypertension and diabetes. 35 However, another study observed no difference by comorbidities (eg, hypertension, diabetes) on the association between exposure to PM2.5 and HF incidence. 9 Nevertheless, it is still important to identify susceptible populations and treat them with effective preventive strategies because diabetes and hypertension may induce hemodynamic and myocardial changes and narrow and block blood vessels, which lead to cardiac dysfunction or an increased predisposition to other HF risk factors. 36 , 37
The major strength of this study is access to a large prospective cohort study with detailed information on historical exposures to several air pollutants (PM2.5 and NO2) and road traffic noise, for the first time, allowing for a detailed study of their independent effects on HF and effect modification of coexposures on the association. We also benefited from detailed information on potential confounders and HF risk factors that improved model fits as well as an objective and valid definition of HF incidence from the nationwide hospital register. The study covered all of Denmark, in contrast to the majority of previous studies based in urban areas, providing large contrasts in exposures to environmental factors. We benefited from a unique air pollution modeling system providing historical estimates of exposure to air pollution and road traffic noise with fine spatial resolution and taking residential mobility into account. Finally, we quantified the possible extent of selection bias and adjusted for it using IPW. The IPW generates weights that affect selection based on collected data of included and excluded participants. 31
The present study also has several limitations. We lacked information on individual‐level socioeconomic status, indoor air pollution sources at work and home, individuals' time activity patterns (eg, time spent outdoors), the residence’s window thickness or direction toward major roads or sound barriers, hearing impairment, annoyance by noise, and occupational noise exposure. Second, because of missing information on covariates and exposures, we excluded 23% of participants from the original DNC data and cannot rule out selection bias. Indeed, the excluded and included participants had different characteristics at baseline (Table S1). We estimated IPW from the logistic regression and estimated IPW‐adjusted HRs to reduce the selection bias. However, we found that the IPW‐adjusted and unweighted HRs showed similar results. Therefore, selection bias caused by missing information on covariates and exposures likely does not play a significant role in our analyses. It is noteworthy to address that the IPW cannot account for differences in selection caused by unmeasured factors, as the IPW generates weights based on collected data. Finally, when we compared the relative associations across pollutants, we did not account for relative measurement errors of exposures that could occur when we reflected participants’ true exposures. These errors may differ by pollutant, which might modify observed associations with health in either direction.
In conclusion, our study suggests that long‐term exposures to air pollution and road traffic noise were independently associated with HF incidence. Associations with HF were strongest and most robust with PM2.5, whereas associations with NO2 attenuated to unity after adjustment for copollutants. We found a positive association between road traffic noise and HF, which attenuated slightly after adjustment for air pollutants. Former smokers and patients who are hypertensive seemed most susceptible to the adverse effects of PM2.5 on HF.
Sources of Funding
This work was funded by a grant from the Danish Council for Independent Research (DFF‐4183‐00353), Region Zealand Fund, and the Novo Nordisk Foundation Challenge Programme (NNF17OC0027812).
Disclosures
None.
Supporting information
Table S1–S10
Figure S1–S2
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021436
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Savarese G, Lund LH. Global public health burden of heart failure. Cardiac Fail Rev. 2017;3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heart Failure Society of America . HFSA 2010 comprehensive heart failure practice guideline. J Cardiac Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 3. Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1 [DOI] [PubMed] [Google Scholar]
- 4. Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93. doi: 10.1093/eurheartj/ehu458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brook RD. Cardiovascular effects of air pollution. Clin Sci. 2008;115:175–187. doi: 10.1042/CS20070444 [DOI] [PubMed] [Google Scholar]
- 6. Miller MR, Shaw CA, Langrish JP. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012;8:577–602. doi: 10.2217/fca.12.43 [DOI] [PubMed] [Google Scholar]
- 7. Shah ASV, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, Newby DE, Mills NL. Global association of air pollution and heart failure: a systematic review and meta‐analysis. Lancet. 2013;382:1039–1048. doi: 10.1016/S0140-6736(13)60898-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. Long‐term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology. 2013;24:44–53. doi: 10.1097/EDE.0b013e318276ccb8 [DOI] [PubMed] [Google Scholar]
- 9. Bai LI, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Goldberg MS, Lavigne E, Copes R, Martin RV, et al. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: a population‐based study of 5.1 million Canadian adults living in Ontario. Environ Int. 2019;132:105004. doi: 10.1016/j.envint.2019.105004 [DOI] [PubMed] [Google Scholar]
- 10. Bai LI, Weichenthal S, Kwong JC, Burnett RT, Hatzopoulou M, Jerrett M, van Donkelaar A, Martin RV, Van Ryswyk K, Lu H, et al. Associations of long‐term exposure to ultrafine particles and nitrogen dioxide with increased incidence of congestive heart failure and acute myocardial infarction. Am J Epidemiol. 2018;188:151–159. doi: 10.1093/aje/kwy194 [DOI] [PubMed] [Google Scholar]
- 11. Carey I, Anderson H, Atkinson R, Beevers S, Cook D, Dajnak D, Gulliver J, Kelly F. Traffic pollution and the incidence of cardiorespiratory outcomes in an adult cohort in London. Occup Environ Med. 2016;73:849–856. doi: 10.1136/oemed-2015-103531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Downward GS, van Nunen EJHM, Kerckhoffs J, Vineis P, Brunekreef B, Boer JMA, Messier KP, Roy A, Verschuren WMM, van der Schouw YT, et al. Long‐term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a Dutch cohort. Environ Health Perspect. 2018;126:127007. doi: 10.1289/EHP3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim H, Heo J, Yi SM, Kim K, Youn TJ. Cardiovascular effects of long‐term exposure to air pollution: a population‐based study with 900 845 person‐years of follow‐up. J Am Heart Assoc. 2017;6:e007170. doi: 10.1161/JAHA.117.007170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sørensen M, Wendelboe Nielsen O, Sajadieh A, Ketzel M, Tjønneland A, Overvad K, Raaschou‐Nielsen O. Long‐term exposure to road traffic noise and nitrogen dioxide and risk of heart failure: a cohort study. Environ Health Perspect. 2017;125:097021. doi: 10.1289/EHP1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stockfelt L, Andersson EM, Molnár P, Gidhagen L, Segersson D, Rosengren A, Barregard L, Sallsten G. Long‐term effects of total and source‐specific particulate air pollution on incident cardiovascular disease in Gothenburg. Sweden. Environmental Research. 2017;158:61–71. doi: 10.1016/j.envres.2017.05.036 [DOI] [PubMed] [Google Scholar]
- 16. To T, Zhu J, Villeneuve PJ, Simatovic J, Feldman L, Gao C, Williams D, Chen H, Weichenthal S, Wall C, et al. Chronic disease prevalence in women and air pollution—a 30‐year longitudinal cohort study. Environ Int. 2015;80:26–32. doi: 10.1016/j.envint.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 17. Yazdi MD, Wang Y, Di Q, Zanobetti A, Schwartz J. Long‐term exposure to PM2. 5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int. 2019;130:104879. doi: 10.1016/j.envint.2019.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beelen R, Hoek G, Houthuijs D, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, Armstrong B, Brunekreef B. The joint association of air pollution and noise from road traffic with cardiovascular mortality in a cohort study. Occup Environ Med. 2009;66:243–250. doi: 10.1136/oem.2008.042358 [DOI] [PubMed] [Google Scholar]
- 19. Vienneau D, Schindler C, Perez L, Probst‐Hensch N, Röösli M. The relationship between transportation noise exposure and ischemic heart disease: a meta‐analysis. Environ Res. 2015;138:372–380. doi: 10.1016/j.envres.2015.02.023 [DOI] [PubMed] [Google Scholar]
- 20. Bai L, Shin S, Oiamo TH, Burnett RT, Weichenthal S, Jerrett M, Kwong JC, Copes R, Kopp A, Chen H. Exposure to road traffic noise and incidence of acute myocardial infarction and congestive heart failure: a population‐based cohort study in Toronto, Canada. Environ Health Perspect. 2020;128:087001. doi: 10.1289/EHP5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seidler A, Wagner M, Schubert M, Dröge P, Römer K, Pons‐Kühnemann J, Swart E, Zeeb H, Hegewald J. Aircraft, road and railway traffic noise as risk factors for heart failure and hypertensive heart disease—a case‐control study based on secondary data. Int J Hyg Environ Health. 2016;219:749–758. doi: 10.1016/j.ijheh.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 22. Hundrup YA, Simonsen MK, Jorgensen T, Obel EB. Cohort profile: the Danish nurse cohort. Int J Epidemiol. 2012;41:1241–1247. doi: 10.1093/ije/dyr042 [DOI] [PubMed] [Google Scholar]
- 23. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mard S, Nielsen FE. Positive predictive value and impact of misdiagnosis of a heart failure diagnosis in administrative registers among patients admitted to a University Hospital cardiac care unit. Clin Epidemiol. 2010;2:235–239. doi: 10.2147/CLEP.S12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jensen SS, Ketzel M, Becker T, Christensen J, Brandt J, Plejdrup M, Winther M, Nielsen O‐K, Hertel O, Ellermann T. High resolution multi‐scale air quality modelling for all streets in Denmark. Transp Res Part D: Trans Environ. 2017;52:322–339. doi: 10.1016/j.trd.2017.02.019 [DOI] [Google Scholar]
- 27. DELTA . Nordic Environmental Noise Prediction Methods, Nord2000: Summary Report. Force Technology. Published December 31, 2021. Accessed January 28, 2020. https://forcetechnology.com/‐/media/force‐technology‐media/pdf‐files/projects/nord2000/nordic‐environmental‐noise‐prediction‐methods‐nord2000‐summary‐report‐‐‐prediction‐methods.pdf
- 28. Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 29. Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–520. doi: 10.1093/ije/dyr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffin BA, Anderson GL, Shih RA, Whitsel EA. Use of alternative time scales in Cox proportional hazard models: implications for time‐varying environmental exposures. Stat Med. 2012;31:3320–3327. doi: 10.1002/sim.5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hernán MA, Hernández‐Díaz S, Robins JMJE. A structural approach to selection bias. Epidemiology. 2004;615–625. doi: 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- 32. Cramer J, Jørgensen JT, Hoffmann B, Loft S, Bräuner EV, Prescott E, Ketzel M, Hertel O, Brandt J, Jensen SS, et al. Long‐term exposure to air pollution and incidence of myocardial infarction: a Danish Nurse Cohort Study. Environ Health Perspect. 2020;128:057003. doi: 10.1289/EHP5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. So R, Jørgensen JT, Lim Y‐H, Mehta AJ, Amini H, Mortensen LH, Westendorp R, Ketzel M, Hertel O, Brandt J, et al. Long‐term exposure to low levels of air pollution and mortality adjusting for road traffic noise: a Danish Nurse Cohort study. Environ Int. 2020;143:105983. doi: 10.1016/j.envint.2020.105983 [DOI] [PubMed] [Google Scholar]
- 34. Lee H, Son Y‐J. Influence of smoking status on risk of incident heart failure: a systematic review and meta‐analysis of prospective cohort studies. Int J Environ Res Public Health. 2019;16:2697. doi: 10.3390/ijerph16152697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shin S, Bai LI, Oiamo TH, Burnett RT, Weichenthal S, Jerrett M, Kwong JC, Goldberg MS, Copes R, Kopp A, et al. Association between road traffic noise and incidence of diabetes mellitus and hypertension in Toronto, Canada: a population‐based cohort study. J Am Heart Assoc. 2020;9:e013021. doi: 10.1161/JAHA.119.013021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moser M, Hebert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214–1218. doi: 10.1016/0735-1097(95)00606-0 [DOI] [PubMed] [Google Scholar]
- 37. Packer M. Pathophysiology of chronic heart failure. Lancet. 1992;340:88–92. doi: 10.1016/0140-6736(92)90405-R [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S10
Figure S1–S2


