Abstract
Background
The data on the differential impact of sex on the utilization and outcomes of valve replacement surgery for infective endocarditis are limited to single‐center and small sample size patient population.
Methods and Results
We utilized the National Inpatient Sample database to identify patients with a discharge diagnosis of infective endocarditis from 2004 to 2015 to assess differences in the characteristics and clinical outcomes of patients hospitalized with infective endocarditis stratified by sex. We also evaluated trends in utilization of cardiac valve replacement and individual valve replacement surgeries in women versus men over a 12‐year period, and compared in‐hospital mortality after surgical treatment in women versus men. A total of 81 942 patients were hospitalized with a primary diagnosis of infective endocarditis from January 2004 to September 2015, of whom 44.31% were women. Women were less likely to undergo overall cardiac valve replacement (6.92% versus 12.12%), aortic valve replacement (3.32% versus 8.46%), mitral valve replacement (4.60% versus 5.57%), and combined aortic and mitral valve replacement (0.85% versus 1.81%) but had similar in‐hospital mortality rates. From 2004 to 2015, the overall rates of cardiac valve replacement increased from 11.76% to 13.96% in men and 6.34% to 9.26% in women and in‐hospital mortality declined in both men and women. Among the patients undergoing valve replacement surgery, in‐hospital mortality was higher in women (9.94% versus 6.99%, P<0.001).
Conclusions
Despite increased utilization of valve surgery for infective endocarditis in both men and women and improving trends in mortality, we showed that there exists a treatment bias with underutilization of valve surgeries for infective endocarditis in women and demonstrated that in‐hospital mortality was higher in women undergoing valve surgery in comparison to men.
Keywords: infective endocarditis, sex differences, underutilization, valve replacement
Subject Categories: Quality and Outcomes, Health Services, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- AVR
aortic valve replacement
- AVR+MVR
combined aortic and mitral valve replacement
- IE
infective endocarditis
- MVR
mitral valve replacement
- NIS
National Inpatient Sample
Clinical Perspective
What Is New?
Female sex was independently associated with decreased likelihood of valve replacement for infective endocarditis.
Among the patients undergoing valve replacement surgery, female sex was associated with significantly increased mortality.
What Are the Clinical Implications?
Treatment bias may account for underutilization of valve surgery in women, which in turn has an adverse impact on the overall outcome.
More women hospitalized with infective endocarditis should be offered early surgical intervention.
Infective endocarditis (IE) is a lethal and potentially devastating complication of heart valve disease and its incidence has increased from 9.3 per 100 000 population in 1998 to 15 per 100 000 in 2011. 1 Early valve replacement surgery can potentially be lifesaving in a selected group of patients with IE. 2 , 3 Surgical intervention within 5 to 7 days of clinical presentation should be considered in patients with new‐onset heart failure, prosthetic valve endocarditis, tissue invasion and destruction, persistent bacteremia, presence of large mobile vegetations, and for the prevention of recurrent embolization. 2
Sex‐related differences in the incidence, clinical presentation, treatment, and outcomes for various cardiovascular pathologies have been studied extensively. These differences may be attributed to a variety of factors including variable risk factors/comorbidities, treatment biases, or inherent physiologic differences. Women are less likely to receive surgical intervention including coronary artery bypass graft, 4 aortic or mitral valve replacement (MVR) than men, 5 , 6 , 7 and when they do, they have worse postoperative outcomes. IE has been shown to be more frequent in men than women, and the presence of estrogen has been proposed to be a protective factor against endothelial damage. 8 , 9 The data on the differential impact of sex on the utilization and outcomes of valve replacement surgery for IE is, however, limited to a single‐center and small sample size patient population. 10 , 11 , 12
To assess this gap, we utilized a large national database to (1) evaluate differences in the characteristics and clinical outcomes of patients hospitalized with IE stratified by sex from 2004 to 2015, (2) assess trends in utilization of cardiac valve replacement and individual valve replacement surgeries (aortic valve replacement [AVR], mitral valve replacement [MVR], combined aortic and mitral valve replacement [AVR+MVR]) in women versus men over a 12‐year period, and (3) analyze in‐hospital mortality after cardiac valve replacement in women versus men in the setting of IE.
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Study Data
We used the Agency for Healthcare Research and Quality's National Inpatient Sample (NIS) database of hospitalized patients in the United States to derive patient‐relevant information between January 2004 and September 2015. The NIS is the largest publicly available all‐payer administrative claims–based database and contains information about patient discharges from ≈1000 nonfederal hospitals in 45 states. Briefly, the NIS is a random 20% sample of all inpatient hospitalizations in the United States each year. Unweighted, it contains data from more than 7 million hospital stays each year; and weighted, it estimates more than 35 million hospitalizations nationally. Discharges are weighted based on the sampling scheme to permit inferences for a nationally representative population. The Institutional Review Board at Cleveland Clinic exempted the study from board approval and waived the requirement for informed consent because the NIS is a publicly available deidentified database.
Study Population
We used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes 4210, 4211, 4219, 11281, 3642, 9884, 11504, 11514, 11594, 42490, 42491, and 42499 to identify all individuals with the principal diagnosis of IE. We then excluded discharges with missing data on age, sex, and in‐hospital death.
Study End Points
The primary end point of the study was cardiac valve replacement in patients hospitalized with IE. The secondary end points were individual valve surgeries (AVR, MVR, AVR+MVR, mitral valve repair, tricuspid valve replacement, and tricuspid valve repair), in‐hospital mortality, acute stroke, length of stay, and hospitalization charges.
Statistical Analysis
Outcome analyses were performed using the actual unweighted sample available in the NIS, whereas trend analysis was performed utilizing the national estimate. 13 Continuous variables are described as medians and interquartile ranges. Categorical variables are described as percentages. To compare the baseline characteristics, Mann–Whitney test/Wilcoxon rank sum test were used for continuous variables, and Pearson χ2 tests were used for categorical variables. Temporal trends in overall cardiac valve replacement, AVR, MVR, AVR+MVR, in‐hospital mortality, and length of stay were examined and were further stratified by sex. Temporal trends over time for cardiac valve replacement, AVR, MVR, AVR+MVR, and in‐hospital mortality were assessed using Cochrane Armitage trend test, whereas length of stay trend was assessed by multivariable linear regression using log‐transformed length of stay as the dependent variable and year as a continuous variable.
The outcomes including the utilization of cardiac valve replacement, in‐hospital mortality, and stroke were evaluated using the multivariable logistic regression, whereas the length of hospital stay was analyzed using multivariable negative binomial regression. Multivariable regression analysis was used to adjust for baseline differences between men and women by adjusting for univariate predictors of examined outcomes (P<0.05). The following variables were included in the multivariable regression adjustment model: age, elective admission, organisms (Staphylococcus aureus endocarditis, Streptococcus endocarditis, gram‐negative endocarditis, Enterococcus endocarditis, fungal endocarditis, and unknown organism), comorbidities (drug abuse, congenital heart disease, hepatitis C, infection of cardiac device/implant, prior valve replacement, prior percutaneous coronary intervention, prior coronary artery bypass graft, congestive heart failure, cardiac arrhythmias, atrial fibrillation, liver cirrhosis, coagulopathy, diabetes, hypertension, peripheral vascular disease, smoking, solid tumor without metastasis, cardiogenic shock, myocardial infarction, acute renal failure, and mechanical ventilation), race/ethnicity and hospital characteristics (hospital bed size, and region). Odds ratio and 95% CI were used to report logistic regression, whereas incidence rate ratios and 95% CI were used for negative binomial regression. Further adjustments were made using the Bonferroni correction method to account for multiple comparisons. All statistical tests were 2‐sided, and a P value of <0.05 was considered to be statistically significant. Statistical analyses were performed with SPSS software (version 25.0; IBM Corp, Armonk, NY) and R version 4.0.3.
Results
A total of 81 942 (weighted national estimates: 405 386) hospitalizations were identified with a primary diagnosis of IE from 2004 to 2015, of which 36 302 (44.31%) were female.
Baseline Characteristics
Baseline characteristics for hospitalizations with IE stratified by sex are shown in Table 1. Men were younger (59.85 [17.85] versus 62.58 [19.62] years) and had increased prevalence of risk factors and comorbidities as compared with women. Men had higher rates of drug abuse, congenital heart disease, hepatitis C, history of prior procedures/interventions including valve replacement, percutaneous coronary intervention, coronary artery bypass graft, congestive heart failure, cardiac arrhythmias, coronary artery disease, liver cirrhosis, coagulopathy, peripheral vascular disease, smoking, uncontrolled hypertension, and solid tumors. Also, there was a higher incidence of infected cardiac device or implant (prosthetic valve endocarditis) (9.02% versus 5.01%, P<0.001), myocardial infarction (3.95% versus 3.50%, P=0.005), cardiogenic shock (1.72% versus 1.08%, P<0.001), and need for mechanical ventilation (3.64% versus 3.36%, P=0.035) among men.
Table 1.
Baseline Patient Characteristics Stratified by Sex for Infective Endocarditis Hospitalizations From 2004 to 2015
| Characteristics | Men (N=45 640) | Women (N=36 302) | P value |
|---|---|---|---|
| Age, mean (SD), y | 59.85 (17.85) | 62.58 (19.62) | <0.001 |
| Elective admission | 15.17% | 15.93% | 0.003 |
| Organisms/microbiology | |||
| Staphylococcus aureus endocarditis | 27.92% | 26.04% | <0.001 |
| Streptococcus endocarditis | 27.66% | 17.99% | <0.001 |
| Gram‐negative endocarditis | 7.69% | 4.84% | <0.001 |
| Enterococcus endocarditis | 4.03% | 3.18% | <0.001 |
| Fungus endocarditis | 0.82% | 0.67% | 0.02 |
| Unknown organism | 19.6% | 28.2% | <0.001 |
| Risk factors and comorbidities | |||
| Drug abuse | 19.73% | 15.54% | <0.001 |
| Congenital heart disease | 5.22% | 3.16% | <0.001 |
| Hepatitis C | 12.69% | 11.39% | <0.001 |
| Chronic rheumatic heart disease | 8.83% | 9.16% | 0.099 |
| Infection of cardiac device/implant (prosthetic valve endocarditis) | 9.02% | 5.01% | <0.001 |
| Prior valve replacement | 7.00% | 5.32% | <0.001 |
| Prior PCI | 2.58% | 1.94% | <0.001 |
| Prior CABG | 6.73% | 3.68% | <0.001 |
| Congestive heart failure | 32.75% | 32.09% | 0.046 |
| Cardiac arrhythmias | 34.26% | 31.84% | <0.001 |
| Atrial fibrillation | 21.13% | 20.40% | 0.009 |
| Coronary artery disease | 23.05% | 18.61% | <0.001 |
| Liver cirrhosis | 3.75% | 2.34% | <0.001 |
| Coagulopathy | 11.85% | 9.55% | <0.001 |
| Diabetes controlled | 16.48% | 18.12% | <0.001 |
| Diabetes uncontrolled | 5.58% | 5.62% | 0.91 |
| Hypertension controlled | 29.27% | 33.51% | <0.001 |
| Hypertension uncontrolled | 18.55% | 17.90% | 0.018 |
| Peripheral vascular disease | 6.46% | 5.64% | <0.001 |
| Smoking | 6.16% | 3.97% | <0.001 |
| Solid tumor without metastasis | 2.91% | 2.41% | <0.001 |
| Metastatic cancer | 1.29% | 1.28% | 0.98 |
| Malnutrition disorder | 7.47% | 7.47% | 0.99 |
| Cardiogenic shock | 1.72% | 1.08% | <0.001 |
| Myocardial infarction | 3.95% | 3.50% | 0.005 |
| Mechanical ventilation | 3.64% | 3.36% | 0.035 |
| Blood transfusion | 17.67% | 17.62% | 0.86 |
| Demographics | |||
| Race/ethnicity | |||
| White | 74.05% | 73.50% | <0.001* |
| Black | 12.72% | 14.60% | |
| Hispanic | 8.09% | 6.81% | |
| Hospital bed size | |||
| Small | 13.98% | 15.39% | <0.001 |
| Medium | 23.21% | 24.56% | |
| Large | 62.80% | 60.05% | |
| Hospital region | |||
| Northeast | 22.90% | 21.68% | <0.001 |
| Midwest | 20.21% | 21.23% | |
| South | 38.76% | 41.30% | |
| West | 18.12% | 15.76% | |
CABG indicates coronary artery bypass graft; and PCI, percutaneous coronary intervention.
P value applies to all three races.
Study Outcomes
Women were less likely to undergo overall cardiac valve replacement (6.92% versus 12.12%, P<0.001), AVR, MVR, and AVR+MVR. There were no significant difference in the rates of mitral valve repair, tricuspid valve surgery, in‐hospital stroke, and mortality rates. In addition, the mean length of stay (11.13 [12.12] versus 10.16 [10.78] days, P<0.001) and hospitalization charges (88 409 [121 179.3] $ versus 71 196 [118 723.2] $, P<0.001) were higher in men (Table 2).
Table 2.
In‐Hospital Outcomes Stratified by Sex for Infective Endocarditis Hospitalizations From 2004 to 2015
| In‐hospital outcomes | Men (N=45 640) | Women (N=36 302) | P value |
|---|---|---|---|
| Cardiac valve replacement | 12.12% | 6.92% | <0.001 |
| Aortic valve replacement | 8.46% | 3.32% | <0.001 |
| Mitral valve replacement | 5.57% | 4.60% | <0.001 |
| AVR+MVR | 1.81% | 0.85% | <0.001 |
| Mitral valve repair | 0.25% | 0.31% | 0.772 |
| Tricuspid valve replacement | 0.32% | 0.36% | 0.990 |
| Tricuspid valve repair | 0.63% | 0.65% | 0.990 |
| In‐hospital mortality | 6.36% | 6.09% | 0.990 |
| Acute stroke | 7.47% | 7.06% | 0.314 |
| Length of stay, mean (SD), d | 11.13 (12.12) | 10.16 (10.78) | <0.001 |
| Total charges, mean (SD), $ | 88 409 (121 179.3) | 71 196 (118 723.2) | <0.001 |
AVR+MVR indicates combined aortic and mitral valve replacement.
Utilization of Valve Replacement Surgery Stratified by Sex
Figure 1 shows the utilization of individual valve surgeries by sex (AVR: 8.46% versus 3.32%, P<0.001, MVR: 5.57% versus 4.60%, P<0.001, AVR+MVR: 1.81% versus 0.85%, P<0.001, mitral valve repair: 0.25% versus 0.31%, P=0.07, tricuspid valve replacement: 0.32% versus 0.36%, P=0.23, tricuspid valve repair: 0.63% versus 0.65%, P=0.59).
Figure 1. Surgical intervention for patients hospitalized with infective endocarditis stratified by sex.
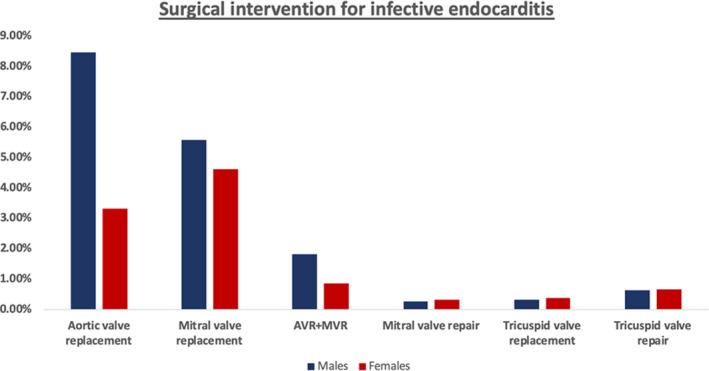
AVR+MVR indicates combined aortic and mitral valve replacement.
Table 3 shows the relationship between sex and likelihood of undergoing valve replacement surgery, in‐hospital mortality, and stroke. After adjustment with multivariable logistic regression analysis, female sex remained associated with less likelihood of overall cardiac valve replacement (0.614 [95% CI, 0.578–0.652], P<0.001), AVR (0.422 [95% CI, 0.390–0.456], P<0.001), and AVR+MVR (0.559 [95% CI, 0.481–0.648], P<0.001); however, there was no difference in the mitral valve replacement rates (0.963 [95% CI, 0.894–1.036], P=0.314).
Table 3.
Unadjusted and Adjusted Association Between Sex and Likelihood of Undergoing Valve Replacement, In‐Hospital Mortality, and Stroke
| Variables | Unadjusted association | P value | Adjusted association | P value |
|---|---|---|---|---|
| Cardiac valve replacement | 0.540 (0.514–0.568) | <0.001 | 0.614 (0.578–0.652) | <0.001 |
| Aortic valve replacement | 0.371 (0.347–0.397) | <0.001 | 0.422 (0.390–0.456) | <0.001 |
| Mitral valve replacement | 0.818 (0.768–0.871) | <0.001 | 0.963 (0.894–1.036) | 0.314 |
| AVR+MVR | 0.473 (0.415–0.539) | <0.001 | 0.559 (0.481–0.648) | <0.001 |
| Mitral valve repair | 1.280 (0.987–1.660) | 0.062 | ||
| Tricuspid valve replacement | 1.163 (0.919–1.470) | 0.208 | ||
| Tricuspid valve repair | 1.052 (0.885–1.248) | 0.563 | ||
| In‐hospital mortality | 0.958 (0.905–1.015) | 0.143 | ||
| Acute stroke | 0.942 (0.893–0.993) | 0.027 | 0.909 (0.856–0.966) | 0.002 |
Adjusted for the following variables: age, elective admission, Staphylococcus aureus endocarditis, Streptococcus endocarditis, Gram‐negative endocarditis, Enterococcus endocarditis, fungal endocarditis, unknown organism, drug abuse, congenital heart disease, hepatitis C, infection of cardiac device/implant (prosthetic valve endocarditis), prior valve replacement, prior PCI, prior CABG, congestive heart failure, cardiac arrhythmias, atrial fibrillation, liver cirrhosis, coagulopathy, diabetes controlled, hypertension controlled, hypertension uncontrolled, peripheral vascular disease, smoking, solid tumor without metastasis, cardiogenic shock, myocardial infarction, acute renal failure, mechanical ventilation, race, hospital bed size, and region. AVR+MVR indicates combined aortic and mitral valve replacement; CABG, coronary artery bypass graft; and PCI, percutaneous coronary intervention.
In addition, there were no significant differences in mortality; however, female sex was associated with decreased stroke rates. Furthermore, after multivariable negative binomial regression adjustment, female sex was not associated with the length of stay (incidence rate ratio, 1.001 [95% CI, 0.986–1.015], P=0.766) in patients hospitalized with IE.
Characteristics and Outcomes of Hospitalizations Undergoing Valve Replacement Surgery
Table 4 shows the baseline characteristics and in‐hospital outcomes of IE admissions who underwent cardiac valve replacement surgery stratified by sex. Among hospitalized patients who underwent valve replacement surgery, women were more likely to have Staphylococcal bacteremia, chronic rheumatic heart disease, coagulopathy, uncontrolled hypertension, malnutrition disorder, and require mechanical ventilation during the hospitalization. Of the admissions undergoing cardiac valve replacement, 11.67% (12.16% in men and 10.57% in women, P=0.040) had an infection of cardiac device/implant (prosthetic valve endocarditis). The overall in‐hospital mortality rates were significantly higher in women who underwent valve replacement surgery in comparison to men (9.94% versus 6.99%, P<0.001).
Table 4.
Baseline Patient Characteristics and In‐Hospital Outcomes Stratified by Sex for Infective Endocarditis Hospitalizations Undergoing Cardiac Valve Replacement Surgery From 2004 to 2015
| Men (N=5529) | Women (N=2518) | P value | |
|---|---|---|---|
| Characteristics (%) | |||
| Mean age, y | 52.83 | 52.97 | 0.38 |
| Elective admission | 19.15% | 19.91% | 0.44 |
| Organisms | |||
| Staphylococcus endocarditis | 22.96% | 27.14% | <0.001 |
| Streptococcus endocarditis | 34.90% | 29.65% | <0.001 |
| Gram‐negative endocarditis | 8.86% | 7.19% | 0.013 |
| Enterococcus endocarditis | 3.94% | 2.65% | 0.005 |
| Fungus endocarditis | 1.20% | 1.18% | >0.99 |
| Risk factors and comorbidities | |||
| Drug abuse | 20.62% | 18.75% | 0.056 |
| Congenital heart disease | 13.07% | 10.57% | <0.001 |
| Hepatitis C | 10.82% | 13.04% | 0.005 |
| Chronic rheumatic heart disease | 16.30% | 19.91% | <0.001 |
| Infection of cardiac device/implant (prosthetic valve endocarditis) | 12.16% | 10.57% | 0.040 |
| Prior valve replacement | 1.86% | 1.47% | 0.246 |
| Prior PCI | 1.13% | 0.96% | 0.526 |
| Prior CABG | 1.94% | 1.02% | 0.004 |
| Congestive heart failure | 45.93% | 46.85% | 0.455 |
| Cardiac arrhythmias | 43.12% | 39.60% | 0.003 |
| Atrial fibrillation | 22.73% | 18.76% | <0.001 |
| Coronary artery disease | 18.16% | 14.12% | <0.001 |
| Liver cirrhosis | 2.46% | 1.28% | 0.001 |
| Coagulopathy | 18.63% | 22.11% | 0.003 |
| Diabetes controlled | 10.93% | 11.28% | 0.665 |
| Diabetes uncontrolled | 3.23% | 3.89% | 0.153 |
| Hypertension controlled | 21.77% | 19.84% | 0.054 |
| Hypertension uncontrolled | 15.58% | 18.06% | 0.006 |
| Peripheral vascular disease | 5.83% | 4.60% | 0.027 |
| Smoking | 5.52% | 3.39% | <0.001 |
| Solid tumor without metastasis | 1.12% | 0.86% | 0.370 |
| Metastatic cancer | 0.42% | 0.44% | >0.99 |
| Malnutrition disorder | 11.54% | 14.70% | <0.001 |
| Cardiogenic shock | 8.78% | 8.37% | 0.590 |
| Myocardial infarction | 6.39% | 5.53% | 0.147 |
| Mechanical ventilation | 10.20% | 14.41% | <0.001 |
| Blood transfusion | 38.99% | 41.13% | 0.071 |
| Demographics | |||
| Race/ethnicity | |||
| White | 72.25% | 70.73% | 0.002* |
| Black | 13.13% | 16.45% | |
| Hispanic | 8.99% | 6.98% | |
| Hospital bed size | |||
| Small | 5.56% | 5.38% | 0.576 |
| Medium | 17.64% | 18.56% | |
| Large | 76.60% | 76.02% | |
| Hospital region | |||
| Northeast | 22.82% | 21.97% | 0.005 |
| Midwest | 21.34% | 18.57% | |
| South | 36.46% | 39.60% | |
| West | 19.36% | 16.57% | |
| In‐hospital outcomes | |||
| Mortality | 6.99% | 9.94% | <0.001 |
| Stroke | 13.34% | 14.22% | 0.307 |
| Length of stay, mean (SD), d | 20.87 (15.84) | 23.21 (17.49) | <0.001 |
CABG indicates coronary artery bypass graft; and PCI, percutaneous coronary intervention.
P value applies to all three races.
Table 5 shows the significant predictors of in‐hospital mortality in those undergoing valve replacement surgery. We note that after adjustment with multivariable logistic regression analysis, female sex remained independently associated with increased in‐hospital mortality (1.312 [95% CI, 1.092–1.575], P=0.003). Other factors associated with increased mortality in patients undergoing surgery included fungal endocarditis, infected cardiac device/implant, presence of coagulopathy, uncontrolled hypertension, liver cirrhosis, myocardial infarction, need for mechanical ventilation, and combined aortic and mitral valve replacement.
Table 5.
Predictors of In‐Hospital Mortality in Patients Undergoing Cardiac Valve Replacement Surgery
| Characteristics (%) | Unadjusted | P value | Adjusted | P value |
|---|---|---|---|---|
| Mean age, y | 1.027 (1.021–1.032) | <0.001 | 1.027 (1.020–1.034) | <0.001 |
| Female sex | 1.470 (1.243–1.735) | <0.001 | 1.312 (1.092–1.575) | 0.003 |
| Elective admission | 0.537 (0.416–0.683) | <0.001 | 0.649 (0.497–0.836) | <0.001 |
| Organisms/microbiology | ||||
| Staphylococcus endocarditis | 1.399 (1.170–1.667) | <0.001 | 1.102 (0.901–1.345) | 0.341 |
| Streptococcus endocarditis | 0.491 (0.401–0.597) | <0.001 | 0.685 (0.538–0.865) | 0.001 |
| Gram‐negative endocarditis | 0.411 (0.264–0.608) | <0.001 | 0.635 (0.389–0.996) | 0.057 |
| Enterococcus endocarditis | 1.122 (0.721–1.671) | 0.589 | ||
| Fungus endocarditis | 2.358 (1.323–3.947) | <0.001 | 2.352 (1.265–4.140) | 0.004 |
| Risk factors and comorbidities | ||||
| Drug abuse | 0.328 (0.242–0.434) | <0.001 | 0.499 (0.361–0.676) | <0.001 |
| Congenital heart disease | 0.513 (0.368–0.696) | <0.001 | 0.753 (0.530–1.044) | 0.100 |
| Hepatitis C | 1.000 (0.770–1.281) | 0.998 | ||
| Chronic rheumatic heart disease | 0.809 (0.641–1.010) | 0.066 | ||
| Infection of cardiac device/implant (prosthetic valve endocarditis) | 1.987 (1.607–2.441) | <0.001 | 1.840 (1.457–2.309) | <0.001 |
| Prior valve replacement | 0.991 (0.502–1.759) | 0.976 | ||
| Prior PCI | 0.412 (0.101–1.104) | 0.132 | ||
| Prior CABG | 1.048 (0.531–1.865) | 0.881 | ||
| Congestive heart failure | 1.192 (1.014–1.402) | 0.033 | 1.033 (0.865–1.233) | 0.720 |
| Cardiac arrhythmias | 0.825 (0.698–0.974) | 0.024 | 0.661 (0.550–0.793) | <0.001 |
| Atrial fibrillation | 0.887 (0.721–1.083) | 0.246 | ||
| Coronary artery disease | 0.775 (0.610–0.974) | 0.032 | 0.784 (0.605–1.007) | 0.061 |
| Liver cirrhosis | 2.385 (1.546–3.551) | <0.001 | 2.886 (1.810–4.466) | <0.001 |
| Coagulopathy | 1.928 (1.611–2.300) | <0.001 | 1.594 (1.313–1.930) | <0.001 |
| Diabetes controlled | 0.699 (0.516–0.927) | 0.016 | 0.918 (0.665–1.245) | 0.593 |
| Diabetes uncontrolled | 0.955 (0.590–1.465) | 0.843 | ||
| Hypertension controlled | 0.338 (0.253–0.443) | <0.001 | 0.464 (0.340–0.622) | <0.001 |
| Hypertension uncontrolled | 1.956 (1.618–2.354) | <0.001 | 1.349 (1.093–1.658) | 0.004 |
| Peripheral vascular disease | 1.182 (0.833–1.633) | 0.328 | ||
| Smoking | 0.265 (0.126–0.485) | <0.001 | 0.406 (0.191–0.755) | 0.009 |
| Solid tumor without metastasis | 0.578 (0.176–1.394) | 0.286 | ||
| Metastatic cancer | 2.011 (0.682–4.781) | 0.150 | ||
| Malnutrition disorder | 1.164 (0.916–1.462) | 0.203 | ||
| Cardiogenic shock | 1.900 (1.493–2.395) | <0.001 | 1.125 (0.862–1.455) | 0.376 |
| Myocardial infarction | 2.562 (1.981–3.277) | <0.001 | ||
| Mechanical ventilation | 4.076 (3.383–4.898) | <0.001 | 2.764 (2.252–3.383) | <0.001 |
| Blood transfusion | 0.726 (0.611–0.861) | <0.001 | ||
| Race | 1.022 (0.944–1.102) | 0.576 | ||
| Hospital bed size | 0.971 (0.845–1.123) | 0.690 | ||
| Hospital region | 0.990 (0.915–1.071) | 0.799 | ||
| Aortic valve replacement | 1.002 (0.848–1.186) | 0.985 | ||
| Mitral valve replacement | 1.511 (1.283–1.782) | <0.001 | 1.044 (0.851–1.279) | 0.678 |
| AVR+MVR | 1.926 (1.578–2.339) | <0.001 | 1.844 (1.446–2.347) | <0.001 |
| Mitral valve repair | 0.755 (0.417–1.256) | 0.313 | ||
| Tricuspid valve replacement | 0.655 (0.370–1.069) | 0.114 | ||
| Tricuspid valve repair | 1.283 (0.806–1.945) | 0.265 | ||
| Acute stroke | 1.752 (1.425–2.141) | <0.001 | 1.486 (1.184–1.853) | <0.001 |
Adjusted for variables with P<0.05 on univariate analysis. AVR+MVR indicates combined aortic and mitral valve replacement; CABG, coronary artery bypass graft; and PCI, percutaneous coronary intervention.
In addition, among the patients undergoing valve replacement surgery, there were no significant differences in the adjusted length of stay based on sex (incidence rate ratio, 0.998 [0.978–1.083], P=0.832).
Temporal Trends in Utilization of Valve Replacement Surgery and In‐Hospital Mortality
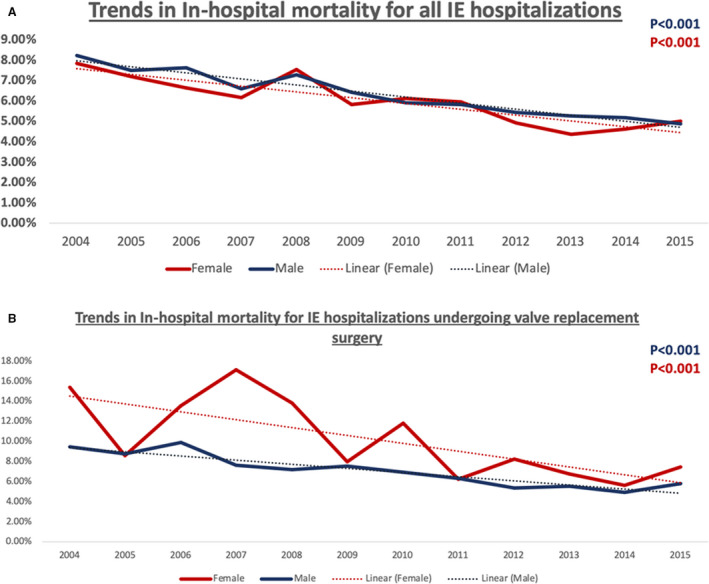
There was an increase in the utilization of valve replacement surgeries for IE during the study period (Figures 2 and 3, Tables S1 through S4). The overall rates of cardiac valve replacement in the setting of endocarditis increased from 11.76% to 13.96% (P<0.001) in men and 6.34% to 9.26% (P<0.001) in women; AVR increased from 7.88% to 9.55% (P<0.001) in men and 2.87% to 4.11% (P<0.001) in women; MVR increased from 5.47% to 6.45% (P<0.001) in men and 4.25% to 5.92% (P<0.001) in women; combined AVR+MVR increased from 1.54% to 1.94% (P<0.001) in men and 0.68% to 1.02% (P<0.001) in women. However, there continued to exist a significant sex difference with decreased utilization of surgery in women. A significant decline in in‐hospital mortality rates for both of the sexes (men, 8.24%–4.90% and women, 7.85%–4.99%) was observed during the study period. Among the hospitalizations undergoing valve replacement surgery, the difference in in‐hospital mortality rates between the 2 groups declined over the time period, with a greater reduction of mortality in women (15.36%–7.50%, P<0.001) in comparison to men (9.46%–5.84%, P<0.001) (Figure 4) (Tables S5 and S6). The trends in the length of stay are shown in Figure S1. The predictors of undergoing valve replacement surgery are shown in Table S7.
Figure 2. Temporal trends in overall cardiac valve replacement by sex for patients hospitalized with infective endocarditis from 2004 to 2015.
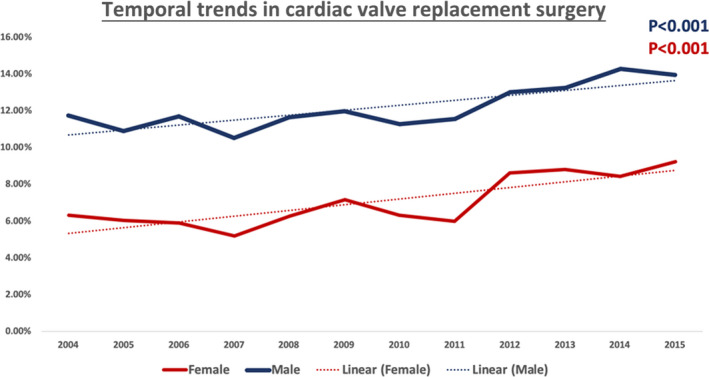
Figure 3. Temporal trends in individual valve replacement surgeries (aortic valve replacement, mitral valve replacement, and aortic+mitral valve replacement) by sex for patients hospitalized with infective endocarditis from 2004 to 2015.
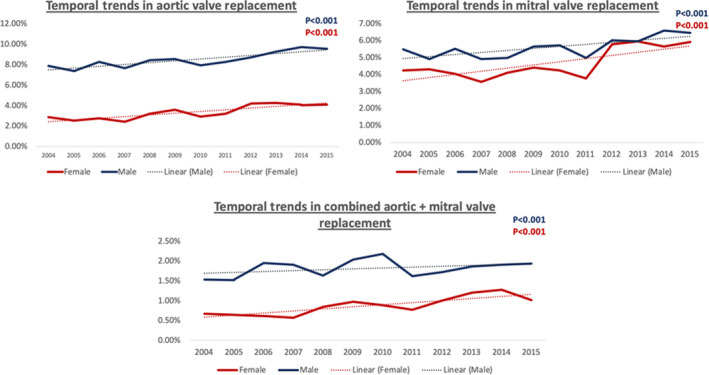
Figure 4. Temporal trends in in‐hospital mortality stratified by sex for (A) all patients hospitalized with infective endocarditis from 2004 to 2015, and (B) patients with infective endocarditis who underwent cardiac valve replacement surgery from 2004 to 2015. IE indicates infective endocarditis.
Discussion
We conducted an analysis exploring the clinical outcomes in the setting of IE and utilization rates of surgery stratified by sex. The salient finding is that female sex was associated with a decreased likelihood of undergoing overall cardiac valve replacement. However, the lower rates of surgery in women did not translate into higher observed in‐hospital mortality. Women selected to undergo surgery had significantly higher in‐hospital mortality rates than their male counterparts. The overall rates of surgery for endocarditis rose during the period of observation but there continued to exist a gap between the 2 sexes. Finally, although in‐hospital mortality rates for IE surgery decreased during the study period, women continued to experience higher in‐hospital mortality.
In line with previous studies, 10 , 12 IE was more common in men. Women hospitalized with IE were older; however, men had more comorbid conditions including history of surgical interventions (valve replacement, coronary artery bypass graft, and percutaneous coronary intervention) and concurrent prosthetic valve endocarditis, liver cirrhosis, coagulopathy, uncontrolled hypertension, and coronary artery disease, but diabetes was more common in women. 14
Our study shows that men were almost twice as likely to undergo valve replacement surgery (12.12% versus 6.92%). In our NIS cohort, women were older than men and since age is 1 of the predominant factors that influence the decision of surgery, one might attribute the decreased likelihood of surgical intervention in women to that. In addition, the potential indications of surgical intervention that could be identified from the nationwide cohort including congestive heart failure, S aureus endocarditis, fungal endocarditis, cardiogenic shock, prosthetic valve endocarditis, and others were more common in men, and that could be attributed as the reason for increased rates of utilization of surgery for IE in men. However, in our study, we demonstrated that after multivariable adjustment of all the mentioned variables, female sex was independently associated with a decreased likelihood of utilization of cardiac valve replacement surgery with a significant margin (adjusted odds ratio of 0.61 [95% CI, 0.58–0.65]), suggesting the presence of treatment bias. The existence of implicit bias among cardiology physicians has been reported in previous studies. 15 It is possible that the decision to operate more on men might have been influenced partly by the fact that IE was more often found to affect the aortic valve in men, which is associated with increased paravalvular complications, thus necessitating surgical intervention. 2 There were significant differences in overall cardiac valve replacement, AVR, and AVR+MVR; however, after adjustment there was no difference in MVR rates between men and women. This could be because the mitral valve is affected more often in women and because of increased prevalence of rheumatic heart disease in women. 16
Some prior studies have shown female sex to be an independent predictor of mortality after valve replacement surgery whereas others have not. Using the Society of Thoracic Surgeons database between 1994 and 2003, Rankin et al 17 demonstrated female sex to be an independent predictor of operative mortality. Similar results with sex disparities were shown by Chaker et al 18 among the patients undergoing surgical AVR using the NIS database. In contrast, Saxena et al 19 showed no difference in the incidence of early and late mortality between men and women after surgical aortic valve replacement. However, there are only small, single‐center studies that have evaluated the sex differences in operative outcomes for patients with IE. 10 , 12 , 20 In our large nationwide study, women undergoing valve replacement surgery had about 1.5 times (9.94% versus 6.99%) higher likelihood of in‐hospital mortality in comparison to men. Early surgery for IE within 48 hours has been shown to have a favorable outcome with reduction in composite end point of death and embolic events. 3 The most common cause of mortality in patients with IE is because of congestive heart failure. Decreased survival in women undergoing valve replacement surgery could be secondary to late presentation and delayed surgical intervention, which increases the risk of having congestive heart failure. The smaller body size and anatomy of women further makes surgical interventions technically more difficult and frequently require the use of smaller prosthetic valves, which is associated with worse postoperative outcomes. 21 , 22
Treatment bias may account for underutilization of valve surgery in women, which in turn has an adverse impact on the overall outcome. In line with previous investigations, more women hospitalized with IE should be offered early surgical intervention. It is encouraging to find that the rates of valve replacement surgeries have increased from 2004 to 2015 and more importantly, the gap in the in‐hospital mortality rates between men and women has narrowed over the mentioned time period.
Study Limitations
Our study has several limitations. First, the NIS is an administrative database that collects data for billing purposes and is subject to erroneous coding. However, we used ICD‐9 CM code for IE that has been well‐validated and shown to have high predictive value. Second, the NIS provides detailed data on in‐hospital mortality, morbidity, and cost but lacks the echocardiographic, laboratory, and outcomes data beyond hospital discharge. Vegetation size has been shown to be an independent predictor of early mortality. Third, the nonprocedural codes are not associated with specific dates in this administrative database, thus introducing the uncertainties about the timing of certain morbidities. For example, ICD codes are unable to distinguish whether acute stroke, blood transfusion, mechanical ventilation, or cardiogenic shock were the reason or a complication of valve replacement surgery. Fourth, we could not determine the indication of valve replacement surgery from the database. Fifth, in our study we did not use the ICD code for methicillin‐resistant S aureus. This is because the ICD coding for methicillin‐resistant S aureus was introduced in 2008 and we looked at the time period from 2004 to 2015. Sixth, using the ICD‐9 codes, we are unable to determine with certainty which valve is affected with IE. Thus, for example, in hospitalizations undergoing combined aortic and mitral valve replacement, it is possible that the mitral valve is infected and the aortic valve is replaced secondary to other reasons. Also, we are unable to ascertain the presence or severity of valvular dysfunction secondary to IE. Seventh, the potential for unmeasured confounders may bias the outcome results; however, we believe that our rigorous multivariable adjustment for the variables adequately addressed the selection bias.
Conclusions
Despite increased utilization of valve surgery for IE in both men and women and improving trends in mortality, in this large, multicenter, population‐based observational study, we showed that there exists a possible treatment bias with underutilization of valve surgeries for IE in women, and observed that in‐hospital mortality was higher in women undergoing valve surgery in comparison to men.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S7
Figure S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020095
For Sources of Funding and Disclosures, see page 12.
References
- 1. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65:2070–2076. DOI: 10.1016/j.jacc.2015.03.518. [DOI] [PubMed] [Google Scholar]
- 2. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8:630–644. DOI: 10.21037/acs.2019.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang D‐H, Kim Y‐J, Kim S‐H, Sun BJ, Kim D‐H, Yun S‐C, Song J‐M, Choo SJ, Chung C‐H, Song J‐K, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366:2466–2473. DOI: 10.1056/NEJMoa1112843. [DOI] [PubMed] [Google Scholar]
- 4. Swaminathan RV, Feldman DN, Pashun RA, Patil RK, Shah T, Geleris JD, Wong S‐C, Girardi LN, Gaudino M, Minutello RM, et al. Gender differences in in‐hospital outcomes after coronary artery bypass grafting. Am J Cardiol. 2016;118:362–368. DOI: 10.1016/j.amjcard.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 5. Fuchs C, Mascherbauer J, Rosenhek R, Pernicka E, Klaar U, Scholten C, Heger M, Wollenek G, Czerny M, Maurer G, et al. Gender differences in clinical presentation and surgical outcome of aortic stenosis. Heart. 2010;96:539–545. DOI: 10.1136/hrt.2009.186650. [DOI] [PubMed] [Google Scholar]
- 6. Hamed O, Persson PJ, Engel AM, McDonough S, Smith JM. Gender differences in outcomes following aortic valve replacement surgery. Int J Surg. 2009;7:214–217. DOI: 10.1016/j.ijsu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 7. Seeburger J, Eifert S, Pfannmüller B, Garbade J, Vollroth M, Misfeld M, Borger M, Mohr FW. Gender differences in mitral valve surgery. Thorac Cardiovasc Surg. 2013;61:42–46. [DOI] [PubMed] [Google Scholar]
- 8. Cabell CH, Abrutyn E. Progress toward a global understanding of infective endocarditis. Early lessons from the International Collaboration on Endocarditis investigation. Infect Dis Clin North Am. 2002;16:255–272. [DOI] [PubMed] [Google Scholar]
- 9. Watanakunakorn C. Changing epidemiology and newer aspects of infective endocarditis. Adv Intern Med. 1977;22:21–47. [PubMed] [Google Scholar]
- 10. Sambola A, Fernández‐Hidalgo N, Almirante B, Roca I, González‐Alujas T, Serra B, Pahissa A, García‐Dorado D, Tornos P. Sex differences in native‐valve infective endocarditis in a single tertiary‐care hospital. Am J Cardiol. 2010;106:92–98. DOI: 10.1016/j.amjcard.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 11. Elamragy AA, Meshaal MS, El‐Kholy AA, Rizk HH. Gender differences in clinical features and complications of infective endocarditis: 11‐year experience of a single institute in Egypt. Egypt Heart J. 2020;72:5. DOI: 10.1186/s43044-020-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aksoy O, Meyer LT, Cabell CH, Kourany WM, Pappas PA, Sexton DJ. Gender differences in infective endocarditis: pre‐ and co‐morbid conditions lead to different management and outcomes in female patients. Scand J Infect Dis. 2007;39:101–107. DOI: 10.1080/00365540600993285. [DOI] [PubMed] [Google Scholar]
- 13. Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the National Inpatient Sample. JAMA. 2017;318:2011–2018. DOI: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benvenga RM, De Rosa R, Silverio A, Matturro R, Zambrano C, Masullo A, Mastrogiovanni G, Soriente L, Ascoli R, Citro R, et al. Infective endocarditis and diabetes mellitus: results from a single‐center study from 1994 to 2017. PLoS One. 2019;14:e0223710. DOI: 10.1371/journal.pone.0223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daugherty SL, Blair IV, Havranek EP, Furniss A, Dickinson LM, Karimkhani E, Main DS, Masoudi FA. Implicit gender bias and the use of cardiovascular tests among cardiologists. J Am Heart Assoc. 2017;6:e006872. DOI: 10.1161/JAHA.117.006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kislitsina ON, Zareba KM, Bonow RO, Andrei AC, Kruse J, Puthumana J, Akhter N, Chris Malaisrie S, McCarthy PM, Rigolin VH. Is mitral valve disease treated differently in men and women? Eur J Prev Cardiol. 2019;26:1433–1443. DOI: 10.1177/2047487319833307. [DOI] [PubMed] [Google Scholar]
- 17. Rankin JS, Hammill BG, Ferguson TB Jr, Glower DD, O'Brien SM, DeLong ER, Peterson ED, Edwards FH. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg. 2006;131:547–557. DOI: 10.1016/j.jtcvs.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 18. Chaker Z, Badhwar V, Alqahtani F, Aljohani S, Zack CJ, Holmes DR, Rihal CS, Alkhouli M. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc. 2017;6:e006370. DOI: 10.1161/JAHA.117.006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saxena A, Dinh DT, Smith JA, Reid CM, Shardey GC, Newcomb AE. Females do not have increased risk of early or late mortality after isolated aortic valve replacement: results from a multi‐institutional Australian study. J Cardiovasc Surg (Torino). 2013;54:297–303. [PubMed] [Google Scholar]
- 20. Castillo JC, Anguita MP, Delgado M, Ruiz M, Mesa D, Romo E, Crespín M, García D, Arizón JM, Suárez de Lezo J. Características clínicas y pronóstico de la endocarditis infecciosa en la mujer [Clinical characteristics and prognosis of infective endocarditis in women]. Rev Esp Cardiol. 2008;61:36–40. DOI: 10.1157/13114955. [DOI] [PubMed] [Google Scholar]
- 21. Nitsche C, Koschutnik M, Kammerlander A, Hengstenberg C, Mascherbauer J. Gender‐specific differences in valvular heart disease. Wien Klin Wochenschr. 2020;132:61–68. DOI: 10.1007/s00508-019-01603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blackstone EH, Cosgrove DM, Jamieson WR, Birkmeyer NJ, Lemmer JH Jr, Miller DC, Butchart EG, Rizzoli G, Yacoub M, Chai A. Prosthesis size and long‐term survival after aortic valve replacement. J Thorac Cardiovasc Surg. 2003;126:783–796. DOI: 10.1016/S0022-5223(03)00591-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figure S1


