Abstract
Background
Long‐term benefit of dual antiplatelet therapy (DAPT) over single antiplatelet therapy (SAPT) for the prevention of recurrent stroke has not been established in patients with intracranial arterial stenosis. We compared the efficacy and safety of DAPT with cilostazol and clopidogrel or aspirin to those of SAPT with clopidogrel or aspirin in patients with intracranial arterial stenosis, who were recruited to the Cilostazol Stroke Prevention Study for Antiplatelet Combination trial, a randomized controlled trial in high‐risk Japanese patients with ischemic stroke.
Methods and Results
We compared the vascular and hemorrhagic events between DAPT and SAPT in patients with ischemic stroke and symptomatic or asymptomatic intracranial arterial stenosis of at least 50% in a major intracranial artery. Patients were placed in two groups: 275 were assigned to receive DAPT and 272 patients SAPT. The risks of ischemic stroke (hazard ratio [HR], 0.47; 95% CI, 0.23–0.95); and composite of stroke, myocardial infarction, and vascular death (HR, 0.48; 95% CI, 0.26–0.91) were lower in DAPT than SAPT, whereas the risk of severe or life‐threatening bleeding (HR, 0.72; 95% CI, 0.12–4.30) did not differ between the 2 treatment groups.
Conclusions
DAPT using cilostazol was superior to SAPT with clopidogrel or aspirin for the prevention of recurrent stroke and vascular events without increasing bleeding risk among patients with intracranial arterial stenosis after stroke.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01995370.
Keywords: antiplatelet therapy, cilostazol, intracranial artery, stroke, vascular event
Subject Categories: Clinical Studies, Platelets
Nonstandard Abbreviations and Acronyms
- CSPS.com
Cilostazol Stroke Prevention Study for Antiplatelet Combination
- DAPT
dual antiplatelet therapy
- ICAS
intracranial arterial stenosis
- SAPT
single antiplatelet therapy
Clinical Perspective
What Is New?
Dual antiplatelet therapy with cilostazol was superior to aspirin or clopidogrel alone for the long‐term prevention of recurrent stroke and vascular events without increasing bleeding risk in patients with ischemic stroke and intracranial arterial stenosis.
What Are the Clinical Implications?
Dual antiplatelet therapy with cilostazol can be a therapeutic option for the long‐term prevention of recurrent stroke and vascular events without concern for increased bleeding in patients with symptomatic or asymptomatic intracranial arterial stenosis of at least 50% after ischemic stroke.
Intracranial arterial stenosis (ICAS) is more common in Asian people than in White people, 1 , 2 , 3 and the risk of first‐ever and recurrent stroke is high in patients with ICAS. 4 , 5 , 6 Based on the results of clinical trials including the WASID 7 (Warfarin‐Aspirin Symptomatic Intracranial Disease) and SAMPRIS 8 (Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trials, the American Heart Association/American Stroke Association guidelines recommend short‐term dual antiplatelet therapy (DAPT) plus aggressive risk factor management in patients with severe ICAS in the vascular territory of ischemic stroke or transient ischemic attack. 9 According to a meta‐analysis of clopidogrel plus aspirin versus aspirin alone for acute ischemic stroke or high‐risk transient ischemic attack, that included the CHANCE 10 (Clopidogrel in High‐risk Patients With Acute Non‐disabling Cerebrovascular Events) and POINT 11 (Platelet‐Oriented Inhibition in New TIA [Transient Ischemic Attack] and Minor Ischemic Stroke) trials, the risk of recurrent stroke was reduced by DAPT only for the first 10 days, there was no benefit at 22 to 90 days after the initiation, and the risk of bleeding increased until 90 days. 12 In addition, clopidogrel resistance in association with CYP2C19 polymorphisms is more common in East Asian people including the Japanese population, than in White people. 13 In reality, according to the genetic analysis of CHANCE trial, the use of clopidogrel plus aspirin, as compared with aspirin alone, reduced the risk of recurrent stroke only in the subgroup of patients who were not carriers of the CYP2C19 loss‐of‐function alleles. 14
We previously conducted the CSPS.com (Cilostazol Stroke Prevention Study for Antiplatelet Combination) trial, a randomized controlled trial that compare DAPT using cilostazol and single antiplatelet therapy (SAPT) with aspirin or clopidogrel in high‐risk Japanese patients with ischemic stroke, including those with ICAS. 15 The results showed that recurrent stroke and vascular events were significantly fewer in the DAPT group than in the SAPT group, whereas serious or life‐threatening bleeding was comparable between both groups. 16
Based on this background, we conducted a subgroup analysis of ICAS in patients recruited in the CSPS.com trial to compare DAPT with cilostazol and aspirin or clopidogrel and SAPT with aspirin or clopidogrel in high‐risk patients with ischemic stroke. 7
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The CSPS.com trial was a multicenter, open‐label, randomized controlled trial that involved patients who were recruited from 292 hospitals across Japan. The design and main results on CSPS.com were described previously. 7 The study was approved by the institutional review board in each participating site and the patients gave written informed consent before being randomly assigned to treatment. Eligible patients were aged between 20 and 85 years, had developed a noncardioembolic ischemic stroke identified on magnetic resonance imaging between 8 and 180 days before the start of the protocol treatment, and were taking either aspirin (81 or 100 mg/day) or clopidogrel (50 or 75 mg/day) alone as antiplatelet therapy when providing informed consent. The patients were required to meet at least 1 of the 3 following criteria indicating a high risk for stroke recurrence: at least 50% stenosis of a major intracranial artery (to the level of A2 [the post‐communicating segment of the anterior cerebral artery], M2 [the Sylvian segment of the middle cerebral artery], or P2 [the ambient segment of the posterior cerebral artery]), at least 50% stenosis of an extracranial artery (the common carotid artery, internal carotid artery, vertebral artery, brachiocephalic artery, or subclavian artery); and 2 or more of the following risk factors including age of 65 years or older, hypertension, diabetes, chronic kidney disease, peripheral artery disease, history of ischemic stroke, history of ischemic heart disease, and current cigarette smoking. Among the recruited patients who met these criteria, those with 50% or more ICAS in a major intracranial artery were selected for this subgroup analysis. Patients were randomly allocated to either the cilostazol group (200 mg/day, 100 mg twice daily) or noncilostazol group. The observation periods lasted for at least a year.
The background characteristics in patients with ICAS included sex, age, body mass index, cigarette smoking, hypertension, diabetes, dyslipidemia, chronic kidney disease, peripheral artery disease, extracranial arterial stenosis, history of stroke, and history of ischemic heart disease. Vascular events included any stroke, ischemic stroke, hemorrhagic stroke (intracerebral hemorrhage and subarachnoid hemorrhage), and composite vascular events (stroke, myocardial infarction, and vascular death). Hemorrhagic events included any hemorrhagic events and severe or life‐threatening bleeding.
Statistical Analysis
Differences in background characteristics between patients with DAPT and SAPT were analyzed using the Wilcoxon rank sum test for continuous variables and the chi‐square test for binary variables. The log‐rank test was used for comparison between the 2 treatment groups, and the hazard ratios (HRs) and 95% CIs were calculated using the Cox‐proportional hazard model. Cumulative event rates in the 2 treatment groups were expressed using the Kaplan‐Meier’s plot and compared using the log‐rank test. Vascular events were analyzed in the intention‐to‐treat population, and hemorrhagic events were analyzed in patients who had received study treatment at least once during the trial. Data with missing values for variables necessary in the analyses were excluded from the analysis data set. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 155 patients were excluded from the overall intention‐to‐treat population (n=1879) because of missing ICAS data (Figure 1). Among the remaining 1724 patients, the numbers of patients with ICAS and without ICAS were 547 (31.7%) and 1177 (68.3%), respectively. Aspirin was used in 198 (36.2%) patients, and clopidogrel was used in 349 (63.8%) patients with ICAS. The median duration of follow‐up was 1.4 years (interquartile range: 0.8–2.2). Finally, 275 patients were assigned to receive DAPT, and 272 to SAPT. Clopidogrel was similarly used between DAPT (58.2%) and SAPT (59.3%) groups (P=0.6575).
Figure 1. Flow chart of patients.
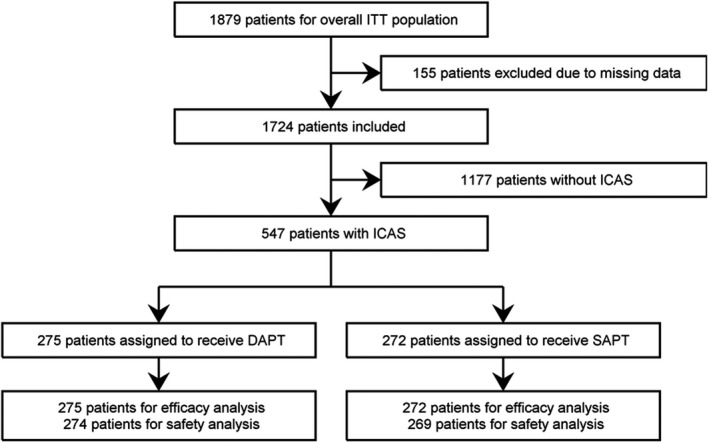
Efficacy analysis was conducted for vascular events including any stroke, ischemic stroke, and composite vascular events of stroke, myocardial infarction, and vascular death in all randomized patients. Safety analysis was conducted for any bleeding and severe or life‐threatening bleeding in patients excluding those who never received a dose (1 in the DAPT group and 3 in the SAPT group). DAPT indicates dual antiplatelet therapy; ICAS, intracranial arterial stenosis; ITT, intention to treat; and SAPT, single antiplatelet therapy.
The background characteristics were comparable between the 2 treatment groups, except for chronic kidney disease, which was more common in DAPT than SAPT (Table 1). The risk of any stroke (HR, 0.47; 95% CI, 0.24–0.93), ischemic stroke (HR, 0.47; 95% CI, 0.23–0.95), and composite vascular events (HR, 0.48; 95% CI, 0.26–0.90) were lower in the DAPT group, whereas the risk of major or life‐threatening bleeding (HR, 0.72; 95% CI, 0.12–4.30) was comparable between 2 groups (Table 2). After adjusting for chronic kidney disease, the risk of any stroke (HR, 0.47; 95% CI, 024–0.94; P=0.033), ischemic stroke (HR, 0.47; 95% CI, 023–0.95; P=0.036), and the composite vascular events (HR, 0.47; 95% CI, 0.25–0.90; P=0.022) remained lower in the DAPT group than in the SAPT group, whereas the risk of major or life‐threatening bleeding (HR, 0.59; 95% CI, 0.09–3.78; P=0.58) remained comparable between the 2 treatment groups in patients with ICAS. Figure 2 shows the Kaplan‐Meier curves to the first events.
Table 1.
Background Characteristics of Patients Treated With Dual Antiplatelet Therapy 458 With Cilostazol and Aspirin or Clopidogrel and Those Treated With Single Antiplatelet Therapy With Aspirin or Clopidogrel Among Patients With Intracranial Arterial Stenosis After Ischemic Stroke
|
DAPT* (n=275) |
SAPT † (n=272) |
P value | |
|---|---|---|---|
| Sex, female | 101 (36.7%) | 79 (29.0%) | 0.056 |
| Age, y, median (IQR*, ‡ ) | 70 (65–76) | 70 (65–76) | 0.74 |
| Body mass index, median (IQR*, ‡ ) | 23.4 (21.6–25.9) | 23.4 (21.6–25.5) | 0.52 |
| Current cigarette smoking | 62 (22.5%) | 68 (25.0%) | 0.50 |
| Hypertension | 219 (79.6%) | 216 (79.4%) | 0.95 |
| Diabetes | 114 (41.5%) | 102 (37.5%) | 0.34 |
| Dyslipidemia | 171 (62.4%) | 162 (59.6%) | 0.49 |
| Chronic kidney disease | 28 (10.2%) | 9 (3.3%) | 0.0014 |
| Extracranial arterial stenosis | 53 (20.8%) | 49 (19.8%) | 0.77 |
| Coronary artery disease | 16 (5.8%) | 16 (5.9%) | 0.97 |
| Peripheral artery disease | 8 (2.9%) | 2 (0.7%) | 0.058 |
| History of ischemic stroke | 29 (10.5%) | 29 (10.7%) | 0.96 |
DAPT indicates dual antiplatelet therapy; IQR, interquartile range; and SAPT, single antiplatelet therapy.
Dual antiplatelet therapy with cilostazol and aspirin or clopidogrel.
Single antiplatelet therapy with clopidogrel or aspirin.
Interquartile range.
Table 2.
Vascular and Hemorrhagic Events in Patients With Intracranial Arterial Stenosis After Ischemic Stroke, Who Were Either in Dual Antiplatelet Therapy or Single Antiplatelet Therapy
| Dual antiplatelet therapy | Single antiplatelet therapy | HR (95% CI) | P value | |
|---|---|---|---|---|
| Vascular events | n=275 | n=272 | ||
| Any stroke | 12 (4.4%) | 27 (9.9%) | 0.47 (0.24–0.93) | 0.027 |
| Ischemic stroke | 11 (4.0%) | 25 (9.2%) | 0.47 (0.23–0.95) | 0.031 |
| Hemorrhagic stroke | 1 (0.4%) | 2 (0.7%) | 0.55 (0.05–6.03) | 0.620 |
| Composite of stroke, myocardial infarction and vascular death | 14 (5.1%) | 31 (11.4%) | 0.48 (0.26–0.90) | 0.020 |
| Hemorrhagic events | n=274 | n=269 | ||
| Any bleeding | 12 (4.4%) | 7 (2.6%) | 1.83 (0.72–4.65) | 0.20 |
| Severe or life‐threatening bleeding | 2 (0.7%) | 3 (1.1%) | 0.72 (0.12–4.30) | 0.72 |
DAPT indicates dual antiplatelet therapy; HR, hazard ratio; and SAPT, single antiplatelet therapy.
Figure 2. The Kaplan‐Meier curves for the time to the first event of ischemic stroke (A), composite of stroke, myocardial infarction, and vascular death (B), and severe or life‐threatening bleeding (C).
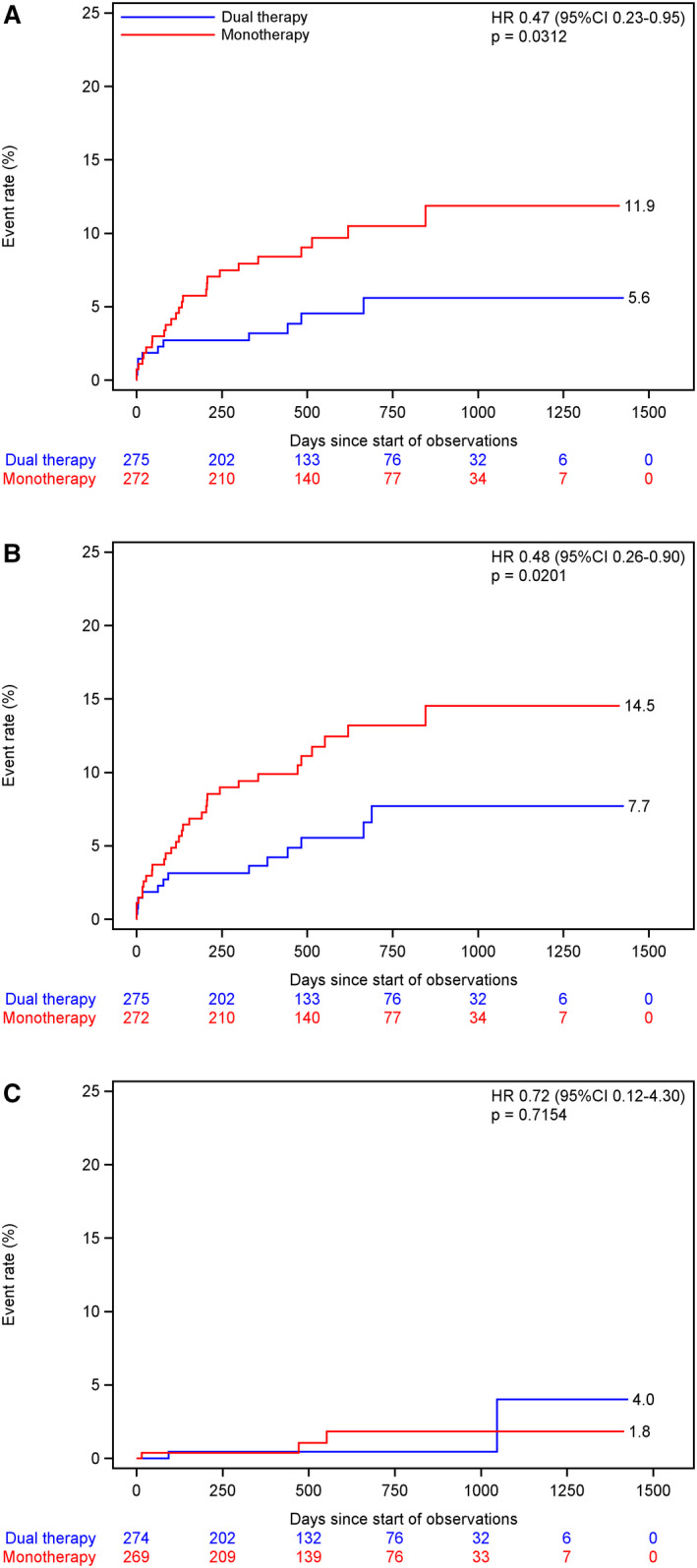
Intention‐to‐treat analysis for (A) and (B) and safety analysis for (C). HR indicates hazard ratio.
There were no interactions for vascular and hemorrhagic events between ICAS/no ICAS and DAPT/SAPT treatment; P=0.8169, 0.9540, 0.7458, 0.8067, 0.4128, and 0.8445 for any stroke, ischemic stroke, composite vascular events, any bleeding, and severe or life‐threatening bleeding, respectively.
Discussion
Among patients with stroke or transient ischemic attack associated with ICAS, the risk of recurrent stroke remains high despite adherence to the current guidelines. 2 , 3 , 4 , 5 , 6 Two randomized clinical trials were conducted to compare DAPT and SAPT in symptomatic ICAS among Asian patients. In the TOSS trial (Trial of Cilostazol in Symptomatic Intracranial Stenosis), involving 135 Korean patients with acute symptomatic ICAS, the progression of ICAS was less in DAPT with aspirin and cilostazol than aspirin alone, and there was no stroke recurrence in either group during the observation period of 6 months. 14 In the CATHARSIS trial (Cilostazol‐Aspirin Therapy against Recurrent Stroke with Intracranial Artery Stenosis), involving 165 Japanese patients with symptomatic ICAS>50%, we failed to prevent the progression of ICAS, which was the primary end point, whereas the composite of all vascular events and silent brain infarcts, which was the secondary end point, was lower in DAPT with aspirin and cilostazol than aspirin alone during a 2‐year observation period. 15
In this CSPS.com subgroup analysis, the risks of stroke, ischemic stroke, and vascular events were less than half in DAPT using cilostazol than SAPT with aspirin or clopidogrel, whereas the bleeding risk did not differ. Stroke rate in this study was lower than those in WASID, 7 SAMPRIS, 8 and other studies 5 including only patients with symptomatic ICAS, partly because CSPS.com also included asymptomatic ICAS. According to the results of the Oxford Vascular Study, asymptomatic ICAS did not increase the short‐ or medium‐term risk of distal recurrent ischemic stroke for patients receiving standard medical treatment. 16 A difference in the definition of stenosis in ICAS between this study and previous trials 7 , 8 would also affect the different stroke rates.
Cilostazol, a phosphodiesterase inhibitor, is known to have pleiotropic effects such as antiplatelet, vasodilating, anti‐inflammatory, and antiatherogenic effects, and protective effects on endothelial function. 17 , 18 , 19 These effects might contribute to the long‐term stroke prevention and help to avoid bleeding risk. 20 , 21 The long‐term effect of cilostazol combined with clopidogrel or aspirin on recurrent stroke is similar to the effect of dipyridamole combined with aspirin. 22 Interestingly, the Kaplan‐Meier curves for the ischemic stroke diverged gradually. Thus, effects of cilostazol might be delayed and presumably more related to the pleiotropic effects rather than direct antiplatelet effects.
This study had some limitations. First, the sample size was relatively small and the evidence level was limited. Second, it remains uncertain whether the present results can be generalized to other ethnicities than Japanese and to patients with acute stroke within 7 days after onset. Third, one cannot tell from these data whether DAPT is preventing recurrent stroke related to ICAS or just preventing stroke in any territory related to other mechanisms of stroke in patients with asymptomatic ICAS.
Conclusions
DAPT with cilostazol and clopidogrel or aspirin would be superior to clopidogrel or aspirin alone for the long‐term prevention of stroke and vascular events without increasing bleeding risk in patients with symptomatic or asymptomatic ICAS after ischemic stroke.
Sources of Funding
Japan Cardiovascular Research Foundation received trial funding from Otsuka Pharmaceutical Co, Ltd. Otsuka did not directly contribute to the trial design, data management, or statistical analysis. Statistical analysis of this article was performed in the Department of Data Science, National Cerebral and Cardiovascular Center. This study was funded mainly by the Japan Agency for Medical Research and Development (AMED, 21lk0201094h0003 and 21lk0201109h0002; PI, Toyoda).
Disclosures
Uchiyama reports grant support, honoraria, advisory board fees, and lecture fees from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Takeda, Healios, Otsuka, Sanofi, and the Japan Cardiovascular Research Foundation. Toyoda reports honoraria from Bayer, Bristol‐Myers Squibb, Daiichi Sankyo, Takeda, and Boehringer Ingelheim. T Yamaguchi reports honoraria from Bristol‐Myers Squibb, Daiichi Sankyo, and Pfizer. Kimura reports lecture fees and research funding from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, and Teijin. Hoshino reports honoraria from Daiichi Sankyo and Pfizer. Sakai reports grant support and lecture fees from Asahi‐Intec, Daiichi Sankyo, Medtronic, NeuroVasc, Stryker, and Terumo. Houkin reports honoraria as trial principal investigator from Healios. K Yamaguchi reports lecture fee from Daiichi Sankyo. Minematsu reports honoraria from Bayer, Bristol‐Myers Squibb, CSL Behring, Daiichi Sankyo, EPS Corporation, Fuji Film Pharma, Healios, Mitsubishi Tanabe, Nippon Chemiphar, Otsuka, Pfizer, Sanofi, and Stryker. Terayama reports grant support, consulting fees, and honoraria from Boehringer Ingelheim, Bristol‐Myers Squibb, and Daiichi Sankyo. Yasuda reports grant support, lecture fees, and clinical trial fees from Abbott, Bristol‐Myers Squibb, Daiichi Sankyo, and Takeda. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 6.
References
- 1. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race‐ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.STR.26.1.14 [DOI] [PubMed] [Google Scholar]
- 2. Hoshino T, Uchiyama S, Wong LKS, Sissani L, Albers GW, Bornstein NM, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, et al. Differences in characteristics and outcomes between Asian and non‐Asian patients in the TIAregistry.org. Stroke. 2017;48:1779–1787. doi: 10.1161/STROKEAHA.117.016874 [DOI] [PubMed] [Google Scholar]
- 3. Uchiyama S, Hoshino T, Sissani L, Linsay MT, Kamiyama K, Nakase T, Kitagawa K, Minematsu K, Todo K, Okada Y, et al. Japanese versus non‐Japanese patients with transient ischemic attack or minor stroke: subanalysis of TIAregistry.org. J Stroke Cerebrovasc Dis. 2019;28:2232–2241. doi: 10.1016/j.jstrokecerebrovasdis.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 4. Amarenco P, Lavallée PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, Anticoli S, Audebert H, Bornstein NM, Caplan LR, et al. Five‐year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182–2190. doi: 10.1056/NEJMoa1802712 [DOI] [PubMed] [Google Scholar]
- 5. Hurford R, Wolters FJ, Li L, Lau KK, Küker W, Rothwell PM, on behalf of the Oxford Vascular Study Phenotyped Cohort . Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischemic attack and minor stroke: a population‐based cohort study. Lancet Neurol. 2020;19:413–421. doi: 10.1016/S1474-4422(20)30079-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uchiyama S. Intensive medical management of intracranial arterial stenosis. Lancet Neurol. 2020;19:371–373. doi: 10.1016/S1474-4422(20)30100-9 [DOI] [PubMed] [Google Scholar]
- 7. Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, Pessin MS, Weichel E, Sila CA, Furlan AJ, et al. The warfarin‐aspirin symptomatic intracranial disease study. Neurology. 1995;45:1488–1493. doi: 10.1212/WNL.45.8.1488 [DOI] [PubMed] [Google Scholar]
- 8. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi‐Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 11. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palsesch YY. Clopidogrel and aspirin in acute ischemic stroke and high‐risk TIA. N Engl J Med. 2018;379:215–225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hao Q, Tampi M, O’Donnell M, Foroutan F, Siemieniuk RAC, Guyatt G. Clopidogrel plus aspirin versus aspirin alone for acute minor ischemic stroke of high risk transient ischemic attack: systematic review and meta‐analysis. BMJ. 2018;363:k5108. doi: 10.1136/bmj.k5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toyoda K, Uchiyama S, Yamaguchi T, Easton JD, Kimura K, Hoshino H, Sakai N, Okada Y, Tanaka K, Origasa H, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high‐risk ischaemic stroke in Japan: a multicentre, open‐label, randomised controlled trial. Lancet Neurol. 2019;18:539–548. doi: 10.1016/S1474-4422(19)30148-6 [DOI] [PubMed] [Google Scholar]
- 14. Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, Lee JH, Kim JS. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–786. doi: 10.1161/01.STR.0000157667.06542.b7 [DOI] [PubMed] [Google Scholar]
- 15. Uchiyama S, Sakai N, Toi S, Ezura M, Okada Y, Takagi M, Nagai Y, Matsubara Y, Minematsu K, Suzuki N, et al. Final results of cilostazol‐aspirin therapy against recurrent stroke with intracranial artery stenosis (CATHARSIS). Cerebrovasc Dis Extra. 2015;5:1–13. doi: 10.1159/000369610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hurford R, Wolters FJ, Linxin Li L, Lau KK, Wilhelm Küker W, Rothwell PM. Prognosis of asymptomatic intracranial stenosis in patients with transient ischemic attack and minor stroke. JAMA Neurol. 2020;77:947–954. doi: 10.1001/jamaneurol.2020.1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, Ouchi Y. Cilostazol inhibits oxidative stress–induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1634–1639. doi: 10.1161/ATVBAHA.108.164368 [DOI] [PubMed] [Google Scholar]
- 18. Lee HR, Park KY, Jeong YJ, Heo TH. Comparative effectiveness of different antiplatelet agents at reducing TNF‐driven inflammatory responses in a mouse model. Clin Exp Pharmacol Physiol. 2020;47:432–438. doi: 10.1111/1440-1681.13211 [DOI] [PubMed] [Google Scholar]
- 19. Katakami N, Kim YS, Kawamori R, Yamasaki Y. The phosphodiesterase inhibitor cilostazol induces regression of carotid atherosclerosis in subjects with type 2 diabetes mellitus. Principle results of the Diabetic Atherosclerosis Prevention by Cilostazol (DAPC) study: a randomized trial. Circulation. 2010;121:2584–2591. doi: 10.1161/CIRCULATIONAHA.109.892414 [DOI] [PubMed] [Google Scholar]
- 20. Uchiyama S, Demaerschalk BM, Goto S, Shinohara Y, Gotoh F, Stone WM, Money SR, Kwon SU. Stroke prevention by cilostazol in patients with atherothrombosis: meta‐analysis of placebo‐controlled randomized trials. J Stroke Cerebrovasc Dis. 2009;18:482–490. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 21. Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, Tanahashi N, Yamamoto H, Genka C, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin‐controlled, double‐blind, randomised non‐inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8 [DOI] [PubMed] [Google Scholar]
- 22. Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time‐course analysis of randomised trials. Lancet. 2016;388:365–375. doi: 10.1016/S0140-6736(16)30468-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


