Abstract
Background
Prior studies have shown an increased risk of ischemic stroke (IS) after myocardial infarction (MI); however, there are limited studies concerning the characteristics, in‐hospital mortality, and complications of patients with IS with a medical history of MI. We hypothesized that patients with IS with a medical history of MI may experience more severe strokes and have a higher risk of in‐hospital mortality and complications than patients with IS without a medical history of MI.
Methods and Results
Consecutive in‐hospital data were extracted from the China Stroke Center Alliance database from August 2015 to July 2019. Patient characteristics, hospital tests, in‐hospital mortality, and complications were analyzed and compared in patients with IS with or without a history of MI. Of 893 429 patients with IS, we identified 81 646 (9.1%) patients with a history of MI (MI group). Compared with patients with IS without MI, MI group patients were older, had a lower prevalence of current smoking, had a higher prevalence of a relative medical history, and took more medications before admission. Compared with the group with IS without MI, the MI group had a higher National Institute of Health Stroke Scale score after onset (4.0 versus 3.0; Hodges‐Lehmann estimator, 22.5) and a higher proportion of severe strokes (National Institute of Health Stroke Scale score ≥15) (7.1% versus 4.4%; absolute standardized difference=11.6%). In the fully adjusted models, the risk of in‐hospital mortality was higher in the MI group (odds ratio [OR], 1.74; 95% CI, 1.57–1.92; P<0.0001). MI group patients also had a higher risk of complications, including urinary tract infection (OR, 1.28; 95% CI, 1.2–1.36; P<0.0001), gastrointestinal bleeding (OR, 1.29; 95% CI, 1.19–1.39; P<0.0001), pneumonia (OR, 1.24; 95% CI, 1.21–1.28; P<0.0001), depression (OR, 1.33; 95% CI, 1.24–1.42; P<0.0001), seizure (OR, 1.35; 95% CI, 1.22–1.49; P<0.0001), atrial fibrillation (OR, 1.78; 95% CI, 1.71–1.86; P<0.0001), and cardiac or respiratory arrest (OR, 1.98; 95% CI, 1.78–2.2; P<0.0001).
Conclusions
Patients with IS with a medical history of MI have an increased risk of severe stroke, in‐hospital mortality, and complications. Studies exploring the underlying mechanisms are needed to improve and tailor stroke treatment strategies.
Keywords: complications, ischemic stroke, mortality, myocardial infarction
Subject Categories: Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- ASD
absolute standardized difference
- CSCA
China Stroke Center Alliance
- HL
Hodges‐Lehmann
- IS
ischemic stroke
- nMI
without medical history of myocardial infarction
Clinical Perspective
What Is New?
This study aims to illustrate the effect of a previous myocardial infarction on the severity of cerebral infarction, mortality, and complications during hospitalization for patients with ischemic stroke.
What Are the Clinical Implications?
This study shows that patients with ischemic stroke with a medical history of myocardial infarction have an increased risk of severe stroke, in‐hospital mortality, and complications, which indicates that special attention and tailored stroke treatment strategies should be paid to and given to this special patient group.
Stroke is the leading cause of death in China, where one fifth of the world’s population resides. 1 In the National Epidemiological Survey of Stroke in China, which involved 480 687 individuals from 31 provinces between 2012 and 2013, the annual mortality rate of stroke was 115 cases per 100 000, 2 most of which were in‐hospital deaths. Myocardial infarction (MI) is another globally leading cause of death, and it shares the same pathogenesis and risk factors with cerebral infarction. 3 , 4 Prior studies have shown an increased risk of ischemic stroke (IS) after MI. 5 , 6 However, although the relationship between MI and cerebral infarction has been studied extensively, the effect of a previous MI on the severity of cerebral infarction, mortality, and complications during hospitalization for IS still lacks effective research. To better address this knowledge gap, in this study, we aimed to illustrate the characteristics, in‐hospital mortality, and complications among patients with IS with or without a history of MI by using registry data from the China Stroke Center Alliance (CSCA), which included 1 006 798 patients from 1476 hospitals.
Methods
Data Source and Study Population
This study was approved by the institutional ethics committee at Beijing Tiantan Hospital, and the subjects gave informed consent. The data that support the findings of this study are available from the corresponding author on reasonable request. Data were obtained from the CSCA database from August 1, 2015, to July 31, 2019. The CSCA is a national, hospital‐based, multicenter, voluntary, multifaceted intervention and continuous quality improvement initiative. The data coordinating center of the CSCA resides at the China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital. Data were collected via the web‐based patient data collection and management tool (Medicine Innovation Research Center, Beijing, China), abstracted via chart review, coded, deidentified, and transmitted in a secure manner to maintain patient confidentiality compliant with national privacy standards. The following data were collected for each hospitalization: patient demographics, disease and medication history, hospital presentation, initial neurological status, medications and interventions, reperfusion strategy, and in‐hospital outcomes and complications. The detailed content of each category and the definition of in‐hospital outcomes and complications can be found in the online CSCA data. 7 As of July 31, 2019, 1476 hospitals in China have contributed detailed clinical information to serve as a benchmark for the stroke care quality of 1 006 798 patients with acute stroke or transient ischemic attack (TIA), which represents approximately 15.3% of all the nation’s hospitalizations in 9617 public secondary and tertiary hospitals.
The enrolled consecutive patients in the participating hospitals in the CSCA should meet the following criteria: (1) are aged ≥18 years; (2) have a primary diagnosis of acute stroke/TIA confirmed by brain computed tomography or magnetic resonance imaging, including IS (TIA was involved), intracerebral hemorrhage, or subarachnoid hemorrhage; (3) are within 7 days of symptom onset; and (4) are admitted either directly toward or through the emergency department. Patients with cerebral venous sinus thrombosis or noncerebrovascular diseases were excluded. In this study, as the subjects were patients with IS (TIA was involved) with or without a history of MI, we excluded patients with subarachnoid hemorrhage and intracranial hemorrhage, as well as patients without MI information (Figure).
Figure 1. Study flowchart diagram.
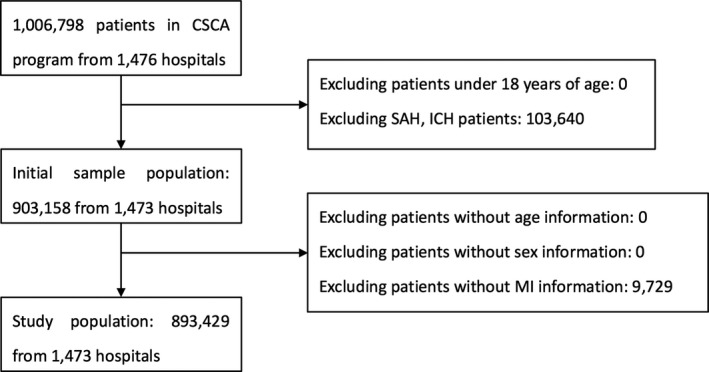
CSCA indicates China Stroke Center Alliance; ICH, intracranial hemorrhage; MI, myocardial infarction; and SAH, subarachnoid hemorrhage.
Patient Characteristics
Baseline patient characteristics included demographics (age, sex, average family income, and education level) and relevant comorbidities, such as smoking, drinking, atrial fibrillation, prior stroke or TIA, hypertension, diabetes, dyslipidemia, heart failure, peripheral vascular disease, prosthetic heart valve, chronic obstructive pulmonary disease, mental disturbance, liver insufficiency, or kidney insufficiency. Medications before admission (antiplatelet, anticoagulation, antihypertensive, cholesterol‐reducing, and diabetic medications and Chinese patent drugs), parameters measured at admission (systolic blood pressure, diastolic blood pressure, pulse rate, and in‐hospital National Institute of Health Stroke Scale [NIHSS] score), expenditures of hospitalization (hospital expenditure, medicine expenditure, and length of stay), and hospital tests and measurements (low‐density lipoprotein, total cholesterol, triglyceride, hemoglobin A1C, fasting glucose, homocysteine, serum creatinine, blood urea nitrogen, international normalized ratio, uric acid, and body mass index) were also included in the statistics. To better evaluate the difference in in‐hospital NIHSS scores between patients with IS with a medical history of MI (MI group) and patients with IS without a medical history of MI (nMI group), we divided the NIHSS scores into 3 grades of 0 to 4 (mild stroke), 5 to 14 (moderate stroke), and ≥15 (severe stroke) and compared the 2 groups.
Outcome Measure
The primary outcome of interest for this study was in‐hospital mortality. The secondary outcome was in‐hospital complications, including urinary tract infection, gastrointestinal bleeding, pneumonia, decubitus ulcer, deep venous thrombosis, pulmonary embolism, depression, seizure, arterial fibrillation, and cardiac or respiratory arrest.
Statistical Analysis
Subjects were divided into 2 groups by the status of MI. The baseline table was produced by %ggBaseline, an SAS macro that can analyze and report baseline characteristics automatically. 8 Specifically, continuous variables are reported as the mean±SD or median (interquartile range), where appropriate. Categorical variables are presented as frequencies and percentages. Given the extensive data set, comparisons in which P<0.05 indicate a statistically significant difference but may not have any clinical significance. Therefore, baseline characteristics were compared using absolute standardized differences (ASDs), with an ASD ≥10 considered to be clinically significant. 9 Multiple logistic regression models were used to assess the association between MI and in‐hospital outcomes and complications. In this study, we defined 3 models. In model 1, there was no adjustment for potential confounding variables. In model 2, the following potential confounding variables were adjusted for: age, sex, a history of smoking, a history of drinking, atrial fibrillation or flutter, hypertension, diabetes, dyslipidemia, heart failure, peripheral vascular disease, prosthetic heart valve, chronic obstructive pulmonary disease, liver insufficiency or kidney insufficiency, antiplatelet medication, anticoagulation medication, antihypertensive medication, cholesterol‐reducing medication, diabetic medication, Chinese patent drugs, diastolic blood pressure, medicine expenditures, and length of stay. In model 3, the in‐hospital NIHSS score variable was added in addition to the variables of model 2. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). A 2‐sided value of P<0.05 was set for statistical significance.
Results
Among 1 006 798 patients in the CSCA program from 1476 hospitals, 103 640 patients with subarachnoid hemorrhage or intracranial hemorrhage and 9729 patients without MI information were excluded. The final study population was 893 429 patients from 1473 hospitals, representing the final cohort included in our analysis (Figure).
Baseline Demographics
Of 893 429 patients with IS hospitalized, we identified 81 646 (9.1%) patients with a history of MI (MI group) and 811 783 (90.9%) patients without a history of MI (nMI group) (Table 1). The mean age of the MI group patients was slightly older than that of the nMI group patients (70.0±11.0 versus 65.6±12.1 years; ASD=38.1%). In these 2 groups, male patients were slightly predominant (56.1% in the MI group and 62.8% in the nMI group). The prevalence of current smoking in the MI group was significantly lower than that in the nMI group (17.7% versus 24.3%; ASD/Hodges‐Lehmann [HL], 16.3). The median in‐hospital NIHSS score in the MI group was higher than that in the nMI group (4.0 versus 3.0; HL estimator=22.5). Further stratified analysis showed that the proportion of patients with mild stroke (NIHSS score, 0–4 points) in the nMI group was significantly higher than that in the MI group (50.5% versus 39.7%; ASD/HL, 21.8), whereas the proportion of patients with severe stroke (NIHSS score ≥15) in the MI group was higher than that in the nMI group (7.1% versus 4.4%; ASD/HL, 11.6). The MI group patients had a higher incidence of other chronic diseases than the nMI group patients in their medical history, such as atrial fibrillation or flutter, prior stroke or TIA, hypertension, diabetes, dyslipidemia, heart failure, peripheral vascular disease, prosthetic heart valve, chronic obstructive pulmonary disease, and liver insufficiency or kidney insufficiency. This trend was also consistent when comparing the medications before admission in these 2 groups. There was no significant difference in most hospital tests between the 2 groups (Table 2). However, the MI group patients spent much more on medical expenditures (6045.1±6463.3 versus 5409.7±5395.5 Chinese monetary unit; ASD=10.7%) and stayed longer in the hospital than the nMI group patients (median, 11.0 versus 10.0 days; HL estimator=10.0).
Table 1.
Demographic and Clinical Characteristics of Patients With Versus Without History of MI
| Variables | Total (N=893 429 [100%]) | With medical history of MI (N=81 646 [9.1%]) | Without medical history of MI (N=811 783 [90.9%]) | ASD/HL estimator |
|---|---|---|---|---|
| Age, mean±SD, y | 66.0±12.0 | 70.0±11.0 | 65.6±12.1 | 38.1 |
| Male, N (%) | 555 579 (62.2) | 45 820 (56.1) | 509 759 (62.8) | 13.7 |
| Average income of family, mean±SD, ×103 RMB | 52.7±47.8 | 52.9±47.8 | 52.7±47.8 | 0.4 |
| Still smoking, N (%) | 211 603 (23.7) | 14 467 (17.7) | 197 136 (24.3) | 16.3 |
| History of drinking, N (%) | 207 889 (23.3) | 15 782 (19.3) | 192 107 (23.7) | 10.7 |
| Diagnosis, N (%) | ||||
| IS without TIA | 829 103 (92.8) | 75 466 (92.4) | 753 637 (92.8) | 1.5 |
| TIA | 64 326 (7.2) | 6180 (7.6) | 58 146 (7.2) | 1.5 |
| In‐hospital NIHSS score | ||||
| Mean±SD | 3.0 (2.0–6.0) | 4.0 (2.0–8.0) | 3.0 (1.0–6.0) | 22.5 |
| In‐hospital NIHSS score, N (%) | ||||
| Undocumented | 212 856 (23.8) | 24 829 (30.4) | 188 027 (23.2) | 16.3 |
| 0–4 (Mild stroke) | 442 491 (49.5) | 32 431 (39.7) | 410 060 (50.5) | 21.8 |
| 5–14 (Moderate stroke) | 196 180 (22.0) | 18 553 (22.7) | 177 627 (21.9) | 1.9 |
| ≥15 (Severe stroke) | 41 902 (4.7) | 5833 (7.1) | 36 069 (4.4) | 11.6 |
| Medical history, N (%) | ||||
| Atrial fibrillation or flutter | 46 214 (5.2) | 11 491 (14.1) | 34 723 (4.3) | 34.4 |
| Prior stroke or TIA | 298 479 (33.4) | 35 083 (43.0) | 263 396 (32.4) | 22.0 |
| Hypertension | 571 655 (64.0) | 58 577 (71.7) | 513 078 (63.2) | 18.2 |
| Diabetes | 188 839 (21.1) | 22 164 (27.1) | 166 675 (20.5) | 15.5 |
| Dyslipidemia | 69 304 (7.8) | 9952 (12.2) | 59 352 (7.3) | 16.6 |
| Heart failure | 9281 (1.0) | 4273 (5.2) | 5008 (0.6) | 27.7 |
| PVD | 15 587 (1.7) | 3348 (4.1) | 12 239 (1.5) | 15.8 |
| Prosthetic heart valve | 1440 (0.2) | 796 (1.0) | 644 (0.1) | 12.2 |
| Dementia | 4305 (0.5) | 636 (0.8) | 3669 (0.5) | 3.7 |
| COPD | 10 959 (1.2) | 2291 (2.8) | 8668 (1.1) | 12.3 |
| Liver insufficiency or kidney insufficiency | 8919 (1.0) | 1818 (2.2) | 7101 (0.9) | 10.5 |
| Medications before admission, N (%) | ||||
| Antiplatelet | 188 757 (21.1) | 30 571 (37.4) | 158 186 (19.5) | 40.5 |
| Anticoagulant | 35 703 (4.0) | 7176 (8.8) | 28 527 (3.5) | 22.2 |
| Antihypertensive | 416 553 (46.6) | 47 009 (57.6) | 369 544 (45.5) | 24.4 |
| Cholesterol reducer | 136 489 (15.3) | 21 325 (26.1) | 115 164 (14.2) | 30.0 |
| Diabetic medication | 147 978 (16.6) | 17 978 (22.0) | 130 000 (16.0) | 15.3 |
| Chinese patent drug | 78 607 (8.8) | 12 578 (15.4) | 66 029 (8.1) | 22.8 |
| Measurement | ||||
| Systolic blood pressure, mean±SD, mm Hg | 149.5±23.1 | 147.9±23.5 | 149.7±23.0 | 7.7 |
| Diastolic blood pressure, mean±SD, mm Hg | 86.7±13.9 | 85.0±14.0 | 86.9±13.8 | 13.7 |
| Pulse rate, mean±SD, /min | 76.7±12.7 | 76.6±14.2 | 76.7±12.5 | 0.7 |
| Hospital expenditure, mean±SD, RMB | 12 372.4±10 681.2 | 13 342.9±12 264.9 | 12 275.1±10 504.3 | 9.4 |
| Medicine expenditure, mean±SD, RMB | 5467.8±5504.7 | 6045.1±6463.3 | 5409.7±5395.5 | 10.7 |
| Length of stay, mean±SD, d | 11.0 (7.0–14.0) | 11.0 (8.0–14.0) | 10.0 (7.0–14.0) | 10.0 |
ASD/HL indicates absolute standardized difference/Hodges‐Lehmann; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; IS, ischemic stroke; MI, myocardial infarction; NIHSS, National Institute of Health Stroke Scale; PVD, peripheral vascular disease; RMB, Chinese monetary unit; and TIA, transient ischemic attack.
Table 2.
Hospital Test and Measurement of Patients With Versus Without MI
| Variables | No. of people used in the actual analysis | Total | With medical history of MI | Without medical history of MI | ASD/HL estimator |
|---|---|---|---|---|---|
| Low‐density lipoprotein, mmol/L | 885 351 | 2.8±1.2 | 2.7±1.2 | 2.8±1.2 | 8.3 |
| Total cholesterol, mg/dL | 211 534 | 36.6±71.3 | 33.7±66.8 | 36.6±71.4 | 4.2 |
| Triglyceride, mg/dL | 211 625 | 30.9±81.0 | 26.9±69.7 | 31.0±81.2 | 5.4 |
| Hemoglobin A1C, % | 795 866 | 6.3±1.8 | 6.4±1.8 | 6.3±1.8 | 5.6 |
| Fasting glucose, mmol/L | 884 263 | 6.4±2.9 | 6.6±3.0 | 6.4±2.9 | 6.8 |
| Homocysteine, mmol/L | 797 766 | 14.4±7.4 | 14.7±7.6 | 14.3±7.4 | 5.3 |
| Serum creatinine, µmol/L | 888 198 | 128.2±1211.9 | 103.8±881.5 | 130.7±1240.2 | 2.5 |
| BUN, mmol/L | 886 332 | 5.7±2.4 | 6.0±2.6 | 5.7±2.4 | 12.0 |
| INR | 878 615 | 1.2±0.9 | 1.2±0.8 | 1.2±0.9 | 0.0 |
| Uric acid, µmol/L | 882 789 | 312.3±121.7 | 319.3±125.9 | 311.6±121.3 | 6.2 |
| BMI, mg/m2 | 879 415 | 24.0±4.4 | 24.1±4.5 | 24.0±4.4 | 2.2 |
ASD/HL indicates absolute standardized difference/Hodges‐Lehmann; BMI, body mass index; BUN, blood urea nitrogen; INR, international normalized ratio; and MI, myocardial infarction.
In‐Hospital Mortality and Complications
The rates of in‐hospital mortality and complications in all enrolled patients with IS were 0.4% (3721/893 429) and 12.0% (106 818/893 429), respectively. Among all the complications reported, pneumonia and atrial fibrillation were the most common, occurring in approximately 8.5% and 4.9% of patients, respectively, followed by depression (1.3%), urinary tract infection (1.2%), deep venous thrombosis (0.9%), gastrointestinal bleeding (0.9%), seizure (0.5%), decubitus ulcer (0.3%), cardiac or respiratory arrest (0.3%), and pulmonary embolism (0.2%) (Table 3). The rate of in‐hospital mortality in the MI group was approximately 1.0%, which was significantly higher than the rate of 0.4% in the nMI group (odds ratio [OR], 2.94; 95% CI, 2.72–3.18; P<0.0001). Similarly, the proportion of complications in the MI group (18.5%) was significantly higher than that in the nMI group (11.3%) (OR, 1.78; 95% CI, 1.74–1.81; P<0.0001). As mentioned above, 3 multiple logistic regression models were used to assess the association between MI and in‐hospital outcomes and complications. In the fully adjusted models (model 3), the MI group that remained had an elevated risk of death (OR, 1.74; 95% CI, 1.57–1.92; P<0.0001) or complications (OR, 1.25; 95% CI, 1.22–1.28; P<0.0001) compared with the nMI group (Table 3). In model 1, the proportions of all kinds of complications mentioned above were higher in the MI group (P<0.0001). However, in model 2, the OR for deep venous thrombosis decreased from 1.48 to 0.98, and the OR for pulmonary embolism decreased from 1.52 to 1.06. In model 3, the in‐hospital NIHSS score was added to the adjusted variables, and the OR for decubitus ulcer decreased from 2.12 to 1.1. The risk of complications, including urinary tract infection, gastrointestinal bleeding, pneumonia, depression, seizure, atrial fibrillation, and cardiac or respiratory arrest, in the MI group was elevated in all 3 models (P<0.0001) (Table 3).
Table 3.
Rate of In‐Hospital Mortality, Outcome, and Complications of Patients With Stroke With or Without History of MI
| Variables | Total (N=893 429 [100%]) | With medical history of MI (N=81 646 [9.1%]) | Without medical history of MI (N=811 783 [90.9%]) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||||
| Mortality, N (%) | 3721 (0.4) | 845 (1.0) | 2876 (0.4) | 2.94 (2.72–3.18) | <0.0001 | 1.8 (1.65–1.95) | <0.0001 | 1.74 (1.57–1.92) | <0.0001 |
| Complication, N (%) | 106 818 (12.0) | 15 074 (18.5) | 91 744 (11.3) | 1.78 (1.74–1.81) | <0.0001 | 1.27 (1.24–1.29) | <0.0001 | 1.25 (1.22–1.28) | <0.0001 |
| UTI, N (%) | 10 667 (1.2) | 1733 (2.1) | 8934 (1.1) | 1.95 (1.85–2.05) | <0.0001 | 1.28 (1.21–1.35) | <0.0001 | 1.28 (1.2–1.36) | <0.0001 |
| Gastrointestinal bleeding, N (%) | 7858 (0.9) | 1239 (1.5) | 6619 (0.8) | 1.87 (1.76–1.99) | <0.0001 | 1.3 (1.21–1.39) | <0.0001 | 1.29 (1.19–1.39) | <0.0001 |
| Pneumonia, N (%) | 76 257 (8.5) | 11 143 (13.6) | 65 114 (8.0) | 1.81 (1.77–1.85) | <0.0001 | 1.28 (1.25–1.31) | <0.0001 | 1.24 (1.21–1.28) | <0.0001 |
| Decubitus ulcer, N (%) | 2685 (0.3) | 470 (0.6) | 2215 (0.3) | 2.12 (1.92–2.34) | <0.0001 | 1.18 (1.06–1.31) | 0.0028 | 1.1 (0.97–1.25) | 0.1324 |
| DVT, N (%) | 7636 (0.9) | 984 (1.2) | 6652 (0.8) | 1.48 (1.38–1.58) | <0.0001 | 0.98 (0.91–1.06) | 0.6455 | 1 (0.92–1.08) | 0.9471 |
| Pulmonary embolism, N (%) | 1707 (0.2) | 226 (0.3) | 1481 (0.2) | 1.52 (1.32–1.75) | <0.0001 | 1.06 (0.92–1.23) | 0.4238 | 1.06 (0.89–1.26) | 0.5075 |
| Depression, N (%) | 11 269 (1.3) | 1519 (1.9) | 9750 (1.2) | 1.56 (1.48–1.65) | <0.0001 | 1.26 (1.19–1.34) | <0.0001 | 1.33 (1.24–1.42) | <0.0001 |
| Seizure, N (%) | 4687 (0.5) | 702 (0.9) | 3985 (0.5) | 1.76 (1.62–1.91) | <0.0001 | 1.32 (1.21–1.44) | <0.0001 | 1.35 (1.22–1.49) | <0.0001 |
| AF, N (%) | 43 980 (4.9) | 10 388 (12.7) | 33 592 (4.1) | 3.38 (3.3–3.46) | <0.0001 | 1.68 (1.62–1.74) | <0.0001 | 1.78 (1.71–1.86) | <0.0001 |
| Cardiac or respiratory arrest, N (%) | 2981 (0.3) | 716 (0.9) | 2265 (0.3) | 3.16 (2.91–3.44) | <0.0001 | 1.97 (1.8–2.17) | <0.0001 | 1.98 (1.78–2.2) | <0.0001 |
In model 1, the OR was not adjusted. In model 2, the following variables were adjusted: age, sex, history of smoking, history of drinking, atrial fibrillation or flutter, hypertension, diabetes, dyslipidemia, heart failure, peripheral vascular disease, prosthetic heart valve, chronic obstructive pulmonary disease, liver insufficiency or kidney insufficiency, antiplatelet, anticoagulation, antihypertensive, cholesterol reducer, diabetic medication, Chinese patent drug, diastolic blood pressure, medicine expenditure, and length of stay. In model 3, the variable “in‐hospital National Institute of Health Stroke Scale score” was added on the basis of the variables of model 2, and model 3 was fitted among 680 564 patients. AF indicates atrial fibrillation; DVT, deep vein thrombosis; MI, myocardial infarction; OR, odds raio; and UTI, urinary tract infection.
Discussion
In the current statistical analysis of registry data from the CSCA, a medical history of MI was associated with a higher NIHSS score after onset and with a higher rate of in‐hospital mortality and complications in patients with IS. This result overturns the misconception of some previous researchers: the risk of cerebral infarction and severity of IS in patients with previous MI might be lower than in other patients because of the good control of high risks and good secondary prevention measures. However, these results support the recent findings by Dabilgou et al, which showed that the predictors of death in patients with acute IS were a history of heart disease, consciousness disorders, hyperglycemia on admission, and pneumonia. 10 In addition, this study also suggests that patients with a previous history of MI should be given closer attention and more active treatment.
In this study, the proportion of admitted patients with IS with MI was ≈9.1%, which is less than the prevalence of 16.8% in National Epidemiological Survey of Stroke in China. The difference in results may be related to the respondents who were residents with stroke in National Epidemiological Survey of Stroke in China. 2 It is easy to accept the findings that MI group patients were older, had more underlying diseases, and took more medications before admission. However, the MI group patients had a lower prevalence of current smoking and drinking than the nMI group patients, which might be related to the good implementation of medical guidance and strict control of poor living habits in patients with MI. Interestingly, the median in‐hospital NIHSS score was higher in patients with IS with MI (4.0 versus 3.0), and the proportion of an NIHSS score ≥15 in the MI group was significantly higher (7.1% versus 4.4%), which means that the severity of stroke in patients with MI was significantly higher than that in the nMI group. An increased NIHSS score is an independent risk factor for death in patients with IS. 11 , 12 , 13 , 14 It is not clear why the NIHSS score of patients with IS with MI is significantly higher than that of patients without MI; however, a possible mechanism might be related to the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification. 15 , 16 Asian patients with MI might have a higher prevalence of large‐artery atherosclerosis, including atherosclerosis of the carotid artery and intracranial arteries, 17 which will lead to a larger territory of infarction when occluded and result in a higher NIHSS score.
Another significant finding of this study was that the in‐hospital mortality and complications of patients with IS with MI were significantly higher than those of patients with IS without MI, even in the fully adjusted models that included potential confounders, such as age, sex, high‐risk factors, related basic diseases in the medical history, and medications before hospitalization. This means that MI might be an independent risk factor to increase the risk of in‐hospital mortality in patients with IS. MI might also be an independent risk factor for in‐hospital complications, such as urinary tract infection, gastrointestinal bleeding, pneumonia, depression, epilepsy, atrial fibrillation, and cardiac or respiratory arrest. The underlying mechanisms of higher complications in patients with IS with MI remain unknown and need further exploration. All these complications are likely to be interrelated and have common mediators. Taking depression as an example, the relationship between cardiovascular disease and depression is likely to be complex, and the bidirectionality of the association between the conditions is important. 18 For example, previous MI predicts incident depression, which can be magnified after stroke onset and subsequently influence the incidence of cardiovascular diseases, such as atrial fibrillation and cardiac arrest, per se. 19 Similarly, patients with epilepsy following stroke are at remarkably high risk for depression and suicide, 20 , 21 and depression also commonly precedes the onset of seizures 22 and has been associated with lower rates of seizure remission following epilepsy surgery, suggesting that biologic links between epilepsy and depression may be bidirectional or reflective of an underlying shared causal mechanism. 23
Three complications, including decubitus ulcer, deep venous thrombosis, and pulmonary embolism, were higher in the MI group in model 1 (P<0.0001); however, there was no significant difference in the fully adjusted models (model 3), including the potential confounder of the NIHSS score. All 3 complications are related to long‐term bed rest, 24 , 25 , 26 whereas most patients with increased NIHSS scores usually have decreased autonomous movement of limbs, which often results in long‐term bed rest. 27 Thus, this also suggests that there are still many areas to be improved in the critical care of patients with stroke in Chinese hospitals, and active nursing and effective rehabilitation should be further strengthened for patients with stroke with high NIHSS scores. 28
Strengths and Limitations
Strengths of the study include the large sample size, affording high statistical power and precision. Most important, as the cases in this study come from all levels of hospitals throughout the country, the conclusions drawn from this study have a wide range of representative significance.
There are several limitations in this study. First, it must be pointed out that hospital participation in the CSCA program is voluntary. As a result, the current participating hospitals are more likely to be larger, tertiary centers with a myriad of resources to which smaller hospitals do not have access. Increasing the number of hospitals participating in the CSCA will optimize the representation of hospitals, patients, and regions. Second, data elements in the CSCA are limited to those that are readily documented in the inpatient medical record. Inpatient data in the CSCA lack follow‐up information. Third, data collected by hospitals were not independently audited by an external chart review. Data reliability depends on training for data abstractors and built‐in automated checks to identify erroneous, illogical data entries. 7 Fourth, in this study, the diagnosis of history of MI is based on patients’ self‐report, not from medical data, which might result in some bias to the results. Fifth, it should be figured out that medication history from database sources did not equate to patient compliance, and this will result in deviations in the analysis results. However, from the data, there is no significant difference between the blood pressure, blood glucose, and blood lipid levels in patients with IS with MI history and those without MI history, suggesting that most patients may take drugs regularly. Sixth, when analyzing the influence of previous history of MI on depression after IS, the lack of data about history of depression before IS may have an impact on the analysis results. And also, the diagnosis of depression was mostly made by clinical physicians but not psychiatrists; therefore, the diagnosis of depression, which might mainly be based on the symptoms and signs of patients and the Hamilton Rating Scale for Depression 6 depression diagnosis related scale, may not fully meet the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for depression, especially the time duration criteria. Furthermore, as the TOAST classification, the location of infarction, and the location of atherosclerotic lesion of each case were not clearly documented in the original data, it is difficult to analyze the underlying mechanism of IS in patients with a history of MI.
Our study also has several clinical implications. Because MI confers an elevated risk of mortality and complications in patients with IS compared with patients with IS without MI, studies to better understand the underlying mechanisms in these patients are necessary to potentially improve stroke treatment strategies. It is possible to reduce the occurrence of complications and improve the prognosis of these patients if we have a clear understanding of the complications and provide active prevention. Furthermore, because cardiac injury may be a marker of severity in patients with IS, evaluating these patients for cerebrovascular disease may improve stroke prevention strategies in these patients.
Conclusions
Patients with IS with a history of MI had increased risks of high NIHSS scores, in‐hospital mortality, and complications compared with patients with IS without a history of MI. Studies exploring the underlying mechanisms in this patient group are needed to improve and tailor stroke treatment strategies.
Sources of Funding
This work was supported by National Key Research and Development Program of China (Nos. 2018YFC1312800 and 2018YFC1312801), Youth Clinical Research Project of Peking University First Hospital (No. 2019CR02), Beijing Natural Science Foundation (Z200016), National Natural Science Foundation of China (92046016), and Beijing Talents Project (2018A13 and 2018000021223ZK03).
Disclosures
None.
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Yongjun Wang, Email: yongjunwang1962@gmail.com.
Dapeng Mo, Email: bjttmodp@163.com.
References
- 1. Wu S, Wu BO, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394–405. doi: 10.1016/S1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- 2. Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population‐based survey of 480 687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 3. GBD 2016 Causes of Death Collaborators . Global, regional, and national age‐sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu S, Bao MY, Miao SM, Zhang X, Jia QQ, Jing SQ, Shan T, Wu XH, Liu Y. Prevalence of hypertension, diabetes, and dyslipidemia, and their additive effects on myocardial infarction and stroke: a cross‐sectional study in Nanjing, China. Ann Transl Med. 2019;7:436. doi: 10.21037/atm.2019.09.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patil S, Gonuguntla K, Rojulpote C, Kumar M, Nadadur S, Nardino RJ, Pickett C. Prevalence and determinants of atrial fibrillation‐associated in‐hospital ischemic stroke in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Am J Cardiol. 2021;144:1–7. doi: 10.1016/j.amjcard.2020.12.066 [DOI] [PubMed] [Google Scholar]
- 6. Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta‐analysis. Am J Med. 2006;119:354.e1–354.e9. doi: 10.1016/j.amjmed.2005.10.058 [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Li Z, Wang Y, Zhao X, Liu L, Yang X, Wang C, Gu H, Zhang F, Wang C, et al. Chinese stroke center alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol. 2018;3:256–262. doi: 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gu HQ, Li DJ, Liu C, Rao ZZ. %ggBaseline: a SAS macro for analyzing and reporting baseline characteristics automatically in medical research. Ann Transl Med. 2018;6:326. doi: 10.21037/atm.2018.08.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 10. Dabilgou AA, Dravé A, Kyelem JMA, Ouedraogo S, Napon C, Kaboré J. Frequency and mortality risk factors of acute ischemic stroke in emergency department in Burkina Faso. Stroke Res Treat. 2020;2020:9745206. doi: 10.1155/2020/9745206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittal SH, Goel D. Mortality in ischemic stroke score: a predictive score of mortality for acute ischemic stroke. Brain Circ. 2017;3:29–34. doi: 10.4103/2394-8108.203256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mihindu E, Mohammed A, Smith T, Brinster C, Sternbergh WC III, Bazan HA. Patients with moderate to severe strokes (NIHSS score >10) undergoing urgent carotid interventions within 48 hours have worse functional outcomes. J Vasc Surg. 2019;69:1471–1481. doi: 10.1016/j.jvs.2018.07.079 [DOI] [PubMed] [Google Scholar]
- 13. Wirtz MM, Hendrix P, Goren O, Beckett LA, Dicristina HR, Schirmer CM, Dalal S, Weiner G, Foreman PM, Zand R, et al. Predictor of 90‐day functional outcome after mechanical thrombectomy for large vessel occlusion stroke: NIHSS score of 10 or less at 24 hours. J Neurosurg. 2021;134:115–121. doi: 10.3171/2019.10.JNS191991 [DOI] [PubMed] [Google Scholar]
- 14. Bhardwaj A, Sharma G, Raina SK, Sharma A, Angra M. Advanced age and higher national institutes of health stroke scale score as predictors of poor outcome in ischemic stroke patients treated with alteplase: a study from a tertiary care centre in rural north‐west India. J Neurosci Rural Pract. 2017;8:236–240. doi: 10.4103/jnrp.jnrp_431_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 16. Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126 [DOI] [PubMed] [Google Scholar]
- 17. Miao Z, Zhang Y, Shuai J, Jiang C, Zhu Q, Chen K, Liu LI, Li B, Shi X, Gao L, et al. Thirty‐day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke. 2015;46:2822–2829. doi: 10.1161/STROKEAHA.115.010549 [DOI] [PubMed] [Google Scholar]
- 18. Daskalopoulou M, George J, Walters K, Osborn DP, Batty GD, Stogiannis D, Rapsomaniki E, Pujades‐Rodriguez M, Denaxas S, Udumyan R, et al. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: data linkage study of 1.9 million women and men. PLoS One. 2016;11:e0153838. doi: 10.1371/journal.pone.0153838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sundbøll J. Depression, stroke, and dementia in patients with myocardial infarction. Dan Med J. 2018;65:B5423. PMID: 29619929. :B5423. [PubMed] [Google Scholar]
- 20. Blumer D, Montouris G, Davies K, Wyler A, Phillips B, Hermann B. Suicide in epilepsy: psychopathology, pathogenesis, and prevention. Epilepsy Behav. 2002;3:232–241. doi: 10.1016/s1525-5050(02)00006-9 [DOI] [PubMed] [Google Scholar]
- 21. Jones JE, Hermann BP, Barry JJ, Gilliam FG, Kanner AM, Meador KJ. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31–S38. doi: 10.1016/j.yebeh.2003.08.019 [DOI] [PubMed] [Google Scholar]
- 22. Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47:246–249. PMID: 10665498. doi: [DOI] [PubMed] [Google Scholar]
- 23. Butler T, Harvey P, Cardozo L, Zhu YS, Mosa A, Tanzi E, Pervez F. Epilepsy, depression, and growth hormone. Epilepsy Behav. 2019;94:297–300. doi: 10.1016/j.yebeh.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kottner J, Cuddigan J, Carville K, Balzer K, Berlowitz D, Law S, Litchford M, Mitchell P, Moore Z, Pittman J, et al. Prevention and treatment of pressure ulcers/injuries: the protocol for the second update of the international Clinical Practice Guideline 2019. J Tissue Viability. 2019;28:51–58. doi: 10.1016/j.jtv.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 25. Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–3073. doi: 10.1016/S0140-6736(16)30514-1 [DOI] [PubMed] [Google Scholar]
- 26. Robert‐Ebadi H, Righini M. Management of distal deep vein thrombosis. Thromb Res. 2017;149:48–55. doi: 10.1016/j.thromres.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 27. Chalouhi N, Daou B, Rincon F, Montano M, Kent A, Barkley K, Starke RM, Tjoumakaris S, Hasan D, Dalyai R, et al. Risk of venous thromboembolism in patients with large hemispheric infarction undergoing decompressive hemicraniectomy. Neurocrit Care. 2016;25:105–109. doi: 10.1007/s12028-016-0252-z [DOI] [PubMed] [Google Scholar]
- 28. Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–464. doi: 10.1016/S1474-4422(07)70004-2 [DOI] [PubMed] [Google Scholar]


