Abstract
Background
The subintimal approach (SA) is widely used in endovascular therapy for femoropopliteal chronic total occlusion lesions. However, when compared with the intraluminal approach (IA), the safety and efficacy of SA in real‐world practice are not well characterized. Furthermore, there is a paucity of data on the clinical impact of subintimal and intraluminal wire passage (SWP and IWP, respectively) assessed by intravascular ultrasound.
Methods and Results
From the IVORY (Intravascular Ultrasound‐Supported Endovascular Therapy in Superficial Femoral Artery) registry, this study included 500 patients undergoing endovascular therapy for femoropopliteal chronic total occlusion lesions (SA, n=67; IA, n=433; and SWP, n=186; IWP, n=314). The primary end point was the cumulative 1‐year incidence of restenosis. The rate of perioperative complications was also assessed. Propensity score matching analysis was performed to adjust for the intergroup differences. After propensity score matching, the final study population consisted of 59 pairs (SA, n=59; IA, n=348) and 170 pairs (SWP, n=170; IWP, n=293), respectively. Cumulative 1‐year incidence of restenosis was comparable between the SA and IA groups (41.0% versus 43.4%, P=0.40). No significant difference in 1‐year restenosis rate between the SWP and IWP groups was observed (48.2% versus 40.8%, P=0.40), although the SWP group tended to be a higher rate of perioperative complications than the IWP group (8.2% versus 4.1%, P=0.07).
Conclusions
At 1 year, both SA and IA showed acceptable results for femoropopliteal chronic total occlusion lesions. Cumulative 1‐year incidence of restenosis was not significantly different between SWP and IWP, whereas perioperative complications occurred more frequently in SWP than in IWP.
Registration
URL: https://www.umin.ac.jp; Unique identifier: UMIN000020472.
Keywords: chronic total occlusion, endovascular therapy, femoropopliteal lesion, intravascular ultrasound
Subject Categories: Peripheral Vascular Disease, Prognosis, Ultrasound
Nonstandard Abbreviations and Acronyms
- CTO
chronic total occlusion
- EVT
endovascular therapy
- IA
intraluminal approach
- IWP
intraluminal wire passage
- POC
perioperative complication
- SA
subintimal approach
- SWP
subintimal wire passage
Clinical Perspective
What Is New?
The discrepancy between the procedural approach (subintimal approach or intraluminal approach) and intravascular ultrasound‐derived wire‐crossing pattern (subintimal wire passage or intraluminal wire passage) was observed in approximately one‐third of the patients with femoropopliteal chronic total occlusion lesions.
At 1 year, both the subintimal and the intraluminal approaches showed acceptable results for femoropopliteal chronic total occlusion lesions (41.0% versus 43.4%, P=0.40).
Cumulative 1‐year incidence of restenosis was not significantly different between subintimal wire passage and intraluminal wire passage (48.2% versus 40.8%, P=0.40), whereas perioperative complications were likely to occur in subintimal wire passage than in intraluminal wire passage (8.2% versus 4.1%, P=0.07).
What Are the Clinical Implications?
Identifying a wire passage within femoropopliteal chronic total occlusion lesions by intravascular ultrasound may result in the reduction of perioperative complications.
Further studies are warranted to assess the clinical implication of intravascular ultrasound‐guided endovascular therapy for femoropopliteal chronic total occlusion lesions in contemporary practice.
Over the past decade, clinical outcomes of endovascular therapy (EVT) for femoropopliteal chronic total occlusion (FP‐CTO) lesions have improved by overcoming several challenges. 1 , 2 In particular, the subintimal approach (SA) was a breakthrough in EVT for FP‐CTO lesions. Since Bolia et al introduced SA using a 0.035‐inch looped guidewire with supported catheter for FP‐CTO lesions, SA has been widely used in EVT for these lesions. 3 Recent studies have compared clinical outcomes between SA and the intraluminal approach (IA) for FP‐CTO lesions, indicating that SA may be preferable because of a shorter procedure time without any increase in the incidence of perioperative complications (POC) or decrease in the primary patency rate. 4 , 5 To date, however, no prospective real‐world data exist on the comparative clinical outcomes of SA versus IA for FP‐CTO lesions.
Intravascular ultrasound (IVUS) has been increasingly used to optimize stent deployment in coronary artery lesions and to precisely determine the path of wire tracking in CTO lesions. 6 The clinical outcomes of subintimal wire passage (SWP) in coronary CTO lesions was comparable to those of intraluminal wire passage (IWP), 6 whereas there is a paucity of data comparing the outcomes of SWP and IWP in FP‐CTO lesions. In the present study, we sought to compare the 1‐year outcomes of EVT for FP‐CTO lesions between the following groups: (1) SA and IA and (2) SWP and IWP.
METHODS
Study Population
This was a subanalysis of the IVORY registry (Intravascular Ultrasound‐Supported Endovascular Therapy in Superficial Femoral Artery Disease Prospective multicenter registry); the details of the study protocol are described elsewhere. 7 For this study, we extracted the data pertaining to patients who successfully underwent unilateral EVT for de novo FP‐CTO lesions with IVUS guidance. Patients with (1) bilateral FP‐CTO lesion, (2) in‐stent restenotic lesion, (3) non‐CTO lesion, or (4) missing data on wire passage were excluded from the current analysis. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of each participating hospital. Written informed consent was obtained from all patients before participation.
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Procedure and Follow‐Up Protocol
Dual antiplatelet therapy for ≥2 days before the procedure was recommended. The choice between SA and IA was left to the physician's discretion. After passing the guidewire, IVUS images were recorded by auto‐ or manual pullback at a constant speed of 10 mm/s through the study segment to analyze the guidewire crossing route. If a suboptimal result was obtained (major flow‐limiting dissection [≥ grade D] or residual stenosis ≥50%) after pre‐balloon angioplasty, a stent was implanted. The stenting strategy (spot or full‐covered stenting) and the device choice was left at the physician's discretion. At the end of the procedure, IVUS images were acquired. Dual antiplatelet therapy was recommended for at least 1 month following the procedure. Cases with drug‐eluting stents and stent grafts were advised according to the package insert. All cases scheduled the evaluation of clinical outcomes at 12±2 months following the procedure.
IVUS Analysis
IVUS analysis was performed by experienced physicians or technicians at their respective institutions. The wire passage route was evaluated after successful guidewire crossing, and the passage route was classified as either subintimal or intraluminal (ie, intraplaque); intramedial wire crossing was included in the SWP group. 8 IVUS parameters were measured according to a consensus. 9
End Points
The primary end point was the cumulative 1‐year incidence of restenosis. The secondary end points were as follows: procedure time, contrast volume, postprocedural residual stenosis on angiogram, postprocedural minimum lumen area by IVUS, postprocedural ankle brachial index, occurrence of POC, and 1‐year clinical events, including all‐cause mortality, major amputation, and major adverse limb events.
Definitions
SWP was defined as having at least part of the subintimal or intramedial wire within the lesions. IWP was defined as having intraplaque wiring all through the lesions. Restenosis was defined as a peak systolic velocity ratio >2.4 according to duplex ultrasound or the recurrence of stenosis ≥50% of the arterial diameter as determined by angiography. 10 The incidence of 1‐year restenosis was assessed by duplex ultrasound or follow‐up angiography. The requirement for reintervention within 1 year was automatically classified as restenosis. POC was defined as the occurrence of at least 1 of the following events within 30 days after the index procedure: all‐cause death, myocardial infarction, stroke, contrast‐induced nephropathy, hemorrhage requiring transfusion, major amputation (ie, surgical limb removal above the ankle), need for any reintervention, acute occlusion, distal embolization, vascular rupture, blue toe syndrome, and infection at the puncture site. Major adverse limb event was defined as any reintervention or major amputation. The severity of calcification was assessed according to the Peripheral Arterial Calcium Scoring System. 11 Full‐covered stenting was defined as a stent implanted throughout the target lesions. 12 The angio‐score was measured according to the previous report. 7
Statistical Analysis
Data on baseline characteristics are presented as means± SD for continuous variables and percentages for categorical variables, unless otherwise mentioned. P values of <0.05 were considered statistically significant. The differences in baseline characteristics between groups were crudely assessed using the Welch's t test for continuous variables, Fisher's exact test for dichotomous variables, and Mann‐Whitney U test for ordinal categorical variables.
In this study, a propensity score (PS) matching was performed to adjust for the differences in baseline characteristics (1) between the SA and IA groups and (2) between the SWP and IWP groups. PS was estimated by a logistic regression model that included patient, lesion, and procedural characteristics listed in Table 1 as exploratory variables. In the comparison between the SWP and IWP groups, SA versus IA was additionally included in the model. Matching was performed on the logit of PS within a caliper of 0.2 SD of the logit of PS. 13 To maximize the statistical power to detect intergroup prognostic differences, we extracted as many matched samples undergoing IA (or IWP) to one undergoing SA (or SWP) as possible. After matching, the intergroup differences were analyzed with stratification by pairs, and the weighted descriptive statistics are reported. The difference in binary outcomes was assessed using the generalized linear mixed model with a logit‐link function, whereas that in continuous outcomes was assessed using the linear mixed model. In these models, a matched pair was treated as a cluster. Because the data on the procedure time and the contrast agent dose were right‐skewed, they were treated after log transformation. Multiple imputation (5 times) was adopted for missing data. Point estimates are reported with their 95% CIs. All statistical analyses were performed with R version 3.1.0 (R Development Core Team, Vienna, Austria). Propensity score matching was performed using the MatchIt package, mixed‐model analysis was performed using the lme4 package, and multiple imputation was performed using the mice package.
Table 1.
Baseline Clinical Characteristics of SA and IA
| Variables | Overall population | Matched population | ||||
|---|---|---|---|---|---|---|
| SA (n=67) | IA (n=433) | SD (%) | SA (n=59) | IA (n=348) | SD (%) | |
| Male sex* | 68.7% | 70.4% | 3.9 | 72.9% | 70.3% | 5.8 |
| Age, y* | 77±9 | 74±9 | 27.3 | 76±9 | 76±8 | 3.6 |
| Current smoking* | 29.9% | 33.0% | 6.8 | 33.9% | 34.4% | 1.0 |
| Diabetes mellitus* | 50.7% | 52.4% | 3.4 | 54.2% | 51.3% | 5.9 |
| Chronic renal failure* | 17.9% | 27.9% | 24.0 | 20.3% | 22.0% | 4.1 |
| On dialysis* | 13.4% | 18.2% | 13.2 | 15.3% | 16.2% | 2.7 |
| Chronic heart failure* | 7.5% | 18.5% | 33.2 | 8.5% | 10.7% | 7.5 |
| Aspirin use* | 67.2% | 80.8% | 31.5 | 69.5% | 71.4% | 4.2 |
| Thienopyridine use* | 82.1% | 80.6% | 3.8 | 81.4% | 83.1% | 4.7 |
| Cilostazol use* | 37.3% | 32.8% | 9.5 | 37.3% | 34.3% | 6.1 |
| Statin use* | 37.3% | 53.1% | 32.2 | 40.7% | 42.0% | 2.7 |
| Anticoagulant use* | 16.4% | 16.4% | 0.1 | 11.9% | 14.8% | 8.6 |
| Critical limb ischemia* | 34.3% | 30.5% | 8.2 | 33.9% | 32.9% | 2.0 |
| Ankle brachial index* | 0.55±0.17 | 0.53±0.21 | 7.6 | 0.55±0.17 | 0.54±0.21 | 4.1 |
| (missing data) | 4.5% | 3.0% | 7.8 | 5.1% | 3.4% | 8.3 |
| TransAtlantic Inter‐Society Consensus II classification* | ||||||
| Class A | 0.0% | 4.4% | 30.3 | 0.0% | 0.0% | 0.0 |
| Class B | 7.5% | 20.8% | 39.0 | 8.5% | 10.0% | 5.3 |
| Class C | 55.2% | 40.0% | 30.9 | 54.2% | 52.8% | 2.9 |
| Class D | 37.3% | 34.9% | 5.1 | 37.3% | 37.2% | 0.2 |
| Popliteal involvement | 26.9% | 32.3% | 12.0 | 28.8% | 28.1% | 1.5 |
| History of aortoiliac revascularization* | 34.3% | 25.2% | 20.1 | 30.5% | 30.3% | 0.4 |
| Below‐the‐knee runoff* | ||||||
| No runoff | 9.0% | 6.0% | 11.2 | 8.5% | 7.7% | 2.7 |
| 1 runoff | 37.3% | 29.6% | 16.5 | 32.2% | 34.0% | 3.9 |
| 2 runoffs | 38.8% | 38.6% | 0.5 | 42.4% | 41.8% | 1.2 |
| 3 runoffs | 14.9% | 25.9% | 27.4 | 16.9% | 16.4% | 1.4 |
| Distal reference vessel diameter, mm* | 5.1±0.8 | 4.9±1.0 | 23.3 | 5.1±0.9 | 5.1±1.0 | 7.5 |
| (missing data) | 1.5% | 0.0% | 17.4 | 0.0% | 0.0% | 0.0 |
| Lesion length, cm* | 23±7 | 21±9 | 25.3 | 23±7 | 22±7 | 4.4 |
| Peripheral Arterial Calcium Scoring System classification* | ||||||
| Grade 0 | 34.3% | 35.8% | 3.1 | 33.9% | 35.3% | 3.0 |
| Grade 1 | 20.9% | 18.7% | 5.5 | 22.0% | 21.8% | 0.5 |
| Grade 2 | 14.9% | 14.3% | 1.7 | 15.3% | 14.3% | 2.7 |
| Grade 3 | 3.0% | 11.1% | 32.1 | 1.7% | 2.2% | 3.4 |
| Grade 4 | 26.9% | 20.1% | 16.0 | 27.1% | 26.4% | 1.6 |
| Angio‐score* | 4.1±1.0 | 4.0±1.2 | 11.7 | 4.1±1.0 | 4.0±1.0 | 5.8 |
| (missing data) | 1.5% | 0.0% | 17.4 | 0.0% | 0.0% | 0.0 |
| Stent implantation* | 91.0% | 85.0% | 18.7 | 89.8% | 89.6% | 0.9 |
| Full‐covered stenting* | 80.6% | 68.4% | 28.3 | 78.0% | 78.9% | 2.3 |
| Stent graft use* | 20.9% | 4.8% | 49.3 | 15.3% | 12.9% | 6.9 |
| Drug‐eluting stent use* | 4.5% | 13.2% | 31.0 | 5.1% | 6.4% | 5.6 |
| Drug‐coated balloon use* | 0.0% | 0.5% | 9.6 | 0.0% | 0.0% | 0.0 |
Data are expressed as means±SD for continuous variables and percentages for categorical variables. IA indicates intraluminal approach; and SA, subintimal approach.
Variables included in a logistic regression model to estimate propensity score.
RESULTS
Study Population
Of the 1766 patients in the IVORY registry, 500 patients were enrolled in the present study (Figure 1). One‐year clinical follow‐up was completed in 389 patients (77.8%).
Figure 1. Patient flow chart.
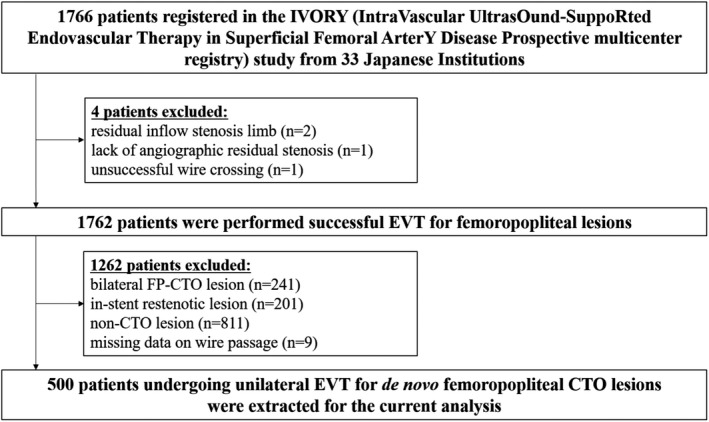
CTO indicates chronic total occlusion; EVT, endovascular therapy; and FP, femoropopliteal.
Baseline Clinical Characteristics Between SA and IA
SA and IA were performed in 67 (13.4%) and 433 (86.6%) of 500 patients, respectively. Baseline clinical characteristics of SA and IA before and after PS matching are shown in Table 1. Before PS matching, no significant differences were observed between the 2 groups except for age, prior chronic heart failure, medication (aspirin and statin use), lesion length, drug‐eluting stent use, stent graft use, and full‐covered stenting strategy. PS matching extracted 59 pairs (SA, n=59; IA, n=348), with no remarkable intergroup differences in baseline characteristics (Table 1). Representative cases of SA and IA are shown in Figures 2 and 3, respectively.
Figure 2. Representative case of subintimal approach.
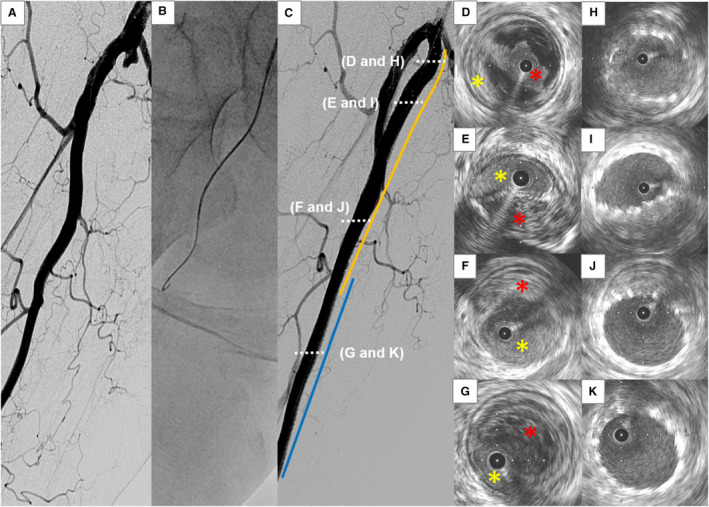
A, Angiography shows a chronic total occlusion (CTO) lesion at the right femoropopliteal artery. B, Subintimal approach is attempted with a 0.035‐inch guidewire. C, Two bare‐metal nitinol stents (orange and blue lines) are successfully implanted. D through G, Intravascular ultrasound (IVUS) images of the wire passage within the CTO lesion from proximal (D) to distal (G). The guidewire is located in an intraplaque space (red asterisk) at the proximal portion of the lesion (D), whereas it goes through a subintimal space (yellow asterisk) thereafter. H through K, IVUS images after stent deployment from proximal (H) to distal (K).
Figure 3. Representative case of intraluminal approach.
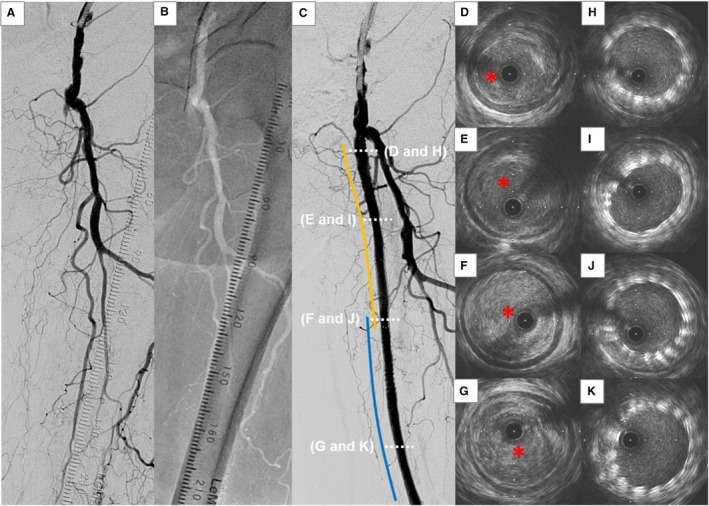
A, Angiography shows a chronic total occlusion (CTO) lesion at the left femoropopliteal (FP) artery. B, Intraluminal approach is attempted with a 0.014‐inch guidewire. C, Two bare‐metal nitinol stents (orange and blue lines) are successfully implanted. D through G, Intravascular ultrasound (IVUS) images of the wire passage within the CTO lesion from proximal (D) to distal (G). The guidewire passes all through the intraplaque space (red asterisk) within the CTO lesion. H through K, IVUS images after stent deployment from proximal (H) to distal (K).
Outcomes Between SA and IA
Table 2 summarizes the endovascular strategy and clinical outcomes between the 2 groups. The 1‐year restenosis rate was comparable between the SA and IA groups (41.0% versus 43.4%, P=0.40). SWP was significantly higher in the SA group than the IA group (61.0% versus 36.3%, P<0.001). Secondary end points did not significantly differ in both groups.
Table 2.
Outcomes of Subintimal and Intraluminal Approach After Matching
| Subintimal approach | Intraluminal approach | P value | |
|---|---|---|---|
| Endovascular treatment | |||
| Subintimal approach in successful wire crossing | 47.5% (34.7%–60.2%) | 2.2% (0.3%–4.1%) | <0.001 |
| Subintimal wire passage | 61.0% (48.6%–73.5%) | 36.3% (29.7%–42.9%) | <0.001 |
| Procedure time, min | 82.9 (74.4–92.4) | 78.6 (73.3–84.3) | 0.54 |
| Contrast volume, mL | 82.6 (64.1–106.3) | 88.3 (80.7–96.6) | 0.86 |
| Postoperative outcomes | |||
| Percentage of residual stenosis | 8.5% (1.4%–15.6%) | 6.3% (3.5%–9.1%) | 0.79 |
| Intravascular ultrasound‐derived minimum stent area, mm2 | 16.1 (14.5–17.6) | 14.9 (14.2–15.6) | 0.10 |
| Ankle‐brachial index | 0.90 (0.84–0.95) | 0.90 (0.84–0.95) | 0.96 |
| Perioperative complications | 9.5% (1.3%–17.7%) | 5.6% (1.3%–9.8%) | 0.47 |
| 1‐year clinical outcomes | |||
| Restenosis | 41.0% (21.2%–60.9%) | 43.4% (18.7%–68.1%) | 0.40 |
| All‐cause mortality | 9.4% (1.2%–16.8%) | 8.7% (0.3%–16.5%) | 0.68 |
| Major amputation | 0.0% (0.0%–0.0%) | 0.9% (0.0%–3.5%) | 0.55 |
| Major adverse limb events | 19.1% (6.8%–29.7%) | 15.0% (3.9%–24.7%) | 0.83 |
Data are presented with estimates and 95% CIs.
Baseline Clinical Characteristics and Outcomes Between SWP and IWP
SWP and IWP were observed in 186 (37.2%) and 314 (62.8%) of the patients, respectively. Baseline clinical characteristics of SWP and IWP before and after PS matching are shown in Table 3. Before PS matching, no significant differences were observed between the 2 groups except for statin use, TransAtlantic Inter‐Society Consensus II classification, lesion length, angio‐score, stent graft use, and full‐covered stenting. PS matching extracted 170 pairs (SWP, n=170; IWP, n=293), with no remarkable differences in baseline characteristics (Table 3). The 1‐year restenosis rate was not significantly different between the SWP and IWP groups (48.2% versus 40.8%, P=0.40), although the POC rate tended to be higher in the SWP group (8.2% versus 4.1%, P=0.07) with significant differences in procedural time and contrast volume (Table 4 and Table S1). Regarding other end points, no significant difference was observed in both groups. Moreover, in the SWP group, the length of subintimal track showed no significant association with clinical outcomes (Table S2).
Table 3.
Baseline Clinical Characteristics of SWP and IWP
| Variable | Overall population | Matched population | ||||
|---|---|---|---|---|---|---|
| SWP (n=186) | IWP (n=314) | SD (%) | SWP (n=170) | IWP (n=293) | SD (%) | |
| Male sex | 72.6% | 68.8% | 8.3 | 72.9% | 72.5% | 1.1 |
| Age, y | 75±8 | 74±9 | 11.7 | 75±8 | 75±8 | 1.8 |
| Current smoking | 31.7% | 33.1% | 3.0 | 32.9% | 33.9% | 2.0 |
| Diabetes mellitus | 46.8% | 55.4% | 17.3 | 47.6% | 49.0% | 2.8 |
| Chronic renal failure | 22.0% | 29.3% | 16.7 | 22.9% | 24.4% | 3.4 |
| On dialysis | 13.4% | 20.1% | 17.8 | 14.1% | 16.2% | 5.7 |
| Chronic heart failure | 12.9% | 19.4% | 17.8 | 13.5% | 14.7% | 3.3 |
| Aspirin use | 80.6% | 78.0% | 6.5 | 80.6% | 80.8% | 0.5 |
| Thienopyridine use | 80.6% | 80.9% | 0.6 | 80.6% | 80.6% | 0.0 |
| Cilostazol use | 35.5% | 32.2% | 7.0 | 35.3% | 33.9% | 3.0 |
| Statin use | 57.5% | 47.1% | 20.9 | 57.1% | 54.5% | 5.1 |
| Anticoagulant use | 16.7% | 16.2% | 1.1 | 15.9% | 17.2% | 3.5 |
| Critical limb ischemia | 29.0% | 32.2% | 6.8 | 27.1% | 29.3% | 4.9 |
| Ankle brachial index | 0.56±0.20 | 0.52±0.21 | 16.0 | 0.56±0.20 | 0.55±0.18 | 7.1 |
| (missing data) | 2.2% | 3.8% | 9.8 | 2.4% | 2.1% | 1.9 |
| TransAtlantic Inter‐Society Consensus II classification | ||||||
| Class A | 1.1% | 5.4% | 24.7 | 1.2% | 1.1% | 0.9 |
| Class B | 13.4% | 22.3% | 23.3 | 14.7% | 17.3% | 7.1 |
| Class C | 43.5% | 41.1% | 5.0 | 42.9% | 42.8% | 0.2 |
| Class D | 41.9% | 31.2% | 22.4 | 41.2% | 38.8% | 4.9 |
| Popliteal involvement | 31.2% | 31.8% | 1.4 | 30.0% | 29.8% | 0.5 |
| History of aortoiliac revascularization | 30.1% | 24.2% | 13.3 | 30.0% | 27.7% | 5.2 |
| Below‐the‐knee runoff | ||||||
| No runoff | 6.5% | 6.4% | 0.3 | 5.9% | 4.8% | 4.8 |
| 1 runoff | 29.6% | 31.2% | 3.6 | 28.8% | 30.2% | 3.1 |
| 2 runoffs | 38.7% | 38.5% | 0.4 | 39.4% | 42.3% | 5.8 |
| 3 runoffs | 25.3% | 23.9% | 3.2 | 25.9% | 22.7% | 7.5 |
| Distal reference vessel diameter, mm | 5.0±0.9 | 4.9±1.0 | 3.8 | 4.9±1.0 | 4.9±1.0 | 3.6 |
| (missing data) | 0.5% | 0.0% | 10.4 | 0.0% | 0.0% | 0.0 |
| Lesion length, cm | 23±8 | 20±9 | 41.1 | 23±8 | 23±8 | 4.0 |
| Peripheral Arterial Calcium Scoring System classification | ||||||
| Grade 0 | 39.2% | 33.4% | 12.1 | 38.8% | 37.0% | 3.7 |
| Grade 1 | 20.4% | 18.2% | 5.8 | 19.4% | 20.0% | 1.4 |
| Grade 2 | 14.5% | 14.3% | 0.5 | 15.9% | 14.1% | 5.1 |
| Grade 3 | 8.6% | 10.8% | 7.5 | 7.6% | 8.9% | 4.7 |
| Grade 4 | 17.2% | 23.2% | 15.1 | 18.2% | 20.0% | 4.5 |
| Angio‐score | 4.2±1.1 | 3.9±1.2 | 32.1 | 4.2±1.1 | 4.1±1.1 | 6.0 |
| (missing data) | 0.5% | 0.0% | 10.4 | 0.0% | 0.0% | 0.0 |
| Subintimal approach | 21.5% | 8.6% | 36.7 | 16.5% | 14.0% | 6.8 |
| Stent implantation | 89.8% | 83.4% | 18.7 | 88.8% | 88.5% | 1.1 |
| Full‐covered stenting | 76.9% | 65.9% | 24.4 | 74.7% | 72.9% | 4.2 |
| Stent graft use | 11.3% | 4.5% | 25.6 | 9.4% | 6.8% | 9.7 |
| Drug‐eluting stent use | 11.3% | 12.4% | 3.5 | 11.2% | 11.6% | 1.4 |
| Drug‐coated balloon use | 0.5% | 0.3% | 3.4 | 0.6% | 0.6% | 0.0 |
Data are expressed as means±SD for continuous variables and percentages for categorical variables. IWP indicates intraluminal wire passage; and SWP, subintimal wire passage.
Table 4.
Outcomes of Subintimal and Intraluminal Wire Passage After Matching
| Subintimal wire passage | Intraluminal wire passage | P value | |
|---|---|---|---|
| Endovascular treatment | |||
| Procedure time, min | 93.0 (86.7–99.8) | 73.5 (68.4–79.0) | <0.001 |
| Contrast volume, mL | 91.8 (80.3–105.1) | 78.5 (70.6–87.4) | 0.02 |
| Postoperative outcomes | |||
| Percentage of residual stenosis | 10.0% (5.5–14.5%) | 6.5% (3.3–9.7%) | 0.23 |
| Intravascular ultrasound‐derived minimum stent area, mm2 | 15.1 (14.3–15.8) | 15.4 (14.6–16.3) | 0.76 |
| Ankle‐brachial index | 0.91 (0.87–0.94) | 0.90 (0.85–0.95) | 0.70 |
| Perioperative complications | 8.2% (3.5–13.0%) | 4.1% (1.5–6.7%) | 0.07 |
| 1‐year clinical outcomes | |||
| Restenosis | 48.2% (33.4–63.1%) | 40.8% (18.3–63.4%) | 0.40 |
| All‐cause mortality | 5.5% (1.9–8.9%) | 8.6% (3.7–13.4%) | 0.70 |
| Major amputation | 1.7% (0.0–4.1%) | 1.3% (0.0–3.0%) | 0.98 |
| Major adverse limb events | 18.8% (11.9–25.2%) | 17.6% (10.7–23.9%) | 0.55 |
Data are estimates and 95% CIs.
DISCUSSION
The main findings of the present study were as follows: (1) both SA and IA showed acceptable results for FP‐CTO lesions at 1 year; (2) the discrepancy between the procedural approach (SA or IA) and IVUS‐derived wire‐crossing pattern (SWP or IWP) was observed in approximately one‐third of all patients; and (3) the 1‐year restenosis rate was not significantly different between the SWP and IWP groups, although the incidence of POC tended to be higher for SWP than IWP.
SA has improved with several advances in techniques and devices, resulting in increased rates of technical success and patency in FP‐CTO lesions. 3 Results of SA for FP‐CTO lesions appeared acceptable in comparison to those of IA, although there are still some concerns regarding the safety and efficacy of SA for FP‐CTO lesions because (1) previous studies could not adjust major differences in baseline clinical characteristics between SA and IA 4 , 5 ; and (2) only bare‐metal stents were used in previous studies. 4 , 5 In the present study, after PS matching, 1‐year clinical outcomes after EVT for FP‐CTO lesions were not significantly different between the SA and IA groups. These results emphasize the safety and efficacy of both approaches for FP‐CTO lesions in clinical practice, whereas some differences between the present and previous studies should be discussed. The 1‐year restenosis rate was slightly higher in the present study than in previous studies. 4 , 5 This could be explained by the fact that the current study population was older and had more comorbidities (eg, chronic kidney disease and diabetes mellitus). Notably, the procedural time was similar between the 2 groups in the present study, unlike previous studies. 4 , 5 Possible explanations for this are as follows: (1) the crossover approach (ie, an approach that switches from IA to SA) was less performed in the present study and (2) bidirectional wiring approaches and reentry devices have been introduced in clinical practice over the past decade. As such, both approaches may be acceptable strategies for treating FP‐CTO lesions.
SA is a technique that recanalizes CTO lesions by intentionally making a subintimal channel. However, because this approach was defined based on the angiographic findings or technical aspects, it remains unclear whether the guidewire truly passes through the subintimal space. The current study showed some discrepancies between the procedural strategy (SA or IA) and IVUS‐detected wire‐crossing pattern (SWP or IWP). Indeed, IWP was detected in 39.0% of SA, whereas SWP accounted for 36.3% of IA. Given that FP‐CTO lesions have greater complexities (eg, severe calcification or long occlusion), our results underscore the difficulty in controlling the wire‐crossing route regardless of the approaches. In coronary arteries, IVUS‐detected subintimal tracking was observed in approximately one‐half of all successful CTO procedures and there were no significant differences in clinical outcomes between the subintimal and intraplaque groups. 6 , 14 However, there is a paucity of data on the clinical impact of IVUS‐detected subintimal tracking in FP‐CTO lesions. In the current study, the 1‐year restenosis rate was numerically higher in the SWP group than in the IWP group but did not reach statistical significance. Intriguingly, Mori et al reported that the proportion of subintimal tracking within FP‐CTO lesions was inversely correlated with the rate of restenosis. 15 Further studies are warranted to assess the clinical implication of IVUS‐detected wire‐crossing route within the FP‐CTO lesions, although subintimal tracking may affect outcomes following EVT.
The POC occurred in ≈5% to 10% of patients undergoing EVT for FP‐CTO lesions, 4 , 5 contributing to an increased risk of mortality and major adverse limb events. 16 Accordingly, preventing POC may assist in improving outcomes of EVT for FP‐CTO lesions. In the present study, the POC rate did not significantly differ between the SA and IA groups, which was in line with previous studies. 4 , 5 In contrast, SWP showed a trend toward a higher incidence of POC than IWP. This is likely attributed to the fact that the procedural approach (SA or IA) does not guarantee the precise position of the wire within the lesions. Several attempts have been made to identify the optimal EVT strategy for FP‐CTO lesions. However, the optimal strategy is yet to be established, mainly owing to the lack of clinical evidence. For example, atherectomy devices are designed to debulk the plaque and, in some cases, to modify the plaque morphology. However, the optimal timing and extent of debulking is unclear. 17 Indeed, the choice of EVT strategy for FP‐CTO lesions in clinical practice remains largely subjective. Currently, IVUS plays a central role in optimizing the results of percutaneous coronary intervention. 18 Although there is a paucity of data regarding the clinical benefits and lack of potential algorithms for IVUS use in FP‐CTO lesions, IVUS may help standardize the EVT strategy for these lesions.
Limitations
There are several limitations in the present study. First, this was a post hoc analysis of the IVORY registry; therefore, the sample size could not be calculated. Although PS analysis was used to adjust for differences between the 2 groups, the potential influence of bias in our results could not be ruled out. Second, the 1‐year follow‐up rate was relatively low in the present study. Third, angiographic and IVUS analyses were not performed by an independent core laboratory in the current study. The location of SWP (eg, the depth of subintimal wire crossing) may affect the outcomes, whereas we could not assess this aspect in the present study. Fourth, we could not collect detailed data regarding the index procedure, such as the size, length, and maximum inflation pressure of balloon. Fifth, we could not obtain any information on the frequency of bidirectional wiring approach and reentry device use in the present study. Finally, the present study included a few cases with contemporary devices (eg, atherectomy, drug‐coated balloon, or drug‐eluting stents). Therefore, it might have difficulty in generalizing our results to the latest clinical practice. Further studies are warranted to assess the clinical implication of IVUS‐guided EVT for FP‐CTO lesions in contemporary practice.
Conclusions
At 1 year, both SA and IA showed acceptable results for FP‐CTO lesions. Cumulative 1‐year incidence of restenosis was not significantly different between SWP and IWP, whereas POC occurred more frequently in SWP than in IWP.
Sources of Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The IVORY study is sponsored by the following companies (in alphabetical order): Boston Scientific Japan K.K.; The Orbusneich Foundation; Johnson & Johnson K.K.; Medicon Inc.; Medtronic Japan Co., Ltd.; MSD K.K.; Otsuka Pharmaceutical Co., Ltd.; Terumo Corp.; and W.L. Gore & Associates, Co., Ltd. The funding companies played no role in the design of the study; the selection of the enrolled patients, treatment strategies, revascularization procedures and equipment; or the collection, analysis, and interpretation of the data.
Disclosures
None.
Supporting information
Appendix S1
Tables S1–S2
Acknowledgments
The authors thank the clinical research coordinators of the participating centers.
For Sources of Funding and Disclosures, see page 10.
See Editorial by Gutierrez and Patel
REFERENCES
- 1. Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, et al.; RESILIENT Investigators . Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve‐month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3:267–276. DOI: 10.1161/CIRCINTERVENTIONS.109.903468. [DOI] [PubMed] [Google Scholar]
- 2. Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, et al.; Zilver PTX Investigators . Paclitaxel‐eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve‐month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. DOI: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 3. London NJ, Srinivasan R, Naylor AR, Hartshorne T, Ratliff DA, Bell PR, Bolia A. Subintimal angioplasty of femoropopliteal artery occlusions: the long‐term results. Eur J Vasc Surg. 1994;8:148–155. DOI: 10.1016/S0950-821X(05)80450-5. [DOI] [PubMed] [Google Scholar]
- 4. Kim K, Ko Y‐G, Ahn C‐M, Min P‐K, Lee J‐H, Yoon C‐H, Yu CW, Lee SW, Lee S‐R, Choi SH, et al. Clinical outcomes of subintimal vs. intraluminal revascularization approaches for long femoropopliteal occlusions in a Korean Multicenter Retrospective Registry Cohort. Circ J. 2018;82:1900–1907. DOI: 10.1253/circj.CJ-17-1464. [DOI] [PubMed] [Google Scholar]
- 5. Soga Y, Iida O, Suzuki K, Hirano K, Kawasaki D, Shintani Y, Suematsu N, Yamaoka T. Initial and 3‐year results after subintimal versus intraluminal approach for long femoropopliteal occlusion treated with a self‐expandable nitinol stent. J Vasc Surg. 2013;58:1547–1555. DOI: 10.1016/j.jvs.2013.05.107. [DOI] [PubMed] [Google Scholar]
- 6. Song L, Maehara A, Finn MT, Kalra S, Moses JW, Parikh MA, Kirtane AJ, Collins MB, Nazif TM, Fall KN, et al. Intravascular ultrasound analysis of intraplaque versus subintimal tracking in percutaneous intervention for coronary chronic total occlusions and association with procedural outcomes. JACC Cardiovasc Interv. 2017;10:1011–1021. DOI: 10.1016/j.jcin.2017.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iida O, Takahara M, Soga Y, Fujihara M, Kawasaki D, Hirano K, Choi D, Mano T. A novel angiographic risk score in femoropopliteal intervention. J Endovasc Ther. 2020;27:967–973. DOI: 10.1177/1526602820948472. [DOI] [PubMed] [Google Scholar]
- 8. Kawasaki D, Iida O, Fukunaga M, Kato M, Ohkubo N. Wire passages of 0.035‐inch looped wire technique for femoropopliteal long total occlusions. J Atheroscler Thromb. 2015;22:1071–1079. DOI: 10.5551/jat.29538. [DOI] [PubMed] [Google Scholar]
- 9. Saito Y, Kobayashi Y, Fujii K, Sonoda S, Tsujita K, Hibi K, Morino Y, Okura H, Ikari Y, Honye J. Clinical expert consensus document on standards for measurements and assessment of intravascular ultrasound from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. 2020;35:1–12. DOI: 10.1007/s12928-019-00625-6. [DOI] [PubMed] [Google Scholar]
- 10. Patel MR, Conte MS, Cutlip DE, Dib N, Geraghty P, Gray W, Hiatt WR, Ho M, Ikeda K, Ikeno F, et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol. 2015;65:931–941. DOI: 10.1016/j.jacc.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rocha‐Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014;83:212–220. DOI: 10.1002/ccd.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomoi Y, Soga Y, Takahara M, Fujihara M, Iida O, Kawasaki D, Ando K. Spot stenting versus full coverage stenting after endovascular therapy for femoropopliteal artery lesions. J Vasc Surg. 2019;70:1166–1176. DOI: 10.1016/j.jvs.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 13. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. DOI: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finn MT, Doshi D, Cleman J, Song L, Maehara A, Hatem R, Redfors B, Kalra S, Fried JA, Liao M, et al. Intravascular ultrasound analysis of intraplaque versus subintimal tracking in percutaneous intervention for coronary chronic total occlusions: one year outcomes. Catheter Cardiovasc Interv. 2019;93:1048–1056. DOI: 10.1002/ccd.27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mori S, Hirano K, Yamawaki M, Kobayashi N, Sakamoto Y, Tsutsumi M, Honda Y, Makino K, Shirai S, Ito Y. Usefulness of ultrasound‐guided intraluminal approach for long occlusive femoropopliteal lesion. Heart Vessels. 2021;36:376–382. DOI: 10.1007/s00380-020-01697-8. [DOI] [PubMed] [Google Scholar]
- 16. Sato K, Iida O, Takahara M, Soga Y, Suzuki K, Tanigawa T, Ito M, Uematsu M. Effect of perioperative complications after endovascular therapy in patients with peripheral artery disease due to femoropopliteal lesions. J Vasc Surg. 2015;61:1272–1277. DOI: 10.1016/j.jvs.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 17. Shishehbor MH, Jaff MR. Percutaneous therapies for peripheral artery disease. Circulation. 2016;134:2008–2027. DOI: 10.1161/CIRCULATIONAHA.116.022546. [DOI] [PubMed] [Google Scholar]
- 18. Sonoda S, Hibi K, Okura H, Fujii K, Honda Y, Kobayashi Y. Current clinical use of intravascular ultrasound imaging to guide percutaneous coronary interventions. Cardiovasc Interv Ther. 2020;35:30–36. DOI: 10.1007/s12928-019-00603-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S2


