Abstract
Background
Although the prognostic importance of pulmonary arterial capacitance (PAC; stroke volume/pulmonary arterial pulse pressure) has been elucidated in heart failure with reduced ejection fraction, whether its significance in patients suffering from heart failure with preserved ejection fraction is not known. We aimed to examine the association of PAC with outcomes in inpatients with heart failure with preserved ejection fraction.
Methods and Results
We prospectively studied 705 patients (median age, 83 years; 55% women) registered in PURSUIT‐HFpEF (Prospective Multicenter Observational Study of Patients With Heart Failure With Preserved Ejection Fraction). We investigated the association of echocardiographic PAC at discharge with the primary end point of all‐cause death or heart failure rehospitalization with a mean follow‐up of 384 days. We further tested the acceptability of the prognostic significance of PAC in a subgroup of patients (167/705 patients; median age, 81 years; 53% women) in whom PAC was assessed by right heart catheterization. The median echocardiographic PAC was 2.52 mL/mm Hg, with a quartile range of 1.78 to 3.32 mL/mm Hg. Univariable and multivariable Cox regression testing revealed that echocardiographic PAC was associated with the primary end point (unadjusted hazard ratio, 0.82; 95% CI, 0.72–0.92; P=0.001; adjusted hazard ratio, 0.86; 95% CI, 0.74–0.99; P=0.035, respectively). Univariable Cox regression testing revealed that PAC assessed by right heart catheterization (median calculated PAC, 2.82 mL/mm Hg) was also associated with the primary end point (unadjusted HR, 0.70; 95% CI, 0.52–0.91; P=0.005).
Conclusions
A prospective cohort study revealed that impaired PAC diagnosed with both echocardiography and right heart catheterization was associated with adverse outcomes in inpatients with heart failure with preserved ejection fraction.
Registration
URL: https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000024414. Unique identifier: UMIN000021831.
Keywords: hemodynamics, HFpEF, prognosis, pulmonary arterial capacitance, pulmonary circulation, right ventricular–pulmonary arterial coupling
Subject Categories: Heart Failure, Echocardiography, Prognosis, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- PAC
pulmonary arterial capacitance
- PAPP
pulmonary artery pulse pressure
- PASP
pulmonary artery systolic pressure
- PAWP
pulmonary artery wedge pressure
- PH
pulmonary hypertension
- RHC
right heart catheterization
- RHC‐PAC
pulmonary arterial capacitance calculated with right heart catheterization
- TAPSE
tricuspid annular plane systolic excursion
Clinical Perspective
What Is New?
This is the first large observational study to evaluate pulmonary arterial capacitance in 705 patients with heart failure with preserved ejection fraction from a prospective multicenter registry in East Asia (PURSUIT‐HFpEF [Prospective Multicenter Observational Study of Patients With Heart Failure With Preserved Ejection Fraction]).
Pulmonary arterial capacitance is estimated using echocardiography as [stroke volume/0.6×pulmonary arterial systolic pressure], which is validated by right heart catheterization among hospitalized patients with heart failure with preserved ejection fraction.
Pulmonary arterial capacitance is negatively correlated with adverse outcomes of all‐cause mortality or heart failure rehospitalization.
What Are the Clinical Implications?
Among right ventricular–pulmonary arterial coupling components, pulmonary arterial capacitance is focused to predict adverse outcomes, which is able to be simply estimated with echocardiography.
Impaired pulmonary arterial capacitance is associated with increased adverse outcomes, which offers further investigations for approaches targeting pulmonary arterial capacitance to establish possible therapeutic strategies for patients with heart failure with preserved ejection fraction.
Approximately one‐half of patients with heart failure (HF) have HF with preserved ejection fraction (HFpEF). The proportion of HF cases that are HFpEF is increasing. 1 The complicated pathophysiology of HFpEF has made it difficult to improve its poor outcome, and a specific strategy is needed for this abnormality. 2
In patients with HF, the attention of researchers has shifted from the relationship between outcomes and systemic arterial compliance 3 to that between outcomes and pulmonary arterial compliance. 4 Recent studies of HFpEF have clarified the pathophysiological importance of right ventricular (RV)–pulmonary artery (PA) uncoupling, which consists of a combination of RV dysfunction and pulmonary hypertension (PH). 5 , 6 In light of these studies, we have reported the tricuspid annular plane systolic excursion (TAPSE) to pulmonary artery systolic pressure (PASP) ratio on echocardiography as an independent predictor of adverse outcomes in inpatients with HFpEF. 7 , 8 The importance of pulmonary effective vascular elastance and pulmonary arterial capacitance (PAC) has also been focused on patients with HFpEF. 9 In left‐sided HF, PAC has been shown to be a strong outcome predictor independent of pulmonary vascular resistance (PVR). 10 Although PAC had been easily calculated at right heart catheterization (RHC) by dividing the right ventricular stroke volume by the pulmonary arterial pulse pressure, 11 invasive tests are limited for patients with HFpEF in clinical settings. Although an echocardiographic method for estimating PAC was previously developed, its usability is limited because of its requirement for a pulmonary regurgitation signal, which is not seen in approximately one‐half of patients. 12 Noordegraaf et al reported that, in left‐sided HF, PA systolic and diastolic pressures are proportional to mean PA pressure. 13 Echocardiographically measured PA pulse pressure (PAPP) in patients with patent pulmonary valves allows a simple calculation of PAPP as 0.6×PASP. 14
The prognostic importance of PAC has been elucidated mainly for advanced HF with reduced ejection fraction (HFrEF). 10 , 15 In a small study (<100 patients), Al‐Naamani et al 5 reported that PAC measured with RHC had prognostic value in patients with HFpEF. Guazzi et al 6 assessed the prognostic significance of pulmonary circulation parameters including PAC in a larger study with 387 patients with HFpEF. While that study looked at the prognostic significance of the echocardiographic TAPSE/PASP ratio, this ratio bears a positive correlation with PAC. Those studies were performed in patients suspected of having PH who were undergoing RHC. 5 , 16 There have been no studies of PAC as a prognostic indicator in patients with HFpEF with recent acute decompensated HF.
In this study, we aimed to elucidate the prognostic importance of echocardiographic PAC in a hospitalized HFpEF population.
Methods
The authors declare that all supporting data are available within the article and the online supplemental files.
The Prospective Multicenter Observational Study of Patients With Heart Failure With Preserved Ejection Fraction Registry
This prospective, multicenter, observational cohort study was performed in 1024 consecutive hospitalized patients with HFpEF. Details of the PURSUIT‐HFpEF (Prospective Multicenter Observational Study of Patients With Heart Failure With Preserved Ejection Fraction) registry have been described previously. 17 Briefly, in collaboration with 31 hospitals in Japan, this large‐scale registry aimed to collect and record a comprehensive range of clinical data to define the pathophysiology and prognostic factors of patients with HFpEF. Inclusion criteria were acute decompensated HFpEF diagnosed by the Framingham criteria for HF 18 and the following: (1) left ventricular ejection fraction ≥50% and (2) NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) ≥400 ng/L or brain natriuretic peptide ≥100 ng/L on admission. Exclusion criteria were aged <20 years, severe valvular disease (aortic valve stenosis, aortic valve regurgitation, mitral valve stenosis, or mitral valve regurgitation) on admission, acute coronary syndrome on admission, life expectancy of <6 months attributable to prognosis of noncardiac diseases, or previous heart transplantation. The anonymized data were transferred to the data center of Osaka University Hospital for analysis via a data capturing system connected with electronic medical records. 19 Written informed consent was received from each participating patient. This study, including the procedure for enrollment, conformed to the principles of the Declaration of Helsinki and was approved by the institutional review board of each participating facility. It was registered under the Japanese UMIN Clinical Trials Registration (UMIN000021831).
Study Population
The 1024 inpatients with HFpEF were registered from June 2016 to July 2020. Of all the participants, we excluded 16 patients who died in the hospital. We excluded an additional 267 patients whose PAC on echocardiography at discharge were missing, comprising 172 with missing stroke volume and 195 with missing estimated PAPP (100 patients missed both), 7 patients who had severe mitral valve regurgitation at discharge for the possible overestimation of stroke volume, 36 patients with greater than moderate aortic valve stenosis, and 2 patients with greater than moderate mitral valve stenosis for the reasons of unignorable effects on hemodynamics. Finally, 705 patients were analyzed for echocardiographic PAC. In the analysis of patients whose PAC was measured with RHC, we excluded an additional 538 patients who had not undergone RHC examination (n=515) or whose PAC at RHC were missing (n=23) and analyzed the remaining 167 patients (Figure 1).
Figure 1. Overview of patients included in this study.
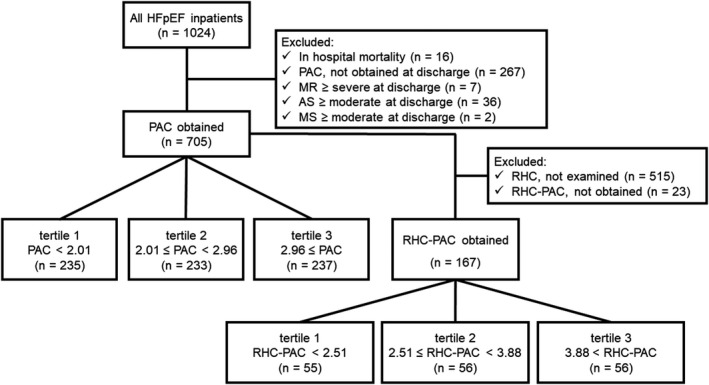
Selection process of this cohort is shown. AS, aortic valve stenosis; HFpEF, heart failure with preserved ejection fraction; MR, mitral valve regurgitation; MS, mitral valve stenosis; PAC, pulmonary artery capacitance estimated with echocardiography; RHC, right heart catheterization; RHC‐PAC, pulmonary artery capacitance measured with RHC.
Echocardiography
Comprehensive echocardiographic examinations were performed by trained cardiac sonographers according to the American Society of Echocardiography guidelines. 20 In patients with atrial fibrillation (AF), recordings of 5 to 7 consecutive beats were recommended. Measurement of systolic or diastolic parameters for 1 beat occurring after 2 serial beats with average RR interval or 1 beat with an average Doppler‐wave contour with an average velocity were also permitted in accordance with previous studies. 21 Left ventricular ejection fraction and stroke volume were calculated with the biplane Simpson’s method using apical 2‐ and 4‐chamber views. Left atrial volume index was also calculated with the biplane Simpson’s method. The ratio of peak E/e′ was calculated with the mean e′ velocity obtained from the septal and lateral sides of the mitral annulus. TAPSE was tracked in the RV‐focused apical 4‐chamber view using M‐mode echocardiography.
PASP (mm Hg) was calculated as:
| (1) |
with the pressure measurement based on the inferior vena cava diameter and collapsibility.
PAPP (mm Hg) was calculated as 14 :
| (2) |
PAC (mL/mm Hg) on echocardiography was calculated as:
| (3) |
Calculation of PAC With Invasive Hemodynamics
The indication and timing of hemodynamic evaluation by RHC were at the discretion of attending physicians. RHC was performed using a standard protocol, 22 and the following measurements were obtained: systolic (PASP), diastolic, and mean pulmonary artery pressures; pulmonary artery wedge pressure (PAWP); right atrial pressure; stroke volume; and cardiac output. Stroke volume and cardiac output were measured by the thermodilution technique. The PAC (mL/mm Hg) on RHC (RHC‐PAC) was calculated as:
| (4) |
PVR (dyne×sec/cm5) was calculated as:
| (5) |
Follow‐up and End Points
Among 705 discharged patients with PAC measured by echocardiography, 225 patients reached the primary end point of all‐cause death or rehospitalization for HF, with a mean±SD follow‐up of 384±360 days. A total of 102 patients with a mean follow‐up of 468±382 days and 162 patients with a mean of 384±360 days suffered the secondary end points of all‐cause death and HF readmission, respectively. Among 167 discharged patients with PAC calculated using RHC, 38 reached the primary end point with a follow‐up of 479±371 days. A total of 15 patients with a mean follow‐up of 540±379 days and 29 patients with a mean follow‐up of 479±371 days suffered the secondary end point of all‐cause death and HF readmission, respectively. The duration of the follow‐up period was calculated from the day of discharge until an end point, or at the time of the last patient contact (including teleconferencing).
Statistical Analysis
Data are presented as medians and interquartile ranges of 25% to 75% for continuous variables and frequency/percentage for categorical variables. Continuous variables were compared using the Kruskal‐Wallis test, and categorical variables were compared using Pearson’s chi‐squared test. Linear regression and Bland‐Altman analyses were performed for testing the concordance between echocardiographic PAC and RHC‐PAC. The clinical end point was assessed with the Kaplan‐Meier method and compared with the log‐rank test. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% CIs for the associations between clinical and hemodynamic factors and each end point. Based on our clinical experience and previous literature, multivariable Cox regression for the primary end point of the echocardiographic PAC assessed population was performed using covariates as follows: age, sex, prior HF hospitalization, history of hypertension and diabetes, systolic blood pressure, heart rate, AF, the Geriatric Nutritional Risk Index, hemoglobin concentration, NT‐proBNP, and estimated glomerular filtration rates at discharge. According to the secondary end point of all‐cause death and HF readmission, examined covariates were restricted to avoid overfitting as follows: age, sex, prior HF hospitalization, AF, Geriatric Nutritional Risk Index, hemoglobin concentration, NT‐proBNP, and estimated glomerular filtration rate. Multivariable Cox regression for the primary end point of RHC‐PAC calculated population was performed using covariates as follows: model 1—hemodynamic parameters of PAWP, right atrial pressure, and PVR; model 2—clinical aspects of age, sex, and NT‐proBNP, respectively.
All statistical tests were 2‐sided, and P<0.05 was regarded as statistically significant. Statistical analysis was performed using JMP Pro 13.2.1 (SAS Institute Inc., Chicago IL) or R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the Echocardiographic PAC Assessed Study Population
Demographic and clinical characteristics of the 705 patients are summarized in Table 1. The study population had a median age of 83 years; 55% were women. Hypertension (85%) was the most prevalent comorbidity followed by dyslipidemia and diabetes (41% and 32%, respectively), and AF was present in 39% at discharge. We divided patients into 3 groups based on the tertiles of PAC (2.01 and 2.96 mL/mm Hg). Important findings in the lower PAC group included older age, more women than men, lower body mass index, and higher presence of prior history of HF admission. Regarding the data examined at discharge, NT‐proBNP and existence of AF were significantly higher in the lower PAC group, while hemoglobin concentration, estimated glomerular filtration rate, and RV size were comparable between the groups.
Table 1.
Baseline Clinical Characteristics Divided With Tertiles of PAC
| All patients |
Tertile 1: PAC<2.01 |
Tertile 2: 2.01≤PAC<2.96 |
Tertile 3: 2.96≤PAC |
P value | |
|---|---|---|---|---|---|
| No. | 705 | 235 | 233 | 237 | |
| Age, y | 83 (78–87) | 85 (80–89) | 83 (78–87) | 81 (75–85) | <0.001 |
| Sex, female | 389 (55) | 166 (71) | 126 (44) | 97 (41) | <0.001 |
| Prior HF hospitalization | 168 (24) | 73 (32) | 55 (24) | 40 (17) | 0.002 |
| Comorbidities | |||||
| Hypertension | 599 (85) | 190 (81) | 196 (84) | 213 (90) | 0.028 |
| Diabetes | 227 (32) | 61 (26) | 80 (35) | 86 (36) | 0.041 |
| Dyslipidemia | 286 (41) | 72 (31) | 103 (44) | 111 (47) | 0.001 |
| Hyperuricemia | 227 (32) | 73 (31) | 68 (29) | 86 (37) | 0.231 |
| CKD | 278 (40) | 89 (38) | 87 (38) | 102 (43) | 0.398 |
| COPD | 55 (8) | 24 (11) | 16 (7) | 15 (7) | 0.245 |
| Malignancy | 84 (12) | 31 (13) | 24 (10) | 29 (13) | 0.635 |
| General condition at discharge | |||||
| BMI, kg/m2 | 21.3 (18.9–23.9) | 20.6 (18.5–23.3) | 21.4 (18.8–23.7) | 21.7 (19.6–24.6) | 0.004 |
| SBP, mm Hg | 119 (107–130) | 116 (103–128) | 117 (107–130) | 124 (110–134) | <0.001 |
| DBP, mm Hg | 65 (57–74) | 65 (58–74) | 66 (58–72) | 64 (56–74) | 0.665 |
| Heart rate | 70 (61–79) | 74 (64–82) | 69 (61–76) | 67 (60–77) | <0.001 |
| AF | 273 (39) | 111 (47) | 89 (38) | 73 (31) | 0.001 |
| GNRI | 92 (85–99) | 90 (84–97) | 91 (85–97) | 93 (86–101) | 0.024 |
| 6MWD, m | 250 (154–335) | 218 (130–300) | 271 (170–330) | 285 (196–376) | <0.001 |
| NYHA I/II/III/IV | 262/433/41/1 | 70/149/15/0 | 86/132/15/0 | 94/132/8/1 | 0.146 |
| Laboratory examination at discharge | |||||
| Hemoglobin, g/dL | 11.3 (10.1–12.8) | 11.1 (10.0–12.7) | 11.5 (10.3–12.9) | 11.4 (10.0–12.8) | 0.478 |
| Hematocrit, % | 34 (31–39) | 34 (31–38) | 34 (31–39) | 35 (30–39) | 0.733 |
| Serum total protein, g/dL | 6.6 (6.2–7.1) | 6.6 (6.2–7.2) | 6.6 (6.1–7.1) | 6.7 (6.2–7.2) | 0.542 |
| Serum albumin, g/dL | 3.4 (3.1–3.7) | 3.4 (3.1–3.7) | 3.4 (3.1–3.7) | 3.4 (3.2–3.7) | 0.602 |
| eGFR, mL/min per 1.73 m2 | 44 (30–55) |
40 (29–53) |
44 (33–55) | 44 (29–59) | 0.236 |
| NT‐proBNP, ng/L | 1057 (466–2372) | 1437 (752–3120) | 892 (461–2041) | 783 (375–1850) | <0.001 |
| CRP, mg/dL | 0.26 (0.11–0.75) | 0.28 (0.10–0.86) | 0.23 (0.11–0.68) | 0.28 (0.12–0.64) | 0.724 |
| Echocardiographic variables at discharge | |||||
| Echocardiography examined day | 14 (10–19) | 14 (10–21) | 13 (9–18) | 14 (10–21) | 0.224 |
| LVDd, mm | 45 (41–50) | 42 (39–46) | 45 (42–49) | 49 (45–52) | <0.001 |
| LVEDV (m‐Simpson), mL | 77 (58–101) | 54 (44–68) | 79 (64–94) | 102 (86–129) | <0.001 |
| LVEDVI (m‐Simpson), mL/m2 | 53 (41–66) | 38 (31–48) | 54 (44–63) | 66 (55–81) | <0.001 |
| LVEF (m‐Simpson), % | 61 (55–66) | 60 (55–66) | 61 (55–65) | 61 (56–66) | 0.279 |
| SV, mL | 46 (35–61) | 32 (26–41) | 46 (39–55) | 64 (53–77) | <0.001 |
| LAD, mm | 44 (39–49) | 45 (40–50) | 44 (39–48) | 44 (39–49) | 0.071 |
| LAVI, mL/m2 | 50 (37–65) | 54 (39–74) | 46 (35–63) | 48 (37–60) | 0.001 |
| E/e′ | 12.5 (9.6–16.6) | 12.6 (9.9–18.3) | 12.2 (9.5–16.5) | 12.5 (9.4–15.9) | 0.351 |
| RVD, mm | 32 (28–36) | 32 (27–36) | 32 (28–36) | 33 (29–37) | 0.324 |
| TAPSE, mm | 17.3 (14.4–20.4) | 16.0 (12.7–19.0) | 17.1 (14.5–20.0) | 18.9 (16.0–22.7) | <0.001 |
| TRPG, mm Hg | 27 (22–32) | 30 (26–39) | 26 (22–32) | 23 (18–27) | <0.001 |
| PASP, mm Hg | 31 (26–38) | 36 (30–45) | 31 (26–37) | 27 (22–32) | <0.001 |
| PAPP, mm Hg | 19 (15–23) | 22 (18–27) | 19 (16–22) | 16 (13–19) | <0.001 |
| TAPSE/PASP, mm/mm Hg | 0.54 (0.42–0.72) | 0.44 (0.33–0.54) | 0.54 (0.43–0.68) | 0.71 (0.56–0.89) | <0.001 |
| PAC, mL/mm Hg | 2.52 (1.78–3.32) | 1.53 (1.24–1.78) | 2.52 (2.24–2.72) | 3.73 (3.29–4.48) | <0.001 |
| Mitral valve regurgitation (none/trace/mild/moderate) | 38/232/310/125 | 7/74/105/49 | 15/67/113/38 | 16/91/92/38 | 0.066 |
| Tricuspid valve regurgitation (none/trace/mild/moderate/severe) | 12/235/301/141/16 | 3/41/100/81/10 | 6/67/121/34/5 | 3/127/80/26/1 | <0.001 |
| Aortic valve stenosis (none/mild) | 657/48 | 221/14 | 216/17 | 220/17 | 0.817 |
| Mitral valve stenosis (none/mild) | 690/15 | 227/8 | 228/5 | 235/2 | 0.156 |
| Medication at discharge | |||||
| Antiplatelet | 193 (27) | 53 (23) | 65 (28) | 75 (32) | 0.089 |
| ACE inhibitor or ARB | 387 (55) | 114 (49) | 130 (56) | 143 (60) | 0.034 |
| Calcium channel blocker | 342 (49) | 83 (35) | 112 (48) | 147 (62) | <0.001 |
| β‐blocker | 397 (56) | 151 (65) | 129 (55) | 117 (49) | 0.004 |
| Loop diuretics | 567 (80) | 201 (86) | 190 (82) | 176 (74) | 0.008 |
| Tolvaptan | 111 (16) | 49 (21) | 33 (14) | 29 (12) | 0.027 |
| Aldosterone antagonist | 295 (42) | 121 (51) | 93 (40) | 81 (34) | 0.001 |
| Anticoagulant | 440 (62) | 176 (75) | 151 (65) | 113 (48) | <0.001 |
| Additional assessment | |||||
| Right heart catheterization | 190 (29) | 52 (25) | 57 (27) | 81 (36) | 0.031 |
Values are given as median (interquartile range) or n (%). Age and comorbidities are given on admission and all the others are at discharge. GNRI was calculated as:
6MWD indicates 6‐minute walking distance; ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; HF, heart failure; LAD, left atrial dimension; LAVI, left atrial volume index; LVDd, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association heart failure functional class; PAC, pulmonary artery capacitance; PAPP, pulmonary artery pulse pressure; PASP, pulmonary artery systolic pressure; RVD, basal right ventricular linear dimension; SBP, systolic blood pressure; SV, stroke volume; TAPSE, tricuspid annular plane systolic excursion; and TRPG, tricuspid valve regurgitation pressure gradient.
Between‐group comparisons were performed using Kruskal‐Wallis test or Pearson’s χ2 test.
PAC and Prognosis
The higher predictability of the composite end point in lower PAC was shown when comparing end points between the patients grouped in tertiles of PAC (log‐rank P=0.046; Figure 2). The univariable Cox regression model revealed that PAC showed significant prognostic predictabilities of the primary end point (HR, 0.82; 95% CI, 0.72–0.92; P=0.001) and of the secondary end points of all‐cause mortality (HR, 0.75; 95% CI, 0.61–0.90; P=0.001) and of HF readmission (HR, 0.82; 95% CI, 0.71–0.94; P=0.003; Table 2). The multivariable Cox regression model revealed that PAC showed significant prognostic predictability of the primary end point in an independent manner from other covariates (HR, 0.86; 95% CI, 0.74–0.99; P=0.035). Although the secondary end point of HF readmission was also proven to be associated with PAC (HR, 0.81; 95% CI, 0.68–0.95; P=0.011), that of all‐cause mortality was not (HR, 0.89; 95% CI, 0.71–1.11; P=0.291).
Figure 2. Kaplan‐Meier survival curves of PAC with echocardiography.
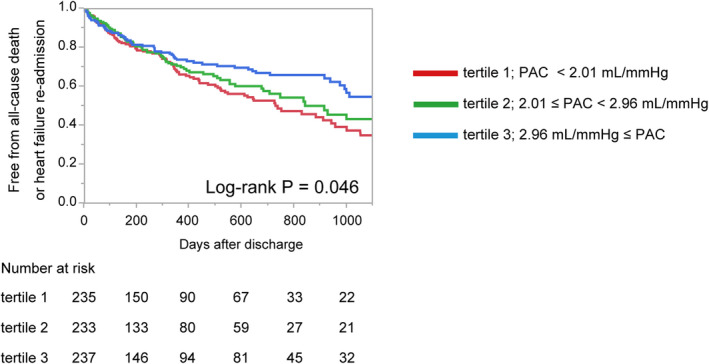
Kaplan‐Meier survival curves for prediction of composite endpoint of PAC tertiles. PAC, pulmonary artery capacitance estimated with echocardiography.
Table 2.
Cox Regression Model for Prognostic Prediction of Adverse Outcomes with PAC
| Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
|---|---|---|---|---|
| Primary end point | ||||
| Age, 5‐y increments | 1.24 (1.13–1.35) | <0.001 | 1.16 (1.04–1.30) | 0.007 |
| Female | 1.01 (0.78–1.32) | 0.938 | 0.74 (0.54–1.03) | 0.072 |
| Prior HF hospitalization | 2.04 (1.54–2.69) | <0.001 | 1.61 (1.18–2.20) | 0.003 |
| Hypertension | 0.95 (0.67–1.41) | 0.799 | 1.06 (0.69–1.71) | 0.814 |
| Diabetes | 1.05 (0.80–1.39) | 0.708 | 1.06 (0.77–1.45) | 0.701 |
| SBP, 5‐mm Hg increments | 1.01 (0.97–1.05) | 0.794 | 1.00 (0.96–1.04) | 0.959 |
| Heart rate, 5‐beat per min increments | 1.05 (1.00–1.10) | 0.056 | 1.07 (1.01–1.13) | 0.027 |
| AF | 1.19 (0.91–1.55) | 0.195 | 1.05 (0.77–1.43) | 0.757 |
| GNRI, 5‐unit increments | 0.91 (0.86–0.97) | 0.002 | 0.97 (0.89–1.04) | 0.393 |
| Hemoglobin, 1‐g/dL increments | 0.84 (0.79–0.91) | <0.001 | 0.93 (0.85–1.01) | 0.093 |
| NT‐proBNP, 1000‐ng/L increments | 1.05 (1.03–1.06) | <0.001 | 1.04 (1.01–1.06) | 0.002 |
| eGFR, 5‐mL/min per 1.73 m2 increments | 0.91 (0.87–0.94) | <0.001 | 0.95 (0.90–0.99) | 0.034 |
| PAC, 1‐mL/mm Hg increments | 0.82 (0.72–0.92) | 0.001 | 0.86 (0.74–0.99) | 0.035 |
| All‐cause mortality | ||||
| Age, 5‐y increments | 1.65 (1.42–1.91) | <0.001 | 1.67 (1.40–2.00) | <0.001 |
| Female | 0.85 (0.58–1.26) | 0.422 | 0.55 (0.34–0.90) | 0.016 |
| Prior HF hospitalization | 1.93 (1.27–2.88) | 0.002 | 2.08 (1.31–3.26) | 0.002 |
| AF | 0.99 (0.66–1.47) | 0.969 | 0.87 (0.54–1.38) | 0.550 |
| GNRI, 5‐unit increments | 0.76 (0.70–0.84) | <0.001 | 0.81 (0.72–0.91) | <0.001 |
| Hemoglobin, 1‐g/dL increments | 0.79 (0.71–0.88) | <0.001 | 0.94 (0.82–1.07) | 0.350 |
| NT‐proBNP, 1000‐ng/L increments | 1.06 (1.03–1.08) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| eGFR, 5‐mL/min per 1.73 m2 increments | 0.93 (0.88–0.98) | 0.010 | 0.98 (0.92–1.05) | 0.656 |
| PAC, 1‐mL/mm Hg increments | 0.75 (0.61–0.90) | 0.001 | 0.89 (0.71–1.11) | 0.291 |
| HF rehospitalization | ||||
| Age, 5‐y increments | 1.11 (1.00–1.22) | 0.041 | 0.98 (0.87–1.12) | 0.787 |
| Female | 1.06 (0.78–1.45) | 0.718 | 0.85 (0.59–1.24) | 0.409 |
| Prior HF hospitalization | 2.32 (1.67–3.19) | <0.001 | 1.79 (1.25–2.56) | 0.002 |
| AF | 1.45 (1.06–1.97) | 0.019 | 1.36 (0.95–1.93) | 0.095 |
| GNRI, 5‐unit increments | 1.00 (0.93–1.08) | 0.947 | 1.04 (0.95–1.13) | 0.422 |
| Hemoglobin, 1‐g/dL increments | 0.87 (0.80–0.94) | 0.001 | 0.90 (0.81–1.00) | 0.043 |
| NT‐proBNP, 1000‐ng/L increments | 1.04 (1.01–1.06) | 0.011 | 1.02 (0.98–1.05) | 0.318 |
| eGFR, 5‐mL/min per 1.73 m2 increments | 0.90 (0.86–0.94) | <0.001 | 0.94 (0.88–0.99) | 0.027 |
| PAC, 1‐mL/mm Hg increments | 0.82 (0.71–0.94) | 0.003 | 0.81 (0.68–0.95) | 0.011 |
Cox proportional hazard models of PAC for composite end point, all‐cause mortality, and heart failure rehospitalization. The composite endpoint was defined as all‐cause mortality or heart failure rehospitalization. AF indicates atrial fibrillation; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; HF, heart failure; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAC, pulmonary artery capacitance estimated with echocardiography; and SBP, systolic blood pressure.
Subgroup Assessment With RHC‐PAC
Characteristics of RHC‐PAC observed patients (including classified groups with tertiles of RHC‐PAC [2.51 and 3.88 mL/mm Hg]) is summarized in Table S1. Among the 705 patients whose echocardiographic PACs were assessed, 167 (23.7%) were able to calculate RHC‐PAC examined on a median hospitalization day of 10 days. Because of the limited number of RHC‐PAC obtained patients, several patient characteristics were different from RHC‐PAC not obtained patients (Table S2). RHC‐PAC obtained patients were younger (RHC‐PAC obtained versus RHC‐PAC not obtained; medians of 81 years versus 84 years; P<0.001) and had fewer comorbidities of AF (29% versus 42%, P=0.004), larger stroke volume (51 mL versus 45 mL, P<0.001), and smaller PAPP (18 mm Hg versus 19 mm Hg, P=0.029). It should be noted that the differences of stroke volume and PAPP between RHC‐PAC obtained and not obtained patients resulted in the significant difference of echocardiographic PAC (2.82 mL/mm Hg versus 2.42 mL/mmHg; P<0.001). RHC‐PAC had weakly concordant relationship with echocardiographic PAC (r=0.455, P<0.001, Figure S1A). At Bland and Altman analysis, the difference between echocardiographic PAC and RHC‐PAC was not significant (mean difference±SD; 0.18±0.13 mL/mm Hg; P=0.162; Figure S1B). Kaplan‐Meier curve analyses showed that RHC‐PAC was also able to categorize the predictability of the primary end point (log‐rank P=0.047; Figure S2). Univariable Cox regression model showed that RHC‐PAC was associated with the primary end point (HR, 0.70; 95% CI, 0.52–0.91; P=0.005), all‐cause mortality (HR, 0.60; 95% CI, 0.34–0.93; P=0.019), and HF readmission (HR, 0.73; 95% CI, 0.52–0.97; P=0.029). Multivariable Cox regression models revealed that RHC‐PAC showed significant prognostic predictability of the primary end point in a manner independent from other hemodynamic covariates as PAWP, right atrial pressure, and PVR (HR, 0.71; 95% CI, 0.50–0.96; P=0.023; Table 3, model 1) and also from clinical aspects as age, sex, and NT‐proBNP (HR, 0.71; 95% CI, 0.050–0.96; P=0.023; Table 3, model 2).
Table 3.
Cox Regression Model for Prognostic Prediction of Adverse Outcomes With RHC‐PAC
| Unadjusted HR (95% CI) | P value |
Adjusted HR (95% CI) |
P value |
Adjusted HR (95% CI) |
P value | |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| Primary End Point | ||||||
| Age, 5‐y increments | 1.14 (0.95–1.42) | 0.164 | 1.12 (0.91–1.42) | 0.312 | ||
| Female | 1.05 (0.55–2.01) | 0.882 | 0.86 (0.44–1.70) | 0.666 | ||
| eGFR, 5‐mL/min per 1.73 m2 increments | 0.93 (0.86–1.01) | 0.098 | ||||
| NT‐proBNP, 1000‐ng/L increments | 1.05 (1.00–1.08) | 0.040 | 1.06 (1.01–1.10) | 0.023 | ||
| PAWP, 1‐mm Hg increments | 1.04 (0.99–1.08) | 0.079 | 1.02 (0.95–1.09) | 0.588 | ||
| RAP, 1‐mm Hg increments | 1.05 (0.97–1.12) | 0.238 | 0.99 (0.89–1.11) | 0.900 | ||
| PVR, 1‐dyne/sec/cm5 increments | 1.01 (0.97–1.03) | 0.736 | 0.99 (0.96–1.02) | 0.657 | ||
| RHC‐PAC, 1‐mL/mm Hg increments | 0.70 (0.52–0.91) | 0.005 | 0.71 (0.50–0.96) | 0.023 | 0.73 (0.53–0.96) | 0.021 |
| All‐cause mortality | ||||||
| RHC‐PAC, 1‐mL/mm Hg increments | 0.60 (0.34–0.93) | 0.019 | ||||
| HF rehospitalization | ||||||
| RHC‐PAC, 1‐mL/mm Hg increments | 0.73 (0.52–0.97) | 0.029 | ||||
Cox proportional hazard models of RHC‐PAC for composite end point, all‐cause mortality, and heart failure rehospitalization. The composite end point was defined as all‐cause mortality or heart failure rehospitalization. Multivariable Cox regression for primary end point was performed using covariates as follows: model 1—hemodynamic parameters of PAWP, RAP, and PVR; model 2—clinical aspects of age, sex, and NT‐proBNP, respectively. eGFR indicates estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; and RHC‐PAC, pulmonary artery capacitance calculated with right heart catheterization.
Discussion
In a large prospective multicenter study for inpatients with HFpEF, we showed the major findings that (1) echocardiographic PAC showed a negative correlation with postdischarge adverse outcomes, which was associated independently from other covariates; and (2) RHC‐PAC was also associated with the adverse outcomes, which reinforces the prognostic predictability of echocardiographic PAC.
Although RV function has been deemphasized in the consideration of left‐sided HF for many years, it is now evident that RV dysfunction is highly prevalent and contributes to poor prognosis in patients with left‐sided HF with HFpEF. 23 Among the components related to RV dysfunction, our findings reinforce the importance of PAC in patients with HFpEF, which may point toward treatment strategies targeting the impaired PAC.
The Prognostic Impact of Impaired PAC Among Patients With HFpEF
The prognostic importance of PAC had been well established in the investigations of HFrEF patients with RHC. Pellegrini et al 15 studied 306 patients with HFrEF (mean age, 55±11 years; left ventricular ejection fraction, 23%±7%) and reported that impaired PAC was more strongly associated with adverse outcomes than other hemodynamic variables such as PAWP, cardiac output, PA mean pressure, and PVR. Dupont et al 10 studied larger sample size of 724 patients with HFrEF (mean age, 55±11 years; left ventricular ejection fraction, 19%±9%), and found that PAC showed a strong inverse relation with PVR and PAWP, and that PAC (but not PVR) remained an independent predictor for adverse outcomes in multivariable analysis. Saito et al 24 studied 30 patients with HFrEF (mean age, 53±16 years; left ventricular ejection fraction, 21%±7%), and investigated PAC with both RHC and echocardiography. They showed that the difference between the echocardiographic PAC and RHC measured PAC was insignificant, and lowered echocardiographic PAC was proven to be associated with the poor outcomes.
Al‐Naamani et al 5 previously described the prognostic importance of invasively measured PAC in HFpEF patients. In contrast with our cohort of patients with acute decompensated HFpEF, the subjects in their single‐center prospective study were patients undergoing diagnostic RHC for suspected PH. A total of 73 patients with suspected PH with HFpEF were studied, and PAC was found to be more discriminating than diastolic pressure gradient (the difference between PA diastolic pressure and PAWP), transpulmonary gradient (between PA mean pressure and PAWP) and PVR for the prediction of survival with receiver operating characteristic curve analyses. The included patients in their study were younger (mean age, 69±12 years versus 82±8 years) and more women‐prevalent (74% versus 55%) compared with those in our study. Because the subjects of their study were suspected of having PH, the hemodynamic measurements of PAWP (21±4 mm Hg versus 14±7 mm Hg), PAPP (38±16 mm Hg versus 20±9 mm Hg), stroke volume (69±29 mL versus 61±22 mL) and PVR (392±296 dyne×sec/cm5 versus 163±105 dyne×sec/cm5) were generally higher than those of our study. It is important to note that our study also revealed that invasively measured PAC had significant prognostic meanings for patients with HFpEF.
Gerges et al 25 and Guazzi et al 6 had shown that invasively measured PAC correlated with echocardiographically measured TAPSE/PASP ratio, and the latter report (a total of 387 HFpEF patients, average age of ≈65 years, and roughly 60% women) also revealed that lowered TAPSE/PASP ratio was an independent predictor of worse outcomes in patients with HFpEF. Following these reports, several studies described the prognostic importance of TAPSE/PASP ratio among patients with HFpEF. 26 However, nothing has been elucidated whether echocardiographic PAC had prognostic significance among inpatients with HFpEF. On this point, it is also important that our study revealed that echocardiographic PAC was directly associated with outcomes in inpatients with HFpEF.
Pathophysiological Importance of PAC in Patients With HFpEF
As described above, several studies have focused on the importance of RV function and pulmonary circulation in patients with HFpEF. TAPSE/PASP was proposed to be a comprehensive echocardiographic parameter for RV‐PA uncoupling and has been proven to have a linear correlation with PAC. 6 While PVR represents the static component of the RV afterload, the pulmonary impedance, effective vascular elastance, and PAC include both static and dynamic pulsatile components. 4 Vascular compliance reflects the arterial distensibility, which decreases with increasing vessel stiffness mainly as a consequence of pulmonary diseases. In patients with HF, the presence of elevated PAWP was found to have a large impact on the relationship between PAC and PVR, since PAC was lower for any PVR value. 27 Pellegrini et al 15 reported that PAC was associated with the outcomes independently from PVR in HFrEF patients. Al‐Naamani et al 5 reported that PAC had more accurate predictability of adverse outcomes than PVR in patients with HFpEF. We also found that PAC in patients with HFpEF had a prognostic value, which was not the case with PAWP and PVR (Table 3, model 1). These findings suggest that structural changes of large vessels precede the increase of PVR in patients with HF, as was observed in patients with pulmonary arterial hypertension, where the early phase of pulmonary vascular disease could not be detected by RHC with elevation of PVR but only with a reduction in PAC. 4 In our current investigation, it seems notable that the Kaplan‐Meier curve of PAC (Figure 2) showed little difference in adverse events in the early phases after discharge, but showed clear differences in the later phases. These findings also suggest that, for patients with HFpEF, impaired PAC is a sensitive initial marker among patients with RV‐PA uncoupling, predictive of the disorders occurring in the later phase.
Suggestions for Future HFpEF Treatment
Considering the prognostic relationship between PAC with adverse outcomes, the result is possible to suggest the necessity of a treatment strategy for patients with HFpEF on the basis of RV function and pulmonary circulation. The beneficial effects of β‐adrenergic agonists for HFpEF have been considered from the point of RV function and pulmonary circulation. Andersen et al 16 reported that dobutamine infusion caused greater pulmonary vasodilatation with enhanced reductions in PA resistance, greater increase in PA compliance, and a more negative slope in the PA pressure‐flow relationship in a prospective trial in patients with HFpEF. Following this study, in a randomized, double‐blind, placebo‐controlled trial for patients with HFpEF, Reddy et al 28 described the beneficial effects of an inhaled β‐adrenergic stimulant that improved the primary end point of exercise pulmonary vascular resistance. Both trials showed that beneficial effects on acute improvement were achieved with the elevation of PAC with β‐adrenergic stimulation. The abilities of these and other potential therapeutic agents to improve PAC in patients with HFpEF need to be explored in further investigations.
Limitations
Several limitations of this study should be mentioned. First, we analyzed 705 enrolled patients (303 patients discharged alive were excluded) whose echocardiographic PACs at discharge were obtained, and 167 patients (as many as 841 were excluded) whose PACs were assessed with RHC. Because RHC was not mandatory in this observational cohort study, we missed a large number of patients who did not undergo RHC examination. These exclusions could have made unavoidable selection biases. Second, the current study was performed mainly using echocardiography, whereas cardiac magnetic resonance imaging is considered the gold standard for RV functional assessment. RV function was assessed only by TAPSE and 2‐dimensional RV dimension, and other parameters such as 3‐dimensional measurement, fractional area change, RV S′, and RV global/free wall systolic strain were not assessed. Third, because we registered patients with HFpEF just based on the echocardiographic data on admission, we were not able to avoid including patients with HF with recovered ejection fraction. Fourth, cardiac sonographers were not blinded to clinical information, which may have caused a measurement bias. Moreover, measurements were done by sonographers and not evaluated by an imaging core laboratory. Fifth, we failed to identify potential diastolic dysfunction in some patients because we did not perform stress testing during echocardiography and RHC testing. Sixth, although a method for estimating PAPP from PASP has been established previously, 13 , 14 this estimation method was proven in patients with pulmonary hypertension caused by left heart failure with a mean age of 66 years. It should be noted that this formula could be applied to older patients with HFpEF in the current study. Seventh, because of the small number of events, the results of Cox regression testing among RHC‐PAC population should be interpreted carefully. We additionally performed Firth’s penalized Cox regression 29 and found that RHC‐PAC was still significantly associated with the adverse outcomes (Table S3). Further investigations are required to confirm the results of this study and to support understanding the pathophysiological meaning of PAC in patients with HFpEF.
Conclusions
We showed in a multicenter observational cohort study that PAC was an important prognostic indicator in patients with decompensated HFpEF. Echocardiographic PAC was proven to be associated with adverse outcomes, which was reinforced with the identical prognostic predictability of invasively measured PAC.
Sources of Funding
This work is funded by Roche Diagnostics K.K. (Minato‐ku, Tokyo, Japan) and Fuji Film Toyama Chemical Co. Ltd. (Chuo‐ku, Tokyo, Japan).
Disclosures
Dr Nakatani has received honoraria from Roche Diagnostics. Dr Hikoso has received personal fees from Daiichi Sankyo Company, Bayer, Astellas Pharma, Pfizer Pharmaceuticals, and Boehringer Ingelheim Japan; and received grants from Roche Diagnostics, FUJIFILM Toyama Chemical, and Actelion Pharmaceuticals. Dr Sakata received personal fees from Otsuka Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Actelion Pharmaceuticals; and received grants form Roche Diagnostic, FUJIFILM Toyama Chemical, Abbott Medical, Japan, Otsuka Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Biotronik. The remaining authors have no disclosures to report.
Supporting information
Appendix
Tables S1–S3
Figures S1–S2
Acknowledgments
The authors thank all the investigators, clinical research coordinators and data managers involved in the PURSUIT‐HFpEF registry for their dedicated contributions. In particular, the authors thank Nagisa Yoshioka, Kyoko Tatsumi, Satomi Kishimoto, Noriko Murakami, and Sugako Mitsuoka for their excellent assistance with data collection. The authors thank Libby Cone, MD, MA, from DMC Corp. (http://www.dmed.co.jp/) for editing a draft of this manuscript.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33:951–958. doi: 10.1016/S0735-1097(98)00679-2 [DOI] [PubMed] [Google Scholar]
- 4. Ghio S, Schirinzi S, Pica S. Pulmonary arterial compliance: how and why should we measure it? Glob Cardiol Sci Pract. 2015;2015:58. doi: 10.5339/gcsp.2015.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al‐Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail. 2015;3:467–474. doi: 10.1016/j.jchf.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guazzi M, Dixon D, Labate V, Beussink‐Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. doi: 10.1016/j.jcmg.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 7. Nakagawa A, Yasumura Y, Yoshida C, Okumura T, Tateishi J, Yoshida J, Abe H, Tamaki S, Yano M, Hayashi T, et al. Prognostic importance of right ventricular‐vascular uncoupling in acute decompensated heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2020;13:e011430. doi: 10.1161/CIRCIMAGING.120.011430 [DOI] [PubMed] [Google Scholar]
- 8. Nakagawa A, Yasumura Y, Yoshida C, Okumura T, Tateishi J, Yoshida J, Tamaki S, Yano M, Hayashi T, Nakagawa Y, et al. Distinctive prognostic factor of heart failure with preserved ejection fraction stratified with admission blood pressure. ESC Heart Fail. 2021;8:3145–3155. doi: 10.1002/ehf2.13420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, Kelemen BW, Houston BA, Kolb TM, Damico R, et al. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail. 2018;11:e004436. doi: 10.1161/CIRCHEARTFAILURE.117.004436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dupont M, Mullens W, Skouri HN, Abrahams Z, Wu Y, Taylor DO, Starling RC, Tang WH. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ Heart Fail. 2012;5:778–785. doi: 10.1161/CIRCHEARTFAILURE.112.968511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tedford RJ. Determinants of right ventricular afterload (2013 Grover Conference series). Pulm Circ. 2014;4:211–219. doi: 10.1086/676020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahapatra S, Nishimura RA, Oh JK, McGoon MD. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr. 2006;19:1045–1050. doi: 10.1016/j.echo.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 13. Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047 [DOI] [PubMed] [Google Scholar]
- 14. Handoko ML, De Man FS, Oosterveer FP, Bogaard HJ, Vonk‐Noordegraaf A, Westerhof N. A critical appraisal of transpulmonary and diastolic pressure gradients. Physiol Rep. 2016;4. doi: 10.14814/phy2.12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 2014;145:1064–1070. doi: 10.1378/chest.13-1510 [DOI] [PubMed] [Google Scholar]
- 16. Andersen MJ, Hwang SJ, Kane GC, Melenovsky V, Olson TP, Fetterly K, Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular: pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:542–550. doi: 10.1161/CIRCHEARTFAILURE.114.002114 [DOI] [PubMed] [Google Scholar]
- 17. Suna S, Hikoso S, Yamada T, Uematsu M, Yasumura Y, Nakagawa A, Takeda T, Kojima T, Kida H, Oeun B, et al. Study protocol for the PURSUIT‐HFpEF study: a prospective, multicenter, observational study of patients with heart failure with preserved ejection fraction. BMJ Open. 2020;10:e038294. doi: 10.1136/bmjopen-2020-038294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–2121. doi: 10.1161/01.CIR.101.17.2118 [DOI] [PubMed] [Google Scholar]
- 19. Matsumura Y, Hattori A, Manabe S, Takahashi D, Yamamoto Y, Murata T, Nakagawa A, Mihara N, Takeda T. Case report form reporter: a key component for the integration of electronic medical records and the electronic data capture system. Stud Health Technol Inform. 2017;245:516–520. doi: 10.3233/978-1-61499-830-3-516 [DOI] [PubMed] [Google Scholar]
- 20. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 21. Abe Y, Akamatsu K, Ito K, Matsumura Y, Shimeno K, Naruko T, Takahashi Y, Shibata T, Yoshiyama M. Prevalence and prognostic significance of functional mitral and tricuspid regurgitation despite preserved left ventricular ejection fraction in atrial fibrillation patients. Circ J. 2018;82:1451–1458. doi: 10.1253/circj.CJ-17-1334 [DOI] [PubMed] [Google Scholar]
- 22. Krishnan A, Markham R, Savage M, Wong YW, Walters D. Right heart catheterisation: how to do it. Heart Lung Circ. 2019;28:e71–e78. doi: 10.1016/j.hlc.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 23. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saito Y, Ohtani T, Kioka H, Onishi T, Tsukamoto Y, Nakamoto K, Taniguchi T, Nakatani S, Hirayama A, Sakata Y. Clinical significance of pulmonary arterial capacitance calculated by echocardiography in patients with advanced heart failure. Circ J. 2017;81:1871–1878. doi: 10.1253/circj.CJ-16-1318 [DOI] [PubMed] [Google Scholar]
- 25. Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, Binder T, Lang IM. Pulmonary hypertension in heart failure. epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192:1234–1246. doi: 10.1164/rccm.201503-0529OC [DOI] [PubMed] [Google Scholar]
- 26. Gorter TM, van Veldhuisen DJ, Voors AA, Hummel YM, Lam CSP, Berger RMF, van Melle JP, Hoendermis ES. Right ventricular‐vascular coupling in heart failure with preserved ejection fraction and pre‐ vs. post‐capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2018;19:425–432. doi: 10.1093/ehjci/jex133 [DOI] [PubMed] [Google Scholar]
- 27. Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reddy YNV, Obokata M, Koepp KE, Egbe AC, Wiley B, Borlaug BA. The β‐adrenergic agonist albuterol improves pulmonary vascular reserve in heart failure with preserved ejection fraction. Circ Res. 2019;124:306–314. doi: 10.1161/CIRCRESAHA.118.313832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57:114–119. doi: 10.1111/j.0006-341X.2001.00114.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Tables S1–S3
Figures S1–S2


