Abstract
Background
Little is known about the impact of center volume on outcomes in acute myocardial infarction complicated by cardiogenic shock. The aim of this study was to investigate the association between center volume, treatment strategies, and subsequent outcome in patients with acute myocardial infarction complicated by cardiogenic shock.
Methods and Results
In this subanalysis of the randomized CULPRIT‐SHOCK (Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock) trial, study sites were categorized based on the annual volume of acute myocardial infarction complicated by cardiogenic shock into low‐/intermediate‐/high‐volume centers (<50; 50–100; and >100 cases/y). Subjects from the study/compulsory registry with available volume data were included. Baseline/procedural characteristics, overall treatment, and 1‐year all‐cause mortality were compared across categories. n=1032 patients were included in this study (537 treated at low‐volume, 240 at intermediate‐volume, and 255 at high‐volume centers). Baseline risk profile of patients across the volume categories was similar, although high‐volume centers included a larger number of older patients. Low‐/intermediate‐volume centers had more resuscitated patients (57.5%/58.8% versus 42.2%; P<0.01), and more patients on mechanical ventilation in comparison to high‐volume centers. There were no differences in reperfusion success despite considerable differences in adjunctive pharmacological/device therapies. There was no difference in 1‐year all‐cause mortality across volume categories (51.1% versus 56.5% versus 54.4%; P=0.34).
Conclusions
In this study of patients with acute myocardial infarction complicated by cardiogenic shock, considerable differences in adjunctive medical and mechanical support therapies were observed. However, we could not detect an impact of center volume on reperfusion success or mortality.
Keywords: acute myocardial infarction, cardiogenic shock, center volume, extracorporeal cardiac life support, intensive care
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Treatment, Mortality/Survival, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- CULPRIT‐SHOCK
Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock
Clinical Perspective
What Is New?
We addressed the issue of center volume in relation to outcomes in patients with cardiogenic shock early after myocardial infarction, and found no difference in 1‐year all‐cause mortality across a wide range of cardiogenic shock cases from <50 to >100 cases per year.
The treatment of cardiogenic shock differed between hospitals according to case volume, with highly significant differences in use of P2Y12 and GP IIb/IIIa inhibitors as well as the use of mechanical cardiac support.
What Are the Clinical Implications?
These findings call for a harmonization of all aspects in cardiogenic shock care ranging from adjunctive medical therapy to mechanical devices, but also challenge the perception that higher annual volume equals better care, when patients are treated in experienced centers dedicated to the treatment of myocardial infarction and its immediate complications.
In the field of invasive cardiology, as with many other disciplines in medicine and surgery, the impact of center volume and experience as well as individual operator volume on subsequent outcomes is often discussed. In general, the data suggest that higher volume/experience leads to improved management, so that pooling more procedures at fewer centers would subsequently improve outcomes in specific patient populations. In this regard, it has previously been suggested that higher center/operator volume translates into better outcome for patients with elective and urgent percutaneous coronary intervention (PCI). 1 , 2 , 3 , 4 , 5 Consequently, treatment of myocardial infarction is often organized in networks of several hospitals to improve procedural aspects, a strategy endorsed by European and American guidelines. 6 , 7
Cardiogenic shock with or without cardiac arrest complicating myocardial infarction is associated with a very high mortality, which exceeds 40% in most series. 8 , 9 , 10 To improve the survival of affected patients, many smaller hospitals without invasive treatment capabilities as well as hospitals organized in networks have protocols in place for the transfer of patients with cardiogenic shock to dedicated hubs with more resources. However, little is known about the association between center volume, PCI, and medical management strategies and subsequent outcome in this patient population.
Based on a subanalysis from the CULPRIT‐SHOCK (Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock) trial, the aim of this study was to analyze the associations between cardiogenic shock center volume and baseline/procedural characteristics, overall use of treatment options, and 1‐year all‐cause mortality in patients with myocardial infarction complicated by cardiogenic shock.
Methods
The data supporting the findings presented in this study are available from the Stiftung Institut für Herzinfarktforschung, Ludwigshafen am Rhein, Germany upon reasonable request.
Setting
The design of the CULPRIT‐SHOCK trial has been reported previously. 11 In short, CULPRIT‐SHOCK was an open‐label, multicenter trial randomizing patients with myocardial infarction complicated by cardiogenic shock and multivessel coronary artery disease to culprit‐lesion‐only PCI (and potentially staged revascularization) versus immediate multivessel PCI. Patients not eligible for randomization could be entered into the CULPRIT‐SHOCK registry. Both were approved by local ethics committees and institutional review boards, and in all subjects written informed consent was obtained.
Center Volume Substudy
For this subanalysis, study sites were categorized based on their annual volume of cardiogenic shock cases into low‐volume (<50 cardiogenic shock cases per year, ≈1 case per week), intermediate‐volume (50–100 cardiogenic shock cases per year, ≈1–2 cases per week), and high‐volume (>100 cardiogenic shock cases per year, more than 2 cases per week) centers. Information on annual volume of cardiogenic shock cases and other center characteristics was self‐reported by each study site based on hospital data and International Classification of Diseases and Related Health Problems, Tenth Revision (ICD) codes.
Subjects enrolled in the main study and registry were then allocated to the low‐volume versus intermediate‐volume versus high‐volume center group based on the study site in which the subjects were enrolled and treated. There were no secondary transfers after enrollment. Variables of interest were baseline, presentation and procedural characteristics, overall treatment, and outcome. For the latter, 1‐year all‐cause mortality was assessed. If the information on the cardiogenic shock center volume was missing for a study site, patients enrolled at this site were excluded from the analysis.
Statistical Analysis
Continuous variables are presented as mean (±SD) or median (interquartile range) and compared using the Kruskal–Wallis test; categorical variables are presented as counts (frequencies) and compared using the Pearson χ2 test. For the outcome of 1‐year all‐cause mortality, all deaths that occurred within 365 days after randomization (patients from the randomized controlled trial) or after screening (patients from the registry) were considered. The Kaplan–Meier method was used to estimate survival functions in subjects enrolled at low‐volume versus intermediate‐volume versus high‐volume centers. To investigate the association between center volume and 1‐year all‐cause mortality, a Cox regression model was fitted adjusted for age, male sex, family history of coronary artery disease, previous congestive heart failure, known peripheral artery disease, mechanical ventilation, resuscitation within 24 hours, left bundle‐branch block, known renal insufficiency, and atrial fibrillation. These variables were selected based on clinical expertise and knowledge from previous analyses. Hazard ratios (HR) and 95% CI were calculated based on this multivariable‐adjusted Cox regression model. All subjects enrolled in the main study or the registry and with available information on cardiogenic shock center volume of the enrollment site were included in this analysis, irrespective of allocated treatment group or actual treatment. Two‐sided P values <0.05 was considered to be statistically significant. All analyses were performed using SAS statistical package, version 9.4, 12 and R version 3.5.3 (R‐package ggplot2) was used for data visualization. 13 , 14
Results
Center Characteristics
Information on cardiogenic shock center volume was available for 74 out of 83 study sites, and a total of 1032 patients, including registry and randomized patients, were included in this substudy (Figure 1). Among these sites, 39 centers enrolling 537 patients were categorized as low‐volume centers, 22 centers enrolling 240 patients were categorized as intermediate‐volume centers, and 13 centers enrolling 255 patients were categorized as high‐volume centers. Other center characteristics were similarly distributed as cardiogenic shock volume, such that high‐volume centers also performed more angiographies per year, more PCI (overall, urgent, and chronic total occlusion) per year, had more cardiogenic shock cases treated with mechanical circulatory support per year, and had a higher intensive care unit capacity (overall beds and cardiology beds; Figure 2). However, high‐volume centers tended to receive more patients as referrals from other centers than low‐ or intermediate‐volume centers (in 63.1% of the high‐volume centers, >50% of the patients are transfers versus 16.5%/5.8%; P<0.001).
Figure 1. Study flow chart.
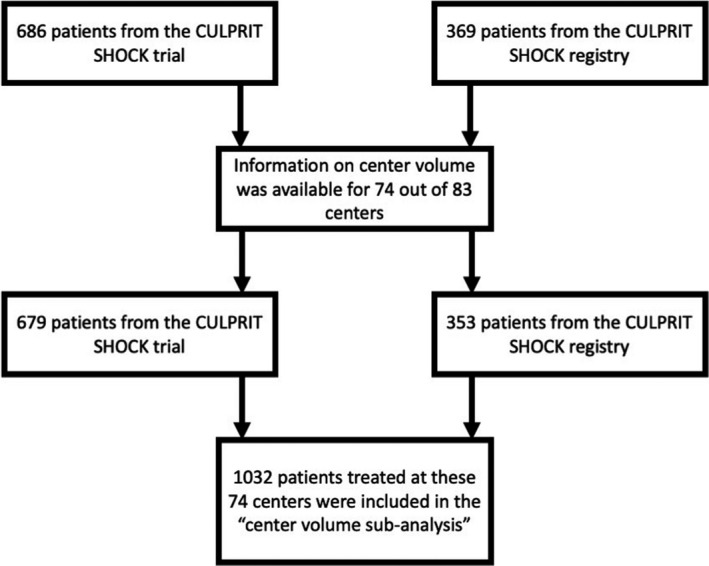
CULPRIT‐SHOCK indicates Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock.
Figure 2. Frequency distribution of invasive coronary procedures, use of mechanical support devices, and intensive care capacity in hospitals according to cardiogenic shock center volume.
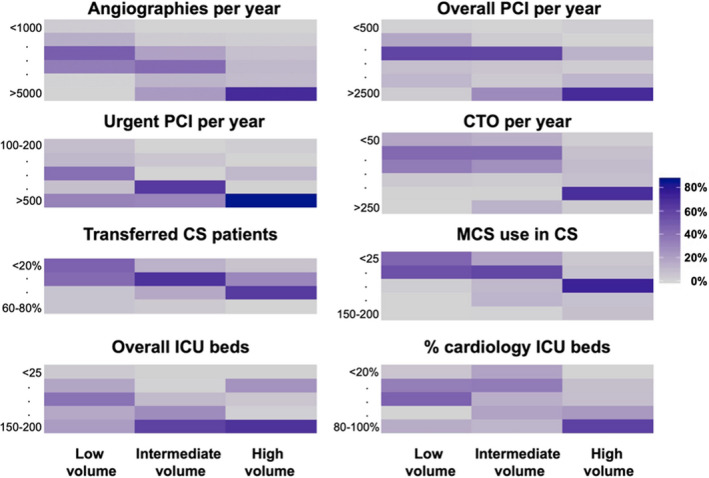
CS indicates cardiogenic shock; CTO, chronic total occlusion; ICU, intensive care unit; MCS, mechanical circulatory support; and PCI, percutaneous coronary intervention.
Patient Characteristics
Patients enrolled at intermediate‐volume centers were younger, had less peripheral artery disease, and were less likely to present with previous β‐blocker therapy. Additionally, the prevalence of a family history of coronary artery disease differed across center volume. However, most other baseline characteristics were equally distributed between the 3 groups (Table 1).
Table 1.
Baseline Characteristics Stratified by Center Volume
|
Low‐volume center (n=537, 52%) |
Intermediate‐volume center (n=240, 23%) |
High‐volume center (n=255, 25%) |
|
|---|---|---|---|
| Demographics | |||
| Age, y | 68 (±12) | 66 (±13) | 69 (±11) |
| Sex, male | 396 (74.4) | 187 (78.6) | 180 (72.6) |
| Body mass index, kg/m2 | 27.4 (±4.8) | 27.5 (±4.4) | 27.1 (±4.0) |
| Cardiovascular risk factors | |||
| Current smoking | 161 (31.4) | 65 (28.0) | 63 (28.6) |
| Hypertension | 319 (61.2) | 128 (54.0) | 146 (65.5) |
| Dyslipidemia | 186 (35.7) | 64 (27.0) | 77 (34.8) |
| Diabetes mellitus | 168 (32.3) | 64 (27.0) | 65 (29.0) |
| Family history of CAD | 82 (16.3) | 25 (11.0) | 18 (8.2) |
| Medical history | |||
| Previous myocardial infarction | 83 (15.9) | 40 (16.9) | 40 (17.7) |
| Previous PCI | 92 (17.6) | 41 (17.3) | 43 (19.0) |
| Previous CABG | 27 (5.1) | 11 (4.6) | 11 (4.8) |
| Previous congestive heart failure | 41 (7.8) | 18 (7.6) | 29 (12.8) |
| Previous stroke | 40 (7.6) | 15 (6.3) | 20 (8.9) |
| Known peripheral artery disease | 59 (11.2) | 17 (7.2) | 33 (14.5) |
| Chronic kidney disease | 27 (5.2) | 16 (6.8) | 20 (8.8) |
| Chronic dialysis | 5 (1.0) | 3 (1.3) | 6 (2.7) |
| Previous drug therapy | |||
| Aspirin | 185 (43.7) | 62 (36.7) | 70 (40.9) |
| Clopidogrel | 47 (11.3) | 17 (10.1) | 20 (11.9) |
| Prasugrel | 7 (1.7) | 4 (2.4) | 4 (2.4) |
| Ticagrelor | 20 (4.8) | 3 (1.8) | 6 (3.6) |
| Vitamin K antagonists | 23 (5.6) | 10 (5.9) | 14 (8.3) |
| β‐Blocker | 158 (38.7) | 53 (31.5) | 81 (48.2) |
| Renin angiotensin system inhibitor | 183 (44.9) | 69 (41.1) | 82 (48.0) |
| Statin | 164 (40.1) | 50 (29.9) | 61 (36.1) |
CABG indicates coronary artery bypass graft; CAD, coronary artery disease; and PCI, percutaneous coronary intervention.
Regarding presentation characteristics, patients enrolled at low‐ or intermediate‐volume centers were more likely to receive mechanical ventilation and to have had prior resuscitation, whereas patients enrolled at high‐volume centers had more severely depressed TIMI (Thrombolysis in Myocardial Infarction trial) flow before revascularization. There were no significant differences in regard to the number of diseased vessels, culprit‐lesion artery, hemodynamics, renal function, or hypoperfusion (Table 2).
Table 2.
Presentation Characteristics Stratified by Center Volume
|
Low‐volume center (n=537, 52%) |
Intermediate‐volume center (n=240, 23%) |
High‐volume center (n=255, 25%) |
|
|---|---|---|---|
| Coronary artery characteristics | |||
| Number of diseased vessels | |||
| Single‐vessel disease | 76 (14.3) | 34 (14.5) | 27 (10.9) |
| Double‐vessel disease | 171 (32.3) | 68 (28.9) | 75 (30.4) |
| Triple‐vessel disease | 283 (53.4) | 133 (56.6) | 145 (58.7) |
| Coronary artery with culprit lesion | |||
| RCA | 154 (29.1) | 62 (26.7) | 68 (27.6) |
| Left main | 51 (9.6) | 14 (6.0) | 16 (6.5) |
| LAD | 214 (40.4) | 108 (46.6) | 113 (45.9) |
| CFX | 102 (19.2) | 46 (19.8) | 48 (19.5) |
| CABG | 9 (1.7) | 2 (0.9) | 1 (0.4) |
| TIMI‐flow pre‐PCI (culprit lesion) | |||
| 0 | 262 (50.5) | 147 (62.6) | 154 (63.4) |
| 1 | 68 (13.1) | 26 (11.1) | 28 (11.5) |
| 2 | 92 (17.7) | 21 (8.9) | 37 (15.2) |
| 3 | 97 (18.7) | 41 (17.4) | 24 (9.9) |
| Clinical characteristics | |||
| Mechanical ventilation | 333 (63.2) | 141 (59.5) | 121 (52.6) |
| Fibrinolysis within 24 h | 33 (6.3) | 12 (5.0) | 16 (7.0) |
| Resuscitation within 24 h | 303 (57.5) | 140 (58.8) | 97 (42.2) |
| Systolic blood pressure, mm Hg | 106 (±30) | 106 (±32) | 106 (±31) |
| Diastolic blood pressure, mm Hg | 65 (±20) | 65 (±23) | 64 (±18) |
| Heart rate, bpm | 91 (±27) | 89 (±24) | 93 (±28) |
| ST‐elevation or LBBB | 375 (73.0) | 174 (74.4) | 159 (71.3) |
| Atrial fibrillation | 63 (12.2) | 22 (9.4) | 37 (16.4) |
| Creatinine, μmol/L | 111 (90, 141) | 106 (88, 139) | 116 (93, 149) |
| Arterial lactate, mmol/L | 5.4 (2.7, 8.9) | 5.2 (2.6, 8.7) | 4.4 (2.6, 8.1) |
| Mechanical circulatory support | 165 (30.7%) | 88 (36.7%) | 49 (19.2%) |
| IABP | 70 (42.4%) | 29 (33.0%) | 21 (42.9%) |
| Impella 2.5 | 30 (18.2%) | 12 (13.6%) | 2 (4.1%) |
| Impella CP | 37 (22.4%) | 20 (22.7%) | 11 (22.4%) |
| VA‐ECMO | 35 (21.2%) | 35 (39.8%) | 23 (46.9%) |
| Other | 4 (2.4%) | 2 (2.3%) | 0 (0%) |
Bpm indicates beats per minute; CABG, coronary artery bypass graft; CFX, circumflex artery; IABP, intra‐aortic balloon pump; LAD, left anterior descending artery; LBBB, left bundle‐branch block; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction; and VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation therapy.
Treatment
Adjunctive medical therapies as well as mechanical circulatory support differed significantly between patients enrolled at low‐ versus intermediate‐ versus high‐volume centers (Figure 3).
Figure 3. Use of treatment stratified by center volume.
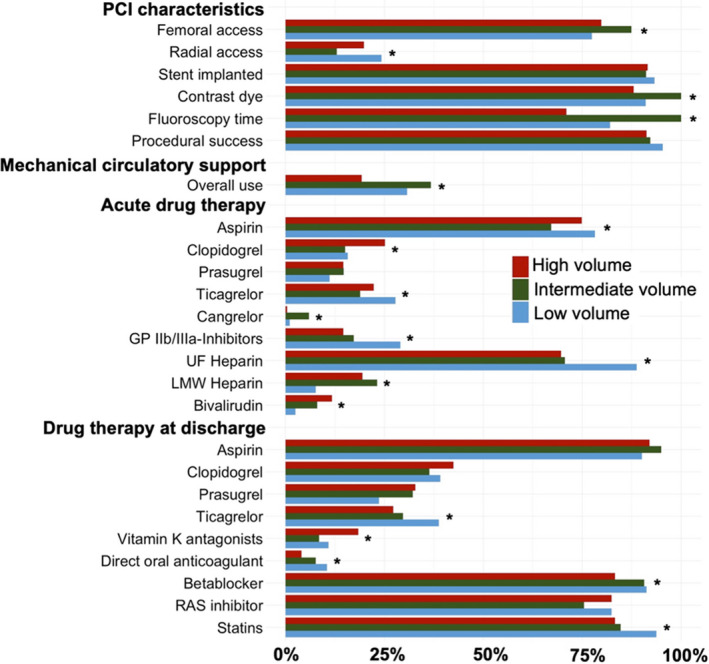
Contrast dye and fluoroscopy time are shown as relative values comparing low‐/high‐volume centers vs intermediate‐volume centers. GP IIb/IIIa‐Inhibitors, glycoprotein IIb/IIIa inhibitors; LMW heparin, low‐molecular weight heparin; PCI, percutaneous coronary intervention; RAS inhibitor, renin‐angiotensin‐aldosterone inhibitor; and UF heparin, unfractionated heparin. * Statistical difference.
Patients enrolled in intermediate‐volume centers were less likely to receive PCI via radial access and more likely to receive more contrast dye and have longer fluoroscopy time as compared with patients enrolled at low‐ or high‐volume centers; but there were no differences in PCI procedural success, defined as either TIMI‐III flow in the culprit artery or complete revascularization. Mechanical circulatory support was more often used in patients enrolled in low‐ or intermediate‐volume centers. Among patients treated with mechanical circulatory support at high‐volume centers, extracorporeal membrane oxygenation was the most frequently used device, and the percentage of use was higher compared with patients treated with mechanical circulatory support at low‐ or intermediate‐volume centers (21.2% [35/165] versus 39.8% [35/88] versus 46.9% [23/49], P<0.01).
Patients enrolled at low‐volume centers were more likely to be treated with unfractionated heparin, glycoprotein IIb/IIIa inhibitors, and ticagrelor, whereas patients enrolled in high‐volume centers were more likely to receive clopidogrel and bivalirudin.
Outcome
During 365 days after randomization or entering the registry, 535 deaths (51.8%) occurred in the overall study cohort. The estimated 1‐year mortality was 50.8% (95% CI, 46.4%–55.0%) in patients enrolled at low‐volume centers, 56.4% (95% CI, 49.8%–62.4%) in patients enrolled at intermediate‐volume centers, and 54.4% (95% CI, 48.0%–60.3%) in patients enrolled at high‐volume centers. After adjustment for relevant confounders, there was no significant difference in the risk of 1‐year mortality between patients enrolled in low‐ versus intermediate‐volume centers (HR, 1.15 [95% CI, 0.92–1.43], P=0.22) and low‐ versus high‐volume centers (HR, 0.95 [95% CI 0.76–1.20], P=0.69) (Figure 4).
Figure 4. Outcome stratified by center volume.
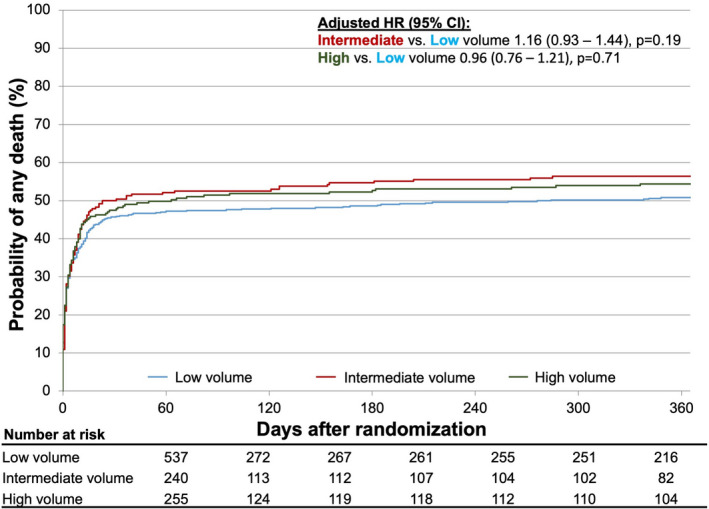
HR indicates hazard ratio.
Discussion
In this subanalysis from the CULPRIT‐SHOCK trial, there were large differences in adjunctive antithrombotic therapies during and after PCI and use of mechanical circulatory support in patients with myocardial infarction complicated by cardiogenic shock treated at low‐ versus intermediate‐ versus high‐volume centers. Despite these variations, 1‐year all‐cause mortality was comparable across centers irrespective of annual cardiogenic shock volume.
The only evidence‐based treatment improving outcomes in myocardial infarction complicated by cardiogenic shock is early revascularization of the culprit artery. 9 , 15 , 16 Nevertheless, cardiogenic shock was almost always an exclusion criterion in acute coronary syndrome trials investigating adjunctive therapies in this field, so that the generated evidence on myocardial infarction itself does not necessarily cover the subpopulation of patients with myocardial infarction complicated by cardiogenic shock. Consequently, the management of patients with cardiogenic shock is much less driven by evidence‐based recommendations.
This study describes a marked variation in the management of patients with myocardial infarction complicated by cardiogenic shock based on annual center volume. This ranges from technical aspects of the PCI (eg, arterial access) to use of mechanical circulatory support and choice of adjunctive antithrombotic therapy. However, this was not associated with differences in outcome, because PCI procedural success and 1‐year all‐cause mortality were comparable across centers with a low‐ versus intermediate versus high annual volume of cardiogenic shock cases.
Our findings differ from 2 previous studies on this topic, although variations in study design might explain the observed differences. In a US‐based administrative database, Shaefi et al did observe a higher in‐hospital mortality in patients with cardiogenic shock treated at hospitals with lower annual case volume. 17 Although this study covers a large sample of patients, there was no adjustment for cardiogenic shock–specific confounders such as prior resuscitation, and patients who died in a nursing home or hospice care were excluded. 17 Hence, differences in cardiogenic shock severity and the proportion of patients discharged to care facilities may help to explain the difference between our findings and those of Shaefi et al. Based on a national PCI registry in Japan, Kubo et al evaluated the volume‐outcome association of cardiogenic shock in patients undergoing PCI. 18 Here, the authors report that lower annual PCI volume was independently associated with worse outcome. 18 In this study, cardiogenic shock was mostly defined via hypotension (not hypoperfusion) and overall mortality was very low, at 13%, suggesting a somewhat selected population differing from a real‐world consecutive cardiogenic shock cohort. 18 Therefore, the findings might be more applicable to patients with less severe/developing cardiogenic shock as described by the recent Society for Cardiovascular Angiography and Interventions cardiogenic shock classification. 19 , 20 , 21 Additionally, hospital volume was defined based on annual PCI procedures with <640 procedures performed over 3 years in the lowest category; so this study supports the volume–outcome association previously described for urgent PCI, rather than conflicting with our findings.
Ultimately, the following conclusions can be drawn from this study: Reperfusion success and 1‐year outcomes did not differ across the spectrum of experience or center volume. This being said, most adjunctive therapies differed depending on center volume, including antithrombotic therapy, renal replacement, and even the use of mechanical support. The lack of evidence‐based treatment protocols might potentially explain the high mortality in the overall cardiogenic shock population and in this study. 22 Additionally, the treatment heterogeneity underscores the urgent need for standardized protocols and accelerated research to answer the open questions (eg, what are the best adjunctive treatments in cardiogenic shock?). In the future, dedicated treatment protocols might help to improve outcomes in these patients (eg, via guiding the use of mechanical circulatory support towards patients who might benefit the most). In this regard, 2 recent studies have already reported encouraging results for standardized team‐based care for cardiogenic shock. 23 , 24 Additionally, the National Cardiogenic Shock Initiative reported promising results based on an algorithm focusing on left ventricular unloading for the treatment of cardiogenic shock, indicating that a more targeted application of these devices could potentially improve survival of affected patients. 25
Nevertheless, our study should not be interpreted as an opportunity to further decentralize the care for patients with cardiogenic shock, because all participating centers in this study had 24/7 availability for coronary angiography/intervention, access to level 3 intensive care unit, which is a minimum requirement as per current guidelines, 16 but also selected based on scientific interest and level of care by the steering committee. Hence, all participating centers are considered Level 1 Cardiogenic Shock Care Centers, 26 which provide the needed expertise and treatment options for this patient population, and thus mainly differ in overall case volume, but not in individual expertise.
Rather, this study should be understood as a reminder that more work is needed in this field, and a call for a harmonization of the therapeutic approach to myocardial infarction complicated by cardiogenic shock.
Limitations
This study is based on the largest randomized trial and its accompanying registry in the field of cardiogenic shock, with near‐complete data capture regarding center volume and complete follow‐up of enrolled patients. The great depth of available data allowed us to provide a clear picture of patient characteristics, management, and outcomes. However, we could only consider the available variables in our analysis, so unknown or unmeasured confounding cannot be ruled out. While the data are extremely valid for the participating centers, generalizability to other centers might be limited. Additionally, 9 trial sites did not participate in this substudy, so that patients enrolled at these centers were not considered, which might have influenced our findings. Data on the duration between onset of shock and treatment were not available for this analysis, so that longer treatment delays (eg, during transfer of patients to high‐volume centers) might have influenced the results. Lastly and most importantly, the present study was conducted within the framework of a network of hospitals and investigators with interest and experience in treating cardiogenic shock and doing complex clinical trials. However, the data do reflect the current reality of triage and treatment of patients with cardiogenic shock, because most of the patients in the low‐ and intermediate‐volume hospitals were directly admitted whereas the high‐volume centers recruited most of their patients through transfers from surrounding spoke hospitals, which is also highlighted by the more severely depressed TIMI flow in those patients.
Conclusions
This CULPRIT‐SHOCK trial and registry analysis revealed major differences in PCI‐related adjunctive antithrombotic therapies and use of mechanical circulatory support between low‐, intermediate‐, and high‐volume centers when treating cardiogenic shock in patients with myocardial infarction. However, the mortality risk was comparable across centers, despite adjustment for several relevant confounders. These observations are provocative and should call for a harmonization of the therapeutic approach to myocardial infarction complicated by cardiogenic shock.
Sources of Funding
The statistical analysis presented in the article was funded by Herzzentrum Leipzig and Stiftung Institut für Herzinfarktforschung. The CULPRIT‐SHOCK trial was funded by European Union, Seventh Framework Programme (FP7/2007–2013) Grant agreement n°602202, German Heart Research Foundation, and the German Cardiac Society.
Disclosures
B. Schrage reports speaker’s fee from Abiomed and AstraZeneca, outside of this study. S. Windecker reports research and educational grants to the institution from Abbott, Amgen, Astra Zeneca, BMS, Bayer, Biotronik, Boston Scientific, Cardinal Health, CardioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Johnson & Johnson, Medicure, Medtronic, Novartis, Polares, OrPha Suisse, Pfizer, Regeneron, Sanofi‐Aventis, Sinomed, Terumo, and V‐Wave.
Stephan Windecker serves as unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, Terumo, V‐Wave, and Xeltis, but has not received personal payments by pharmaceutical companies or device manufacturers. He is also a member of the steering/executive committee group of several investigator‐initiated trials that receive funding by industry without impact on his personal remuneration. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Kontos MC, Wang Y, Chaudhry SI, Vetrovec GW, Curtis J, Messenger J, Ncdr . Lower hospital volume is associated with higher in‐hospital mortality in patients undergoing primary percutaneous coronary intervention for ST‐segment‐elevation myocardial infarction: a report from the ncdr. Circ Cardiovasc Qual Outcomes. 2013;6:659–667. doi: 10.1161/CIRCOUTCOMES.113.000233 [DOI] [PubMed] [Google Scholar]
- 2. Strom JB, Wimmer NJ, Wasfy JH, Kennedy K, Yeh RW. Association between operator procedure volume and patient outcomes in percutaneous coronary intervention: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2014;7:560–566. doi: 10.1161/CIRCOUTCOMES.114.000884 [DOI] [PubMed] [Google Scholar]
- 3. Badheka AO, Patel NJ, Grover P, Singh V, Patel N, Arora S, Chothani A, Mehta K, Deshmukh A, Savani GT, et al. Impact of annual operator and institutional volume on percutaneous coronary intervention outcomes: a 5‐year United States experience (2005–2009). Circulation. 2014;130:1392–1406. doi: 10.1161/CIRCULATIONAHA.114.009281 [DOI] [PubMed] [Google Scholar]
- 4. Post PN, Kuijpers M, Ebels T, Zijlstra F. The relation between volume and outcome of coronary interventions: a systematic review and meta‐analysis. Eur Heart J. 2010;31:1985–1992. doi: 10.1093/eurheartj/ehq151 [DOI] [PubMed] [Google Scholar]
- 5. West RM, Cattle BA, Bouyssie M, Squire I, de Belder M, Fox KA, Boyle R, McLenachan JM, Batin PD, Greenwood DC, et al. Impact of hospital proportion and volume on primary percutaneous coronary intervention performance in england and wales. Eur Heart J. 2011;32:706–711. doi: 10.1093/eurheartj/ehq476 [DOI] [PubMed] [Google Scholar]
- 6. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 7. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the american college of emergency physicians and society for cardiovascular angiography and interventions. Catheter Cardiovasc Interv. 2013;82:E1–27. [DOI] [PubMed] [Google Scholar]
- 8. Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139:1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614 [DOI] [PubMed] [Google Scholar]
- 9. Thiele H, Akin I, Sandri M, de Waha‐Thiele S, Meyer‐Saraei R, Fuernau G, Eitel I, Nordbeck P, Geisler T, Landmesser U, et al. One‐year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379:1699–1710. doi: 10.1056/NEJMoa1808788 [DOI] [PubMed] [Google Scholar]
- 10. Aissaoui N, Puymirat E, Delmas C, Ortuno S, Durand E, Bataille V, Drouet E, Bonello L, Bonnefoy‐Cudraz E, Lesmeles G, et al. Trends in cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail. 2020;22:664–672. [DOI] [PubMed] [Google Scholar]
- 11. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer‐Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 12. SAS Institute (Cary NC, U.S.) . Statistical analysis software (sas), version 9.4.
- 13. R Development Core Team . R: A language and environment for statistical computing. 2010.
- 14. Hadley W. Ggplot2: Elegant Graphics for Data Analysis. New York: Springer‐Verlag; 2016. [Google Scholar]
- 15. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 17. Shaefi S, O'Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J, Mahmood E, Talmor D, Shahul S. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015;4:e001462. doi: 10.1161/JAHA.114.001462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubo S, Yamaji K, Inohara T, Kohsaka S, Tanaka H, Ishii H, Uemura S, Amano T, Nakamura M, Kadota K. In‐hospital outcomes after percutaneous coronary intervention for acute coronary syndrome with cardiogenic shock (from a Japanese Nationwide Registry [J‐PCI Registry]). Am J Cardiol. 2019;123:1595–1601. doi: 10.1016/j.amjcard.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 19. Schrage B, Dabboura S, Yan I, Hilal R, Neumann JT, Sorensen NA, Gossling A, Becher PM, Grahn H, Wagner T, et al. Application of the scai classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020;96:E213–E219. [DOI] [PubMed] [Google Scholar]
- 20. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, et al. Scai clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 21. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019:74: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 22. Schrage B, Weimann J, Dabboura S, Yan I, Hilal R, Becher PM, Seiffert M, Bernhardt AM, Kluge S, Reichenspurner H, et al. Patient characteristics, treatment and outcome in non‐ischemic vs. ischemic cardiogenic shock. J Clin Med. 2020;9:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taleb I, Koliopoulou AG, Tandar A, McKellar SH, Tonna JE, Nativi‐Nicolau J, Alvarez Villela M, Welt F, Stehlik J, Gilbert EM, et al. Shock team approach in refractory cardiogenic shock requiring short‐term mechanical circulatory support: a proof of concept. Circulation. 2019;140:98–100. doi: 10.1161/CIRCULATIONAHA.119.040654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, et al. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659–1669. doi: 10.1016/j.jacc.2018.12.084 [DOI] [PubMed] [Google Scholar]
- 25. Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, Khandelwal AK, Ohman EM, O'Neill WW. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol. 2017;119:845–851. doi: 10.1016/j.amjcard.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 26. Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB III, O'Neill W. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol. 2018;72:1972–1980. doi: 10.1016/j.jacc.2018.07.074 [DOI] [PubMed] [Google Scholar]


