Abstract
Background
Microvascular dysfunction might be a major determinant of clinical deterioration and outcome in patients with hypertrophic cardiomyopathy (HCM). However, long‐term prognostic value of transthoracic Doppler echocardiography (TDE) coronary flow velocity reserve (CFVR) on clinical outcome is uncertain in HCM patients. Therefore, the aim of our study was to assess long‐term prognostic value of CFVR on clinical outcome in HCM population.
Methods and Results
We prospectively included 150 HCM patients (82 women; mean age 48±15 years). Patients’ clinical characteristics, echocardiographic and CFVR findings (both for left anterior descending [LAD] and posterior descending artery [PD]), were assessed in all patients. The primary outcome was a composite of: HCM related death, heart failure requiring hospitalization, sustained ventricular tachycardia and ischemic stroke. Patients were stratified into 2 subgroups depending on CFVR LAD value: Group 1 (CFVR LAD>2, [n=87]) and Group 2 (CFVR LAD≤2, [n=63]). During a median follow‐up of 88 months, 41/150 (27.3%) patients had adverse cardiac events. In Group 1, there were 8/87 (9.2%), whereas in Group 2 there were 33/63 (52.4%, P<0.001 vs. Group 1) adverse cardiac events. By Kaplan‐Meier analysis, patients with preserved CFVR LAD had significantly higher cumulative event‐free survival rate compared to patients with impaired CFVR LAD (96.4% and 90.9% versus 66.9% and 40.0%, at 5 and 8 years, respectively: log‐rank 37.2, P<0.001). Multivariable analysis identified only CFVR LAD≤2 as an independent predictor for adverse cardiac outcome (HR 6.54; 95% CI 2.83–16.30, P<0.001), while CFVR PD was not significantly associated with outcome.
Conclusions
In patients with HCM, impaired CFVR LAD (≤2) is a strong, independent predictor of adverse cardiac outcome. When the aim of testing is HCM risk stratification and CFVR LAD data are available, the evaluation of CFVR PD is redundant.
Keywords: adverse cardiac outcome, coronary flow velocity reserve, hypertrophic cardiomyopathy, microvascular dysfunction, prognosis
Subject Categories: Echocardiography, Prognosis, Imaging, Cardiomyopathy, Hypertrophy
Nonstandard Abbreviations and Acronyms
- HCM
hypertrophic cardiomyopathy
- SCD
sudden cardiac death
- CFVR
coronary flow velocity reserve
- TDE
transthoracic Doppler echocardiography
- LVOTG
left ventricular outflow tract gradient
- PD
posterior descending coronary artery
- LA
left atrial
- IVS
interventricular septum
- PW
posterior wall
- LAVI
left atrial volume indexed for body surface area
- RVSP
right ventricular systolic pressure
- PH
pulmonary hypertension
- NSVT
nonsustained ventricular tachycardia
- MBF
myocardial blood flow
- SE
stress echocardiography
Clinical Perspective
What Is New?
Our main finding is that impaired transthoracic Doppler echocardiography coronary flow velocity reserve of left anterior descending artery represents an independent and strong predictor of adverse long‐term outcome in patients with hypertrophic cardiomyopathy.
What Are the Clinical Implications?
The identification of patients with reduced coronary flow velocity reserve of left anterior descending artery might be of great clinical value in order to improve risk stratification of hypertrophic cardiomyopathy patients and to potentially include microvascular dysfunction in more comprehensive risk models.
Further large longitudinal follow‐up studies are needed to determine the prognostic value of coronary flow velocity reserve of left anterior descending artery as an independent predictor of cardiac death or sudden cardiac death in hypertrophic cardiomyopathy patients.
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiac disease which is associated with increased cardiovascular morbidity and mortality. 1 , 2 , 3 , 4 As a result of heterogeneous clinical course and phenotypes of HCM, risk stratification remains challenging, and is mainly focused on sudden cardiac death (SCD). 2 , 5 However, certain echocardiographic and clinical parameters used for SCD prediction have limited prognostic value in predicting other HCM related cardiovascular events such as progressive deterioration of left ventricular (LV) systolic function with heart failure development or an ischemic stroke. 6 , 7 Furthermore, it is notable that some HCM patients without "traditional" risk markers can nevertheless experience fatal events or significant clinical deterioration. 4 Therefore, clinical research has focused on identifying other potential predictors in order to optimize HCM risk assessment and patient management. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20
Over the past 3 decades, several groups have shown that the response of myocardial blood flow (MBF) to vasodilators or stress is impaired and that microvascular ischemia might be a major determinant of clinical deterioration and outcome in patients with HCM. 7 , 8 , 9 , 11 , 21 So far, the assessment of microvascular ischemia in HCM patients remains challenging and has not become a part of a routine clinical practice nor it has been incorporated in the contemporary guidelines.
Coronary flow velocity reserve (CFVR) acquired by transthoracic Doppler echocardiography (TDE) has proved to be valuable, inexpensive, noninvasive tool with high concordance with positron emission tomography (PET) derived coronary flow reserve. 22 In fact, whenever local expertise, practice, and available technology allow, TDE CFVR may be used as a highly reproducible imaging technique for functional evaluation of microcirculation in the absence of epicardial stenosis. 8 , 21 , 23 , 24 , 25 However, the long‐term predictive value of TDE CFVR on clinical outcome has not been investigated in HCM patients. Therefore, the aim of our study was to assess the long‐term prognostic value of TDE CFVR on clinical outcome in this population.
METHODS
All data and supporting materials have been provided with the published article.
Study Population
From January 2008 until July 2017, we have prospectively included 150 patients (68 men, 82 women; mean age 48±15 years) with HCM, (41 patients with significant left ventricular outflow tract gradient [LVOTG], and 109 patients without obstruction) at the Clinic for cardiology, Clinical Center of Serbia. Patients fulfilled the following inclusion criteria; (1) echocardiographic evidence of asymmetric myocardial hypertrophy, defined as a LV myocardial wall thickness ≥15 mm and septum/posterior wall ratio>1.3, in the absence of another cardiac or systemic cause of LV hypertrophy 1 , 2 ; (2) preserved LV ejection fraction (>55%); (3) CFVR assessment of both left anterior descending (LAD) and posterior descending (PD) coronary artery; (4) clinical follow‐up. The exclusion criteria were: (1) presence of significant valvular disease; (2) poor acoustic window (for CFVR and echo assessment); (3) second and third degree atrioventricular block; (4) chronic obstructive pulmonary disease, (5) New York Heart Association (NYHA) class III or IV; (6) diabetes; (7) presence of significant coronary artery stenosis (quantitatively assessed coronary diameter reduction ≥50%) on coronary angiography or history of coronary artery disease; (8) poor life expectancy due to other concomitant disease. From the 171 patients initially selected, 10 patients were excluded due to presence of angiographically significant coronary artery stenosis or prior coronary interventions, 4 patients were excluded due to cancer disease that was diagnosed at the time of the initial examination, while 7 patients were excluded due to inability to obtain technically adequate CFVR—2 for LAD (overall feasibility 98.7%) and 5 for PD (overall feasibility 96.8%), thus, the final study population consisted of 150 patients. CFVR LAD value of 2.0 was considered as a cut‐off point, 8 , 11 , 23 , 25 , 26 , 27 and consequently patients were divided into 2 groups: Group 1 with preserved CFVR LAD > 2 (87 patients) and Group 2 with impaired CFVR LAD ≤ 2 (63 patients).
Patients’ clinical characteristics, echocardiographic and CFVR findings, arrhythmias (paroxysmal atrial fibrillation [AF] or nonsustained VT [NSVT]) on 24‐hour ECG Holter monitoring, and medical therapy were assessed for each patient at the moment of the first outpatient visit or hospitalization (i.e., study entry). Also, the 5‐year risk of SCD for each patient was calculated using the HCM risk SCD formula. 2 Coronary angiography was performed in 105 patients who had either anginal symptoms or other indications outlined in existing guidelines, 2 and none of them had significant coronary stenosis. The remaining 45 patients had either less than 5% probability for having coronary artery disease 28 or negative stress echocardiography (SE) test. 29
Study was approved by the Institutional Ethical Committee and written informed consent was obtained from all participants involved.
Echocardiographic Examination
Echocardiographic studies were performed with available digital ultrasound system (Acuson Sequoia C256; Siemens Medical Solutions, Inc., Mountain View, CA, USA) with a 3V2C multifrequency transducer using second‐harmonic technology. Standard 2 dimensional, M‐Mode, LV ejection fraction measurements were acquired according to the ASE guidelines. 30 The average of 3 cardiac cycles was used for measurements of cardiac dimensions. Following parameters were obtained in M‐mode parasternal long axis view: LV end‐diastolic diameter, LV end‐systolic diameter, end‐systolic left atrial (LA) diameter, end‐diastolic LV interventricular septum (IVS) and left posterior wall (PW) thickness. In addition, we have also calculated IVS/PW ratio, as a LV morphological parameter. Ejection fraction, LV and LA volumes were assessed using the modified Simpson biplane method. 30 LA volume was indexed for body surface area (LAVI). LAVI was considered enlarged if >34 ml/m2. 30 LVOTG was examined by the combined use of color Doppler, pulsed‐wave Doppler and continuous‐wave Doppler echocardiography at rest and during Valsalva maneuver in each patient. Additionally, exercise induced LVOTG was also evaluated in 52 patients during SE test. Maximally induced LVOTG values were taken in further analysis. 2 , 11 Hypertrophic obstructive cardiomyopathy was defined if systolic gradient at rest was ≥30 mm Hg in the LV outflow tract. 1 , 2 Right ventricular systolic pressure (RVSP) was calculated on the basis of trans‐tricuspid gradient derived from the modified Bernoulli equation with the addition of estimated right atrial pressure. 13 Pulmonary hypertension (PH) was defined as a RVSP >36 mm Hg. 13
Pulsed‐wave Doppler with the sample volume placed at the mitral leaflet tips was used for early (E) and late (A) diastolic peak velocity measurements. Tissue Doppler imaging was used in order to obtain early (e’) and late (a’) diastolic peak velocities from the lateral part of the mitral annulus. Filters were set in order to exclude high frequency signals, while the direction of annulus motion was aligned with the scan line direction. Signals were obtained at end‐expiratory cycle. Ratio of early transmitral flow velocity to early diastolic lateral mitral annular velocity (E/e’) has been shown to be reasonably accurate noninvasive predictor of elevated LV filling pressure. 31 , 32
Coronary Flow Velocity Reserve
CFVR was assessed by TDE using the 4‐MHz transducer. For color Doppler flow mapping the velocity range was set at 16–24 cm/s. Visualization of the distal segment of LAD artery was done in a modified 3‐chamber view. Evaluation of PD coronary artery was done in the apical 2‐chamber view. From this position, probe was slightly rotated anticlockwise and tilted anteriorly, until the coronary blood flow in the posterior interventricular groove was identified by color Doppler. Blood flow velocity was measured by pulsed wave Doppler using a sample volume of 3 to 5 mm wide. Alignment of ultrasound beam direction with coronary flow was as parallel as possible, with the stable transducer position at rest and during maximal hyperemia. Peak diastolic coronary flow velocity was measured in basal conditions and during maximal hyperemia, which was induced with adenosine (0.14 mg/kg/min intravenously, during 2 minutes). Three optimal diastolic flow profiles at rest and during hyperemia were measured, and results were averaged. CFVR was calculated as the ratio of hyperemic to basal peak diastolic flow velocities, 8 , 21 , 23 , 24 , 25 , 27 (Figure 1). Based on previously defined diagnostic and prognostic cutoff values, CFVR ≤2.0 was considered abnormal. 8 , 11 , 25 , 26 CFVR measurements were done offline, using the integrated software package of the ultrasound system, by 2 experienced investigators. We have previously reported interobserver agreement for CFVR evaluation of 96%. 33
Figure 1. Examples of noninvasively derived coronary flow velocity reserve (CFVR) for left anterior descending artery (LAD) and posterior descending artery (PD).
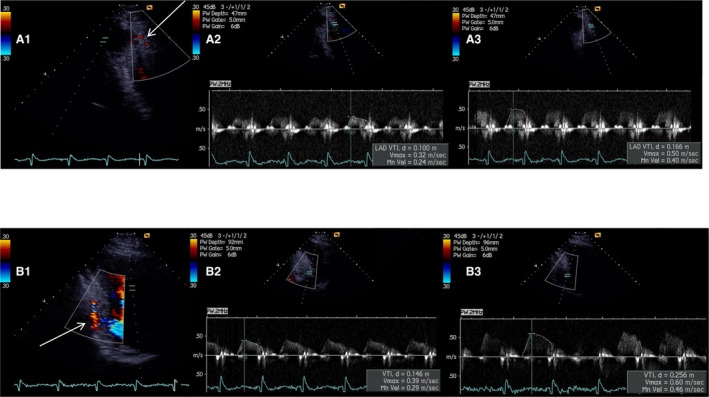
(A1) Color Doppler signal of coronary flow for the distal segment of LAD in modified 3 chamber view (arrow). Peak baseline (A2) and hyperemic (A3) diastolic flow velocities with impaired CFVR LAD (0.50/0.32) – 1.56. (B1) Color Doppler signal of coronary flow for the PD, in modified 2 chamber view (arrow). Peak baseline (B2) and hyperemic (B3) diastolic flow velocities with impaired CFVR PD (0.60/0.39) – 1.54.
Assessment of Outcome
Follow up was performed by outpatient medical visit or telephone contact in all patients. In case of an adverse event, all hospital records were obtained. The primary outcome was a composite of: (1) HCM related death ‐ considered in the case of heart failure (occurring in the setting of cardiac decompensation, pulmonary edema or a progressive course to end stage disease), sudden cardiac death (including cardiac arrest with resuscitation after cardiac arrest) or fatal ischemic stroke; (2) heart failure requiring hospitalization (in the setting of pulmonary congestion on chest X ray); (3) sustained ventricular tachycardia (VT) or appropriate shocks by an implanted defibrillator; (4) ischemic stroke (judged to be a direct consequence of embolic events usually in the setting of paroxysmal or chronic atrial fibrillation). As secondary exploratory outcomes, we analyzed all‐cause mortality and cardiac death.
Any unexplained sudden death was regarded as cardiac and attributed to adverse events. All events were clinically adjudicated by the 2 senior cardiologists.
Statistical Analysis
All data were entered into a database, and then processed in the statistical programs SPSS version 21 (SPSS, Chicago, IL) and R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). All numeric data were expressed as mean±standard deviation (SD), and categorical as frequencies, or percentages. Differences in continuous variables were assessed with the Student’s t test. Categorical data were compared using the chi‐square test or Fisher exact test, as appropriate. Survival rates were estimated with Kaplan‐Meier curves and compared by the log‐rank test. The association of selected variables with outcome was assessed with the Cox proportional hazards regression model with a Firth's correction. Univariable analysis included all available major clinical and echocardiographic markers (together with evaluated CFVRs, presence of enlarged LAVI and moderate MR) that were used to judge increased risk in HCM according to the current American College of Cardiology/American Heart Association (ACC/AHA) guidelines. 1 , 34 Variables that were significantly related to the primary outcome in univariable analysis (P<0.05) were included in the multivariable model. Hazard ratios (HR) with the corresponding 95% CIs were estimated. Statistical significance was defined as P<0.05.
RESULTS
The main clinical data for the whole study population and between the 2 analyzed groups are presented in Table 1. The mean age for whole group was 48±15 years, without significant differences between the subgroups. Female patients and NYHA functional class II were more prevalent in group with reduced CFVR LAD. There were no significant differences regarding the presence of family history of HCM or SCD, angina, syncope, arrhythmias on 24‐hour ECG Holter monitoring, HCM risk SCD score, or hemodynamic parameters except for the baseline heart rate (which was higher in Group 2). Concerning the medical treatment, there were no differences between the groups, except for the use of diuretics which was more frequent in patients with reduced CFVR LAD.
Table 1.
Clinical Characteristics of Patients With Hypertrophic Cardiomyopathy
| Variables | Total (n=150) | Group 1 (CFVR >2) (n=87) | Group 2 (CFVR ≤2) (n=63) |
P value Group 1 vs. Group 2 |
|---|---|---|---|---|
| Age, y | 48±15 | 47±15 | 50±16 | 0.180 |
| Female sex, no. (%) | 82 (54.7) | 40 (46) | 42 (66.7) | 0.012 |
| BSA, m2 | 1.84±0.18 | 1.87±0.16 | 1.81±0.20 | 0.059 |
| Angina, no. (%) | 83 (55.3) | 43 (49.4) | 40 (63.5) | 0.087 |
| Hypertension, no. (%) | 51 (34) | 26 (29.9) | 25 (39.7) | 0.211 |
| Syncope, no. (%) | 21 (14) | 8 (12.7) | 13 (14.9) | 0.696 |
| Family history of HCM, no. (%) | 53 (35.3) | 31 (35.6) | 22 (34.9) | 0.928 |
| Family history of SCD, no. (%) | 17 (11.3) | 8 (9.2) | 9 (14.3) | 0.332 |
| HCM Risk SCD score | 3.13±2.10 | 2.97±2.09 | 3.35±2.10 | 0.266 |
| NYHA functional class, no. (%) | 0.003 | |||
| I | 92 (61.3) | 62 (71.3) | 30 (47.6) | |
| II | 58 (38.7) | 25 (28.7) | 33 (52.4) | |
| Unsustained ventricular tachycardia on Holter ECG, no. (%) | 28 (18.7) | 15 (17.2) | 13 (20.6) | 0.599 |
| Paroxysmal atrial fibrillation, no. (%) | 28 (18.7) | 14 (16.1) | 14 (22.2) | 0.342 |
| Medical therapy, no. (%) | ||||
| Beta blockers | 126 (84) | 72 (82.8) | 54 (85.7) | 0.626 |
| Ca antagonists | 25 (16.7) | 11 (12.6) | 14 (22.2) | 0.120 |
| ACEI/ARB | 38 (25.3) | 22 (25.3) | 16 (25.4) | 0.988 |
| Diuretic | 27 (18) | 8 (9.2) | 19 (30.2) | 0.001 |
| Amiodarone | 19 (12.7) | 10 (11.5) | 9 (14.3) | 0.612 |
| Anticoagulants | 28 (18.7) | 14 (16.1) | 14 (22.2) | 0.342 |
| Baseline heart rate, beats/min | 70±14 | 67±12 | 73±16 | 0.008 |
| Peak heart rate during hyperemia, beats/min | 76±16 | 74±15 | 78±18 | 0.135 |
| Diastolic blood pressure, mm Hg | 77±9 | 76±9 | 78±9 | 0.225 |
| Systolic blood pressure, mm Hg | 120±15 | 118±14 | 121±16 | 0.213 |
ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BSA, body surface area; CFVR, coronary flow velocity reserve; HCM, hypertrophic cardiomyopathy; NYHA, New York Heart Association; Plus–minus values are means ±SD; and SCD, sudden cardiac death.
Echocardiographic Parameters
Echocardiographic parameters for the whole study population and the 2 analyzed groups are summarized in Table 2. Patients with reduced CFVR LAD had significantly thicker IVS, PW and maximal wall thickness, larger LA dimension and LAVI, as well as higher RVSP compared to patients with preserved CFVR LAD. There were no significant differences between the groups concerning LV dimensions, LV ejection fraction and median value of maximal LVOTG. Furthermore, patients with reduced CFVR LAD had lateral mitral annular e’ velocities significantly decreased, while E/e’ was higher. In HCM patients with significant LVOTG at rest, a regional CFVR difference was observed—CFVR LAD was significantly lower in comparison to CFVR PD (2.00±0.37 vs. 2.39±0.38, P<0.001 respectively), while there was no significant difference between CFVR LAD and CFVR PD in HCM patients without obstruction (2.19±0.45 vs. 2.23±0.43, P=0.070, respectively).
Table 2.
Echocardiographic Characteristics of Patients With Hypertrophic Cardiomyopathy
| Variables | Total (n=150) | Group 1 (CFVR > 2) (n=87) | Group 2 (CFVR ≤ 2) (n=63) |
P value Group 1 vs. Group 2 |
|---|---|---|---|---|
| LV end‐diastolic dimension, mm | 46.1±5.0 | 46.4±5.0 | 45.7±4.9 | 0.438 |
| LV end‐systolic dimension, mm | 27.4±5.1 | 27.3±4.5 | 27.5±5.8 | 0.826 |
| IVS thickness, mm | 19.4±4.8 | 18.5±4.2 | 20.5±5.3 | 0.013 |
| PW thickness, mm | 10.5±2.7 | 9.9±2.0 | 11.3±3.2 | 0.002 |
| IVS/PW ratio | 1.96±0.58 | 1.97±0.5 | 1.93±0.64 | 0.664 |
| Maximal wall thickness, mm | 21.1±4.8 | 20.2±4.3 | 22.6±5.2 | 0.004 |
| LV wall thickness ≥ 30 mm, no. (%) | 9 (6) | 4 (4.6) | 5 (7.9) | 0.493 |
| LV ejection fraction, % | 68±7.7 | 69.6±8.0 | 69.4±8.9 | 0.839 |
| LVOTG at rest ≥ 30 mm Hg, no. (%) | 41 (27.3) | 21 (24.1) | 20 (31.7) | 0.302 |
| Maximal induced LVOTG – median (IQR), mm Hg | 11 (7–54) | 10 (7–48) | 12 (7–68) | 0.090 |
| Maximal induced LVOTG ≥ 50 mm Hg, no. (%) | 42 (28%) | 21 (24.1) | 21 (33.3) | 0.216 |
| Left atrial dimension, mm | 43±6.4 | 41.5±6.2 | 44.5±6.2 | 0.004 |
| LAVI, ml/m2 | 38±15 | 35.3±12.8 | 42.0±16.8 | 0.009 |
| LAVI > 34 ml/m2, no. (%) | 79 (52.7) | 38 (43.7) | 41 (65.1) | 0.010 |
| Systolic anterior motion, no. (%) | 65 (43.3) | 35 (40.2) | 30 (47.6) | 0.367 |
| Mitral regurgitation, no. (%) | 0.056 | |||
| Mild | 101 (67.3) | 64 (73.6) | 37 (58.7) | |
| Moderate | 49 (32.7) | 23 (26.4) | 26 (41.3) | |
| RVSP, mm Hg | 33.6±8.1 | 32±6.6 | 35±9.3 | 0.025 |
| PH, no. (%) | 38 (25.3) | 18 (20.7) | 20 (31.7) | 0.124 |
| Baseline diastolic flow velocity (m/sec) – LAD | 0.36±0.10 | 0.33±0.07 | 0.41±0.11 | <0.001 |
| Hyperemic diastolic flow velocity (m/sec) – LAD | 0.75±0.19 | 0.79±0.18 | 0.69±0.19 | 0.002 |
| CFVR LAD | 2.13±0.44 | 2.42±0.30 | 1.73±0.23 | <0.001 |
| Baseline diastolic flow velocity (m/sec) – PD | 0.33±0.07 | 0.32±0.06 | 0.36±0.08 | 0.001 |
| Hyperemic diastolic flow velocity (m/sec) – PD | 0.74±0.16 | 0.77±0.14 | 0.70±0.17 | 0.008 |
| CFVR PD | 2.28±0.42 | 2.48±0.32 | 2.00±0.38 | <0.001 |
| E wave, m/s | 0.73±0.22 | 0.73±0.19 | 0.74±0.25 | 0.750 |
| A wave, m/s | 0.67±0.26 | 0.63±0.23 | 0.72±0.29 | 0.049 |
| E/A | 1.26±0.69 | 1.31±0.71 | 1.19±0.65 | 0.271 |
| E deceleration time, m/sec | 219±68 | 217±62 | 222±75 | 0.634 |
| Mitral lateral annular e’, m/s | 0.103±0.033 | 0.111±0.031 | 0.092±0.033 | 0.001 |
| Mitral lateral annular a’, m/s | 0.115±0.038 | 0.117±0.037 | 0.111±0.040 | 0.383 |
| Mitral lateral annular s, m/s | 0.094±0.030 | 0.094±0.028 | 0.094±0.031 | 0.927 |
| E/e’ | 7.71±3.11 | 7.05±2.79 | 8.62±3.32 | 0.003 |
Plus–minus values are means ±SD; CFVR indicates coronary flow velocity reserve; IQR, inter quartile range; IVS, interventricular septum; LV, left ventricular; LAVI, Left atrial volume indexed for body surface area; LVOTG, left ventricular outflow tract gradient; LAD, left anterior descending artery; PD, posterior descending coronary artery; PH, Pulmonary hypertension; PW, posterior wall; and RVSP, Right ventricular systolic pressure.
Long‐term Clinical Outcome
During a median follow‐up of 88 months (interquartile range [IQR; 60–112]), primary composite outcome occurred in 41/150 (27.3%) patients. CFVR was significantly lower in patients with events of the composite outcome compared to those who were without events, both for LAD (1.88±0.47 vs. 2.23±0.38, P<0.001, respectively) and PD (2.16±0.51 vs. 2.32±0.37, P<0.001, respectively). In patients with preserved CFVR LAD there were 8/87 (9.2%) events of the composite outcome; cardiac death occurred in 3 patients (SCD in 2, while one was a result of heart failure), 2 patients had ischemic stroke, while 3 patients experienced sustained VT. However, in patients with impaired CFVR LAD there were 33/63 (52.4%, P<0.001 vs. Group 1) events of the composite outcome; cardiac cause of death was determined in 15 patients (SCD in 7, 5 were due to heart failure, and 3 were a result of ischemic stroke), heart failure requiring hospitalization in 15 patients, one patient had stroke and 2 patients experienced sustained VT. Notably, prevalence of heart failure (both fatal and non‐fatal) was significantly higher in Group 2 compared to Group 1 (20 [31.7%] vs. 1 [1.1%], P<0.001, respectively).
Regarding secondary explanatory outcomes, during follow up there were 3 more non‐cardiac deaths (1 due to breast cancer and 2 due to complications of colitis and pneumonia). All‐cause mortality (16 [25.4%] vs. [5 (5.7%]), P=0.001) and cardiac death (15 [23.8%] vs. 3 [3.4%], P<0.001) were significantly higher in Group 2 in comparison to Group 1.
By Kaplan‐Meier analysis for primary composite outcome, patients with preserved CFVR LAD had significantly higher cumulative event‐free survival rate compared to patients with impaired CFVR LAD (96.4% and 90.9% vs. 66.9% and 40.0%, at 5 and 8 years, respectively: log‐rank 37.2, P<0.001) (Figure 2). Furthermore, there was no difference in primary composite outcome between subgroups of patients with reduced both CFVRs and those with reduced CFVR LAD but preserved CFVR PD (18/37 [48.6%] vs. 15/26 [57.7%], P=0.479 respectively).
Figure 2.
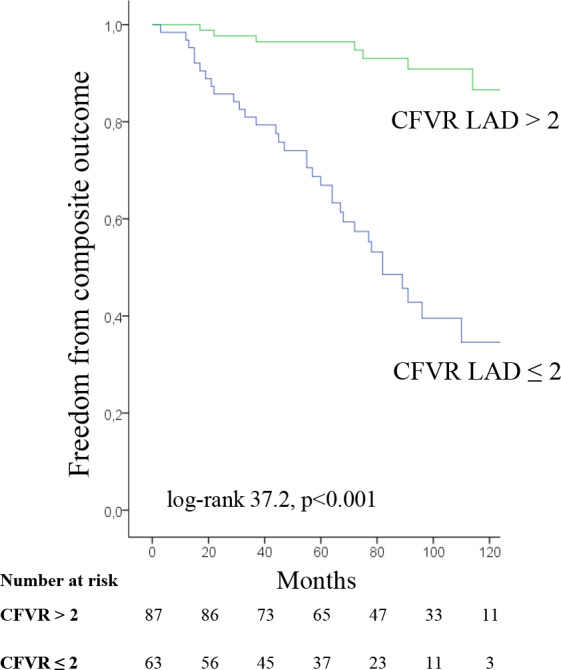
Kaplan‐Meier survival curves for composite outcome in patients with coronary flow velocity reserve (CFVR) for left anterior descending artery (LAD) >2 or ≤2
In addition, patients with preserved CFVR LAD had higher estimated freedom from all‐cause mortality (96.4% and 94.7% vs. 86.6% and 69.5%, at 5 and 8 years, respectively; log‐rank 11.1, P=0.001) (Figure 3A) and cardiac death (97.7% and 96.0% vs. 88.0% and 70.6%, at 5 and 8 years, respectively; log‐rank 13.6, P<0.001) (Figure 3B) compared to the patients with impaired CFVR LAD.
Figure 3.
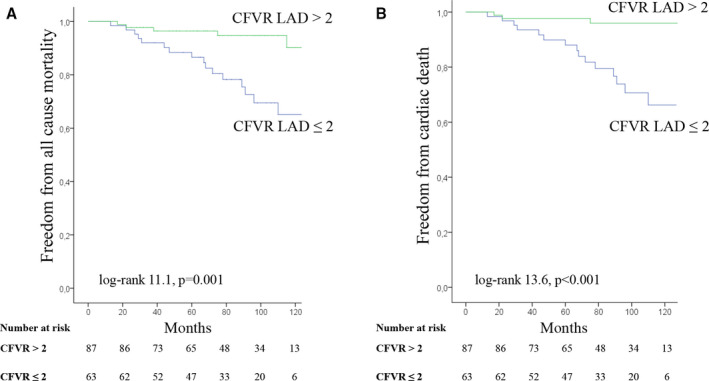
Kaplan‐Meier survival curves for all‐cause mortality (A) and cardiac death (B) in patients with coronary flow velocity reserve (CFVR) for left anterior descending artery (LAD) >2 or ≤2
Univariable Cox proportional hazard regression analysis showed that female sex, age, presence of LAVI > 34 ml/m2 and moderate MR, maximal induced LVOTG ≥50 mm Hg, CFVR LAD ≤2 and CFVR PD ≤2 were all significantly associated with the primary outcome (Table 3). However, multivariable analysis identified only CFVR LAD ≤2 as an independent predictor for adverse cardiac outcome (HR 6.54; 95% CI 2.83–16.30, P<0.001), while CFVR PD was not significantly associated with outcome (Table 3).
Table 3.
Univariable and Multivariable Prognostic Predictors of Composite Outcome
| Variables | Univariable analysis multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | P value | 95% CI | HR | P value | 95% CI | |
| Female sex | 2.29 | 0.016 | 1.17–4.49 | 1.39 | 0.346 | 0.71–2.87 |
| Age, y | 1.03 | 0.022 | 1.00–1.05 | 1.01 | 0.315 | 0.99–1.04 |
| Family history of SCD | 1.73 | 0.216 | 0.73–4.14 | / | ||
| NSVT | 1.46 | 0.303 | 0.71–2.98 | / | ||
| Syncope | 0.60 | 0.328 | 0.21–1.68 | / | ||
| Maximal wall thickness, mm | 1.04 | 0.181 | 0.98–1.11 | / | ||
| LV wall thickness ≥30 mm | 1.15 | 0.821 | 0.35–3.72 | / | ||
| Maximal induced LVOTG ≥50 mm Hg | 2.07 | 0.022 | 1.11–3.86 | 1.29 | 0.557 | 0.56–3.05 |
| LAVI >34 ml/m2 | 2.54 | 0.006 | 1.31–4.95 | 1.44 | 0.378 | 0.64–3.29 |
| Moderate MR | 2.34 | 0.007 | 1.27–4.33 | 1.08 | 0.877 | 0.41–2.74 |
| CFVR LAD ≤2 | 7.91 | <0.001 | 3.62–17.28 | 6.54 | <0.001 | 2.83–16.30 |
| CFVR PD ≤2 | 2.61 | 0.002 | 1.40–4.85 | 0.94 | 0.863 | 0.45–1.98 |
CFVR indicates coronary flow velocity reserve; HR, hazard ratio; LV, left ventricular; LAVI, left atrial volume indexed for body surface area; LVOTG, left ventricular outflow tract gradient; LAD, left anterior descending artery; MR, mitral regurgitation; NSVT, nonsustained ventricular tachycardia; PD, posterior descending coronary artery; and SCD, sudden cardiac death.
DISCUSSION
To our knowledge this is the largest prospective single center study with the long‐term follow‐up emphasizing the role of microvascular dysfunction, as assessed by TDE CFVR, considering HCM risk stratification and clinical outcome. Our main finding is that TDE CFVR LAD represents an independent and strong predictor of adverse long‐term outcome in HCM patients. The presence of reduced CFVR LAD enabled the identification of a subgroup of patients who had 6.5‐fold increase in the risk of adverse cardiac events, whereas preserved CFVR identified patients with favorable prognosis. Notably, CFVR LAD ≤ 2 appeared to be a marker of an increased risk for both all‐cause mortality and cardiac death, as well as development of heart failure either requiring hospitalization or leading to fatal outcome. In particular, microvascular dysfunction could be detected years before the profound clinical deterioration or death occur. Thus, CFVR LAD may be considered as an additional marker of an adverse cardiac prognosis along with the well‐known clinical (age, sex, family history of SCD, presence of syncope, or NSVT) and echocardiographic determinants (presence of maximal induced LVOTG ≥50 mm Hg, moderate MR, enlarged LAVI, maximal wall thickness, or massive hypertrophy). 1 , 11 , 12 , 35 , 36 , 37 , 38
According to the current guidelines, 1 , 2 several clinical, echocardiographic and cardiovascular magnetic resonance markers have been assembled into a risk‐stratification algorithm that is mainly focused on primary prevention of SCD, as the most severe event. Although the new risk models 2 , 4 have improved risk stratification for SCD and subsequent identification of patients who might benefit from implantable cardioverter–defibrillators, current guidelines are still lacking recommendations concerning the risk stratification of other cardiac adverse events, such as development of heart failure or ischemic stroke. Several prior studies have tried to identify additional prognostic markers of worse clinical outcome (other than those associated with SCD), such as LV mass and the presence of late gadolinium enhancement and fibrosis on cardiovascular magnetic resonance, 15 , 16 elevated values of E/e' and brain natriuretic peptide, 10 , 39 abnormal LV global longitudinal strain values, 14 , 17 as well as LA volume enlargement. 12
Presence of microvascular dysfunction, as assessed by PET, has been proposed as an important risk marker which was shown to be associated with cardiac adverse events, including progression to heart failure, development of ventricular arrhythmias, and death in HCM patients. 7 , 9 Marked structural abnormalities of intramyocardial arteries, 3 , 40 , 41 , 42 as well as extravascular compression forces on coronary vessels, 3 , 21 , 23 , 41 , 42 are most likely primary pathophysiological substrate responsible for blunted MBF during stress. 7 , 9 , 43 In a cohort of 51 HCM patients prospectively followed for more than 8 years, PET dipyridamole MBF value in the lowest tertile (<1.11 mL/min/gr) proved to be the most powerful independent predictor of cardiovascular mortality, with an almost 20‐fold increase in relative risk for the composite end point of death, stroke, or progression to NYHA class III–IV. 7 Similarly, Olivotto et al., 9 demonstrated that patients within the lowest tertile of dipyridamole MBF as assessed by PET, had significantly higher incidence of long‐term adverse LV remodeling and decline in systolic function. 9
TDE CFVR has an excellent correlation with coronary flow reserve quantified by PET which has been validated as a noninvasive reference standard for CFVR measurement. 22 Furthermore, we showed that CFVR LAD enabled effective risk stratification of HCM patients, particularly in regard to either all‐cause mortality or cardiac death as well as the onset of heart failure, during a long term follow up. The only TDE CFVR study by Cortigiani et al., 8 that included 68 HCM patients, during a shorter follow‐up of 22 months, showed that CFVR LAD ≤2 was strong and independent predictor of unfavorable outcome.
Previous reports have used SE to predict outcomes in HCM patients. 11 , 18 , 19 , 20 In addition to imaging information obtained during SE, heart rate reserve represents an imaging‐independent parameter which was shown to be associated with mortality as a result of blunted sympathetic reserve in HCM. 44 , 45 Furthermore, HCM patients with impaired exercise capacity were 3 times more likely to have adverse events in comparison to those with a preserved functional capacity. 20 The significant increase in LVOTG during exercise, 2 , 46 as well as the onset of new wall motion abnormalities during SE, 18 , 19 were previously shown to have a role in HCM risk stratification. However, in a recent study of Ciampi et al., 11 SE related clinical/hemodynamic criteria (symptoms, exercise induced hypotension and exercise induced LVOTG) did not predict events during the follow‐up, whereas ischemia‐related criteria (new wall motion abnormalities and/or CFVR reduction when available) appeared as the best predictors for risk stratification. Our data confirm and extend previous findings, 8 , 11 with representative cohort of HCM patients, longer follow‐up and hard, well defined and adjudicated outcome variables. Furthermore, this is the first study to our knowledge where CFVR was obtained both for LAD and PD, demonstrating comprehensive evaluation of coronary microcirculation pattern in patients with HCM, with CFVR LAD as adequate and sufficient predictor of adverse events.
We have previously shown that patients without significant LVOTG at rest had similar CFVR values in different coronary territories in both hypertrophic and non‐hypertrophic regions of LV. 21 Contrary, in patients with significant LVOTG at rest (HCM with obstruction) there was significant regional difference of CFVR LAD in comparison to PD. 21 As a result of LV outflow tract obstruction, there is an increase in extravascular compression forces on coronary vessels, especially in the region of marked hypertrophy (LAD territory), contributing to the coronary flow disturbances throughout the cardiac cycle, that further diminish coronary flow. 47 Consequently, in order to maintain perfusion at rest, there is a decrease in coronary resistance during diastole that leads to higher basal diastolic coronary flow velocity in LAD, followed by the consequent decrease of CFVR in LAD territory in comparison to PD. 21 , 47 Therefore, CFVR LAD completely reflects all pathophysiological aspects of HCM irrespective of its’ type (with or without obstruction). As a result, the prognostic value of CFVR LAD is superior to CFVR PD, thus the evaluation of CFVR LAD can be regarded sufficient to represent the integrity of microvascular function. To the contrary, it was recently shown that in patients with known or suspected coronary artery disease and negative SE tests further evaluation of both CFVR for LAD and PD has an important additive prognostic value. 48 Patients with CFVR LAD >2 were identified as low risk subset, while patients with preserved CFVRs for both coronary arteries were shown to have very low risk for hard events (death and myocardial infarction). 48 Although lower feasibility for the evaluation of CFVR PD was reported in this study (around 58%), CFVR acquisition for this coronary artery may play a role in further risk stratification especially in patients with preserved CFVR LAD and negative SE tests. 48
The identification of patients with reduced CFVR LAD might be of great clinical value in order to improve risk stratification of HCM patients and to potentially include microvascular dysfunction in more comprehensive risk models. In comparison to PET as a gold standard for evaluation of coronary flow reserve, 22 TDE CFVR LAD offers an inexpensive, feasible, and highly reproducible method for evaluation of microvascular dysfunction with significant discriminative and predictive value in patients with HCM.
Study Limitations
Coronary angiography was performed in all HCM patients who had an indication according to the current guidelines 2 ; the rest had either negative stress echocardiography test or low likelihood for having coronary artery disease (mean age 39±14 years). Furthermore, patients without coronary angiography had significantly higher CFVR LAD compared to those with coronary angiography (2.24±0.45 vs. 2.08±0.42, P=0.046, respectively), while CFVR PD values were similar (2.34±0.43 vs. 2.24±0.41, P=0.212, respectively). Therefore, in this group of patients without coronary angiography, coronary artery stenosis cannot be ruled out completely, but is unlikely, especially considering that patients without angiography had significantly higher CFVR for LAD, whereas CFVR for PD was similar in both groups.
Although TDE CFVR assessment might be occasionally challenging because of the poor acoustic window, in our stress echo laboratory it represents a routine and everyday diagnostic tool with a high feasibility of over 95% for the LAD. 33
Even though we showed that patients with impaired CFVR LAD are in greater risk for cardiac death, large longitudinal follow‐up studies are needed to definitely establish CFVR LAD as an independent predictor of cardiac death or SCD in HCM patients.
CFVR might be incorporated as a part of a SE imaging protocol, 25 but SE test was not assessed systematically in this study. Therefore additional prognostic value of CFVR over established SE parameters such are dynamic LVOTG, 2 , 46 regional wall motion abnormalities, 18 , 19 functional evaluation of symptoms and capacity, 20 integrity of cardiac autonomic function 44 and other SE derived echocardiographic parameters needs to be determined.
Defibrillator implantations can reduce mortality in HCM patients, thus we have included onset of sustained ventricular tachycardia and appropriate shocks delivered by the implantable defibrillator as an event of the composite outcome in order not to underestimate the number of events. Finally, we have enrolled in the study patients with no or with mild symptoms, but during follow up one alcohol septal ablation, one surgical myectomy and 2 mitral valves replacements with myectomy have occurred. Although infrequent, these procedures might have influenced the outcome and therefore limited the potential prognostic value of LVOTG.
CONCLUSIONS
The present study demonstrated that impaired TDE CFVR LAD (≤2) is a strong, independent predictor of adverse cardiac outcome in patients with asymmetric HCM. Severe microvascular dysfunction is often present even in asymptomatic or mildly symptomatic patients with preserved LV systolic function, and may precede clinical deterioration by years. Therefore, identification of patients with impaired CFVR LAD might be of a great clinical value in order to improve risk stratification of HCM patients. When the aim of testing is HCM risk stratification and CFVR LAD data are available, the evaluation of CFVR PD is redundant.
Sources of Funding
The authors are grateful for the support of the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant numbers III41022 and ON175086).
Disclosures
None.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e558–e631. DOI: 10.1161/CIR.0000000000000938. [DOI] [PubMed] [Google Scholar]
- 2. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. DOI: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ. Hypertrophic cardiomyopathy. JAMA. 2002;287:1308–1320. DOI: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 4. Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. DOI: 10.1056/NEJMra1710575. [DOI] [PubMed] [Google Scholar]
- 5. Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. DOI: 10.1016/S0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ, Casey SA, Chan RH, Garberich RF, Rowin EJ, Maron MS. Independent assessment of the European society of cardiology sudden death risk model for hypertrophic cardiomyopathy. Am J Cardiol. 2015;116:757–764. DOI: 10.1016/j.amjcard.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 7. Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–1035. DOI: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 8. Cortigiani L, Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E. Prognostic implications of coronary flow reserve on left anterior descending coronary artery in hypertrophic cardiomyopathy. Am J Cardiol. 2008;102:1718–1723. DOI: 10.1016/j.amjcard.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 9. Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, Torricelli F, Camici PG. Relevance of coronary microvascular flow impairment to long‐term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1043–1048. DOI: 10.1016/j.jacc.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 10. Kitaoka H, Kubo T, Hayashi K, Yamasaki N, Matsumura Y, Furuno T, Doi YL. Tissue Doppler imaging and prognosis in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. European Heart Journal ‐ Cardiovascular Imaging. 2013;14(6):544–549. DOI: 10.1093/ehjci/jes200. DOI: 10.1093/ehjci/jes200. [DOI] [PubMed] [Google Scholar]
- 11. Ciampi Q, Olivotto I, Gardini C, Mori F, Peteiro J, Monserrat L, Fernandez X, Cortigiani L, Rigo F, Lopes LR, et al. Prognostic role of stress echocardiography in hypertrophic cardiomyopathy: the International Stress Echo Registry. Int J Cardiol. 2016;219:331–338. DOI: 10.1016/j.ijcard.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 12. Losi M‐A, Betocchi S, Barbati G, Parisi V, Tocchetti C‐G, Pastore F, Migliore T, Contaldi C, Caputi A, Romano R, et al. Prognostic significance of left atrial volume dilatation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22:76–81. DOI: 10.1016/j.echo.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 13. Ong KC, Geske JB, Hebl VB, Nishimura RA, Schaff HV, Ackerman MJ, Klarich KW, Siontis KC, Coutinho T, Dearani JA, et al. Pulmonary hypertension is associated with worse survival in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17:604–610. DOI: 10.1093/ehjci/jew024. [DOI] [PubMed] [Google Scholar]
- 14. Tower‐Rader A, Mohananey D, To A, Lever HM, Popovic ZB, Desai MY. Prognostic value of global longitudinal strain in hypertrophic cardiomyopathy: A systematic review of existing literature. JACC Cardiovasc Imaging. 2018;12:1930–1942. DOI: 10.1016/j.jcmg.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 15. Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, et al. Prognostic value of quantitative contrast‐enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. DOI: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 16. Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, De Santis M, Quarta G, Nistri S, Cecchi F, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. DOI: 10.1016/j.jacc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 17. Tower‐Rader A, Betancor J, Popovic ZB, Sato K, Thamilarasan M, Smedira NG, Lever HM, Desai MY. Incremental prognostic utility of left ventricular global longitudinal strain in hypertrophic obstructive cardiomyopathy patients and preserved left ventricular ejection fraction. J Am Heart Assoc. 2017;6:e006514. DOI: 10.1161/JAHA.117.006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peteiro J, Bouzas‐Mosquera A, Fernandez X, Monserrat L, Pazos P, Estevez‐Loureiro R, Castro‐Beiras A. Prognostic value of exercise echocardiography in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2012;25:182–189. DOI: 10.1016/j.echo.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 19. Peteiro J, Fernandez X, Bouzas‐Mosquera A, Monserrat L, Mendez C, Rodriguez‐Garcia E, Soler R, Couto D, Castro‐Beiras A. Exercise echocardiography and cardiac magnetic resonance imaging to predict outcome in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:423–432. DOI: 10.1093/ehjci/jeu225. [DOI] [PubMed] [Google Scholar]
- 20. Desai MY, Bhonsale A, Patel P, Naji P, Smedira NG, Thamilarasan M, Lytle BW, Lever HM. Exercise echocardiography in asymptomatic HCM: exercise capacity, and not LV outflow tract gradient predicts long‐term outcomes. JACC Cardiovasc Imaging. 2014;7:26–36. DOI: 10.1016/j.jcmg.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 21. Tesic M, Djordjevic‐Dikic A, Beleslin B, Trifunovic D, Giga V, Marinkovic J, Petrovic O, Petrovic M, Stepanovic J, Dobric M, et al. Regional difference of microcirculation in patients with asymmetric hypertrophic cardiomyopathy: transthoracic Doppler coronary flow velocity reserve analysis. J Am Soc Echocardiogr. 2013;26:775–782. DOI: 10.1016/j.echo.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 22. Saraste M, Koskenvuo J, Knuuti J, Toikka J, Laine H, Niemi P, Sakuma H, Hartiala J. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol. 2001;21:114–122. DOI: 10.1046/j.1365-2281.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 23. Tesic M, Seferovic J, Trifunovic D, Djordjevic‐Dikic A, Giga V, Jovanovic I, Petrovic O, Marinkovic J, Stankovic S, Stepanovic J, et al. N‐terminal pro‐brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. J Cardiol. 2017;70:323–328. DOI: 10.1016/j.jjcc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 24. Jovanovic I, Tesic M, Giga V, Dobric M, Boskovic N, Vratonjic J, Orlic D, Gudelj O, Tomasevic M, Dikic M, et al. Impairment of coronary flow velocity reserve and global longitudinal strain in women with cardiac syndrome X and slow coronary flow. J Cardiol. 2020;76:1–8. DOI: 10.1016/j.jjcc.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 25. Ciampi Q, Zagatina A, Cortigiani L, Gaibazzi N, Borguezan Daros C, Zhuravskaya N, Wierzbowska‐Drabik K, Kasprzak JD, de Castro e Silva Pretto JL, D'Andrea A, et al. Functional, anatomical, and prognostic correlates of coronary flow velocity reserve during stress echocardiography. J Am Coll Cardiol. 2019;74:2278–2291. DOI: 10.1016/j.jacc.2019.08.1046. [DOI] [PubMed] [Google Scholar]
- 26. Cortigiani L, Ciampi Q, Lombardo A, Rigo F, Bovenzi F, Picano E. Age‐ and gender‐specific prognostic cutoff values of coronary flow velocity reserve in vasodilator stress echocardiography. J Am Soc Echocardiogr. 2019;32:1307–1317. DOI: 10.1016/j.echo.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 27. Tesic M, Djordjevic‐Dikic A, Giga V, Stepanovic J, Dobric M, Jovanovic I, Petrovic M, Mehmedbegovic Z, Milasinovic D, Dedovic V, et al. Prognostic value of transthoracic doppler echocardiography coronary flow velocity reserve in patients with nonculprit stenosis of intermediate severity early after primary percutaneous coronary intervention. J Am Soc Echocardiogr. 2018;31:880–887. DOI: 10.1016/j.echo.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 28. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary‐artery disease. N Engl J Med. 1979;300:1350–1358. DOI: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 29. Lazzeroni E, Picano E, Dodi C, Morozzi L, Chiriatti GP, Lu C, Botti G. Dipyridamole echocardiography for diagnosis of coexistent coronary artery disease in hypertrophic cardiomyopathy. Echo‐Persantine International Cooperative (EPIC) Study Group‐Subproject Hypertrophic Cardiomyopathy. Am J Cardiol. 1995;75:810–813. DOI: 10.1016/S0002-9149(99)80417-2. [DOI] [PubMed] [Google Scholar]
- 30. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1–39):e14. DOI: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 31. Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH 3rd, Zoghbi WA, Quinones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–261. DOI: 10.1161/01.CIR.99.2.254. [DOI] [PubMed] [Google Scholar]
- 32. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. DOI: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 33. Djordjevic Dikic A, Tesic M, Boskovic N, Giga V, Stepanovic J, Petrovic M, Dobric M, Aleksandric S, Juricic S, Dikic M, et al. Prognostic value of preserved coronary flow velocity reserve by noninvasive transthoracic doppler echocardiography in patients with angiographically intermediate left main stenosis. J Am Soc Echocardiogr. 2019;32:74–80. DOI: 10.1016/j.echo.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 34. Maron MS, Rowin EJ, Wessler BS, Mooney PJ, Fatima A, Patel P, Koethe BC, Romashko M, Link MS, Maron BJ. Enhanced American College of Cardiology/American Heart Association Strategy for Prevention of Sudden Cardiac Death in High‐Risk Patients With Hypertrophic Cardiomyopathy. JAMA Cardiol. 2019;4:644–657. DOI: 10.1001/jamacardio.2019.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spirito P, Autore C, Rapezzi C, Bernabò P, Badagliacca R, Maron MS, Bongioanni S, Coccolo F, Estes NAM, Barillà CS, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119:1703–1710. DOI: 10.1161/CIRCULATIONAHA.108.798314. [DOI] [PubMed] [Google Scholar]
- 36. Adabag AS, Casey SA, Kuskowski MA, Zenovich AG, Maron BJ. Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45:697–704. DOI: 10.1016/j.jacc.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 37. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. DOI: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 38. Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left‐ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–424. DOI: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 39. Kitaoka H, Kubo T, Okawa M, Takenaka N, Sakamoto C, Baba Y, Hayashi K, Yamasaki N, Matsumura Y, Doi YL. Tissue doppler imaging and plasma BNP levels to assess the prognosis in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2011;24(9):1020–1025. DOI: 10.1016/j.echo.2011.05.009. DOI: 10.1016/j.echo.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 40. Kofflard MJ, Michels M, Krams R, Kliffen M, Geleijnse ML, Ten Cate FJ, Serruys PW. Coronary flow reserve in hypertrophic cardiomyopathy: relation with microvascular dysfunction and pathophysiological characteristics. Neth Heart J. 2007;15:209–215. DOI: 10.1007/BF03085982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology. 2004;44:412–427. DOI: 10.1111/j.1365-2559.2004.01835.x. [DOI] [PubMed] [Google Scholar]
- 42. Ho CY, López B, Coelho‐Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–563. DOI: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, Camici PG. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:866–875. DOI: 10.1016/j.jacc.2009.04.072. [DOI] [PubMed] [Google Scholar]
- 44. Ciampi Q, Olivotto I, Peteiro J, D’Alfonso M, Mori F, Tassetti L, Milazzo A, Monserrat L, Fernandez X, Pálinkás A, et al. Prognostic value of reduced heart rate reserve during exercise in hypertrophic cardiomyopathy. J Clin Med. 2021;10:1347. DOI: 10.3390/jcm10071347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. DOI: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maron MS, Rowin EJ, Olivotto I, Casey SA, Arretini A, Tomberli B, Garberich RF, Link MS, Chan RHM, Lesser JR, et al. Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2016;67:1399–1409. DOI: 10.1016/j.jacc.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 47. Raphael CE, Cooper R, Parker KH, Collinson J, Vassiliou V, Pennell DJ, de Silva R, Hsu LY, Greve AM, Nijjer S, et al. Mechanisms of myocardial ischemia in hypertrophic cardiomyopathy: insights from wave intensity analysis and magnetic resonance. J Am Coll Cardiol. 2016;68:1651–1660. DOI: 10.1016/j.jacc.2016.07.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cortigiani L, Rigo F, Bovenzi F, Sicari R, Picano E. The prognostic value of coronary flow velocity reserve in two coronary arteries during vasodilator stress echocardiography. J Am Soc Echocardiogr. 2019;32:81–91. DOI: 10.1016/j.echo.2018.09.002. [DOI] [PubMed] [Google Scholar]


