Abstract
Background
There is wide variability in cardiac rehabilitation (CR) dose (ie, number of sessions) delivered, and no evidence‐based recommendations regarding what dose to prescribe. We aimed to test what CR dose impacts major adverse cardiovascular events (MACEs).
Methods and Results
This is an historical cohort study of all patients who had coronary artery disease and who initiated supervised CR between 2002 and 2012 from a single major CR center. CR dose was defined as number of visits including exercise and patient education. Follow‐up was performed using record linkage from the Rochester Epidemiology Project. MACEs included acute myocardial infarction, unstable angina, ventricular arrhythmias, stroke, revascularization, or all‐cause mortality. Dose was analyzed in several ways, including tertiles, categories, and as a continuous variable. Cox models were adjusted for factors associated with dose and MACE. The cohort consisted of 2345 patients, who attended a mean of 12.5±11.1 of 36 prescribed sessions. After a mean follow‐up of 6 years, 695 (29.65%) patients had a MACE, including 231 who died. CR dose was inversely associated with MACE (hazard ratio, 0.66 [95% CI]; 0.55–0.91) in those completing ≥20 sessions, when compared with those not exposed to formal exercise sessions (≤1 session; log‐rank P=0.007). We did not find evidence of nonlinearity (P≥0.050), suggesting no minimal threshold nor ceiling. Each additional session was associated with a lower rate of MACE (fully adjusted hazard ratio, 0.98 [95% CI, 0.97–0.99]). Greater session frequency was also associated with lower MACE risk (fully adjusted hazard ratio, 0.74 [95% CI, 0.58–0.94]).
Conclusions
CR reduces MACEs, but the benefit appears to be linear, with greater risk reduction with higher doses, and no upper threshold.
Keywords: cardiac rehabilitation, major adverse cardiovascular events, mortality
Subject Categories: Heart Failure, Cardiovascular Disease, Exercise, Lifestyle, Primary Prevention
Nonstandard Abbreviations and Acronyms
- CR
cardiac rehabilitation
- MACE
major adverse cardiovascular event
Clinical Perspective
What Is New?
This study confirms the dose–response association between cardiac rehabilitation (CR) session attendance or dose and reduced risk of major adverse cardiovascular events including death.
For the first time to our knowledge, a minimum dose was explored; results suggested no ceiling to dose benefit, as well as major adverse cardiovascular event reductions with every session attended.
Given that offering patients some unsupervised sessions may increase adherence and hence dose received, a minimum dose of unsupervised CR should be explored.
What Are the Clinical Implications?
Results of this study should encourage CR programs to consider both the sufficiency of the dose they offer each patient, and also degree of patient adherence to all prescribed sessions.
Effective interventions to increase program adherence should be applied, so patients can derive maximal benefit from CR.
Patients should be encouraged to be active on non‐CR days, and comprehensive secondary prevention should be sustained for the longest duration possible.
Cardiovascular disease is estimated to become the leading cause of disability worldwide by 2020. 1 Cardiac rehabilitation (CR) is an outpatient chronic disease management program designed to reduce the mortality and morbidity burden in patients with cardiovascular disease. It is well established that CR is a cost‐effective model of care, 2 which reduces cardiovascular mortality by ≈25% and hospital re‐admissions by 18%. 3
CR programs around the world are of varying durations, and sessions are offered at varying frequencies. 4 For example, in a recent review of CR guidelines, 5 the recommended duration ranged from a minimum of 3 weeks in Germany (although this is often residential) to a maximum of 12 months in Austria. The frequency recommended by the American Association of Cardiovascular and Pulmonary Rehabilitation, as well as the Canadian and European Associations of Cardiovascular Prevention and Rehabilitation was a minimum of 3 sessions per week, whereas guidelines for Austria, Australia, Japan, and the United Kingdom recommend 3 or fewer per week. Therefore, the “dose,” or the number of sessions per week multiplied by the number of weeks, is not standard, and is generally based on funding policies and past practice. This variation significantly affects costs to deliver CR, capacity to serve patients, and also outcomes achieved. Indeed, previous work has shown that the more CR patients receive, the better the outcomes. 6
To our knowledge, there is no evidence on which CR programs can base decisions on what dose should be offered to patients to achieve optimal clinical outcomes. The effect of CR dose on morbidity and mortality has been scantly examined in the literature previously, 7 , 8 , 9 , 10 , 11 , 12 , 13 with inconsistent definitions, and not often as a primary objective. Moreover, what investigation has been done has generally considered prescribed dose, but not the actual number of sessions patients attended; given adherence rates of 66%, 14 dose received should be used. 6 The purpose of this study was to identify the minimally effective dose of CR to reduce major adverse cardiovascular events (MACEs).
Methods
In consideration of the privacy of patients, the data, the analytic methods, and the study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Design and Data Sources
This is a population‐based, historical cohort study of all Olmsted County, Minnesota residents over the age of 18 years that enrolled in phase II, outpatient, CR for coronary artery disease at Mayo Clinic between the years 2002 and 2012. Patients were identified using the CR registry. Outcomes were ascertained using the Rochester Epidemiology Project, 15 , 16 a federally funded record linkage system that indexes medical records, medications, procedures, and other health‐related information from the primary providers of medical care in Olmsted County, namely, Olmsted Medical Center, the Mayo Clinic, and a few other individual private providers. All tertiary care, cardiovascular procedures, and CR for the population occurred at the Mayo Clinic during the study period. Baseline patient characteristics, CR attendance, and outcomes were passively ascertained through record linkage.
The study protocol was reviewed and approved by the Institutional Review Board of both the Mayo Clinic and Olmsted Medical Center. All included patients provided research authorization, as required by the state of Minnesota.
Setting
The comprehensive outpatient CR program at Mayo Clinic is based on the American Association of Cardiovascular and Pulmonary Rehabilitation guidelines. 17 With regard to dose, most CR‐eligible patients were prescribed three 1‐hour supervised sessions of CR per week, which on average consisted of 45 minutes of exercise and 15 minutes of counseling/education over 12 weeks. Participants could elect to come less frequently over a longer period, and in some cases they elected to stop their sessions because of weather, family obligations, or travel and later re‐initiated their remaining sessions. Thus, most patients were prescribed 36 sessions as per insurance regulations, although a small subset had additional insurance coverage that funded more sessions.
Participants
We included patients referred for CR because of coronary artery disease, defined as history of a myocardial infarction (MI), either ST‐ or non‐ST–segment elevation, unstable angina, coronary revascularization by either coronary artery bypass grafting or percutaneous coronary intervention, or chronic angina with documented evidence of stress‐induced myocardial ischemia. Patients who had MACE within the first 6 months of follow‐up were excluded to mitigate survivorship bias.
Measures
Sociodemographic and clinical characteristics, including the Charlson comorbidity index, 18 and number of diseased vessels treated with percutaneous coronary intervention, were extracted electronically from the Rochester Epidemiology Project within 3 months of CR entry. Clinical variables were operationalized as per the International Classification of Diseases, Ninth Revision (ICD‐9). 19 The data extraction approach has been previously validated. 15 , 16 A random sample of these variables was reviewed in duplicate for validation (JMI, FLJ). Interobserver agreement for sociodemographic and clinical characteristics was excellent (all κ>0.80).
Independent Variable
Dose was operationalized as actual CR attendance or number of sessions. It was ascertained from administrative data using Current Procedural Terminology (CPT) codes (93797–93798); a random subsample was verified in the Electronic Medical Record by an investigator (JMI) to confirm validity.
Dependent Variables
MACEs were ascertained through December 2014, and included any of the following events: acute coronary syndrome (MI [ICD‐9, 410.x] or unstable angina [ICD‐9, 411.x]), ventricular arrhythmias that required in‐hospital management (ICD‐9 427.X), stroke ([CPT/ICD‐9 433.X]), coronary revascularization (coronary artery bypass grafting [CPT/ICD‐9 337700‐337735/V45.81] or percutaneous coronary intervention [CPT/ICD‐9 2980‐92982/V45.82]), or death from any cause. Mortality information was obtained directly from the Rochester Epidemiology Project, which records vital status from state vital statistics offices and the National Death Index. 20 Follow‐up data were complete for this cohort. Only the first event was considered.
All outcome information was followed passively, through electronic ascertainment using diagnostic codes, an approach that has been extensively validated in the Rochester Epidemiology Project. 15 , 16 A physician‐investigator/co‐author (JMI), who was blinded to baseline characteristics, reviewed a fraction of the records in the record‐linkage system to confirm the outcome and validate the research strategy. Additionally, a random 10% of the outcomes were reviewed in duplicate and blinded by a clinician expert/senior author (FLJ) to ascertain interobserver agreement. Interobserver agreement over MACE outcome assessments was excellent (all κ>0.85).
Statistical Analysis
CR dose was operationalized 4 ways: (1) as a continuous variable; (2) as a binomial variable (ie, <12 versus ≥12 sessions, given this was the mean number of sessions, and a previous review suggests this may be a key threshold for mortality) 6 ; (3) categorically; and (4) on the basis of session frequency (ie, <2 versus ≥2 per week). For the third, the first category consisted of participants who did not enroll or only attended the orientation session, and thus had no exposure to formal exercise sessions (referent category; 0 or 1 session); another 3 additional categories were created based on tertiles of CR session attendance (ie, 2–7, 8–20, and >20).
Patient characteristics at the time of enrollment were compared across CR dose categories with χ2 tests or ANOVA, as appropriate. To further assess factors associated with CR participation, linear regression was used to estimate the associations between sociodemographic and clinical characteristics, with the number of CR sessions patients attended (continuous CR dose). The association between MACEs and patient characteristics was also tested, using Cox proportional hazards regression models. This was undertaken to inform adjustment of subsequent models.
To assess the association between CR dose and the time to first recorded MACE, Kaplan–Meier curves with the log‐rank test were used to test for difference in outcomes rates (to avoid survival bias, last recorded CR participation date was defined as baseline). Cox proportional hazards models were then run, adjusted for factors associated with CR dose and MACE, as follows: age and sex (model 1); age, sex, CR indication, sociodemographic and clinical characteristics significantly associated with CR participation and MACE in the univariate analysis (model 2); and sex, ethnocultural background, any former tobacco use, hypertension, dyslipidemia, plus the disease severity indicators that could impact dose received (ie, Charlson comorbidity index and number of diseased vessels, model 3). Because the Charlson comorbidity index 18 includes age, diabetes, myocardial infarction, and heart failure, we did not include these variables in the final model. CR session frequency (greater than average weekly and monthly sessions) was also modeled to assess its association with MACEs. Finally, aiming to identify the presence of a minimum threshold, we modeled the CR dose as continuous using penalized smoothing splines to better understand the potential nonlinear relationship. We also ran Cox models (as above) to test the association of each 1‐session dose increase with MACE.
Findings were summarized using hazard ratios and 95% CI. Cox models were also used to test the association between dose as a binomial variable and MACE as well as session frequency and MACE. The assumption of proportionality for the Cox proportional hazards models was assessed graphically (and fulfilled).
Two‐sided P values <0.05 were considered statistically significant. All analyses were completed using JMP, Version 14.1 (SAS Institute Inc., Cary, NC) and R (www.r‐project.org).
Results
Of the 2507 patients enrolled in CR in the study period, we excluded 162 (6.5%) patients who had MACEs within 6 months of CR enrollment (a comparison of baseline characteristics between excluded and included patients is presented in Table S1; no significant differences).
The final analytic cohort consisted of 2345 patients. Baseline characteristics are shown in Table 1. The mean number of CR sessions attended was 12.5±11.1, median was 9, and ranged from 1 to 49. Participants attended a mean of 1.12±1.19 sessions per week, 4.04±1.21 per month, over a median of 12±14.85 weeks, and the range was 1 to 25 weeks. The average number of CR sessions attended was similar for men and women (12.8±11.3 and 11.7±10.7, respectively, P=0.060). The mean number of sessions in which patients participated in the highest tertile was 27.00±7.31 (median=26).
Table 1.
Precardiac Rehabilitation Patient Sociodemographic and Clinical Characteristics by Cardiac Rehabilitation Dose Category
| ≤1 Session | 2 to 7 Sessions | 8 to 20 Sessions | >20 Sessions | Total | P value* | |
|---|---|---|---|---|---|---|
| (n=514) | (n=548) | (n=641) | (n=642) | (N=2345) | ||
| Sociodemographic | ||||||
| Age, y | 66.5±11.6 | 64.1±12.2 | 63.33±12.5 | 63.6±14.3 | 64.3±12.8 | <0.001 |
| Sex (female) | 147 (28.60%) | 151 (27.55%) | 192 (30.39%) | 225 (35.05%) | 715 (30.49%) | 0.025 |
| Ethnocultural background (White race) | 490 (95.33%) | 515 (93.98%) | 591 (92.20%) | 586 (91.28%) | 2182 (93.05%) | 0.030 |
| Clinical | ||||||
| Angina | 2 (0.39%) | 5 (0.91%) | 3 (0.47%) | 8 (1.25%) | 18 (0.877%) | <0.001 |
| Percutaneous coronary intervention | 165 (32.10%) | 230 (41.97%) | 279 (43.53%) | 338 (52.73%) | 1012 (43.17%) | |
| Coronary artery bypass graft surgery | 127 (24.71%) | 132 (24.09%) | 168 (26.21%) | 177 (27.61%) | 604 (25.77%) | |
| Myocardial infarction | 220 (42.80%) | 181 (33.03%) | 191 (29.80%) | 118 (18.41%) | 710 (30.29%) | |
| Hypertension | 363 (70.62%) | 293 (53.47%) | 336 (52.42%) | 382 (59.50%) | 1374 (58.59%) | <0.001 |
| Dyslipidemia | 471 (91.63%) | 512 (93.43%) | 590 (92.04%) | 575 (89.56%) | 2148 (91.60%) | 0.113 |
| Diabetes | 222 (43.19%) | 239 (43.61%) | 281 (43.84%) | 271 (42.21%) | 1013 (43.20%) | 0.940 |
| Any former tobacco use | 252 (49.03%) | 234 (42.70%) | 262 (40.87%) | 273 (42.52%) | 1021 (43.54%) | 0.036 |
| BMI, kg/m2 | 30.0±6.3 | 29.4±6.0 | 29.3±5.6 | 29.6±6.2 | 29.6±6.0 | 0.311 |
| Heart failure | 115 (22.37%) | 111 (20.26%) | 126 (19.66%) | 154 (23.99%) | 506 (21.58%) | 0.2267 |
| Charlson comorbidity index | 3.5±2.8 | 3.8±2.8 | 3.8±2.8 | 3.5±2.7 | 3.7±2.8 | 0.121 |
| Number of diseased vessels | 1.13±0.35 | 1.15±0.41 | 1.15±0.39 | 1.12±0.34 | 1.14±0.37 | 0.665 |
Values are mean±SD or n (%). BMI indicates body mass index.
P value for χ2 or ANOVA across dose category, as appropriate.
Pre‐CR characteristics by CR dose category are shown in Table 1. The table also shows sociodemographic and clinical characteristics. Factors associated with CR dose (continuous) were age, ethnocultural background, hypertension, dyslipidemia, indication, and Charlson comorbidity index (Table S2).
During a median follow‐up of 6.0 years (interquartile range, 3.16–8.56), there were 695 (29.64%) participants who had at least 1 MACE (Table 2). In univariate analysis, factors associated with MACEs included age, indication, history of hypertension, and tobacco use (Table 3).
Table 2.
Major Adverse Cardiovascular Events by Cardiac Rehabilitation Dose Category
| ≤1 Session | 2 to 7 Sessions | 8 to 20 Sessions | >20 Sessions | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n=514) | (n=548) | (n=641) | (n=642) | (N=2345) | ||||||
| Ventricular arrhythmias | 1 | 0.19% | 3 | 0.55% | 5 | 0.78% | 2 | 0.31% | 11 | 0.47% |
| CABG | 10 | 1.95% | 9 | 1.64% | 6 | 0.94% | 10 | 1.56% | 35 | 1.49% |
| Angina | 25 | 4.86% | 25 | 4.56% | 26 | 4.06% | 13 | 2.02% | 89 | 3.80% |
| Death | 50 | 9.73% | 46 | 8.39% | 64 | 9.98% | 71 | 11.06% | 231 | 9.85% |
| Stroke | 6 | 1.17% | 20 | 3.65% | 21 | 3.28% | 15 | 2.34% | 62 | 2.64% |
| Myocardial infarction | 20 | 3.89% | 19 | 3.47% | 13 | 2.03% | 12 | 1.87% | 64 | 2.73% |
| PCI | 66 | 12.84% | 45 | 8.21% | 57 | 8.89% | 35 | 5.45% | 203 | 8.66% |
| Total MACE | 178 | 34.63% | 167 | 30.47% | 192 | 29.95% | 158 | 24.61% | 695 | 29.64% |
CABG indicates coronary artery bypass graft; MACE, major adverse cardiovascular event; and PCI, percutaneous coronary intervention.
Table 3.
Association Between Patient Characteristics and Having Any Major Adverse Cardiovascular Event
| HR | 95% CI | P value | |
|---|---|---|---|
| Sociodemographic | |||
| Age, y | 1.02 | 1.01 to 1.03 | <0.001 |
| Sex (women) | 1.15 | 0.98 to 1.35 | 0.077 |
| Ethnocultural background (White) | 1.29 | 0.93 to 1.79 | 0.123 |
| Clinical | |||
| Indication | |||
| Angina | 3.44 | 1.89 to 6.25 | <0.001 |
| Percutaneous coronary intervention | 0.98 | 0.85 to 1.15 | 0.859 |
| Coronary artery bypass graft surgery | 1.01 | 0.85 to 1.20 | 0.856 |
| Myocardial infarction | 1.65 | 1.41 to 1.92 | <0.001 |
| Risk factors | |||
| Hypertension | 1.22 | 1.05 to 1.41 | 0.011 |
| Dyslipidemia | 0.88 | 0.67 to 1.17 | 0.409 |
| Diabetes | 1.12 | 0.96 to 1.29 | 0.147 |
| Any former tobacco use | 1.14 | 1.07 to 1.33 | 0.012 |
| BMI (kg/m2) per 1‐unit increase | 1.00 | 0.99 to 1.01 | 0.995 |
| Disease severity indicators | |||
| Heart failure | 1.11 | 0.94 to 1.33 | 0.211 |
| Charlson comorbidity index | 1.01 | 0.99 to 1.05 | 0.163 |
| Number of diseased vessels | 1.10 | 0.88 to 1.39 | 0.384 |
Cox proportional hazard models shown. BMI indicates body mass index; and HR, hazard ratio.
Association Between Dose and MACEs
CR dose category was associated with a decreased risk of MACEs (Figure). The Kaplan–Meier MACE‐free median survival rates across CR dose categories were 71.16%, 75.35%, 75.37%, and 79.23% for ≤1, 2 to 7, 8 to 20, and >20 sessions, respectively. After adjusting for sociodemographic and clinical characteristics, CR dose categories remained associated with MACE (Table 4); this association was unaffected by further adjustment for disease severity indicators. No significant interactions were observed when considering sex, age, heart failure, or diabetes (patient groups that often adhere less to CR and have more MACEs).
Figure 1. Kaplan–Meier survival curve by cardiac rehabilitation dose category.
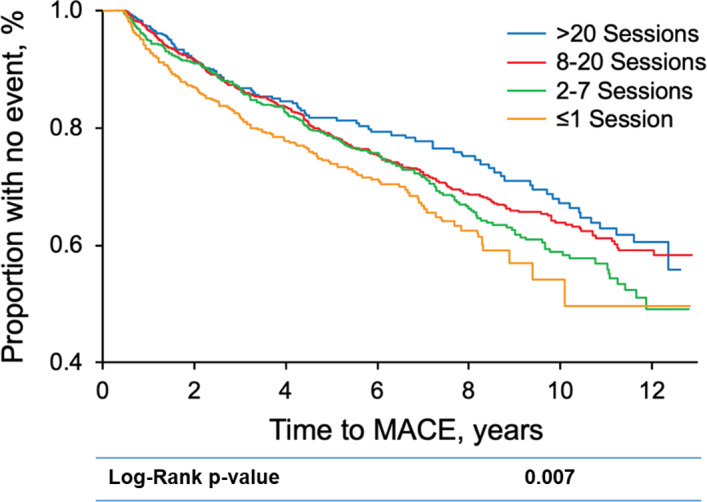
A significant dose–response association between cardiac rehabilitation session attendance and reductions in MACEs is observed. MACE indicates major adverse cardiovascular event.
Table 4.
Cox Proportional Hazard Models Testing the Association Between CR Dose and Having a Major Adverse Cardiovascular Event
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| ≤1 Session | Referent | 0.008† | Referent | 0.006† | Referent | 0.010† | Referent | 0.001† | ||||
| 2 to 7 Sessions | 0.88 | (0.69–1.05) | 0.139 | 0.85 | (0.69–1.05) | 0.150 | 0.84 | (0.68–1.05) | 0.125 | 0.81 | (0.65–1.01) | 0.060 |
| 7 to 20 Sessions | 0.76 | (0.61–0.91) | 0.005 | 0.75 | (0.60–0.91) | 0.005 | 0.74 | (0.62–0.94) | 0.010 | 0.70 | (0.57–0.87) | 0.001 |
| >20 Sessions | 0.68 | (0.53–0.87) | 0.002 | 0.67 | (0.52–0.86) | 0.002 | 0.66 | (0.55–0.91) | 0.007 | 0.64 | (0.50–0.82) | 0.001 |
| Per 1 session increase* | 0.98 | (0.97–0.99) | 0.008 | 0.98 | (0.97–0.99) | 0.007 | 0.98 | (0.97–0.99) | 0.031 | 0.98 | (0.97–0.99) | 0.005 |
Cox proportional hazard models shown. Model 1 adjusted for age and sex; Model 2 adjusted for age, sex, ethnocultural background, history of hypertension, any former tobacco use, diabetes, dyslipidemia, and CR indication; Model 3 adjusted for sex, ethnocultural background, any former tobacco use, hypertension, dyslipidemia, the Charlson comorbidity index, and number of treated vessels during PCI.
CR indicates cardiac rehabilitation; HR, hazard ratio; and PCI, percutaneous coronary intervention.
Adjusted models that include this variable do not included session quartile.
Represents P value for trend.
When considering the median number of sessions completed, the risk of MACE was significantly lower (adjusted hazard ratios, 0.83 [95% CI, 0.71–0.99]; P=0.020) for those completing ≥12 sessions versus less. Moreover, those who attended on average >2 session a week also had a decreased risk for MACE (hazard ratio, 0.74 [95% CI, 0.58–0.94]; P=0.010), and those attending more than the average 4 monthly sessions also had a decreased risk for MACE (hazard ratio, 0.60 [95% CI, 0.48–0.76]; P<0.001) than those attending fewer monthly sessions, independent of the total dose.
The P for trend was <0.05 in all models, suggesting that a greater CR dose is associated with better outcomes (Table 4). To determine a minimum number of sessions impacting MACE, a penalized smoothing spline analysis was used, using CR dose as a continuous (Figure S1) variable (P for linearity >0.05). As shown, we could not find evidence of nonlinearity, and there appeared to be no minimum threshold, nor a ceiling to benefit. Using the continuous range from 1 to 49 sessions attended, the impact of an increase in CR sessions on overall MACEs was also modeled (Table 4). A continuous increase in 1 session was significantly associated with a 1% to 2% reduction in MACE risk, in all models.
Discussion
In this study we demonstrate for the first time an uninterrupted ceiling effect of CR dose in reduction of MACE risk, and that continuous single session increases may even have an impact. Results confirm the dose–response association between session attendance and MACEs 8 , 9 , 10 , 11 , 12 , 13 ; the linear relationship precluded determination of a minimum threshold. Because we did not find a ceiling effect as is generally observed in studies relating aerobic exercise and outcomes, this suggests the doses we currently recommend may still be suboptimal; patients may benefit from longer programs or programs with more frequent exercise sessions a week. 21 , 22 The results underscore the need to develop novel strategies to motivate patients to attend as many sessions as possible, including a greater number of sessions each week, although it is needed to establish proven means of improving CR adherence. 23 , 24
Results are consistent with previous, albeit limited, results in this area. Subgroup analyses in the meta‐analysis by Lawler showed lower cardiovascular‐related mortality (cause of death could not be distinguished in this study) and MI with programs longer than 3 months (no other outcomes reported), 25 and lower cardiovascular‐related mortality, and MI with greater doses was reported in the latest Cochrane review subgroup analyses. 3 There was no impact of dose observed on all‐cause mortality, cardiovascular‐related hospitalization, or revascularization in the latter review. They also considered exercise intensity in their operationalization of dose; this may not be adequately reported and varies over time, rendering quantification problematic.
In terms of primary studies, Whellan et al. and Kuo et al. reported lower all‐cause mortality with only 6 sessions and Suaya et al. reported the same with >24 sessions attended, although no other outcomes were reported and only 2 dose levels were considered in each study. 8 , 9 , 13 Hammill et al. 10 considered dose tertiles in terms of actual (not just prescribed) attendance, but on all‐cause mortality and MI only, and to our knowledge that represents the greatest number of dose categories reported in the literature until this study. They, too, found an association between dose and outcomes. Doll et al. reported 13% lower mortality and 31% less hospitalization for every 5 CR sessions. 12 None of these primary studies stated as their aim to ascertain the minimum dose to achieve mortality and morbidity reductions.
When prescribed 36 sessions, patients attended on average 13, or only one third. Clearly to ensure patients receive maximum dose to achieve reduced morbidity and mortality, proven interventions to improve program adherence need to be applied. 23 This may involve offering unsupervised sessions; however, caution is warranted because previous research has not done a good job comparing adherence across settings equivalently (ie, it is much easier to answer a phone call than to fight traffic and pay for parking at the CR center, yet studies often consider both of these session “attendance”). 24 No research, however, has yet established the dose of home‐based CR (and would need to consider objectively measuring unsupervised secondary prevention behaviors) to reduce MACEs so it is not known at this time what to prescribe in that setting to optimize outcomes. Programs should be encouraging patients to be active on non‐CR days, 26 given that greater session frequency was associated with lower MACE risk and that some programs may not have capacity to increase session frequency. Programs should also be encouraging patients to be active postprogram, 27 given the lack of ceiling effect and limits on program resources to extend duration. Given that results of this study suggest that participating in even 1 additional session is associated with significant MACE reductions, this could serve as a minimal clinically important difference for trials of adherence interventions.
The results herein leave unanswered questions about the sufficiency of CR dose delivered in practice around the globe. The recent global survey of CR programs revealed that programs in the Western Pacific and in middle‐income countries offer lower doses to patients. 4 Augmenting CR dose across countries where standard prescriptions are low should be advocated, which would require significant policy, capacity, and funding changes. The global survey also revealed that programs offered in home‐based settings prescribe many fewer sessions than supervised programs; while as outlined above we do not know what dose is needed to achieve reductions in MACEs in these settings, given the lack of ceiling identified herein, likely we should be advocating for higher doses and assessing more closely a patient’s exercise and other secondary prevention behaviors at home. Reimbursement policies should be revisited to ensure coverage for a sufficient number of CR sessions to achieve optimal benefit, regardless of setting. Results also re‐emphasize the importance of getting all indicated patients into programs so these benefits are achieved.
Caution is warranted in interpreting the results. First, the design was observational, and hence causal associations between CR dose and MACEs cannot be drawn. For example, patients who are healthy because of adhering to medical advice may also have been more likely to adhere to the CR program, and hence have lower mortality (ie, lower MACEs and higher dose). 28 It could also be the case that sicker patients cannot continue CR for clinical reasons and hence receive a lower dose 7 ; however, our analyses did adjust for disease severity indicators (it was shown that those with a higher comorbidity burden [Charlson] did participate in fewer sessions, but there was no association of heart failure or number of diseased vessels with CR dose) and only included those without MACEs to 6 months. Alternatively, it could be that sicker patients (eg, heart failure, diabetes) need less dose to achieve MACE benefits, but exploratory analyses ruled this out; replication is warranted because this is a clinically meaningful question. Clearly, herein lie some important directions for future research. Second, despite adjustment for sociodemographic and clinical characteristics, we were not able to adjust for other social determinants of CR attendance (ie, transportation, income, and medication use), and (other social determinants of health); thus additional sources of residual confounding are possible.
With regard to generalizability, data are limited to a single center and to individuals who have coronary artery disease and who are attending supervised CR. However, the CR center was the only one available in the region, and therefore findings should be fairly representative of patients who attend supervised CR in general. The generalizability of the cohort more broadly has been established elsewhere. 29 Furthermore, patients in this cohort could overlap with other cohorts from this community deployed to explore the benefits of CR, nonetheless with different scope and design. 30
Finally, while it was assumed that all participants were exercising based on an individual exercise prescription that was progressed to meet guideline recommendations, sessions attended is a surrogate for “true” dose. For example, degree of unsupervised (ie, non‐CR days), and hence total, exercise during CR that could also impact MACEs was not captured, and hence could not be considered. Moreover, we did not measure the extent of lifestyle changes such as smoking cessation, and degree of exercise after patients completed their CR program, which could impact MACEs; degree of postprogram exercise might also have been related to degree during CR. However, CR programs are comprehensive, so it likely is not just the structured exercise that is associated with reduced MACE risk, but also the medical risk factor management and psychosocial counseling, among other components that drive the effect. 31
Conclusions
There is a dose–response association between CR participation and MACEs with no apparent lower threshold or ceiling. Policies, clinical guidelines, and practices must be revised to ensure patients are receiving CR, adhering to their programs (which offer ample sessions), and encouraged to exercise on non‐CR days as well as postprogram.
Sources of Funding
This work was supported in part by the European Regional Development Fund‐FNUSA‐ICRC (No. Z.1.05/1.1.00/02.0123) by project no. LQ1605 from the National Program of Sustainability II (MEYS CR), by the project ICRC‐ERA‐Human Bridge (No. 316345) funded by the 7th Framework Programme of the European Union. This publication was also made possible in part by CTSA Grant Number UL1TR000135, resources of the Rochester Epidemiology Project (REP) medical records‐linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. However, the content of this article is solely the responsibility of the authors and does not represent the official views of the funding sources.
Disclosures
Dr Stokin reports grants from ICRC during the conduct of the study. Dr Bonikowske received research royalties from Viome, Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1
For Sources of Funding and Disclosures, see page 8.
References
- 1. Fuster V, Kelly BB, Vedanthan R. Promoting global cardiovascular health: moving forward. Circulation. 2011;123:1671–1678. doi: 10.1161/CIRCULATIONAHA.110.009522 [DOI] [PubMed] [Google Scholar]
- 2. Shields GE, Wells A, Doherty P, Heagerty A, Buck D, Davies LM. Cost‐effectiveness of cardiac rehabilitation: a systematic review. Heart. 2018;104:1403–1410. doi: 10.1136/heartjnl-2017-312809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta‐analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 4. Chaves G, Turk‐Adawi K, Supervia M, de Araújo S, Pio C, Abu‐Jeish AH, Mamataz T, Tarima S, Lopez Jimenez F, Grace SL. Cardiac rehabilitation dose around the world. Circ Cardiovasc Qual Outcomes. 2020;13:e005453. doi: 10.1161/CIRCOUTCOMES.119.005453 [DOI] [PubMed] [Google Scholar]
- 5. Price KJ, Gordon BA, Bird SR, Benson AC. A review of guidelines for cardiac rehabilitation exercise programmes: is there an international consensus? Eur J Prev Cardiol. 2016;23:1715–1733. doi: 10.1177/2047487316657669 [DOI] [PubMed] [Google Scholar]
- 6. de Araújo S, Pio C, Marzolini S, Pakosh M, Grace SL. Effect of cardiac rehabilitation dose on mortality and morbidity: a systematic review and meta‐regression analysis. Mayo Clin Proc. 2017;92:1644–1659. doi: 10.1016/j.mayocp.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 7. Pardaens S, Willems AM, Clays E, Baert A, Vanderheyden M, Verstreken S, Du Bois I, Vervloet D, De Sutter J. The impact of drop‐out in cardiac rehabilitation on outcome among coronary artery disease patients. Eur J Prev Cardiol. 2017;24:1490–1497. doi: 10.1177/2047487317724574 [DOI] [PubMed] [Google Scholar]
- 8. Whellan DJ, Shaw LK, Bart BA, Kraus WE, Califf RM, O’Connor CM. Cardiac rehabilitation and survival in patients with left ventricular systolic dysfunction. Am Heart J. 2001;142:160–166. doi: 10.1067/mhj.2001.115785 [DOI] [PubMed] [Google Scholar]
- 9. Suaya JA, Stason WB, Ades PA, Normand SLT, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. doi: 10.1016/j.jacc.2009.01.078 [DOI] [PubMed] [Google Scholar]
- 10. Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long‐term risks of death and myocardial infarction among elderly medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/CIRCULATIONAHA.109.876383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beauchamp A, Worcester M, Ng A, Murphy B, Tatoulis J, Grigg L, Newman R, Goble A. Attendance at cardiac rehabilitation is associated with lower all‐cause mortality after 14 years of follow‐up. Heart. 2013;99:620–625. doi: 10.1136/heartjnl-2012-303022 [DOI] [PubMed] [Google Scholar]
- 12. Doll JA, Hellkamp A, Thomas L, Ho PM, Kontos MC, Whooley MA, Boyden TF, Peterson ED, Wang TY. Effectiveness of cardiac rehabilitation among older patients after acute myocardial infarction. Am Heart J. 2015;170:855–864. doi: 10.1016/j.ahj.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 13. Kuo LY, Shen SL, Hsu CL, Chen BY, Lin YS, Tsai HY, Cheng FH, Lai YL, Lin WH, Huang HY. Effect of cardiac rehabilitation on cardiovascular events after coronary artery bypass grafting in a 6‐year follow‐up study study population. Health Sci J. 2016;10:1–7. [Google Scholar]
- 14. Oosenbrug E, Marinho RP, Zhang J, Marzolini S, Colella TJ, Pakosh M, Grace SL. Sex differences in cardiac rehabilitation adherence: a meta‐analysis. Can J Cardiol. 2016;32:1316–1324. doi: 10.1016/j.cjca.2016.01.036 [DOI] [PubMed] [Google Scholar]
- 15. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ III, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melton LJ. History of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17. American Association of Cardiovascular and Pulmonary Rehabilitation . Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs, 5th ed. Human Kinetics; 2013. [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19. National Center for Health Statistics, Council on Clinical Classifications, Commission on Professional and Hospital Activities & WHO . The International Classification of Diseases. 9th ed. (Commission on Professional and Hospital Activities, editor.). 1978. Available at: https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed August 31, 2021.
- 20. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ III, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester epidemiology project (REP) medical records‐linkage system. Int J Epidemiol. 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamm LF, Kavanagh T, Campbell RB, Mertens DJ, Beyene J, Kennedy J, Shephard RJ. Timeline for peak improvements during 52 weeks of outpatient cardiac rehabilitation. J Cardiopulm Rehabil. 2004;24:374–382. doi: 10.1097/00008483-200411000-00002 [DOI] [PubMed] [Google Scholar]
- 22. Giannuzzi P, Temporelli PL, Marchioli R, Maggioni AP, Balestroni G, Ceci V, Chieffo C, Gattone M, Griffo R, Schweiger C, et al. Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Arch Intern Med. 2008;168:2194–2204. doi: 10.1001/archinte.168.20.2194 [DOI] [PubMed] [Google Scholar]
- 23. de Araújo S, Pio C, Chaves GS, Davies P, Taylor RS, Grace SL. Interventions to promote patient utilisation of cardiac rehabilitation. Cochrane Database Syst Rev. 2019;2:CD007131. doi: 10.1002/14651858.CD007131.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Araújo S, Pio C, Beckie TM, Varnfield M, Sarrafzadegan N, Babu AS, Baidya S, Buckley J, Chen SY, Gagliardi A, et al. Promoting patient utilization of outpatient cardiac rehabilitation: a joint International Council and Canadian Association of Cardiovascular Prevention and Rehabilitation position statement. Int J Cardiol. 2019;35(10):S206–S207. doi: 10.1016/j.cjca.2019.07.390 [DOI] [PubMed] [Google Scholar]
- 25. Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise‐based cardiac rehabilitation post‐myocardial infarction: a systematic review and meta‐analysis of randomized controlled trials. Am Heart J. 2011;162:571–584. doi: 10.1016/j.ahj.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 26. Taylor C, Tsakirides C, Moxon J, Moxon JW, Dudfield M, Witte K, Ingle L, Carroll S. Exercise dose and all‐cause mortality within extended cardiac rehabilitation: a cohort study. Open Heart. 2017;4:1–7. doi: 10.1136/openhrt-2017-000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chase J‐AD. Systematic review of physical activity intervention studies after cardiac rehabilitation. J Cardiovasc Nurs. 2011;26:351–358. doi: 10.1097/JCN.0b013e3182049f00 [DOI] [PubMed] [Google Scholar]
- 28. Alter DA, Zagorski B, Marzolini S, Forhan M, Oh PI. On‐site programmatic attendance to cardiac rehabilitation and the healthy‐adherer effect. Eur J Prev Cardiol. 2015;22:1232–1246. doi: 10.1177/2047487314544084 [DOI] [PubMed] [Google Scholar]
- 29. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123(7):2344–2352. doi: 10.1161/CIRCULATIONAHA.110.983536 [DOI] [PubMed] [Google Scholar]
- 31. Kabboul NN, Tomlinson G, Francis TA, Grace SL, Chaves G, Rac V, Daou‐Kabboul T, Bielecki JM, Alter DA, Krahn M. Comparative effectiveness of the core components of cardiac rehabilitation on mortality and morbidity: a systematic review and network meta‐analysis. J Clin Med. 2018;7:514. doi: 10.3390/jcm7120514 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


