Abstract
While a variety of meditation techniques are increasingly employed as health interventions, the fact that meditation requires a significant commitment of time and effort may limit its potential widespread utility. In the current study, we ask whether baseline subjective reports or brain activity in response to a “Pain for Self and Others” paradigm predicts subsequent engagement in mindfulness and compassion meditation. The study also investigated whether compassion training would impact neural responses when compared to an active health education control group. Prior to training, activation of the left and right anterior insula, an area thought to be important for empathy, in response to the Other pain task was positively related to engagement with compassion meditation as measured by practice time (n=13). On the other hand, activity in the left amygdala during the Self pain task was negatively correlated with mindfulness practice time. Following the study intervention, there was no difference between the compassion group (n=13), and the control group (n=8), in brain responses to either the Self or Other task. These results are the first to indicate that baseline neural responses may predict engagement with meditation training and suggest that pre-existing neurobiological profiles differentially predispose individuals to engage with disparate meditation techniques.
Keywords: Meditation, Compassion, Mindfulness, Anterior insula, Amygdala, Empathy
Introduction
Meditation is increasingly incorporated into clinical treatments for a variety of mental and physical ailments (Hofmann et al., 2011; Marchand, 2012) and is widely believed to enhance well-being even in individuals not suffering from any specific mental or physical disorder (Sedlmeier et al., 2012). However, despite its apparent promise, the commitment of time and effort required to learn meditative techniques may limit their potential widespread utility. This may be particularly true of meditative practices designed to enhance compassionate feelings and behaviors toward others since these practices pose the additional challenge of requiring trainees to contemplate deeply and for extended periods about the suffering of other people, including those they love. Because health-relevant emotional and physiological effects of compassion meditation appear to be positively associated with practice time (Fredrickson et al., 2008; Pace et al., 2009, 2010, 2012), identifying pre-existing variables that predict people’s differential ability or willingness to engage with the practice has clear therapeutic relevance. The current study sought to identify whether self-report or neurobiological responses to suffering in oneself or others prior to learning compassion meditation would predict subsequent engagement with the practice over an 8-week training period. An additional aim was to investigate whether the training program impacted neural responses upon repeated exposure to “Pain for Self and Others” when compared to an active health education control group.
Cognitively-Based Compassion Training (CBCT), the protocol used in the current investigation, reduces stress responsivity (Pace et al., 2009), depression (Desbordes et al., 2012), and enhances empathic accuracy and related neural activity (Mascaro et al., 2012; Pace et al., 2009). Increased amount of practice time has also been shown to reduce levels of c-reactive protein–an important biomarker for disease development–in traumatized/neglected youth (Pace et al., 2012). CBCT is a secularized training derived from the 11th century Tibetan Buddhist lojong tradition. As such, it commences with two-weeks of training in focused attention (shamatha) and non-judgmental awareness (vipassana) prior to one week of self compassion training and five weeks of specific compassion training designed to enhance interpersonal equanimity, increase feelings of gratitude toward others, and finally to induce strong feelings of empathy toward all people. Importantly, these two aspects of the training (i.e. shamatha/vipassana during the first two weeks and compassion during the last five weeks), are widely divergent in terms of the meditative techniques they employ. Because of this, CBCT provided a novel opportunity to examine the specificity with which subjective and neurobiological responses to the suffering of self and others predict the ability to practice mindfulness based techniques (i.e. shamatha/vipassana) vs. compassion techniques.
The mindfulness-based technique employed in CBCT (i.e. shamatha/vipassana) has been a target of extensive scientific investigation in the last decade, in part because of its apparent promise as a relatively brief and cost-effective practice for alleviating anxiety as well as for enhancing well-being (Baer, 2003; Grossman et al., 2004; Marchand, 2012; Sedlmeier et al., 2012). In particular, it is thought that by promoting a type of awareness in which phenomena are experienced in a non-analytical and non-evaluative manner, mindfulness techniques allow individuals to experience aversive events with less emotional reactivity (Creswell et al., 2007). The mindfulness component of the protocol used in this study was designed to be explicitly devoid of any compassion related content and required that practitioners attend to their sensations, thoughts, and emotions in a non-evaluative manner. We therefore hypothesized that baseline aversiveness ratings and neural activity in regions important for the affective and evaluative response to pain, such as the mid cingulate cortex (MCC), anterior insula, and amygdala (Peyron et al., 1999; Price, 2000), would be inversely associated with the ability or willingness to subsequently engage with the mindfulness component of the CBCT protocol.
Given that the compassion-specific elements within CBCT require extensive contemplation of the suffering of others, we hypothesized that individuals with high levels of baseline empathy would be more likely to engage in this portion of the CBCT protocol. The neural systems related to empathy, defined as an affective reaction similar to, and evoked by, another’s affective state (de Vignemont and Singer, 2006) have been investigated using an empathy for pain (EFP) paradigm. This paradigm, in which participants imagine or observe other people receiving a painful stimulus, commonly elicits neural activation in the affective component of the pain matrix, including the anterior mid-cingulate cortex (aMCC), as well as the bilateral anterior insula (AI) and the ventral frontal operculum (Botvinick et al., 2005; Jackson et al., 2005; Lamm et al., 2010; Simon et al., 2006; Singer et al., 2004). Activity in the AI may represent a simulated mapping of the observed individual’s body state onto one’s own (Singer et al., 2009), which is important for an empathic response. Importantly, activity in the AI predicts later helping behavior, suggesting that its activity is related to prosocial emotions and motivation rather than to the type of distress that has been shown to precede more self-serving behavior (Batson, 1998; Hein et al., 2010). We therefore hypothesized that the activity in the AI in response to an empathy inducing task as well as self-reported empathy levels would predict amount of subsequent engagement with the compassion-specific elements of the CBCT protocol.
Materials and methods
This study was approved by the Institutional Review Board of Emory University and all participants gave written informed consent prior to inclusion. To test the study hypotheses participants underwent functional magnetic resonance imaging (fMRI) while both receiving (Self) and watching videos of others receiving (Other) painful stimulations both prior to and upon completion of the study interventions (for design, see Supplementary figure S1).
Study participants
Twenty-nine (16 males) participants from the Atlanta area were recruited using a combination of fliers and electronic notifications posted at several local universities, as well as electronic advertisements on Craigslist as part of a larger study that assessed the effects of meditation on stress physiology and social cognition. Participants were aged 25–55 (M=31.0; SD=6.02) and were screened and excluded for (self-reported) use of any psychotropic medication within 1 year of screening, for regular use of any medications that might influence activity of the autonomic nervous system, HPA axis, or inflammatory pathways, and for any ongoing medical or psychiatric condition.
Compassion meditation
The compassion meditation training protocol used here (Cognitively-Based Compassion Training [CBCT]) was designed by one of us (LTN). Although secular in presentation, CBCT derives from the 11th century Tibetan Buddhist lojong tradition. In its operationalization, CBCT made two important modifications to traditional lojong teachings. First, all discussions of soteriological or existential themes (e.g. the attainment of Buddhahood, Karma) were omitted. Second, participants were taught one week each of concentrative (i.e. shamatha) and mindful-awareness (i.e. vipassana) practices at the beginning of the course. While not specifically included in traditional lojong curricula, these basic meditation practices were an assumed prerequisite for commencing lojong training in a traditional Buddhist context (HHDL, 2001). For simplicity, we have subsumed these attention practices under the term ‘mindfulness practice’, in accordance with the general Western and clinical understanding of mindfulness, although it is important to note that the attentional practices that are trained in the first two weeks of CBCT are different than those employed when MBSR training is taken as a whole, because they are without any compassion related content. A complete description of the weekly schedule can be found in the Supporting information.
The compassion meditation courses were taught by two graduate students from the Emory Religion department who are experienced meditators and who had undergone extensive training with Lobsang Tenzin Negi. Study participants were asked to attend 2 h of class time per week for eight weeks. Class sessions combined a didactic teaching and discussion section with approximately 20 min of meditation per hour class time. Participants were provided with a meditation compact disk to guide “at-home” practice sessions that reflected in-class material, and were asked to keep track of practice time each day. In calculating practice time for the current study, only “at-home” practice was included, as it was determined a priori that in-class practice did not reflect engagement with the material because participants did not know the specific techniques they would practice each week prior to attending class.
Health discussion control group
Participants randomized to the control condition attended 2 h of a discussion group per week. Classes were designed and taught by graduate students from the Emory Rollins School of Public Health. Topics included history of medicine, nutrition, sleep, mental health, exercise, stress, infectious disease, and complementary and alternative medicine. The health discussion group was designed to control for the non-specific effects of the meditation class, including education and social engagement with a collective group. Subjects were not asked to do any “at home” work.
Protocol for preparing the Other pain stimuli video
The empathy for pain video stimuli set was created using the following protocol. Twenty participants (10 males) were recruited from the Emory campus and we explicitly solicited a diverse population in terms of age and ethnicity (11 Caucasian, 9 non-Caucasian). Volunteers were seated such that they could both view a laptop computer and face directly toward the video camera. Participants were told that the video clips would be used as stimuli in an fMRI study of empathy and were asked to make facial expressions that came naturally.
Volunteers were outfitted with 2 electrode pads on the inside of their right wrist and connected to the GRASS SD-9 stimulator. First, we indexed participants’ pain tolerance by asking them to rate stimulations on a 10-point intensity rating scale (0=‘don’t feel anything’, 1=‘can feel something but not painful’, 8=‘maximum tolerable pain’, and 10=‘worst imaginable pain’). The 1 setting was used for the ‘no pain’ stimuli and the 8 setting was used for the ‘pain’ stimuli. Upon finding the settings, 3 of each were administered in pseudorandom order. Prior to each stimulation, the laptop screen next to the subjects showed a colored screen for 6 s indicating which level they were about to receive (a red screen indicated that they would receive a painful stimulation, a blue screen indicated that they would receive a non-painful stimulation). Each stimulation lasted approximately 3 s.
Data collection
After providing informed consent, participants completed the Interpersonal Reactivity Index (IRI) (Davis, 1983a, 1983b), a 28 item Likert-scale measure (0=Does not describe me very well, 4=Describes me very well). The primary IRI subscale of interest was the empathic concern subscale (example item, ‘I often have tender, concerned feelings for people less fortunate than me’), which assesses other-oriented affective responses to those who are suffering.
Experimental paradigm
The EFP task used here followed other fMRI paradigms that have successfully identified an empathy-for-pain response (Botvinick et al., 2005; Singer et al., 2004) (Fig. 1). First, subjects completed the Self pain task, in which they received moderately painful and nonpainful stimulations to the inside of their wrist. Prior to entering the scanner, subjects’ individual pain levels were found using the same method as was used with the video subjects. During the Self pain task, the subject saw either a green or a yellow colored screen (the anticipation cue) for 6 s, which indicated whether they were about to receive a painful or a non-painful stimulus. The stimulus was then delivered for 3 s, and was followed by a fixation period of 12–16 s (14+/−2 jittered). Pain and no-pain stimuli were each presented 10 times. Null trials were also included (3 painful, 3 non-painful), in which subjects saw the anticipation cue but did not receive a stimulation.
Fig. 1.
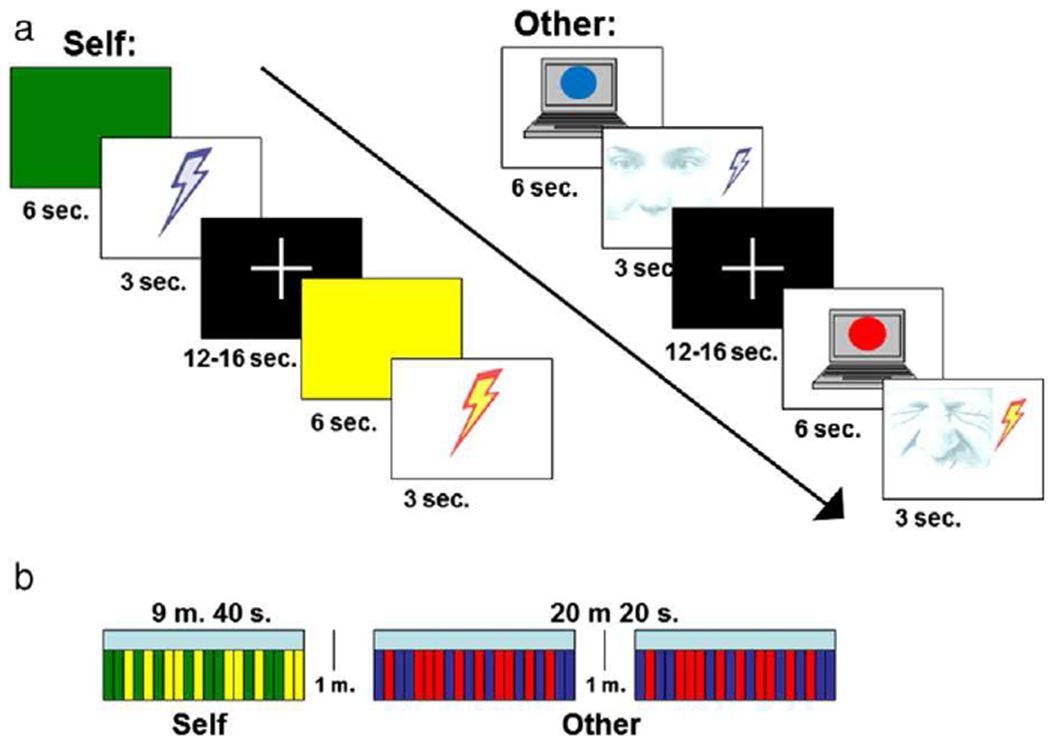
Schematic of the empathy for pain (EFP) (Self pain and Other pain) paradigm. (a.) Trial structure for Self pain and Other pain trials; (b.) Design of Self pain and Other pain tasks.
Following the Self pain task, subjects completed the Other pain task, in which they saw the previously described video clips of other people anticipating and receiving the same stimuli that they received. Both the subject and the person in the video saw the anticipation cue (red=painful, blue=nonpainful), signifying whether the pending stimulus would be painful or not. The video clips then showed the person receiving the stimulus for 3 s, followed by a 12–16 s (14+/−2 s jittered) fixation period. The Other pain task consisted of 2 blocks of stimuli, each comprised 10 pain and 10 no-pain events presented pseudo-randomly. Thus, each subject viewed 20 other pain and 20 other no-pain events in total. Subjects saw 1 pain event and 1 no-pain event for each person. There was a one minute break between blocks, during which the subject saw a fixation cross. Again, 12 null trials (6 pain, 6 non-pain) were included in which the subject saw a video clip of a person viewing the anticipation cue, but they did not see the pain epoch of the trial.
Upon completion of the Self and Other tasks, subjects were asked to rate on a scale from 1 to 5 how aversive they found it to: 1. Receive the nonpainful stimulations, 2. Receive the painful stimulations, 3. Watch others receive the nonpainful stimulations, and 4. Watch others receive the painful stimulations.
During a second visit approximately 10 weeks after the first, subjects were scanned a second time using the same EFP paradigm. In the interim, participants were randomized to 8 weeks of either compassion meditation or to a health control course (described in more detail below). Sixteen participants were randomized to the meditation group and 13 to the control group. Due to subject attrition, 21 (12 males) subjects were scanned the second time (M age=31.9; SD=6.70). Of these, 13 (M age=29.4; SD=4.43) were in the meditation group and 8 (M age=35.9; SD=8.06) were in the control group. Study drop-outs are described in detail in the Supplemental information.
Image acquisition
All MR images were acquired on a Siemens 3 T Trio scanner. Functional images were acquired using an EPI sequence with the following parameters: TR=2000 ms, TE=28 ms, matrix=64 × 64, FOV=192 mm, slice thickness=3 mm, gap=0.45 mm, and 34 axial slices. A 4.5 minute T1-weighted MPRAGE scan (TR=2600 ms, TE=3.02 ms, matrix=256 × 256, FOV=256 mm, slice thickness=1.00 mm, gap=0 mm) was acquired for anatomical localization of fMRI activations.
fMRI image preprocessing and analysis
Image preprocessing was conducted using Brain Voyager QX (version 2.0.8) software (Brain Innovation, Maastricht, The Netherlands). The first 6 volumes of each run were discarded in order to allow the tissue magnetization to equilibrate. Preprocessing involved slice scan time correction, 3D motion correction and temporal filtering by linear trend removal and high pass filtering of frequencies below three cycles per run length. Next, images were normalized into Talairach space (Talairach and Tournoux, 1988), and spatially smoothed with a 5-mm full width at half maximum (FWHM) Gaussian kernel.
A separate general linear model (GLM) was defined for each subject. We defined two regressors for both the Self and Other runs: Pain and NoPain. Null anticipation events were included in the model in order to disambiguate the anticipation and pain epochs. The following contrasts were specified and, for the sake of clarity, will be referred to as follows:
Self pain: [Self Pain–Self NoPain]
Other pain: [Other Pain–Other NoPain]
For each of these contrasts, a one-sample t test was used to identify voxels in which the average contrast for the whole group (n=29 subjects) differed significantly from 0 (i.e. a random-effect analysis). The resulting map of the t statistic was thresholded at p<.001, with a spatial extent threshold of 10 contiguous voxels.
Functional regions of interest (ROIs) were defined from each of the activation maps (Self Pain–Self NoPain, Other Pain–Other NoPain) using the following method. For each run, the activation map was thresholded at p<.001. For each activation of interest, the peak voxel was identified and all contiguously activated voxels within 15 voxels in the X, Y, and Z direction from the peak voxel were included in the ROI. Given the small anatomical volume of the amygdala, the functional ROIs comprised all contiguously active voxels within 10 mm of the peak activation. In the event that a functional activation spanned multiple functional regions, local maxima were identified and used to generate the ROI in the manner described above. In order to minimize the number of multiple comparisons, the ROI analysis was limited to regions hypothesized in advance to be important for the Self pain contrast, the MCC and bilateral anterior insula and amygdala, and for the Other pain contrast, the bilateral anterior insula.
All ROIs were then explored in correlation analyses with self-reported aversiveness scores (Pain–NoPain ratings) during the Self task and state (Pain–NoPain ratings) and trait empathy scores. Because there were no regions during the Self and Other tasks that correlated with subjective ratings, activity within a priori regions of interest was entered into bivariate correlation analyses with practice time. MCC, anterior insula, and amygdala BOLD contrast values during Self pain were entered into bivariate correlation analyses with mindfulness practice time, and anterior insula BOLD contrast values during Other pain were entered into bivariate correlation analyses with compassion practice time. These ROIs were also used in longitudinal analyses to investigate changes related to meditation training.
Predicting practice time using baseline brain activity
To explore whether baseline brain activity during the empathy for pain task predicted practice time, the sample was limited to those randomized to the meditation group who completed the study (n=13). Total practice time was broken down into mindfulness practice time (practice during the first two weeks of the study) and compassion practice time (practice during weeks 4–8 of the study). Notably, the lessons and meditations during week 3, in which self-compassion was the topic, are meant to develop a strongly-felt determination to improve one’s sense of emotional and mental well-being. While this is traditionally considered to be a necessary prerequisite for developing compassion, neither the pedagogical material nor the meditations introduced during this week call on the practitioners to contemplate the suffering of others, and for this reason week 3 was not included in the compassion practice time value. Mindfulness and compassion practice times were entered into bivariate correlation analyses with the self report measures and with contrast values from each ROI described above.
Results
Self-report measures
At both the pre- and post-intervention assessments, subjects rated the Self Pain (n=29) and Other Pain (n=29) conditions as more aversive than the Self NoPain (Time 1 paired t(28)=8.65; p<0.001; Time 2 paired t(20)=9.82; p<0.001) and Other NoPain conditions (Time 1 paired t(28)=4.52; p<0.001; Time 2 paired t(20)=4.25; p<0.001). Subjects found the Self Pain more aversive than the Other Pain (Time 1 paired t(28)=7.33; p<0.001; Time 2 paired t(20)=5.34; p<0.001).
Self-reported mindfulness and compassion practice times for subjects randomized to CBCT are plotted in Fig. 2. The mean mindfulness and compassion practice times were 53.1 min (SD=51.5) and 212.3 min (SD=190.3), respectively, yielding a total mean practice time for the entire eight-week CBCT training period of 315.9 min (SD=228.9). No participants fell beyond 3 standard deviations from the mean for any practice time measures. Mindfulness and compassion practice times were not correlated (r(11)=0.35; p=0.25). Neither was there a relationship between age or gender and mindfulness or compassion practice time.
Fig. 2.

Histogram of self-report practice time, showing mindfulness (green) and compassion (purple) practice times for each participant.
Subjective ratings of Self and Other Pain were unrelated to either mindfulness or compassion practice time (mindfulness with Self Pain ratings: r(11)=−0.29; p=0.33; compassion with Other Pain ratings: r(11)=0.03; p=0.93), nor were empathic concern scores related to either mindfulness or compassion practice time (mindfulness: r(11)=−0.19, p=0.53; compassion: r(11)=−0.05, p=0.86).
Pre-intervention fMRI findings related to Self and Other pain
Self task
The contrast Self [Pain–NoPain] revealed expected neural activation in areas related to the perception of pain, including contralateral S1 and posterior insula. Also active were areas related to the affective and evaluative dimensions of pain, including the anterior insula, MCC, and amygdala (Supplementary table 1). None of the functional regions active during the Self task were correlated with subjectively reported aversiveness to self pain.
Other task
The contrast Other [Pain–NoPain] revealed neural activation patterns known to be associated with empathy for pain such as the anterior insula and inferior frontal gyrus bilaterally, the MCC, and the dorsomedial prefrontal cortex (PFC) (Supplementary table 2). None of the functional regions active during the Other task were correlated with state or trait empathy ratings.
Neural activity predicts meditation practice
While no regions that were active during the Self pain task correlated with aversiveness ratings, we investigated the relationship between practice time and activation within brain regions often implicated in the affective and evaluative response to self pain, including the mid cingulate, and bilateral anterior insula and amygdala (Bingel et al., 2002; Peyron et al., 1999; Price, 2000; Ziv et al., 2010). Of these regions tested, neural activity in the left amygdala was correlated with mindfulness practice time (r(11)=−0.59; p<0.05) (see Fig. 3), but not compassion practice time (r(11)=−0.25, p=0.42). While no regions active during the Other pain task correlated with self-reported state or trait empathy, we investigated the relationship between practice time and activation within bilateral anterior insula ROIs, given the wealth of previous evidence implicating the anterior insula in empathic responses to pain (Botvinick et al., 2005; Jackson et al., 2005; Lamm et al., 2010; Singer et al., 2004). Neural activity in the left and right anterior insula during the Other pain task was positively correlated with practice time during the compassion-specific portion of the CBCT protocol (left: r(11)=0.64; p<0.05; right: r(11)=0.56; p<0.05) (see Fig. 4), but not with mindfulness practice time (left: r(11)=0.21, p=0.49; right: r(11)=0.19, p=0.53). In order to confirm this apparent double dissociation, we tested whether left amygdala activity during Other pain predicted compassion practice time or anterior insula activity during Self pain predicted mindfulness practice time, and neither was the case (left amygdala correlation with compassion practice: r(11)=0.23, p=0.44; anterior insula correlation with mindfulness practice time: r(11)=−0.24, p=0.43).
Fig. 3.
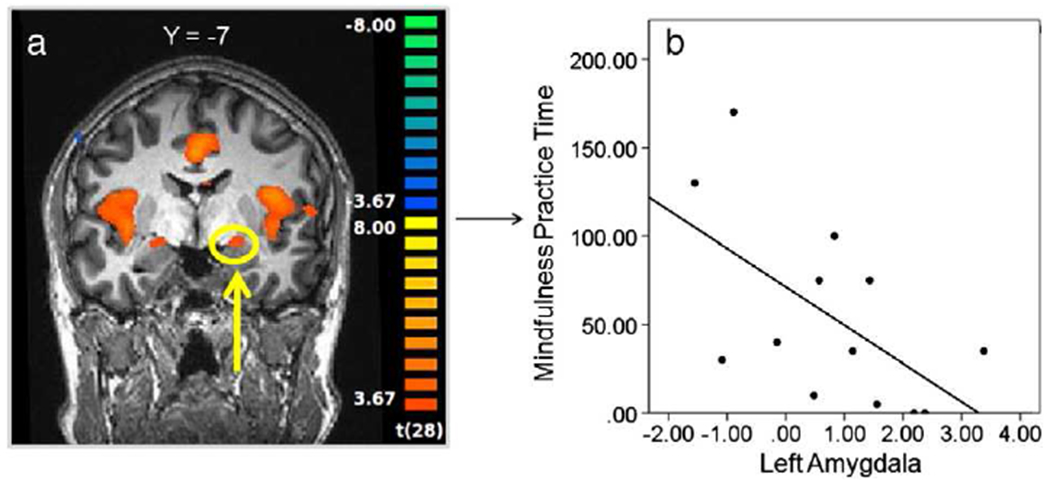
Relationship between neural activity during Self pain and mindfulness practice time. a.) Functional ROI in the left amygdala during Self pain [Pain–NoPain] (thresholded at p<0.001); b.) plot of bivariate correlation between beta contrast values [Pain–NoPain] in the left amygdala and mindfulness meditation practice time (r(11)=−0.59; p<0.05).
Fig. 4.
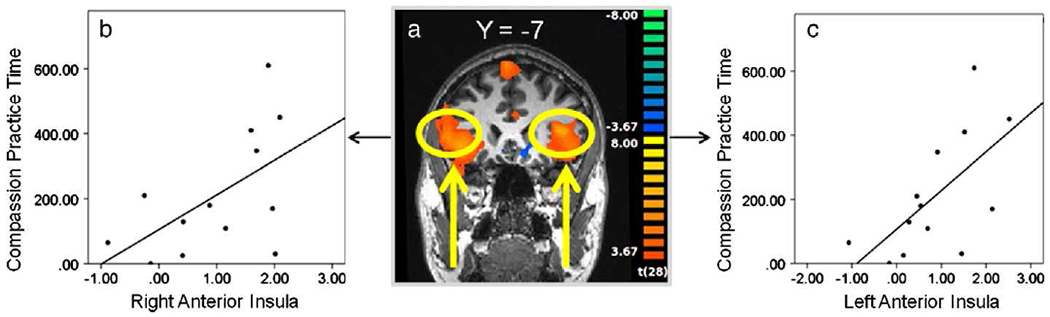
Relationship between neural activity during Other pain and compassion practice time. a.) Functional ROI in the left anterior insula during Other pain [Pain–NoPain] (thresholded at p<0.001); plot of bivariate correlation between beta contrast values [Pain–NoPain] in the b.) right and c.) left anterior insula and compassion meditation practice time (right: r(11)=0.56; p<0.05; left: r(11)=0.64; p<0.05).
Longitudinal investigation
Following the study interventions, there was not a significant group by time interaction in neural responses to either the Self or Other pain tasks. This lack of effect was observed both in the ROIs generated based on the Time 1 main effects and in a whole brain analysis (threshold of p<0.001). Given these null findings and in order to mitigate type 2 errors, we tested more widely for a group by time interaction effect in other functionally defined ROIs generated based on the Time 1 main effects that are arguably important for empathy, including dmPFC, and bilateral amygdala and inferior frontal gyrus and found no significant interaction effects. Similarly, self-reported measures of state and trait empathy did not change from pre- to post-intervention assessments in the study population as a whole, and no differences were observed between those randomized to CBCT vs. the control condition. Linear regression analyses indicated that neither mindfulness nor compassion practice time accounted for a significant amount of the variance in post-intervention amygdala activation during the Self task (b=0.00, R2 change=0.01, F (1, 10)=0.12, p=0.73) or anterior insula activation during the Other task (left: b=0.00, R2 change=0.02, F (1, 10)=0.17, p=0.69; right: b=0.00, R2 change=0.06, F (1, 10)=0.63, p=0.45), when controlling for Time 1 brain activity.
Discussion
In this study, we tested the hypothesis that different patterns of neural activity in response to receiving painful stimulations or watching others receive a similar painful stimulus would uniquely predict subsequent engagement with different types of meditation training (mindfulness vs. compassion). Activation of the left amygdala during Self pain was inversely associated with subsequent mindfulness practice time. During the Other pain task, activity in the anterior insula was positively correlated with subsequent compassion practice time. Interestingly, however, training in CBCT had no effect on brain activity in any of these areas and did not impact self-reported measures of either pain aversiveness or empathy. Moreover, within the group randomized to CBCT, practice time had no effect on patterns of brain activity or self-reported measures of pain aversiveness or empathy as assessed following training. Despite these negative findings, other evidence speaks to the effectiveness of CBCT (Desbordes et al., 2012; Mascaro et al., 2012; Pace et al., 2009, 2010, 2012) and the importance of practice time in attaining these effects. These findings highlight the potential importance of identifying predictors of practice time, because these predictors might help identify individuals most likely to benefit from learning CBCT and/or other mediation-based interventions.
Self-report
The hypothesis that baseline levels of self-reported aversiveness to pain would predict subsequent mindfulness practice time is not supported by the data presented here, nor was there a relationship between state or trait empathy and subsequent compassion practice time. These data are consistent with a larger trend found here, which was a general lack of correlation between self-report data and objective measures, since brain activity was also unrelated to any of the self report measures. While the finding that brain activity predicted subsequent behavior is striking (discussed in more detail below), the fact that subjective reports were not related to brain activity or to practice time means that we cannot definitively determine how these neural states were relevant to the subjective states of the participants. However a large body of previous studies that used very similar tasks found relationships between amygdala and anterior insula activation and subjective states. The discrepancy between previous studies and the data presented here may be due to limitations in our self-report data. For example, it may be that because subjective reports were probed upon completion of both tasks, participants’ reports did not actually reflect what they felt during the task. With respect to the empathy for pain task, the state empathy measure, in which participants were asked how “aversive” they found it to watch others receive the painful stimulations, may have probed something more akin to personal distress than to empathy (Batson et al., 1987). In addition, while state empathy measures are often found to correlate with anterior insula activity, trait empathy, as measured by the empathic concern subscale of the IRI (used here) is far less reliably correlated with anterior insula activity (Lamm et al., 2010). Future studies should include more comprehensive assessments of state and trait empathy, mindfulness, and pain-induced reactivity to more systematically investigate the relationship between subjective states and meditation engagement as well as the eventual effects of meditation training.
Amygdala activity predicts mindfulness practice
The finding that left amygdala activation during the Self task predicted subsequent mindfulness meditation practice time is consistent with previous mindfulness meditation research. Individuals who scored higher on a dispositional mindfulness scale had less bilateral amygdala activation during an affect labeling task (Creswell et al., 2007), and a recent longitudinal study found that participants in a mindfulness based stress reduction (MBSR) intervention reported decreases in perceived life stress that correlated with decreases in gray matter in the right amygdala (Holzel et al., 2010). Moreover, studies of Zen, a similar style of non-evaluative meditation, show that long-term practitioners had less activity in the bilateral amygdala while receiving painful stimuli (Grant et al., 2011). These previous studies suggest that the amygdala is likely an important region for many of the evaluative judgments that mindfulness training is intended to mitigate, and may be one of the key neural regions mediating the stress ameliorating effects of mindfulness practices. The findings presented here elaborate on the role of the amygdala in mindfulness practices by indicating that left amygdala hyperreactivity may impede an individual’s ability to practice mindfulness meditation. While these results do not indicate that mindfulness training is harmful to those who are particularly reactive to their own painful sensations, the data point to a potential “Catch-22”; specifically that mindfulness training may be most difficult precisely for those people most in need of its positive effects.
While the correlation between mindfulness practice time and activity in the right amygdala was in the same direction, it did not reach the level of significance. This is not surprising given the extensive evidence for lateralized amygdala function in human subjects (Baas et al., 2004; Sergerie et al., 2008; Stevens and Hamann, 2012; Wager et al., 2003). Consistent with our findings, a meta-analysis concluded that the left amygdala is more strongly implicated in negative emotion, particularly those negative emotions that tend to elicit a withdrawal response (Wager et al., 2003). The specific subregion identified in that meta-analyses closely matches the dorsal amygdala activation we report here. The idea that individuals with a more robust withdrawal-related response to their own pain are not as likely to engage in mindfulness has face validity and is a hypothesis that warrants more careful testing in the future.
Anterior insula activity predicts compassion practice
Despite the fact that there were no observed correlations in the current study between self-reported state or trait empathy and neural activity when observing another person receive a painful stimulus, the empathy for pain task elicited robust activity in regions previously identified as important for empathy. Given the consistency with which neuroimaging investigations have implicated the anterior insula in empathic responses (Lamm et al., 2010), the finding that anterior insula activity was positively correlated with subsequent compassion-specific meditation practice time may suggest that more empathic individuals are able to engage more fully with compassion meditation. This correlation between anterior insula responses to observing others in pain and subsequent practice time is particularly interesting in light of the traditional Tibetan works upon which CBCT is based. In the late 14th Century Tsong-Kha-Pa (Tsong-kha-pa, 2004) emphasized the importance of having empathy and compassion at the beginning of a practice, since one will not be moved to commit to being compassionate toward others if his or her empathy and compassion are weak to begin with. Our findings support and sharpen Tsong-Kha-Pa’s observation by suggesting that a certain amount of activity in a brain region involved in empathic responses may be important to successfully embark on compassion training.
Several broad points should be made about these practice-time findings. First, the fact that neural activity differentially predicts an individual’s propensity to practice two different meditative techniques supports the assertion that the term ‘meditation’ subsumes heterogeneous techniques with a broad variety of methods and goals (Lutz et al., 2008b). In fact, these data point to a double dissociation of predictive effects since anterior insula activity during the Self pain task does not predict subsequent mindfulness practice time nor does amygdala activity during the Other pain task predict subsequent compassion practice time. Because of this, future meditation studies will benefit from the direct comparison of multiple, well-characterized and operationalized styles of meditation training. Moreover, while this is the first study to show that pre-existing neural activity predicts meditation practice time, the findings presented here are analogous to those coming out of psychiatric research, in which neuroimaging has been used to predict responses to both pharmacological and behavioral treatments (Bryant et al., 2008; Mayberg et al., 1997). Given the increasing use of meditation for clinical indications, we believe the study design utilized here has similar utility for determining the efficacy of meditation for the treatment of psychiatric pathology.
Longitudinal investigation
Longitudinal findings from the current study suggest that the eight-week CBCT protocol used here does not attenuate neural responses associated with pain aversiveness. With respect to the Self pain findings, these results are consistent with a recent study by Zeidan et al. (2011). While this group found that a 4-day mindfulness training program altered brain activation during a meditation plus pain condition, the amygdala was not one of the regions modified by the brief training. While we are duly cautious to lump studies of Zen and mindfulness, the fact that long-term Zen practitioners had less amygdala activity during pain (Grant et al., 2011), while the current study and that by Zeidan et al. (2011) did not find amygdala changes after shorter training periods, may suggest that amygdala changes require a relatively longer period of training.
Additionally, longitudinal analysis of the empathy task suggests that CBCT does not amplify the neural correlates of empathy for pain. With respect to our ability to detect meditation-induced changes in the neural systems important for empathy, we believe that the fMRI paradigm used here was sufficient for investigating the potential of CBCT to change the neurobiology of empathy for pain given the robust pattern of activations elicited by the EFP task at Time 1. In fact, it appears that individuals habituated to the video clips of others in pain, given that activation in the anterior insula, independent of group, showed significant attenuation from the pre- to the post-intervention assessments (see Supplementary figure S5). It remains possible that participants would not have habituated had they been presented at both assessments with people experiencing pain in real time rather than a video. At the least, however, the data presented here suggest that compassion training did not diminish habituation to seeing video clips of others in pain, which in itself suggests the practice had no effect on either the self-reported or neural correlates of empathy. Nonetheless, it is important to note that empathy is a broad construct subserved by complex neural systems, and the empathy measures used here do not tap into the related but distinct prosocial emotion of compassion. One recent theoretical discussion of compassion training suggests that compassion is an emergent process that may be enhanced by training one or more of the multiple “noncompassion” processes from which it arises (Halifax, 2012). If so, then CBCT may differentially affect these underlying, noncompassion processes such that the ability to identify relevant change will rest squarely on the use of appropriate assessments in future studies. Importantly, CBCT does appear to affect empathic accuracy (Mascaro et al., 2012), depressive symptoms (Desbordes et al., 2012), and stress physiology (Pace et al., 2009, 2010, 2012).
Results from the current study may also provide a novel perspective on previous cross-sectional studies reporting an association between amount of engagement with compassion meditation and enhanced activation in neural circuits important for empathy, including in the anterior insula (Lutz et al., 2008a). Our results suggest that rather than being a result of compassion meditation training, high levels of anterior insula activation in response to the suffering of others may actually contribute to the foundation for engaging in compassion meditation in the first place. Indeed, while the population studied in the current project is different in numerous ways from the meditation adepts investigated in other studies, our findings are consistent with the notion that adepts may be a self-selected population who likely begin with extraordinary personality and biological profiles. Studies exploring the underlying features that render such individuals able to attain high levels of expertise are an unexplored but highly promising avenue for meditation research.
Limitations
Some limitations of the present study are worth noting. First, practice time was self-reported and it remains possible that some participants were biased or inaccurate in their reporting. In addition, the control group was relatively small and the study may have been underpowered to find a group by time interaction effect. However, given that both groups showed significantly attenuated brain activity to the EFP task at Time 2, we do not believe that the small sample size contributed to the null results of the longitudinal analyses. Moreover, it is important to note that the same study participants showed a group by time interaction effect for neural activity during an empathic accuracy task and participants randomized to CBCT were significantly more likely to have enhanced empathic accuracy scores (Mascaro et al., 2012), reducing the likelihood that the study was underpowered. Additionally, while this investigation was an initial attempt at investigating individual differences in the propensity to adopt meditation techniques, there are important aspects related to this theme that were glossed for the current study. For example, it remains possible that the pedagogical material or training was differently understood or received across individuals, or that practice time differed in importance at different points of CBCT training. While we use amount of practice time here as a measure of the level of engagement with CBCT due to the fact that other studies have shown the importance of practice for outcomes of interest (Fredrickson et al., 2008; Pace et al., 2009, 2012), future studies should analyze other metrics of engagement such as self-reported connection to the practice and physiological changes that occur during the practice. A fascinating prospective study could interrogate the relationship between practice time and individual differences in the subjective meaning of the training and investigate whether baseline variables are differentially related to these various measures of meditation engagement. Current findings highlight the need to conduct such a study. Moreover, while the control group for the current study was designed to help account for expectancy bias and non-specific effects of group interaction and health-relevant education, future studies should include a randomized comparison of CBCT training with another, similar mental training intervention. These important inquiries will be critical for future investigations of CBCT and for meditation research more broadly.
Supplementary Material
Acknowledgments
We thank Carol Worthman and Nathan Mascaro for the design and statistical advice. We also thank Brooke Dodson-Lavelle and Brendan Ozawa-de Silva for training participants using CBCT protocol, Teri Sivilli for the help in recruiting and screening study participants, and Todd Preuss for the helpful comments on this paper. This work was supported by an Emory University Neuroscience Initiative Seed Grant and by NIH NCCAM (AT004698) (C.R.). J. S. M. was supported by the Center for Behavioral Neuroscience and by a Ruth L. Kirschstein National Research Service Award (NRSA) for Individual Predoctoral Fellows through the National Center for Complementary and Alternative Medicine (NCCAM) (1F31AT004878-01).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2012.12.021.
References
- Baas D, Aleman A, Kahn RS, 2004. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res. Rev 45 (2), 96–103. [DOI] [PubMed] [Google Scholar]
- Baer RA, 2003. Mindfulness training as a clinical intervention: a conceptual and empirical review. (Review) Clin. Psychol. Sci. Pract 10 (2), 125–143. [Google Scholar]
- Batson CD, 1998. Altruism and prosocial behavior. In: Gilbert D, Fiske S, Lindzey G (Eds.), The Handbook of Social Psychology, vol. 2. McGraw-Hill, Boston, pp. 282–316. [Google Scholar]
- Batson CD, Fultz J, Schoenrade PA, 1987. Distress and empathy – 2 qualitatively distinct vicarious emotions with different motivational consequences. J. Personal 55 (1), 19–39. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Büchel C, 2002. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain 99 (1–2), 313–321. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM, 2005. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. NeuroImage 25 (1), 312–319. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, et al. , 2008. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol. Med 38 (04), 555–561. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD, 2007. Neural correlates of dispositional mindfulness during affect labeling. (Article) Psychosom. Med 69 (6), 560–565. [DOI] [PubMed] [Google Scholar]
- Davis MH, 1983a. The effects of dispositional empathy on emotional-reactions and helping – a multidimensional approach. J. Personal 51 (2), 167–184. [Google Scholar]
- Davis MH, 1983b. Measuring individual-differences in empathy – evidence for a multidimensional approach. J. Personal. Soc. Psychol 44 (1), 113–126. [Google Scholar]
- de Vignemont F, Singer T, 2006. The empathic brain: how, when and why? Trends Cogn. Sci 10 (10), 435–441. [DOI] [PubMed] [Google Scholar]
- Desbordes G, Negi LT, Pace TWW, Wallace AB, Raison C, Schwartz EL, 2012. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front. Hum. Neurosci 6 (292), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM, 2008. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. J. Personal. Soc. Psychol 95 (5), 1045–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Rainville P, 2011. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain 152 (1), 150–156. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H, 2004. Mindfulness-based stress reduction and health benefits: a meta-analysis. J. Psychosom. Res 57 (1), 35–43. [DOI] [PubMed] [Google Scholar]
- Halifax J, 2012. A heuristic model of enactive compassion. Curr. Opin. Support. Palliat. Care 6 (2), 228–235 (210.1097/SPC.1090b1013e3283530fbe). [DOI] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, Singer T, 2010. Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron 68 (1), 149–160. [DOI] [PubMed] [Google Scholar]
- HHDL, 2001. An Open Heart. Little Brown and Company, New York. [Google Scholar]
- Hofmann SG, Grossman P, Hinton DE, 2011. Loving-kindness and compassion meditation: psychological interventions. (Review) Clin. Psychol. Rev 31 (7), 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, et al. , 2010. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect. Neurosci 5 (1), 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J, 2005. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage 24 (3), 771–779. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T, 2010. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 54 (3), 2492–2502. [DOI] [PubMed] [Google Scholar]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ, 2008a. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS One 3 (3), e1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ, 2008b. Attention regulation and monitoring in meditation. Trends Cogn. Sci 12 (4), 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand WR, 2012. Mindfulness-based stress reduction, mindfulness-based cognitive therapy, and zen meditation for depression, anxiety, pain, and psychological distress. J. Psychiatr. Pract 18 (4), 233–252 (210.1097/1001.pra.0000416014.0000453215.0000416086). [DOI] [PubMed] [Google Scholar]
- Mascaro JS, Rilling JK, Negi LT, Raison C, 2012. Compassion meditation enhances empathic accuracy and related neural activity. Soc. Cogn. Affect. Neurosci http://scan.oxfordjournals.org/content/early/2012/09/28/scan.nss095 (Electronic publication ahead of print Sept 29 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. , 1997. Cingulate function in depression: a potential predictor of treatment response. NeuroReport 8 (4), 1057–1061. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, et al. , 2009. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology 34 (1), 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Sivilli TI, Issa MJ, Cole SP, Adame DD, et al. , 2010. Innate immune, neuroendocrine and behavioral responses to psychosocial stress do not predict subsequent compassion meditation practice time. Psychoneuroendocrinology 35 (2), 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Dodson-Lavelle B, Ozawa-de Silva B, Reddy SD, Cole SP, et al. , 2012. Engagement with Cognitively-Based Compassion Training is associated with reduced salivary C-reactive protein from before to after training in foster care program adolescents. Psychoneuroendocrinology. 10.1016/j.psyneuen.2012.05.019 (Electronic publication ahead of print July 3 2012). [DOI] [PubMed] [Google Scholar]
- Peyron R, García-Larrea L, Grégoire M-C, Costes N, Convers P, Lavenne F, et al. , 1999. Haemodynamic brain responses to acute pain in humans. Brain 122 (9), 1765–1780. [DOI] [PubMed] [Google Scholar]
- Price DD, 2000. Psychological and neural mechanisms of the affective dimension of pain. Science 288 (5472), 1769–1772. [DOI] [PubMed] [Google Scholar]
- Sedlmeier P, Eberth J, Schwarz M, Zimmermann D, Haarig F, Jaeger S, et al. , 2012. The psychological effects of meditation: a meta-analysis. Psychol. Bull 138 (6), 1139–1171. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL, 2008. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev 32 (4), 811–830. [DOI] [PubMed] [Google Scholar]
- Simon D, Craig KD, Miltner WHR, Rainville P, 2006. Brain responses to dynamic facial expressions of pain. Pain 126 (1–3), 309–318. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD, 2004. Empathy for pain involves the affective but not sensory components of pain. Science 303 (5661), 1157–1162. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K, 2009. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci 13 (8), 334–340. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Hamann S, 2012. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia 50 (7), 1578–1593. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical, New York. [Google Scholar]
- Tsong-kha-pa, 2004. The Great Treatise on the Stages of Enlightenment, vol. 2. Snow Lion Publications. [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF, 2003. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage 19 (3), 513–531. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC, 2011. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci 31 (14), 5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv M, Tomer R, Defrin R, Hendler T, 2010. Individual sensitivity to pain expectancy is related to differential activation of the hippocampus and amygdala. (Article) Hum. Brain Mapp 31 (2), 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


