Abstract
Background
Coronary artery calcium (CAC) is well‐validated for cardiovascular disease risk stratification in middle to older–aged adults; however, the 2019 American College of Cardiology/American Heart Association guidelines state that more data are needed regarding the performance of CAC in low‐risk younger adults.
Methods and Results
We measured CAC in 13 397 patients aged 30 to 49 years without known cardiovascular disease or malignancy between 1997 and 2009. Outcomes of myocardial infarction (MI), stroke, major adverse cardiovascular events (MACE; MI, stroke, or cardiovascular death), and all‐cause mortality were assessed using Cox proportional hazard models, controlling for baseline risk factors (including atrial fibrillation for stroke and MACE) and the competing risk of death or noncardiac death as appropriate. The cohort (74% men, mean age 44 years, and 76% with ≤1 cardiovascular disease risk factor) had a 20.6% prevalence of any CAC. CAC was independently predicted by age, male sex, White race, and cardiovascular disease risk factors. Over a mean of 11 years of follow‐up, the relative adjusted subhazard ratio of CAC >0 was 2.9 for MI and 1.6 for MACE. CAC >100 was associated with significantly increased hazards of MI (adjusted subhazard ratio, 5.2), MACE (adjusted subhazard ratio, 3.1), stroke (adjusted subhazard ratio, 1.7), and all‐cause mortality (hazard ratio, 2.1). CAC significantly improved the prognostic accuracy of risk factors for MACE, MI, and all‐cause mortality by the likelihood ratio test (P<0.05).
Conclusions
CAC was prevalent in a large sample of low‐risk young adults. Those with any CAC had significantly higher long‐term hazards of MACE and MI, while severe CAC increased hazards for all outcomes including death. CAC may have utility for clinical decision‐making among select young adults.
Keywords: calcium score, coronary artery calcium, coronary artery disease, heart disease risk factors, multidetector computed tomography, myocardial infarction, primary prevention, stroke
Subject Categories: Cardiovascular Disease, Risk Factors, Epidemiology, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- ACC/AHA
American College of Cardiology/Americann Heart Association
- aSHR
adjusted subhazard ratio
- CARDIA
Coronary Artery Risk Development in Young Adults
- DoD
Department of Defense
- MACE
major adverse cardiovascular events
- MASALA
Mediators of Atherosclerosis in South Asians Living in America
- MDR
Military Data Repository
- MESA
Multi‐Ethnic Study of Atherosclerosis
- NHANES
National Health and Nutrition Examination Survey
Clinical Perspective
What Is New?
In the second largest study of coronary artery calcium (CAC) in US young adults to date, the number needed to screen to find any CAC was 5, and the presence and severity of CAC was associated with significantly higher hazards of major adverse cardiovascular events and myocardial infarction over 11 years of follow‐up.
What Are the Clinical Implications?
CAC was prevalent in a large sample of low‐risk young adults.
Those with any CAC had significantly higher long‐term hazards of major adverse cardiovascular events and myocardial infarction, while severe CAC increased hazards for all outcomes including death.
CAC may have utility for clinical decision‐making among select young adults.
Multiple cardiovascular guidelines recommend use of 10‐year absolute atherosclerotic cardiovascular disease (ASCVD) risk estimates to guide treatment and allocation of preventive therapies. 1 , 2 Because these scores rely heavily on patient age, nearly all young adults (younger than 50 years) are estimated to have a low 10‐year ASCVD risk, even those who have nonoptimal risk factors. 3 Consequently, young adults are often not offered or adherent to preventive treatments despite the possibilities of subclinical atherosclerosis and elevated lifetime risk. 4 The need for a clinical decision‐making tool that identifies younger individuals at elevated cardiovascular disease (CVD) risk has led to interest in coronary artery calcification (CAC) as a direct marker of coronary artery disease.
The 2019 American College of Cardiology/Americann Heart Association (ACC/AHA) guidelines for primary prevention of ASCVD recommend CAC scoring for further risk assessment in borderline to intermediate‐risk individuals—typically middle‐aged adults—in whom management is uncertain. 1 , 5 Although most studies of CAC have focused on the yield of testing middle to older‐aged adults, the utility of CAC in younger populations is still not clear. 6 The few studies in young adults examining the relationship between CAC and disease outcomes have been limited by small sample sizes, short duration of follow‐up, or lack of cause‐specific mortality, or were conducted in selected populations with a high prevalence of CVD risk factors. 7 , 8 , 9 In response to increasing interest in CAC for risk assessment of young adults, 6 we sought to provide data from the Walter Reed Cohort, a relatively healthy, young, low‐risk population, to help further elucidate the potential role of CAC in identification of young adults at elevated risk, so that preventive measures might be employed to alter their atherosclerotic trajectories and long‐term risks for CVD.
Methods
Study Population
The Walter Reed Cohort is a study of 31 303 low‐risk adult patients (13 397 patients aged 30–49 years) who underwent CAC testing at Walter Reed Army Medical Center between January 1997 and August 2009, with a mean of 11.1 years (SD, 3.4 years) of follow‐up among the younger adult subset. Patients consisted of military health care system beneficiaries, including active duty military, retirees, and other Department of Defense (DoD) beneficiaries, as well as their dependents. To establish baseline covariates, patients were excluded if they did not have at least 1 year of enrollment in the military health care system before their CAC scan. Additional exclusion criteria included patients with preexisting coronary artery disease, myocardial infarction (MI), stroke, cerebral revascularization, peripheral arterial disease, malignancy, or no follow‐up after their CAC scan, or who were foreign military members. This study was approved by the local institutional review board and informed consent was waived because of the retrospective study design. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Baseline comorbidities were extracted using International Classification of Diseases, Ninth Revision (ICD‐9), codes from the DoD Military Data Repository (MDR) for any outpatient or inpatient diagnoses entered before the date of CAC score, as previously described. 10 , 11 The MDR contains comprehensive medical care claims and administrative information—including demographics, diagnoses, procedures, prescriptions, and vitals—for active duty military, retirees, and other DoD health care beneficiaries, as well as their dependents. The database includes services that are provided directly at military treatment facilities worldwide and at civilian facilities paid by the DoD.
Calcium Scoring
Electron beam computed tomography was performed using Imatron C‐150 and C‐300 LXP scanners (Imatron Inc.) to measure and score CAC using the Agatston method, as previously described. 12 Coronary calcium tests were ordered at the discretion of the health care provider. Results were reported in the electronic health record, as per routine clinical care.
Outcome Measures
Patients were assessed for the primary outcomes of all‐cause mortality, incident MI, stroke, and major adverse cardiovascular events (MACE)—defined as stroke, MI, or cardiovascular death—during a mean follow‐up of 11.1 years (SD, 3.4 years). ICD‐9 codes from inpatient records were used to identify study outcomes, as previously described. 11 In line with prior studies by the US Food and Drug Administration, codes for stroke were limited to the primary diagnosis, and codes for MI were obtained from the first 2 positions. 13 , 14 Previous studies have demonstrated that ICD‐9 codes for MI and stroke have a ≥90% positive predictive value for adjudicated MI and stroke outcomes and therefore have been used for assessing large‐scale outcomes within the MDR. 15 , 16 , 17 Death data and cause of death were obtained for all patients from the MDR and National Death Index and cross‐referenced to the Social Security Death Index and the Veterans Affairs Beneficiary Identification Records Locator Subsystem.
Statistical Analysis
Patients were classified by the presence or absence of calcium and further subdivided into CAC score groups of 0 (none), 1 to 10 (mild), 11 to 100 (moderate), and >100 (severe) for this lower‐aged cohort. Demographics and baseline characteristics were compared across the CAC groups using chi‐square tests for categorical variables and analysis of variance accounting for unbalanced data for continuous variables. Independent predictors of any CAC (CAC >0) and severe CAC (CAC >100) were determined using multivariable logistic regression models, which included age (continuous), sex, race (compared with white race), and baseline cardiovascular risk factors including hypertension, diabetes, hyperlipidemia, and tobacco dependence (current or prior). 18 , 19
Cumulative incidence functions and univariable and multivariable Cox proportional hazards models were used to compare time to events. Traditional cardiovascular risk factors were forced into the multivariable Cox model. These models included age (continuous), sex, and risk factors of baseline hypertension, diabetes, hyperlipidemia, and tobacco dependence (current or prior). Baseline atrial fibrillation was also included in the models for stroke and MACE. The Fine‐Gray model was used to account for the competing risk of noncardiovascular death when assessing MACE‐free survival and the competing risk of death when assessing MI and stroke. 20 Gray test was used to compare the cumulative incidence between groups. 21 The first event was used in the survival analysis for patients with >1 incidence of a particular outcome. Patients were followed for each outcome until the outcome occurred, the end of the study, or disenrollment from the health care system.
Harrell C statistic was used to assess the area under the curve for the primary outcomes accounting for censoring of events. 22 The ability of CAC score to improve the predictive ability of baseline risk factors was further evaluated using the likelihood ratio test to compare the difference in the –2 log L fit statistics. CAC score was input into the model using the ln(CAC+1) transformation to account for its non‐normal distribution, consistent with previous studies. 23 Age was input into the models using the natural log transformation consistent with the pooled risk equation.
Given the large number of low‐risk patients in the study, subgroup analyses were performed to explore the association of CAC and MACE in patients with 0, 1, or ≥2 traditional risk factors. Risk factors were counted based on the presence of hypertension, hyperlipidemia, tobacco use, diabetes, and age 45 years and older in men. MACE was chosen as the preferred end point given the overall low mortality for young patients, and subhazard ratios were further adjusted for atrial fibrillation and competing risk of death.
A 2‐tailed value of P<0.05 was considered significant for all comparisons. Statistics were computed using SAS version 9.4 (SAS Institute Inc).
Results
The baseline characteristics of the 13 397 participants aged 30 to 49 years who met inclusion criteria (73.5% men; mean age, 43.6 years [SD, 3.5 years]; 73.8% White race) are shown in Table 1, stratified by CAC score category. The prevalence of hyperlipidemia was 32%, hypertension was 20%, tobacco use was 4.9%, and diabetes was 3.4%.
Table 1.
Baseline Characteristics of 13 397 Asymptomatic Adults Aged 30 to 49 Years
| CAC 0 (n=10 638) | CAC 1–10 (n=916) | CAC 11–100 (n=1358) | CAC >100 (n=485) | P value | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 43.4 (3.5) | 43.9 (3.4) | 44.6 (3.2) | 45 (3.0) | <0.001 |
| Women, n (%) | 2841 (27) | 94 (10) | 135 (10) | 32 (7) | <0.001 |
| Race, n (%) | <0.001 | ||||
| White | 7664 (72) | 713 (78) | 1109 (82) | 395 (81) | |
| Black | 1864 (18) | 120 (13) | 126 (9) | 54 (11) | |
| Native American | 30 (0) | 8 (1) | 3 (0) | 3 (0) | |
| Asian | 278 (3) | 28 (3) | 38 (3) | 14 (3) | |
| Other | 369 (3) | 34 (4) | 61 (4) | 11 (2) | |
| Missing | 433 (4) | 13 (1) | 21 (2) | 8 (2) | |
| Hypertension, n (%) | 1996 (19) | 190 (21) | 353 (26) | 143 (29) | <0.001 |
| Hyperlipidemia, n (%) | 3141 (29) | 355 (39) | 544 (40) | 216 (45) | <0.001 |
| Diabetes, n (%) | 349 (3) | 32 (3) | 50 (4) | 24 (5) | 0.225 |
| Tobacco use, n (%) | 493 (5) | 51 (6) | 73 (5) | 41 (8) | 0.001 |
| Charlson Comorbidity Index score, n (%) | 0.39 | ||||
| 0 | 8468 (80) | 719 (78) | 1071 (79) | 388 (80) | |
| 1 | 1912 (18) | 175 (19) | 250 (18) | 77 (16) | |
| 2 | 178 (2) | 13 (1) | 25 (2) | 12 (2) | |
| 3+ | 80 (1) | 9 (1) | 12 (1) | 8 (2) | |
| Follow‐up, mean (SD), y | 11.0 (3.4) | 11.3 (3.5) | 11.2 (3.4) | 10.8 (3.7) | 0.109 |
Demographics and baseline characteristics were compared across the coronary artery calcium (CAC) groups using chi‐square tests for categorical variables and ANOVA accounting for unbalanced data for age (continuous).
The prevalence of any CAC (>0) for the total sample was 20.6% (n=2759; number needed to scan to detect any CAC was 5), while 3.6% had a CAC score >100 (n=485; number needed to scan to detect CAC >100 was 28). The relative prevalence of CAC was higher in men, older individuals, and those with increased burden of CVD risk factors (Figure 1A through 1C). The odds of any CAC increased with male sex (odds ratio [OR], 3.56; 95% CI, 3.08–4.11), older age (OR, 4.91 per 10 years; 95% CI, 3.62–6.66), hypertension (OR, 1.31; 95% CI, 1.17–1.46), and hyperlipidemia (OR, 1.45; 95% CI, 1.32–1.59) at the P<0.001 level. Similarly, the odds of severe CAC >100 increased with male sex (OR, 4.80; 95% CI, 3.25–7.11), age (OR, 2.97; 95% CI, 2.60–3.40), and hyperlipidemia (OR, 1.47; 95% CI, 1.21–1.78) at the P<0.001 level and hypertension (OR, 1.43; 95% CI, 1.15–1.78) and tobacco use (OR, 1.67; 95% CI, 1.19–2.35) at the P<0.05 level. Black race was associated with significantly lower odds of any CAC (OR, 0.53; 95% CI, 0.46–0.61 [P<0.001]) or severe CAC (OR, 0.58; 95% CI, 0.43–0.78 [P=0.004]) than White race (Table 2).
Figure 1. Prevalence of coronary artery calcium (CAC) in men vs women aged 30 to 49 years (A), prevalence of CAC in individuals aged 30 to 39 years vs 40 to 49 years (B), prevalence of CAC with increasing number of cardiovascular disease (CVD) risk factors (hypertension, hyperlipidemia, diabetes, tobacco dependence, and age 45 years and older in men) (C), and rates of myocardial infarction (MI), stroke, major adverse cardiovascular events (MACE), and all‐cause mortality (D).
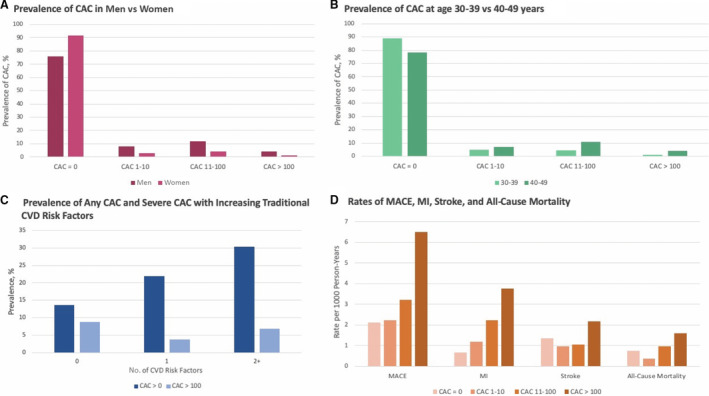
Table 2.
Independent Predictors of Any CAC (>0) and Severe CAC (>100) Among 13 397 Asymptomatic Adults Aged 30 to 49 Years
| Risk factors | CAC >0 (n=2759) | CAC >100 (n=485) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age, per 10‐y increase | 4.91 | (3.62–6.66) | <0.001 | 2.97 | (2.60–3.40) | <0.001 |
| Male sex | 3.56 | (3.08–4.11) | <0.001 | 4.80 | (3.25–7.11) | <0.001 |
| Black (vs White) race | 0.53 | (0.46–0.61) | <0.001 | 0.58 | (0.43–0.78) | 0.004 |
| Hypertension | 1.31 | (1.17–1.46) | <0.001 | 1.43 | (1.15–1.78) | 0.001 |
| Hyperlipidemia | 1.45 | (1.32–1.59) | <0.001 | 1.47 | (1.21–1.78) | <0.001 |
| Tobacco use | 1.21 | (1.00–1.46) | 0.052 | 1.67 | (1.19–2.35) | 0.003 |
| Diabetes | 1.05 | (0.83–1.33) | 0.68 | 1.20 | (0.77–1.85) | 0.43 |
Multivariable logistic regression models were created to determine factors associated with coronary artery calcium (CAC) >0 and CAC >100, forcing age (continuous), race, sex, hypertension, hyperlipidemia, tobacco use, and diabetes into the model. All independent variables except diabetes significantly predicted presence of CAC >100 after adjusting for other risk factors in the model. OR indicates odds ratio.
Over a mean follow‐up of 11.1 years (3.4 years), there were 418 MACE (2.87 events per 1000 person‐years; 95% CI, 2.61–3.16 events per 1000 person‐years), 185 MIs (44% of MACE; 1.26 events per 1000 person‐years; 95% CI, 1.09–1.46 events per 1000 person‐years), 225 strokes (53% of MACE; 1.54 events per 1000 person‐years; 95% CI, 1.35–1.75 events per 1000 person‐years), and 141 deaths (0.96 deaths per 1000 person‐years; 95% CI, 0.81–1.13 deaths per 1000 person‐years), with 30 deaths attributable to cardiovascular causes (7% of MACE; 0.20 cardiovascular deaths per 1000 person‐years; 95% CI, 0.14–0.29 cardiovascular deaths per 1000 person‐years). The rates of all incident CVD events increased with increasing CAC scores (Figure 1D). The cumulative incidence of all events is shown in Figure 2 and the hazard ratios (HRs) in Table 3. Compared with patients without CAC, those with any detectable CAC (>0) had significantly increased hazards of MACE (adjusted subhazard ratio [aSHR], 1.64; 95% CI, 1.33–2.03) and MI (aSHR, 2.94; 95% CI, 2.18–3.95) when adjusted for differences in baseline risk factors, competing mortality, and atrial fibrillation (for MACE). CAC scores >100 were associated with significantly increased hazards of MI (aSHR, 5.16; 95% CI, 3.29–8.10), MACE (aSHR, 3.14; 95% CI, 2.26–4.36), stroke (aSHR, 1.73; 95% CI, 1.01–2.97), and all‐cause mortality (HR, 2.08; 95% CI, 1.13–3.82). CAC scores of 11 to 100 were significantly associated with MI (aSHR, 3.09; 95% CI, 2.16–4.41) and MACE (aSHR, 1.53; 95% CI, 1.15–2.02).
Figure 2. Cumulative incidence of myocardial infarction, stroke, major adverse cardiovascular events (MACE), and all‐cause mortality stratified by coronary artery calcium (CAC) severity among young adults aged 30 to 49 years.
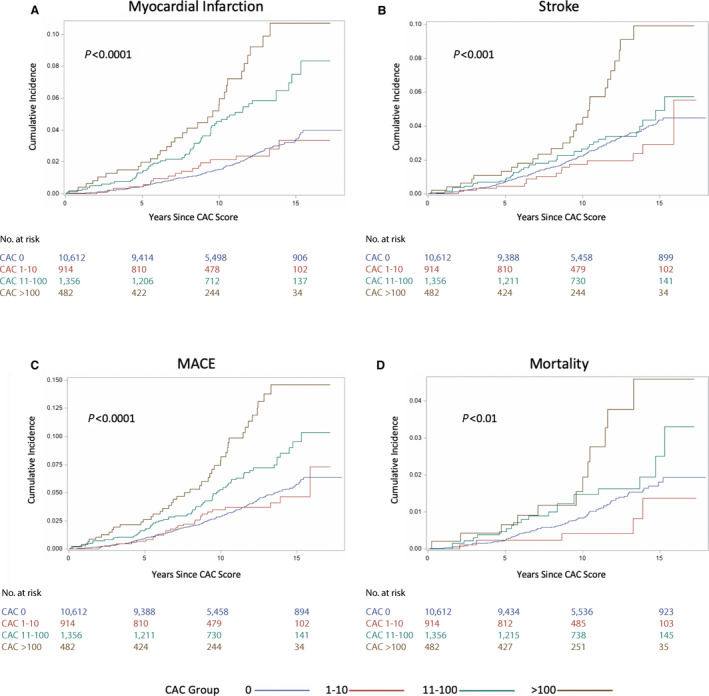
A, Myocardial infarction; B, stroke; C, MACE; and D, all‐cause mortality.
Table 3.
Prevalence and HRs for Cardiovascular Outcomes and Mortality by CAC Group Among 13 397 Asymptomatic Adults Aged 30 to 49 Years
| CAC score | No CAC vs any CAC | CAC subgroups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (n=10 638) | >0 (n=2759) | P value | 1–10 (n=916) | P value | 11–100 (n=1358) | P value | >100 (n=485) | P value | |
| MACE, n (%) | 284 (2.7) | 134 (4.8) | … | 28 (3.1) | … | 61 (4.5) | … | 45 (9.3) | … |
| Unadjusted SHR | … | 1.78 (1.45–2.19) | <0.001 | 1.10 (0.75–1.62) | 0.63 | 1.64 (1.23–2.16) | <0.001 | 3.62 (2.65–4.96) | <0.001 |
| Adjusted SHR | … | 1.64 (1.33–2.03) | <0.001 | 0.95 (0.71–1.55) | 0.81 | 1.53 (1.15–2.02) | 0.003 | 3.14 (2.26–4.36) | <0.001 |
| Stroke, n (%) | 178 (1.7) | 32 (1.2) | … | 12 (1.3) | … | 20 (1.5) | … | 15 (3.1) | … |
| Unadjusted SHR | … | 0.99 (0.72–1.36) | 0.94 | 0.75 (0.42–1.35) | 0.34 | 0.85 (0.53–1.34) | 0.48 | 1.87 (1.11–3.16) | 0.020 |
| Adjusted SHR | … | 0.97 (0.7–1.4) | 0.84 | 0.76 (0.42–1.37) | 0.36 | 0.83 (0.52–1.34) | 0.45 | 1.73 (1.01–2.97) | 0.047 |
| MI, n (%) | 98 (0.9) | 87 (3.2) | … | 16 (1.7) | … | 44 (3.2) | … | 27 (5.6) | … |
| Unadjusted SHR | … | 3.34 (2.50–4.47) | <0.001 | 1.82 (1.07–3.09) | 0.027 | 3.41 (2.39–4.88) | <0.001 | 6.20 (4.05–9.49) | <0.001 |
| Adjusted SHR | … | 2.94 (2.18–3.95) | <0.001 | 1.64 (0.96–2.81) | 0.07 | 3.09 (2.16–4.41) | <0.001 | 5.16 (3.29–8.10) | <0.001 |
| Death, n (%) | 104 (1.0) | 37 (1.3) | 5 (0.5) | … | 20 (1.5) | … | 12 (2.5) | … | |
| Unadjusted HR | … | 1.33 (0.91–1.93) | 0.14 | 0.53 (0.22–1.31) | 0.17 | 1.45 (0.90–2.34) | 0.13 | 2.56 (1.41–4.65) | 0.002 |
| Adjusted HR | … | 1.15 (0.78–1.69) | 0.48 | 0.49 (0.20–1.21) | 0.12 | 1.25 (0.77–2.04) | 0.37 | 2.08 (1.13–3.82) | 0.019 |
| Cardiac death, n (%) | 19 (0.2) | 11 (0.4) | … | 1 (0.1) | … | 2 (0.1) | … | 8 (1.6) | … |
Univariable and multivariable Cox proportional hazard models evaluated the risk of cardiovascular outcomes and death for patients with coronary artery calcium (CAC) compared with those without CAC. Values are number (percentage) or subhazard ratio (SHR)/hazard ratio (HR) (95% CI). Adjusted HRs and SHRs account for the presence of hypertension, hyperlipidemia, diabetes, age (continuous), sex, and tobacco use. Baseline atrial fibrillation is also included in the models for major adverse cardiovascular events (MACE) and stroke. SHRs for myocardial infarction (MI) and stroke account for the competing risk of death. MACE accounts for the competing risk of noncardiac death. Please note the events in the CAC >0 column are the sum of those in the CAC 1 to 10, 11 to 100, and >100 categories.
The addition of CAC severity to baseline standard cardiovascular risk factors, including atrial fibrillation for stroke and MACE, significantly improved the predictive accuracy of the model for MACE (P<0.001), MI (P<0.001), and all‐cause mortality (P=0.028) by the likelihood ratio test. The C statistic with 95% CI for each model is listed in Table 4.
Table 4.
C Statistic for Outcomes With Risk Factors Alone and With Addition of CAC Score Among Adults Aged 30 to 49 Years
| Risk factors | CAC+risk factors | P value* | |
|---|---|---|---|
| All‐cause mortality | 0.57 (0.52–0.63) | 0.59 (0.54–0.64) | 0.028 |
| MACE | 0.60 (0.57–0.63) | 0.63 (0.60–0.66) | <0.001 |
| MI | 0.61 (0.57–0.65) | 0.70 (0.66–0.74) | <0.001 |
| Stroke | 0.58 (0.54–0.62) | 0.59 (0.54–0.63) | 0.32 |
Harrell C statistic was used to assess the area under the curve for the primary outcomes accounting for censoring of events. Values are C statistic (95% CI). MACE indicates major adverse cardiovascular events (myocardial infarction [MI], stroke, and cardiac death).
Reported P values are derived from the likelihood ratio test comparing the predictive ability of the models with and without coronary artery calcium (CAC) as measured by the −2 log L fit statistics. CAC score was input into the model using ln(CAC+1) transformation. Risk factors consist of the presence of hypertension, hyperlipidemia, diabetes, tobacco use, and ln(age).
In subgroup analyses comparing those with and without traditional cardiovascular risk factors, the presence of any CAC and severe CAC scores >100 were independently predictive of incident MACE across all risk factor strata (Figure 3). Among patients without traditional risk factors (n=5475), the presence of any CAC (n=748, 14%) was associated with an increased risk of MACE (aSHR, 1.51; 95% CI, 1.01–2.28 [P=0.046]).
Figure 3. Forest plot of incident major adverse cardiovascular events (MACE) within risk factor subgroups.
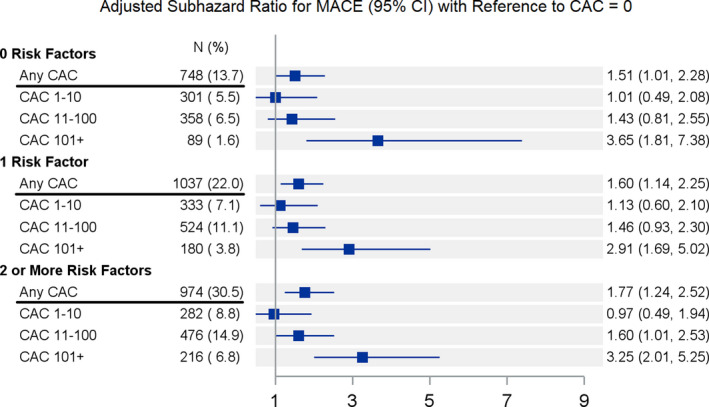
Subhazard ratios (vs no coronary artery calcification [CAC]) for MACE adjusted for age (continuous), traditional risk factors, and stroke. Please note “Any CAC” includes all of the subsequent CAC categories within each risk factor subgroup. The adjusted subhazard ratio for MACE also accounts for the competing risk of noncardiac death.
Discussion
In this large observational study of mostly low‐risk young adults (mean age, 44 years) without baseline CVD or malignancy, we made several observations. First, despite their young age and low prevalence of traditional risk factors, CAC was relatively common, prevalent in 20.6% of patients (number needed to scan to detect CAC=5), with 3.6% having significantly elevated CAC scores (>100). Second, the assessment of CAC more accurately predicted future CVD events and all‐cause mortality than traditional risk factors alone over a mean 11‐year follow‐up. Even CAC scores between 11 and 100, considered a low burden of CAC in older populations, carried considerable risk in this younger population, with a 3‐fold higher hazard of MI and 1.5‐fold higher hazard of MACE. While uncommon in this cohort, CAC scores >100 were associated with a 5‐fold increased hazard for MI and significantly increased hazards for all studied end points. The high prevalence of premature coronary atherosclerosis in this real‐world sample of young adults with a relatively low burden of CVD risk factors highlights the importance of adopting healthy lifestyle behaviors early in life. In addition, these findings suggest that CAC testing may be a reasonable method of further risk stratification for select young adults with elevated CVD risk.
Comparisons of Risk Profile With the US Population
Table S1 summarizes the risk profiles of the other major studies that have investigated the prevalence of CAC and cardiovascular risk factors in US young adults without known CVD. While the prevalence of risk factors in the general population is difficult to determine, most CAC studies had risk profiles that were similar or slightly higher than those in adults with a mean age of 44 years without CVD from the National Health and Nutrition Examination Survey (NHANES). 24 Our cohort, which, to our knowledge, is the second largest of CAC in US young adults to date, had an overall similar prevalence of risk factors to NHANES but slightly lower rates of diabetes and smoking and higher rates of dyslipidemia. 25 The latter may be attributable to differing definitions of dyslipidemia between centers; we do not have access to the lipid values for our cohort.
Comparisons of Long‐Term Risks With Prior Data
Our findings are consistent with and complimentary to the limited prior studies examining the associations of CAC and outcomes in young adults. Tota‐Maharaj et al 7 published a study on a multicenter referral‐based cohort of 8143 individuals (mean age, 40 years), which found a prevalence of 30% for any CAC, with 4% of individuals having CAC >100 and a graded increase in risk of all‐cause mortality across CAC categories. Compared with the present study, the cohort had a greater proportion of women (46.4%) and a lower prevalence of hypertension (15.6%) and dyslipidemia (23.7%), but the study was limited by short‐term follow‐up (mean, 5.8 years) and lack of data on MACE. 7 An analysis of the CAC Consortium, a retrospective multicenter cohort study of 22 346 individuals aged 30 to 49 years without baseline CVD who underwent CAC testing, similarly found a higher total prevalence of CAC (34.4%) with 7.2% of patients having a CAC score >100. 8 They additionally demonstrated a 5‐fold increased risk of coronary heart disease (CHD) mortality and 3‐fold increased risk of CVD mortality for CAC >100 compared with no CAC. The CAC Consortium boasted a large sample size and long‐term (mean, 12.7 years) follow‐up; however, the referral‐based population was 87.7% White race and had a high prevalence of hyperlipidemia (50%) and family history of CHD (49%), thus possibly decreasing the generalizability of findings.
At a mean age 45 years (n=3141), participants in the CARDIA (Coronary Artery Risk Development in Young Adults) prospective cohort study had an overall prevalence of any CAC of 20.1%. 9 The presence of any CAC was associated with a 5‐fold higher risk for incident CHD events and a 1.6‐fold higher risk for all‐cause mortality during 12.5 years of follow‐up. Comparatively, we found a similar CAC prevalence of 20.6% at a mean age of 44 years, with a 5‐fold increased risk of MI and 2‐fold increased risk of all‐cause mortality associated with CAC scores >100 compared with 0. The CARDIA study benefits from being an unselected data set with ethnic and socioeconomic diversity, hence increasing the generalizability of results, but is limited by a small sample size. Nevertheless, both the CARDIA and the present study underscore the important conclusion that there is a nonnegligible prevalence of CAC in young adults and even low scores are associated with higher risk of CVD events by middle age.
Predictors of Premature CAC
Although age is a dominant factor associated with atherogenesis, multiple traditional risk factors and lifestyle behaviors have been associated with the development of premature CAC. 26 In addition to age, the present study found strong independent associations of male sex, hypertension, hyperlipidemia, and tobacco use with any CAC and severe CAC. A substudy of participants in the CARDIA study demonstrated that age, male sex, and baseline characteristics including systolic blood pressure, low‐density lipoprotein cholesterol, cigarette smoking, blood glucose level, and body mass index were all independently correlated with the development of CAC 15 years later. 27 Other studies have determined similar associations in young adults, eg, a large study (n=33 637) of South Korean adults younger than 40 years found a graded increase in CAC prevalence with increases in systolic blood pressure. 28 Interestingly, the prevalence of any CAC in the CARDIA study nearly doubled from 10% at a mean age of 40 years to 20% at a mean age of 45 years. 9 Recent efforts to predict the conversion to CAC >0 using genetic risk scores may have utility to identify high‐risk young adults for early CAC scanning. 29 , 30
Our finding that Black race was associated with lower odds of CAC than White race is consistent with data from the CARDIA study, which demonstrated that White race was associated with higher odds of CAC among nearly equal numbers of Black and White participants. 27 Likewise, MESA (Multi‐Ethnic Study of Atherosclerosis) found the relative risk of having CAC was 0.78 in Black compared with White patients, with Black patients having a significantly lower prevalence and severity of CAC. 31 Adequate representation of historically underrepresented groups in medical research cohorts has long been a challenge, 32 despite significant efforts to combat these disparities in CAC research through studies such as MESA and the MASALA (Mediators of Atherosclerosis in South Asians Living in America) study, 33 among others. Further measures to investigate CAC among underrepresented groups are warranted. 34
Previous studies have demonstrated an association with higher intake of fruits and vegetables, high levels of cardiorespiratory fitness, and lack of abdominal obesity with a lower likelihood of premature CAC. 35 , 36 , 37 Multiple studies, including the present study, have demonstrated an increased prevalence of CAC among individuals with more CVD risk factors. 8 , 11 , 38 The number of CVD risk factors could be a potential method to predict the likelihood of a positive CAC examination. In this study, for example, the number needed to scan for the entire cohort to detect severe CAC was 28, which decreased to 11 for individuals with 4 risk factors. Despite this, we found that 748 of 2759 (27%) individuals with CAC had no risk factors and still had a significantly increased hazards of MACE. A study in MESA demonstrated that individuals with CAC but no traditional CVD risk factors were at significantly elevated risk for CHD events compared with those with traditional risk factors but no CAC. 39 In the CAC Consortium, Grandhi et al 40 found that CAC was a more reliable predictor of long‐term mortality than traditional CVD risk factors. Our study contributes to the literature in support of CAC as one of the strongest tools for risk prediction in primary prevention. 41
CAC Testing in Young Adults
The 2019 ACC/AHA primary prevention guidelines recommend CAC scoring for adults at intermediate risk (≥7.5% to <20% 10‐year ASCVD risk) and select adults at borderline risk (5% to <7.5% 10‐year ASCVD risk) in cases where risk‐based decisions for initiating statin therapy are uncertain. Specifically, risk can be reclassified upward in cases of CAC >100 or age/sex/race percentile score ≥75, or downward if CAC=0. However, no percentile scores exist for individuals under the age of 45 years and the guidelines conclude that more data are needed to support use of CAC scoring in the subgroup of patients younger than 45 years. 1 Prior analyses have suggested that CAC testing may be the best option to determine the magnitude of potential benefit from initiating cardiovascular medications, including statins, 10 , 42 , 43 aspirin, 37 and antihypertensive therapy. 44 We demonstrated a nonnegligible prevalence of CAC in young adults, which was associated with a clear graded increase in the incidence and hazards of CVD events with increasing CAC scores. While these findings suggest that the clinical utility of CAC testing may extend to select younger adults, particularly those with risk factors, future development of age‐sex‐race percentile scores for young adults could be useful for guiding interpretation of CAC scores and subsequent intensity of preventive interventions, as they have been for middle‐ and older‐aged adults. 45
Strengths and Limitations
The present study benefits from being a large, real‐world sample of relatively healthy young adults with long‐term follow‐up, which may increase the generalizability of results. The study has several limitations, many that are inherent to large‐scale observational studies. First, the study population consisted of mostly White men from a single tertiary medical center of military healthcare beneficiaries, which entails broad, comprehensive access to medical care. This may reduce the generalizability of findings. Baseline covariates were assessed using the DoD MDR to include use of ICD‐9 claims, which did not allow for direct measurement of several risk factors, such as blood pressure, lipid values, or height and weight. Consequently, it was impossible to calculate accurate 10‐year or lifetime global risk scores for study participants. We used the Charlson Comorbidity Index to get a general sense of mortality risk in our sample, but this score is not optimal for risk assessment in individuals younger than 50 years. Furthermore, the exact clinical indications for CAC scoring were not documented and family history data could not be ascertained. While additional CAC parameters such as density, 46 distribution, location, 47 and radiomics‐based advanced image analysis techniques 48 have been shown to add prognostic value to CAC score, this deidentified data set only has Agatston CAC score documented without access to the original scans. Outcomes were assessed within the military health system using ICD‐9 codes for MI and stroke, which have been shown to have a ≥90% positive predictive value for representing adjudicated clinical MI and stroke events. 49 Although this methodology has been broadly used for large‐scale outcome studies, there remains a risk for imprecision in accounting of outcomes. While all deaths and their causes were ascertained using the National Death Index, it is possible that some deaths may have been misclassified (cardiac versus noncardiac). Our cohort also had a relatively low number of deaths attributable to cardiac causes and we did not differentiate between deaths caused by CHD and CVD events. Last, Harrel C statistic <0.7 could be interpreted as subsatisfactory performance even with the addition of CAC. However, this only underscores the necessity for better markers of risk in this young demographic and emphasizes the take‐home message that CAC improves the predictive performance of ASCVD events compared with traditional risk factors alone.
Conclusions
In a large, low‐risk cohort of young adults with long‐term follow‐up, there was a nonnegligible prevalence of CAC. Despite their young age, the presence and severity of CAC was strongly and independently associated with incident adverse events such as MACE, MI, stroke, and death when controlling for age, sex, and cardiovascular risk factors. CAC scoring significantly improved prognostic accuracy for long‐term CVD and mortality outcomes. These results may support the utility of CAC scoring in selected younger individuals despite low 10‐year CVD risk scores for guiding risk assessment and intensity of preventive interventions.
Sources of Funding
Research reported in this publication was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR002345.
Disclosures
Dr Mitchell reports grants and nonfinancial support from the National Center for Advancing Translational Sciences of the National Institutes of Health during the conduct of the study; personal fees and nonfinancial support from Pfizer; and grants from Longer Life Foundation and Children's Discovery Institute, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
For Sources of Funding and Disclosures, see page 10.
References
- 1. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation. 2019;74:e596–e646. doi: 10.1016/j.jacc.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bibbins‐Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Kemper AR, Krist AH, Kurth AE, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997–2007. doi: 10.1001/jama.2016.15450 [DOI] [PubMed] [Google Scholar]
- 3. Singh A, Collins BL, Gupta A, Fatima A, Qamar A, Biery D, Baez J, Cawley M, Klein J, Hainer J, et al. Cardiovascular risk and statin eligibility of young adults after an MI: partners YOUNG‐MI Registry. J Am Coll Cardiol. 2018;71:292–302. doi: 10.1016/j.jacc.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahtta D, Ramsey DJ, Al Rifai M, Nasir K, Samad Z, Aguilar D, Jneid H, Ballantyne CM, Petersen LA, Virani SS. Evaluation of aspirin and statin therapy use and adherence in patients with premature atherosclerotic cardiovascular disease. JAMA Netw Open. 2020;3:e2011051. doi: 10.1001/jamanetworkopen.2020.11051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dzaye O, Dudum R, Reiter‐Brennan C, Kianoush S, Tota‐Maharaj R, Cainzos‐Achirica M, Blaha MJ. Coronary artery calcium scoring for individualized cardiovascular risk estimation in important patient subpopulations after the 2019 AHA/ACC primary prevention guidelines. Prog Cardiovasc Dis. 2019;62:423–430. doi: 10.1016/j.pcad.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 6. Saad M, Pothineni NV, Thomas J, Parikh R, Kovelamudi S, Elsayed D, Nairooz R, Feit F. Coronary artery calcium scoring in young adults: evidence and challenges. Curr Cardiol Rep. 2018;20:10. doi: 10.1007/s11886-018-0951-5 [DOI] [PubMed] [Google Scholar]
- 7. Tota‐Maharaj R, Blaha MJ, McEvoy JW, Blumenthal RS, Muse ED, Budoff MJ, Shaw LJ, Berman DS, Rana JS, Rumberger J, et al. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33:2955–2962. doi: 10.1093/eurheartj/ehs230 [DOI] [PubMed] [Google Scholar]
- 8. Miedema MD, Dardari ZA, Nasir K, Blankstein R, Knickelbine T, Oberembt S, Shaw L, Rumberger J, Michos ED, Rozanski A, et al. Association of coronary artery calcium with long‐term, cause‐specific mortality among young adults. JAMA Netw Open. 2019;2:e197440. doi: 10.1001/jamanetworkopen.2019.7440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carr JJ, Jacobs DR Jr, Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–399. doi: 10.1001/jamacardio.2016.5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitchell JD, Fergestrom N, Gage BF, Paisley R, Moon P, Novak E, Cheezum M, Shaw LJ, Villines TC. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72:3233–3242. doi: 10.1016/j.jacc.2018.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell JD, Paisley R, Moon P, Novak E, Villines TC. Coronary artery calcium and long‐term risk of death, myocardial infarction, and stroke: the Walter Reed Cohort Study. JACC Cardiovasc Imaging. 2018;11:1799–1806. 10.1016/j.jcmg.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 12. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 13. Graham DJ, Ouellet‐Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920 [DOI] [PubMed] [Google Scholar]
- 14. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061 [DOI] [PubMed] [Google Scholar]
- 15. Villines TC, Schnee J, Fraeman K, Siu K, Reynolds MW, Collins J, Schwartzman E. A comparison of the safety and effectiveness of dabigatran and warfarin in non‐valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost. 2015;114:1290–1298. doi: 10.1160/TH15-06-0453 [DOI] [PubMed] [Google Scholar]
- 16. Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.STR.0000032240.28636.BD [DOI] [PubMed] [Google Scholar]
- 17. Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of medicare claims‐based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 20. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 21. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. doi: 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 22. Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. doi: 10.1001/jama.1982.03320430047030 [DOI] [PubMed] [Google Scholar]
- 23. Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krittanawong C, Kumar A, Wang Z, Narasimhan B, Mahtta D, Jneid H, Baber U, Mehran R, Tang W, Ballantyne CM, et al. Coronary artery disease in the young in the US population‐based cohort. Am J Cardiovasc Dis. 2020;10:189–194. [PMC free article] [PubMed] [Google Scholar]
- 25. Egan BM, Li J, Sutherland SE, Jones DW, Ferdinand KC, Hong Y, Sanchez E. Sociodemographic determinants of life's simple 7: implications for achieving cardiovascular health and health equity goals. Ethn Dis. 2020;30:637–650. doi: 10.18865/ed.30.4.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Head T, Daunert S, Goldschmidt‐Clermont PJ. The aging risk and atherosclerosis: a fresh look at arterial homeostasis. Front Genet. 2017;8:216–216. doi: 10.3389/fgene.2017.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2007;49:2013–2020. doi: 10.1016/j.jacc.2007.03.009 [DOI] [PubMed] [Google Scholar]
- 28. Kang J, Chang Y, Kim S, Sung KC, Shin H, Ryu S. Increased burden of coronary artery calcium from elevated blood pressure in low‐risk young adults. Atherosclerosis. 2019;282:188–195. doi: 10.1016/j.atherosclerosis.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 29. Severance LM, Carter H, Contijoch FJ, McVeigh ER. Targeted coronary artery calcium screening in high‐risk younger individuals using consumer genetic screening results. JACC Cardiovasc Imaging. 2021;14:1398–1406. doi: 10.1016/j.jcmg.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blaha MJ. Predicting age of conversion to CAC >0: a role for polygenic risk scores? JACC Cardiovasc Imaging. 2021;14:1407–1409. doi: 10.1016/j.jcmg.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 31. Osawa K, Nakanishi R, Budoff M. Coronary artery calcification. Glob Heart. 2016;11:287–293. doi: 10.1016/j.gheart.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Konkel L. Racial and ethnic disparities in research studies: the challenge of creating more diverse cohorts. Environ Health Perspect. 2015;123:A297–A302. doi: 10.1289/ehp.123-A297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mehta A, Patel J, Al Rifai M, Ayers CR, Neeland IJ, Kanaya AM, Kandula N, Blaha MJ, Nasir K, Blumenthal RS, et al. Inflammation and coronary artery calcification in South Asians: the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Atherosclerosis. 2018;270:49–56. doi: 10.1016/j.atherosclerosis.2018.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gooding HC, Gidding SS, Moran AE, Redmond N, Allen NB, Bacha F, Burns TL, Catov JM, Grandner MA, Harris KM, et al. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: report from a National Heart, Lung, and Blood Institute Working Group. J Am Heart Assoc. 2020;9:e016115. doi: 10.1161/JAHA.120.016115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miedema MD, Petrone A, Shikany JM, Greenland P, Lewis CE, Pletcher MJ, Gaziano JM, Djousse L. Association of fruit and vegetable consumption during early adulthood with the prevalence of coronary artery calcium after 20 years of follow‐up: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circulation. 2015;132:1990–1998. doi: 10.1161/CIRCULATIONAHA.114.012562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee CD, Jacobs DR Jr, Hankinson A, Iribarren C, Sidney S. Cardiorespiratory fitness and coronary artery calcification in young adults: the CARDIA Study. Atherosclerosis. 2009;203:263–268. doi: 10.1016/j.atherosclerosis.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reis JP, Loria CM, Lewis CE, Powell‐Wiley TM, Wei GS, Carr JJ, Terry JG, Liu K. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–288. doi: 10.1001/jama.2013.7833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bild DE, Folsom AR, Lowe LP, Sidney S, Kiefe C, Westfall AO, Zheng ZJ, Rumberger J. Prevalence and correlates of coronary calcification in black and white young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol. 2001;21:852–857. doi: 10.1161/01.ATV.21.5.852 [DOI] [PubMed] [Google Scholar]
- 39. Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, Agatston A, Blumenthal RS, Nasir K. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35:2232–2241. doi: 10.1093/eurheartj/eht508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grandhi GR, Mirbolouk M, Dardari ZA, Al‐Mallah MH, Rumberger JA, Shaw LJ, Blankstein R, Miedema MD, Berman DS, Budoff MJ, et al. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD mortality: the CAC Consortium. JACC Cardiovasc Imaging. 2020;13:1175–1186. 10.1016/j.jcmg.2019.08.024 [DOI] [PubMed] [Google Scholar]
- 41. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–447. doi: 10.1016/j.jacc.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–147. doi: 10.1016/j.jacc.2015.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, Miedema MD, Sibley CT, Shaw LJ, Blumenthal RS, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66:1657–1668. doi: 10.1016/j.jacc.2015.07.066 [DOI] [PubMed] [Google Scholar]
- 44. Elshazly MB, Abdellatif A, Dargham SR, Rifai MA, Quispe R, Cainzos‐Achirica M, Martin SS, Yeboah J, Psaty BM, Post WS, et al. Role of coronary artery and thoracic aortic calcium as risk modifiers to guide antihypertensive therapy in stage 1 hypertension (from the Multiethnic Study of Atherosclerosis). Am J Cardiol. 2020;126:45–55. doi: 10.1016/j.amjcard.2020.02.036 [DOI] [PubMed] [Google Scholar]
- 45. McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696 [DOI] [PubMed] [Google Scholar]
- 46. Osei AD, Mirbolouk M, Berman D, Budoff MJ, Miedema MD, Rozanski A, Rumberger JA, Shaw L, Al Rifai M, Dzaye O, et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non‐users: the Coronary Artery Calcium Consortium. Atherosclerosis. 2021;316:79–83. doi: 10.1016/j.atherosclerosis.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Foldyna B, Eslami P, Scholtz JE, Baltrusaitis K, Lu MT, Massaro JM, D’Agostino RB, Ferencik M, Aerts HJ, O’Donnell CJ, et al. Density and morphology of coronary artery calcium for the prediction of cardiovascular events: insights from the Framingham Heart Study. Eur Radiol. 2019;29:6140–6148. doi: 10.1007/s00330-019-06223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eslami P, Parmar C, Foldyna B, Scholtz J‐E, Ivanov A, Zeleznik R, Lu MT, Ferencik M, Vasan RS, Baltrusaitis K, et al. Radiomics of coronary artery calcium in the Framingham Heart Study. Radiol Cardiothorac Imaging. 2020;2:e190119. doi: 10.1148/ryct.2020190119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Floyd JS, Blondon M, Moore KP, Boyko EJ, Smith NL. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of veterans with diabetes. Pharmacoepidemiol Drug Saf. 2016;25:467–471. doi: 10.1002/pds.3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Osei AD, Uddin SMI, Dzaye O, Achirica MC, Dardari ZA, Obisesan OH, Kianoush S, Mirbolouk M, Orimoloye OA, Shaw L, et al. Predictors of coronary artery calcium among 20–30‐year‐olds: the Coronary Artery Calcium Consortium. Atherosclerosis. 2020;301:65–68. doi: 10.1016/j.atherosclerosis.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lichtenstein G, Perlman A, Shpitzen S, Durst R, Shaham D, Leitersdorf E, Szalat A. Correlation between coronary artery calcification by non‐cardiac CT and Framingham score in young patients. PLoS One. 2018;13:e0195061. doi: 10.1371/journal.pone.0195061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paixao ARM, Ayers CR, Rohatgi A, Das SR, de Lemos JA, Khera A, Lloyd‐Jones D, Berry JD. Cardiovascular lifetime risk predicts incidence of coronary calcification in individuals with low short‐term risk: the Dallas Heart Study. J Am Heart Assoc. 2014;3:e001280. doi: 10.1161/JAHA.114.001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


