Abstract
Background
The long‐term burden of cardiovascular disease after repair of coarctation of the aorta (CoA) has not been elucidated. We aimed to determine the incidence of and risk factors for cardiovascular events in adult patients with repaired CoA. Additionally, mortality rates were compared between adults with repaired CoA and the general population.
Methods and Results
Using the Dutch Congenital Corvitia (CONCOR) registry, patients aged ≥16 years with previous surgical or transcatheter CoA repair from 5 tertiary referral centers were included. Cardiovascular events were recorded, comprising coronary artery disease, stroke/transient ischemic attack, aortic complications, arrhythmias, heart failure hospitalizations, endocarditis, and cardiovascular death. In total, 920 patients (median age, 24 years [range 16–74 years]) were included. After a mean follow‐up of 9.3±5.1 years, 191 patients (21%) experienced at least 1 cardiovascular event. A total of 270 cardiovascular events occurred, of which aortic complications and arrhythmias were most frequent. Older age at initial CoA repair (hazard ratio [HR], 1.017; 95% CI, 1.000–1.033 [P=0.048]) and elevated left ventricular mass index (HR, 1.009; 95% CI, 1.005–1.013 [P<0.001]) were independently associated with an increased risk of cardiovascular events. The mortality rate was 3.3 times higher than expected based on an age‐ and sex‐matched cohort from the Dutch general population (standardized mortality ratio, 3.3; 95% CI, 2.3–4.4 [P<0.001]).
Conclusions
This large, prospective cohort of adults with repaired CoA showed a high burden of cardiovascular events, particularly aortic complications and arrhythmias, during long‐term follow‐up. Older age at initial CoA repair and elevated left ventricular mass index were independent risk factors for the occurrence of cardiovascular events. Mortality was 3.3‐fold higher compared with the general population. These results advocate stringent follow‐up after CoA repair and emphasize the need for improved preventive strategies.
Keywords: adult congenital heart disease, aortic coarctation, cardiovascular events, survival
Subject Categories: Congenital Heart Disease, Complications, Mortality/Survival, Prognosis
Nonstandard Abbreviations and Acronyms
- CoA
coarctation of the aorta
- CONCOR
Congenital Corvitia
- MHV
mechanical heart valve
- NHLBI/ACHA
National Heart, Lung, and Blood Institute/Adult Congenital Heart Association
Clinical Perspective
What Is New?
There is a high incidence of cardiovascular events, particularly aortic complications and arrhythmias, in adult patients with repaired coarctation of the aorta.
Older age at initial repair and elevated left ventricular mass index were identified as independent risk factors for the occurrence of cardiovascular events.
In this study, mortality was 3.3‐fold higher in adults with repaired coarctation of the aorta compared with an age‐ and sex‐matched cohort from the general population.
What Are the Clinical Implications?
These findings advocate stringent follow‐up after repair of coarctation of the aorta, preferably in a specialized center for adult congenital heart disease.
Future studies are needed to improve preventive strategies in this high‐risk patient population.
Coarctation of the aorta (CoA) is a common congenital heart defect, accounting for ≈5% to 7% of all congenital heart disease (CHD). 1 Attributable to improved detection and management, the majority of patients currently survive into adulthood. However, the risk of adverse cardiovascular events and consequent premature death remains remarkably high. More than 70% of patients with long‐term follow‐up dies from cardiovascular complications. 2 Accelerated coronary artery disease (CAD) was previously identified as the most common cause of death, although arrhythmias, heart failure, and aortic aneurysms are also prevalent. 3 , 4 , 5 Importantly, complications are not limited to the heart and aorta. Ischemic and hemorrhagic stroke occur 15.9 years and 28.5 years earlier, respectively, compared with the general population, suggesting that CoA is the expression of an underlying diffuse arteriopathy. 6 However, there is limited information on the exact burden of cardiovascular disease (CVD) in the adult CoA population. This knowledge gap was recognized by the National Heart, Lung, and Blood Institute/Adult Congenital Heart Association (NHLBI/ACHA) Working Group and was therefore marked as a high‐priority research topic in adult CHD. 7 Furthermore, it is unclear how to identify patients with CoA who are at the highest risk of cardiovascular complications. In addition to the suggested underlying arteriopathy, hypertension is thought to play an important role, as up to 60% of patients develops hypertension late after initial repair. 8
To our knowledge, we present the largest prospective cohort of adults with repaired CoA to date. In this study, we aimed to determine the incidence of and risk factors for cardiovascular events in this patient population. In addition, we aimed to compare mortality rates of adult CoA patients with those of the general population.
Methods
Study Design
The study cohort consisted of adult patients with repaired CoA from 5 tertiary referral centers in the Netherlands, who were included in the Congenital Corvitia (CONCOR) registry between 2002 and 2018. CONCOR is a prospective, nationwide registry of adults with CHD. Informed consent was obtained upon enrollment. Patients were included when they had previously undergone surgical or transcatheter repair of CoA. Exclusion criteria were a functionally univentricular circulation and transposition of the great arteries, which was not repaired by an arterial switch procedure. Patients were followed until the last clinic visit, death, or the end of the study (April 2020). The study was approved by the Medical Research Ethics Committee of the University Medical Center Utrecht. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Collection and Outcome Measures
Collected baseline data included demographics, medical history, blood pressure (BP) status, medication use, renal function, and echocardiographic parameters. BP was measured on the right arm at the outpatient clinic. When the arm was unspecified or when only the left arm BP was available, it was decided to use these values to minimize missing BP recordings. Hypertension was defined as an office systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg and/or the use of any antihypertensive medication. 9 Cigarette smoking was regarded as current or former smoking. Left ventricular (LV) hypertrophy was assessed at baseline by echocardiography and defined as an LV mass indexed for body surface area >115 g/m2 for men and >95 g/m2 for women. 10
Cardiovascular events that were considered clinically important in this adult CoA population were recorded. These consisted of CAD, stroke or transient ischemic attack (TIA), thoracic aortic complications (ie, aneurysm and dissection), arrhythmias, hospitalizations for heart failure, endocarditis, and cardiovascular death. Cardiovascular events were recorded by 2 researchers (T.A.M., S.C.S.M.) based on the information provided in the medical correspondence by the treating cardiologist. CAD comprised myocardial infarction, coronary revascularization, and medical treatment for stable angina. An aortic aneurysm was defined as a 50% diameter increase compared with the expected diameter based on aortic segment and sex, and/or surgical treatment of the aneurysm. 11 Aortic dissection included dissection, pseudoaneurysm, intramural hematoma, and rupture of the thoracic aorta. Arrhythmias were subdivided into 3 types of arrhythmias: supraventricular arrhythmias, ventricular arrhythmias, and conduction disturbances. Supraventricular arrhythmias included atrial fibrillation, atrial flutter, and atrioventricular (nodal) reentrant tachycardia. Ventricular arrhythmias comprised ventricular fibrillation/flutter (if survived) and sustained and nonsustained ventricular tachycardia. Nonsustained ventricular tachycardia was only recorded as a cardiovascular event when subsequent medical or device treatment was initiated. Conduction disturbances included sick sinus syndrome, sinoatrial block, third‐degree atrioventricular block, and Mobitz type II second‐degree atrioventricular block. Subsequent episodes of the same type of arrhythmia were not recorded as a new event. Cardiovascular events occurring <30 days after an invasive procedure were considered postprocedural and were therefore excluded. Mortality was recorded regardless of any potential association with an invasive procedure and was categorized as cardiovascular or noncardiovascular based on the definitions provided by the SCTI/FDA consensus report. 12 In case of uncertainty regarding the exact cause of death, there was discussion with a third researcher (M.V.) until consensus was reached.
Statistical Analysis
Baseline characteristics were compared between patients who developed a cardiovascular event and patients who did not. Fisher exact test was used for categorical variables, and independent samples t test or Mann‐Whitney U test were used for continuous variables, where appropriate. The yearly risk of a cardiovascular event and specific types of cardiovascular events were estimated by smoothed estimates based on B‐splines. Cox proportional hazards regression was performed to identify factors associated with the risk of cardiovascular events. Patients were followed until the occurrence of a cardiovascular event. If no cardiovascular event occurred, they were censored at the time of the last clinic visit. Potential risk factors were included by forced entry and selected based on their clinical relevance, effect on pathophysiological mechanisms, and previous reports. 4 , 13 , 14 , 15 These included sex, age at initial CoA repair, bicuspid aortic valve, ventricular septal defect, prior CAD, prior stroke/TIA, prior arrhythmia, systolic and diastolic BP, body mass index, hypercholesterolemia, diabetes, cigarette smoking, family history of premature CVD, and LV mass index at baseline. Of all patients, 7% had incomplete data. As deleting patients with missing information from the analysis may introduce bias in results, we used multiple imputation. Imputation included all potential risk factors in the analysis. Other factors were also incorporated based on clinical relevance, including the presence of a left‐sided mechanical heart valve (MHV), antihypertensive agents, renal function, and echocardiographic parameters. Multiple imputation was performed with fully conditional specification, with logistic regression for categorical variables and predictive mean matching for continuous variables. The number of imputed data sets was based on the percentage of patients with missing data. 16 Twenty‐eight imputed data sets were created, the analysis was performed in each imputed data set, and results were subsequently pooled using Rubin's rules. To correct for left‐truncated, right‐censored data, a delayed entry approach was used with age as the time scale. The proportional hazards assumption was evaluated for the multivariable model and each separate variable by graphical inspection of fitted penalized B‐spline curves and the goodness‐of‐fit test based on scaled Schoenfeld residuals. The assumption of linearity was assessed for continuous variables using restrictive cubic splines. Sensitivity analysis was performed by adding age at baseline to the multivariable model, as the measurements of certain potential risk factors, eg, BP and body mass index, may be affected by age. Overall survival was compared with Dutch general population data from Statistics Netherlands (CBS [Central Agency for Statistics]) by matching for age, sex, year of study entry, and observation time. Accordingly, there was a similar left‐truncated data pattern between the study cohort and the reference population.
Statistical analyses were performed using IBM SPSS Statistics 25, RStudio version 1.2.5001 (packages: survival, relsurv, survminer, survexp.fr, bshazard), and SAS version 9 (SAS Institute Inc). A P value <0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 920 patients were included in the study, of whom 191 patients (21%) developed a cardiovascular event. Baseline characteristics are shown in Table 1. Median age at study entry was 24 years (range, 16–74 years). Hypertension was more common in patients with an event compared with patients without an event (75% and 52%, respectively). Sixty patients had a left‐sided MHV, of whom 49 patients had an aortic MHV, 5 patients had a mitral MHV, and 6 patients had both an aortic and mitral MHV. Mean follow‐up duration was 9.3±5.1 years and was longer in the event group (11.3±4.5 years versus 8.7±5.1 years; P<0.001).
Table 1.
Baseline Characteristics
|
All patients n=920 |
Cardiovascular event n=191 |
No cardiovascular event n=729 |
P value* | |
|---|---|---|---|---|
| Age, median (range), y | 24 (16–74) | 31 (17–72) | 23 (16–74) | <0.001 |
| Women, n (%) | 365 (40) | 58 (30) | 307 (42) | 0.004 |
| Age at initial CoA repair, median (range), y | 4 (0–67) | 6 (0–67) | 3 (0–61) | <0.001 |
| Type of initial CoA repair, n (%) | 0.02 | |||
| Surgery | 863 (94) | 173 (91) | 690 (95) | |
| Balloon angioplasty | 19 (2) | 9 (5) | 10 (1) | |
| Stenting | 38 (4) | 9 (5) | 29 (4) | |
| Type of surgical repair, n (%) † | 0.005 | |||
| End‐to‐end anastomosis | 474 (55) | 87 (50) | 387 (56) | |
| Patch angioplasty | 88 (10) | 17 (10) | 71 (10) | |
| Subclavian flap angioplasty | 89 (10) | 9 (5) | 80 (12) | |
| Graft interposition | 33 (4) | 11 (6) | 22 (3) | |
| Ascending‐to‐descending bypass graft | 7 (1) | 2 (1) | 5 (1) | |
| Unknown | 172 (20) | 47 (27) | 125 (18) | |
| Intervention for re‐CoA, n (%) | 178 (19) | 29 (15) | 149 (20) | 0.12 |
| Associated congenital defects, n (%) | ||||
| Bicuspid aortic valve | 519 (56) | 130 (68) | 389 (53) | <0.001 |
| Patent ductus arteriosus | 141 (15) | 25 (13) | 116 (16) | 0.37 |
| Ventricular septal defect | 208 (23) | 35 (18) | 173 (24) | 0.12 |
| Atrial septal defect | 53 (6) | 10 (5) | 43 (6) | 0.86 |
| Patent foramen ovale | 22 (2) | 3 (2) | 19 (3) | 0.60 |
| Turner syndrome | 24 (3) | 4 (2) | 20 (3) | 0.80 |
| Left‐sided MHV, n (%) | 60 (7) | 36 (19) | 24 (3) | <0.001 |
| Prior cardiovascular events, n (%) | ||||
| Prior CAD | 10 (1) | 5 (3) | 5 (1) | 0.04 |
| Prior stroke/TIA | 20 (2) | 10 (5) | 10 (1) | 0.003 |
| Prior arrhythmia | 49 (5) | 18 (9) | 31 (4) | 0.01 |
| Systolic BP, mean±SD, mm Hg | 135±19 | 140±20 | 133±18 | <0.001 |
| Diastolic BP, mean±SD, mm Hg | 76±11 | 79±11 | 75±11 | <0.001 |
| Hypertension, n (%) | 522 (57) | 143 (75) | 379 (52) | <0.001 |
| Use of any antihypertensive medication, n (%) | 299 (33) | 91 (48) | 208 (29) | <0.001 |
| Other cardiovascular risk factors | ||||
| BMI, mean±SD, kg/m2 | 24.0±4.5 | 25.6±5.4 | 23.6±4.1 | <0.001 |
| Hypercholesterolemia, n (%) | 85 (9) | 38 (20) | 47 (6) | <0.001 |
| Diabetes, n (%) | 21 (2) | 7 (4) | 14 (2) | 0.17 |
| Cigarette smoking, n (%) | 169 (18) | 49 (26) | 120 (17) | 0.005 |
| Family history of premature CVD, n (%) | 55 (6) | 16 (8) | 39 (5) | 0.12 |
| eGFR <60 mL/min per 1.73 m2, n (%) | 29 (3) | 10 (5) | 19 (3) | 0.10 |
| LVEF <40%, n (%) | 7 (1) | 4 (2) | 3 (0.4) | 0.04 |
| LV mass index, mean±SD, g/m2 | 94±31 | 111±36 | 90±28 | <0.001 |
| LV hypertrophy, n (%) | 249 (27) | 89 (47) | 160 (22) | <0.001 |
| Follow‐up duration, mean±SD, y | 9.3±5.1 | 11.3±4.5 | 8.7±5.1 | <0.001 |
BMI indicates body mass index; BP, blood pressure; CAD, coronary artery disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; LV, left ventricular; LVEF, left ventricular ejection fraction; MHV, mechanical heart valve; and TIA, transient ischemic attack.
Indicates the difference between patients who developed a cardiovascular event vs patients who did not, as determined by the independent samples t test, Mann‐Whitney U test, or Fisher exact test, where appropriate.
Among patients with surgery as initial coarctation of the aorta (CoA) repair (n=863).
Incidence of Cardiovascular Events
Table 2 provides an overview of the observed cardiovascular events. In 191 patients (21%), at least 1 cardiovascular event occurred. In total, 270 events occurred, of which arrhythmias and aortic complications were most frequent. A substantially higher incidence of events was observed in patients with important comorbidity, ie, the presence of a bicuspid aortic valve, MHV, and/or Turner syndrome. The estimated yearly risk of developing a cardiovascular event increased from 0.8% (95% CI, 0.6%–1.1%) at 20 years to 8.2% (95% CI, 5.5%–12.1%) at 70 years (Figure 1A). Figure 1B presents a yearly risk estimation for various types of cardiovascular events, with detailed information supplied in Table S1. Fourteen CoA site aneurysms were observed. In these patients, initial repair consisted of end‐to‐end anastomosis in 4 patients, patch angioplasty in 3 patients, balloon angioplasty (without subsequent surgical repair) in 2 patients, and an unknown surgical technique in 5 patients.
Table 2.
Overview of Cardiovascular Events
| All patients (n=920) | Low‐risk CoA* (n=376) | High‐risk CoA † (n=544) | ||||
|---|---|---|---|---|---|---|
| No. of cases | Incidence per 1000 patient‐y | No. of cases | Incidence per 1000 patient‐y | No. of cases | Incidence per 1000 patient‐y | |
| CAD | 14 | 1.6 | 4 | 1.3 | 10 | 1.9 |
| Myocardial infarction | 6 | 0.7 | 1 | 0.3 | 5 | 0.9 |
| Coronary revascularization | 6 | 0.7 | 2 | 0.6 | 4 | 0.7 |
| Stable angina, medically treated | 2 | 0.2 | 1 | 0.3 | 1 | 0.2 |
| Stroke/TIA | 34 | 4.0 | 9 | 2.8 | 25 | 4.7 |
| Ischemic stroke | 18 | 2.1 | 5 | 1.6 | 13 | 2.4 |
| Hemorrhagic stroke, intracerebral | 1 | 0.1 | 1 | 0.3 | 0 | 0 |
| Hemorrhagic stroke, subarachnoidal | 1 | 0.1 | 0 | 0 | 1 | 0.2 |
| TIA | 14 | 1.6 | 3 | 0.9 | 11 | 2.1 |
| Aortic complication | 84 | 9.9 | 16 | 5.1 | 68 | 12.7 |
| Aneurysm | 77 | 9.0 | 15 | 4.7 | 62 | 11.6 |
| Dissection | 7 | 0.8 | 1 | 0.3 | 6 | 1.1 |
| Arrhythmia | 84 | 9.9 | 22 | 6.9 | 62 | 11.6 |
| Supraventricular arrhythmia | 58 | 6.8 | 15 | 4.7 | 43 | 8.0 |
| Ventricular arrhythmia | 18 | 2.1 | 4 | 1.3 | 14 | 2.6 |
| Conduction disturbance | 8 | 0.9 | 3 | 0.9 | 5 | 0.9 |
| Heart failure hospitalization | 15 | 1.8 | 6 | 1.9 | 9 | 1.7 |
| Endocarditis | 15 | 1.8 | 2 | 0.6 | 13 | 2.4 |
| Cardiovascular death | 24 | 2.8 | 8 | 2.5 | 16 | 3.0 |
| Total cardiovascular events | 270 | 31.7 | 67 | 21.1 | 203 | 38.0 |
| No. of individual patients with cardiovascular event | 191 | NA | 48 | NA | 143 | NA |
CAD indicates coronary artery disease; NA, not applicable; and TIA, transient ischemic attack.
Includes patients with isolated coarctation of the aorta (CoA) or patients with any of the following associated lesions: closed or small ventricular septal defect, atrial septal defect, patent foramen ovale, or patent ductus arteriosus.
Includes patients with a bicuspid aortic valve, left‐sided mechanical heart valve, and/or Turner syndrome.
Figure 1. Estimated yearly risk of a cardiovascular event (A) and specific types of cardiovascular events (B) by age.
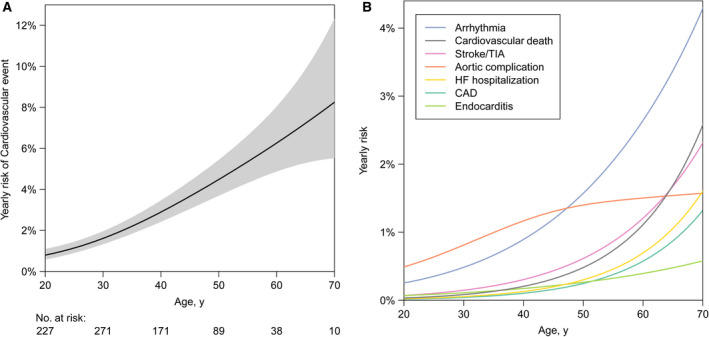
Hazard functions and corresponding 95% CIs are smoothed estimates based on B‐splines. Detailed information regarding the hazard of specific event types is provided in Table S1. CAD indicates coronary artery disease; HF, heart failure; and TIA, transient ischemic attack.
Among the 60 patients with a left‐sided MHV at baseline, 36 patients (60%) experienced at least 1 cardiovascular event, as shown in Table S2. In this group, there were 24 arrhythmias (of which 16 supraventricular arrhythmias), 11 cases of ischemic stroke or TIA, 7 cardiovascular deaths, 6 heart failure hospitalizations, 5 aortic complications, 4 cases of endocarditis, and 2 cases of CAD. Of the 4 cases of endocarditis in patients with a left‐sided MHV, 2 had echocardiographic evidence of prosthetic valve endocarditis. In the 2 other cases, there was a clinical suspicion for prosthetic valve endocarditis, despite the absence of evidence on cardiac imaging.
Factors Associated With the Risk of Cardiovascular Events
The results of Cox proportional hazards regression are presented in Table 3. In univariable analysis, factors associated with an increased risk of cardiovascular events were older age at initial CoA repair (hazard ratio [HR], 1.018; 95% CI, 1.003–1.033 [P=0.02]), bicuspid aortic valve (HR, 1.45; 95% CI, 1.07–1.96 [P=0.02]), increased body mass index (HR, 1.04; 95% CI, 1.01–1.07 [P=0.004]), and elevated LV mass index (HR, 1.010; 95% CI, 1.007–1.014 [P<0.001]), whereas female sex (HR, 0.59; 95% CI, 0.44–0.81 [P=0.001]) was associated with a decreased risk. In multivariable analysis, older age at initial CoA repair (HR, 1.017; 95% CI, 1.000–1.033 [P=0.048]) and elevated LV mass index (HR, 1.009; 95% CI, 1.005–1.013 [P<0.001]) were identified as independent risk factors for the occurrence of cardiovascular events. The use of restrictive cubic splines for continuous variables did not result in improvement of the model fit. Similar associations were observed when patients with a left‐sided MHV were excluded from the analysis. The unadjusted difference in cardiovascular event incidence between patients with and without LV hypertrophy at baseline is graphically shown in Figure 2. The risk of cardiovascular events appeared to increase when initial repair was performed beyond 10 years of age, although this was not statistically significant (P=0.08; Figure S1).
Table 3.
Results of Cox Proportional Hazards Regression to Identify Factors Associated With the Risk of Cardiovascular Events
| Cardiovascular event | ||||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Female sex | 0.59 (0.44–0.81) | 0.001 | 0.82 (0.59–1.15) | 0.25 |
| Age at initial CoA repair, y | 1.018 (1.003–1.033) | 0.02 | 1.017 (1.000–1.033) | 0.048 |
| Bicuspid aortic valve | 1.45 (1.07–1.96) | 0.02 | 1.34 (0.98–1.83) | 0.07 |
| Ventricular septal defect | 0.98 (0.67–1.42) | 0.92 | 1.04 (0.71–1.54) | 0.83 |
| Prior CAD | 1.98 (0.76–5.14) | 0.16 | 2.18 (0.82–5.81) | 0.12 |
| Prior stroke/TIA | 1.74 (0.88–3.41) | 0.11 | 1.74 (0.86–3.53) | 0.12 |
| Prior arrhythmia | 1.55 (0.94–2.54) | 0.09 | 1.46 (0.87–2.45) | 0.15 |
| Systolic BP, mm Hg | 1.006 (0.999–1.014) | 0.11 | 1.004 (0.995–1.013) | 0.37 |
| Diastolic BP, mm Hg | 1.012 (0.998–1.026) | 0.09 | 1.010 (0.994–1.026) | 0.21 |
| BMI, kg/m2 | 1.04 (1.01–1.07) | 0.004 | 1.03 (1.00–1.06) | 0.08 |
| Hypercholesterolemia | 1.36 (0.93–2.00) | 0.11 | 1.41 (0.94–2.11) | 0.10 |
| Diabetes | 0.67 (0.30–1.51) | 0.33 | 0.51 (0.21–1.24) | 0.14 |
| Cigarette smoking | 1.30 (0.94–1.81) | 0.11 | 1.14 (0.81–1.61) | 0.46 |
| Family history of premature CVD | 1.37 (0.82–2.29) | 0.23 | 1.21 (0.70–2.11) | 0.49 |
| LV mass index, g/m2 | 1.010 (1.007–1.014) | <0.001 | 1.009 (1.005–1.013) | <0.001 |
A total of 920 patients were included in the analysis, of whom 191 patients developed a cardiovascular event. BMI indicates body mass index; BP, blood pressure; CoA, coarctation of the aorta; CAD, coronary artery disease;, cardiovascular disease; HR, hazard ratio; LV, left ventricular; and TIA, transient ischemic attack.
Figure 2. Freedom from a cardiovascular event by the presence or absence of left ventricular hypertrophy at baseline.
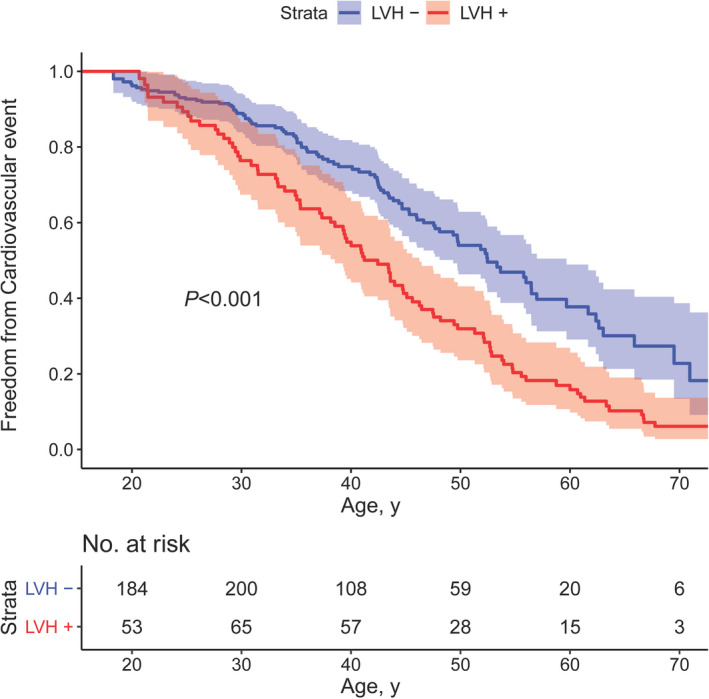
Kaplan‐Meier graph showing the freedom from a cardiovascular event in patients with and without left ventricular hypertrophy (LVH) at baseline (LVH+ and LVH–, respectively) with corresponding 95% CIs.
Sensitivity analyses showed that the addition of age at baseline did not result in substantial changes to the multivariable model (Table S3). In this model, age at initial CoA repair (HR, 1.021; 95% CI, 1.004–1.039 [P=0.01]) and LV mass index (HR, 1.009; 95% CI, 1.005–1.013 [P<0.001]) remained the only factors associated with the occurrence of cardiovascular events.
Risk of Mortality
A total of 42 patients died during the study period, of which 24 patients (57%) were attributed to a cardiovascular cause (Table S4). As illustrated by Figure 3, all‐cause mortality was 3.3 times higher than expected based on a cohort from the Dutch general population matched for age, sex, year of study entry, and observation time (standardized mortality ratio, 3.3; 95% CI, 2.3–4.4 [P<0.001]). When the analysis was limited to patients with isolated CoA (n=215), ie, patients without other congenital defects or left‐sided MHV, the mortality difference persisted (standardized mortality ratio, 4.5; 95% CI, 2.5–7.3 [P<0.001]).
Figure 3. Overall survival compared with a cohort from the Dutch general population (reference).
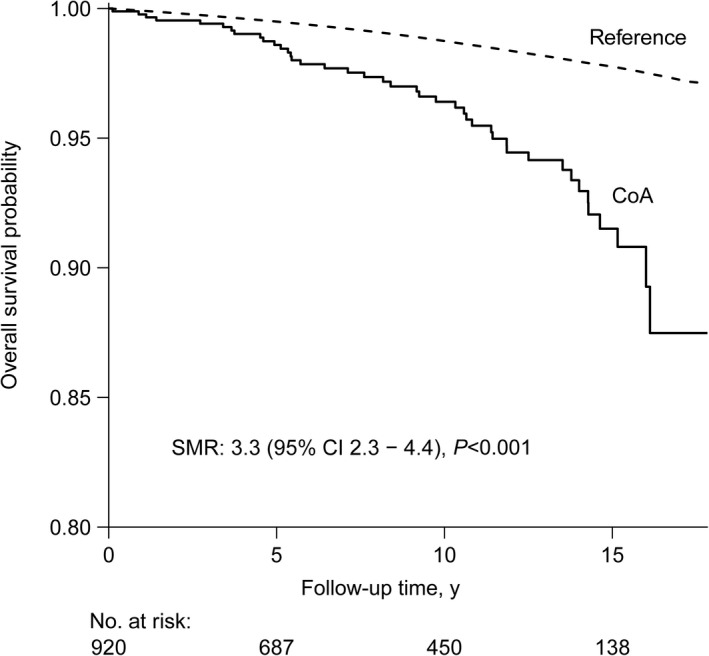
The reference cohort was matched for age, sex, year of study entry, and observation time. The standardized mortality ratio (SMR) is provided, which represents the ratio between the observed number of deaths in the coarctation of the aorta (CoA) population and the expected number of deaths based on the reference population.
Discussion
Repaired CoA is often regarded as a relatively benign condition. However, the cardiovascular burden of CoA in adulthood has not been well characterized, as most previous studies were small or focused on particular complications. To our knowledge, we present the long‐term clinical outcome of the largest prospective cohort of this patient population to date. In our cohort, consisting of patients with a median age of 24 years who were followed for a mean of 9.3 years, the most important findings were that: (1) 21% of patients developed at least 1 cardiovascular event with particularly high incidences of aortic complications and arrhythmias; (2) older age at initial CoA repair and elevated LV mass index were independently associated with an increased risk of cardiovascular events; and (3) all‐cause mortality was 3.3 times higher compared with an age‐ and sex‐matched cohort from the Dutch general population.
Although surgical and transcatheter techniques have improved drastically over the past decades, the observed all‐cause mortality in our cohort was more than 3‐fold higher than the population‐based expected mortality. This is in accordance with other recent findings. 15 The majority of these deaths were of cardiovascular origin. However, increased mortality is not the only concern in this patient cohort, as cardiovascular morbidity is also frequent. The incidence of cardiovascular events rapidly increased with age, up to a yearly incidence of 8% in patients aged 70 years. This is consistent with previous results in adults with CHD, who show a substantially higher burden of morbidity beyond the sixth decade. 17 Aortic aneurysm formation was one of the most common cardiovascular complications in our study. Although aneurysms may occur at the CoA repair site, the majority were located in the ascending aorta, which can largely be attributed to the high prevalence of an associated bicuspid aortic valve. However, it is likely that CoA contributes to ascending aortic dilatation, as the prevalence of ascending aortic complications was previously reported to be significantly higher in patients with both a bicuspid aortic valve and CoA compared with patients with an isolated bicuspid aortic valve. 18 In addition, arrhythmias were frequently observed in this CoA cohort, particularly supraventricular arrhythmias. The impact of arrhythmias on morbidity and mortality in CoA should not be underestimated. The incidence of atrial fibrillation in the general population younger than 50 years is <0.5 per 1000 patient‐years, whereas in our CoA cohort, the incidence of supraventricular arrhythmias was more than 10‐fold higher (6.8 per 1000 patient‐years). 19 The frequent presence of hypertension in patients with CoA may contribute to this high incidence, as a history of hypertension is strongly associated with the development of atrial fibrillation. 19 Furthermore, in patients with CoA already known to have supraventricular arrhythmias, hypertension may have a substantial impact on the risk of stroke and, consequently, the need for prolonged anticoagulant therapy. A previous large study showed that supraventricular arrhythmias were associated with increased all‐cause and cardiovascular mortality in adults with CHD, which emphasizes the prognostic consequences of this type of arrhythmia. 20
Prior studies have identified CAD as the most common cause of late death in patients with CoA. 4 , 21 However, the incidence of CAD in our cohort was significantly lower in comparison to cerebrovascular events. In particular, ischemic cerebrovascular events were frequent. Interestingly, we observed only 2 cases of hemorrhagic stroke, even though intracranial aneurysms have been reported in ≈10% of adults with CoA. 22 The low incidence of hemorrhagic stroke in our study suggests that intracranial aneurysms may either be less prevalent than previously thought or may carry a low risk of rupture, at least in this relatively young CoA population. Hence, our results do not support routine screening for intracranial aneurysms in patients with CoA, which has been subject of debate for decades. 3 We hypothesize that the high incidence of ischemic stroke is likely related to hypertension in patients with CoA post‐repair, even though hypertension is a stronger predictor for hemorrhagic stroke than for ischemic stroke. 23 In the general population, the incidence of ischemic stroke is 6‐ to 7‐fold higher compared with hemorrhagic stroke, which is similar to our findings. 24 In patients with native CoA, who often have more severe hypertension, the distribution of stroke subtypes may be different.
Considering the increased risk of acquired CVD in adults with CoA, it is important to identify patients who are at the highest risk. Our analyses show that LV mass index is strongly associated with the occurrence of cardiovascular events, which is consistent with the known prognostic importance of LV mass index in the general population. 25 Interestingly, this association was observed irrespectively of BP. This is in line with recent findings indicating that LV mass index is elevated in patients with CoA compared with controls despite similar systolic BP. 26 Perhaps, BP should not be the only measure to guide the antihypertensive regimen in these patients. Other arterial load indices, including backward compression waves and total arterial compliance, have been shown to better correlate with LV mass index than systolic BP. 26 , 27 The BP response to exercise may also play a role in assessing the need for antihypertensive therapy. 28 However, the therapeutic and prognostic implications of a hypertensive response to exercise, particularly in patients with a normal resting BP, are not yet fully elucidated. 29 Naturally, recoarctation should be ruled out as a cause of LV overload. Recoarctation can be effectively treated by stent implantation, which has been shown to result in substantial hemodynamic improvement during medium‐term follow‐up. 30 Remarkably, both systolic and diastolic BP did not correlate with the risk of cardiovascular events. This may be caused by the fact that office BP measurements were used, while 24‐hour ambulatory BP monitoring correlates more accurately with LV afterload and remodeling indices in patients with CoA and is superior in predicting cardiovascular events in the general population. 31 , 32 Although the importance of ambulatory BP monitoring has become widely recognized over the past years, this method was not yet routinely performed at the initiation of this study.
Older age at initial CoA repair was identified as another risk factor for the occurrence of cardiovascular events in our study. It has been previously reported that late CoA repair contributes to the development of vascular dysfunction and hypertension. 4 , 33 , 34 In contrast, another study showed no association between the time of repair and arterial wall stiffness. 35 Despite these conflicting results, several studies have highlighted the prognostic impact of age at initial repair on mortality. 4 , 14 , 21 Particularly, mortality caused by CAD is frequent among patients with late CoA repair. 21 Although intervening at a young age may increase the need for subsequent reinterventions, our findings underline the importance of early repair to prevent late cardiovascular sequelae. Our data suggest a particular increase in cardiovascular risk when repair is performed beyond the age of 10 years. To ensure timely detection of CoA, careful attention should be paid to hypertension in childhood, which is still frequently undiagnosed. 36
In addition, our results indicate that patients with CoA and a concomitant aortic and/or mitral MHV represent a group highly susceptible to cardiovascular complications, as 60% of these patients experienced at least 1 cardiovascular event and the events in this group accounted for 19% of all events observed in the study. In particular, thromboembolic events (ischemic stroke, TIA), endocarditis, and supraventricular arrhythmias, which are complications known to be associated with left‐sided MHVs, were considerably more frequent in these patients compared with patients without a left‐sided MHV. 37 Interestingly, the incidence of stroke/TIA (1.8% per patient‐year) and endocarditis (0.7% per patient‐year) in patients with MHV were comparable to those reported in the literature. 37 , 38 These findings suggest that the risk of complications in patients with CoA who have a left‐sided MHV is largely determined by the MHV, whereas the additional risk associated with the repaired CoA appears to be limited.
The results of this study provide clinicians insight into the risk of (specific) cardiovascular events in the adult CoA population and how clinical characteristics of an individual patient may affect this risk. The high incidence of acquired CVD necessitates regular follow‐up after CoA repair, preferably in a specialized center for adults with CHD. Furthermore, a better understanding of the mechanisms responsible for the increased susceptibility of cardiovascular complications is needed. This may lay the foundation for improved preventive strategies.
Limitations
The incidence of cardiovascular events in our study may be underestimated, as patients may have presented with a cardiovascular event in a different (nonparticipating) medical center, which was therefore unknown to the investigators. Additionally, traditional risk factors for CVD were extracted from the medical correspondence, which were infrequently reported in some cases. This study may also be limited by the fact that the primary end point was a composite of various types of cardiovascular events with differences in pathophysiology. However, it was not considered feasible to perform analyses on specific types of cardiovascular events because of limited power and potential overfitting.
Conclusions
A substantial burden of CVD was observed in this large, prospective cohort of adults with prior CoA repair, which illustrates that repaired CoA is not a condition as benign as previously assumed. Aortic complications and arrhythmias were the most common cardiovascular events. Independent risk factors for the occurrence of cardiovascular events were older age at initial CoA repair and elevated LV mass index. A 3.3‐fold increase in all‐cause mortality was observed compared with an age‐ and sex‐matched cohort from the general population. These results advocate stringent follow‐up after repair of CoA, preferably in a specialized center for adult CHD, and underline the importance of improving preventive strategies in this high‐risk patient population.
Sources of Funding
None.
Disclosures
Dr Krings serves as a consultant for Edwards Lifesciences and has received other support from Siemens. The remaining authors have no disclosures to report.
Supporting information
Table S1–S4
Figure S1
Acknowledgments
The authors thank Lia Engelfriet‐Rijk, Sylvia Mantels, and Wybo Hoekstra for their dedicated contribution to the project and the CONCOR registry.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023199
For Sources of Funding and Disclosures, see page 9.
References
- 1. Torok RD, Campbell MJ, Fleming GA, Hill KD. Coarctation of the aorta: management from infancy to adulthood. World J Cardiol. 2015;7:765–775. doi: 10.4330/wjc.v7.i11.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vriend JW, Mulder BJ. Late complications in patients after repair of aortic coarctation: implications for management. Int J Cardiol. 2005;101:399–406. doi: 10.1016/j.ijcard.2004.03.056 [DOI] [PubMed] [Google Scholar]
- 3. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. doi: 10.1161/CIR.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 4. Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC. Coarctation of the aorta. Long‐term follow‐up and prediction of outcome after surgical correction. Circulation. 1989;80:840–845. doi: 10.1161/01.CIR.80.4.840 [DOI] [PubMed] [Google Scholar]
- 5. Choudhary P, Canniffe C, Jackson DJ, Tanous D, Walsh K, Celermajer DS. Late outcomes in adults with coarctation of the aorta. Heart. 2015;101:1190–1195. doi: 10.1136/heartjnl-2014-307035 [DOI] [PubMed] [Google Scholar]
- 6. Pickard SS, Gauvreau K, Gurvitz M, Gagne JJ, Opotowsky AR, Jenkins KJ, Prakash A. Stroke in adults with coarctation of the aorta: a national population‐based study. J Am Heart Assoc. 2018;7:e009072. doi: 10.1161/JAHA.118.009072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gurvitz M, Burns KM, Brindis R, Broberg CS, Daniels CJ, Fuller SM, Honein MA, Khairy P, Kuehl KS, Landzberg MJ, et al. Emerging research directions in adult congenital heart disease: a report from an NHLBI/ACHA working group. J Am Coll Cardiol. 2016;67:1956–1964. doi: 10.1016/j.jacc.2016.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown ML, Burkhart HM, Connolly HM, Dearani JA, Cetta F, Li Z, Oliver WC, Warnes CA, Schaff HV. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62:1020–1025. doi: 10.1016/j.jacc.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 9. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 10. Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, Gottdiener J, Haluska B, Ofili E, Segers P, et al. Recommendations on the use of echocardiography in adult hypertension: a Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28:727–754. doi: 10.1016/j.echo.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 11. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121:e266–369. doi: 10.1016/j.jacc.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 12. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 13. Bambul Heck P, Pabst von Ohain J, Kaemmerer H, Ewert P, Hager A. Survival and cardiovascular events after coarctation‐repair in long‐term follow‐up (COAFU): predictive value of clinical variables. Int J Cardiol. 2017;228:347–351. doi: 10.1016/j.ijcard.2016.11.164 [DOI] [PubMed] [Google Scholar]
- 14. Hager A, Kanz S, Kaemmerer H, Schreiber C, Hess J. Coarctation Long‐term Assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg. 2007;134:738–745. doi: 10.1016/j.jtcvs.2007.04.027 [DOI] [PubMed] [Google Scholar]
- 15. Lee MGY, Babu‐Narayan SV, Kempny A, Uebing A, Montanaro C, Shore DF, d'Udekem Y, Gatzoulis MA. Long‐term mortality and cardiovascular burden for adult survivors of coarctation of the aorta. Heart. 2019;105:1190–1196. doi: 10.1136/heartjnl-2018-314257 [DOI] [PubMed] [Google Scholar]
- 16. Bodner TE. What improves with increased missing data imputations? Struct Equ Modeling. 2008;15:651–675. doi: 10.1080/10705510802339072 [DOI] [Google Scholar]
- 17. Tutarel O, Kempny A, Alonso‐Gonzalez R, Jabbour R, Li W, Uebing A, Dimopoulos K, Swan L, Gatzoulis MA, Diller GP. Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J. 2014;35:725–732. doi: 10.1093/eurheartj/eht257 [DOI] [PubMed] [Google Scholar]
- 18. Oliver JM, Alonso‐Gonzalez R, Gonzalez AE, Gallego P, Sanchez‐Recalde A, Cuesta E, Aroca A, Lopez‐Sendon JL. Risk of aortic root or ascending aorta complications in patients with bicuspid aortic valve with and without coarctation of the aorta. Am J Cardiol. 2009;104:1001–1006. doi: 10.1016/j.amjcard.2009.05.045 [DOI] [PubMed] [Google Scholar]
- 19. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9 [DOI] [PubMed] [Google Scholar]
- 20. Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, van Dijk AP, Vliegen HW, Grobbee DE, Mulder BJ. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220–1229. doi: 10.1093/eurheartj/ehq032 [DOI] [PubMed] [Google Scholar]
- 21. Toro‐Salazar OH, Steinberger J, Thomas W, Rocchini AP, Carpenter B, Moller JH. Long‐term follow‐up of patients after coarctation of the aorta repair. Am J Cardiol. 2002;89:541–547. doi: 10.1016/S0002-9149(01)02293-7 [DOI] [PubMed] [Google Scholar]
- 22. Connolly HM, Huston J, Brown RD, Warnes CA, Ammash NM, Tajik AJ. Intracranial aneurysms in patients with coarctation of the aorta: a prospective magnetic resonance angiographic study of 100 patients. Mayo Clin Proc. 2003;78:1491–1499. doi: 10.4065/78.12.1491 [DOI] [PubMed] [Google Scholar]
- 23. Zia E, Hedblad B, Pessah‐Rasmussen H, Berglund G, Janzon L, Engström G. Blood pressure in relation to the incidence of cerebral infarction and intracerebral hemorrhage. Hypertensive hemorrhage: debated nomenclature is still relevant. Stroke. 2007;38:2681–2685. doi: 10.1161/STROKEAHA.106.479725 [DOI] [PubMed] [Google Scholar]
- 24. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 25. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203 [DOI] [PubMed] [Google Scholar]
- 26. Egbe AC, Reddy YN, Obokata M, Borlaug BA. Doppler‐derived arterial load indices better reflect left ventricular afterload than systolic blood pressure in coarctation of aorta. Circ Cardiovasc Imaging. 2020;13:e009672. doi: 10.1161/CIRCIMAGING.119.009672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quail MA, Short R, Pandya B, Steeden JA, Khushnood A, Taylor AM, Segers P, Muthurangu V. Abnormal wave reflections and left ventricular hypertrophy late after coarctation of the aorta repair. Hypertension. 2017;69:501–509. doi: 10.1161/HYPERTENSIONAHA.116.08763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luijendijk P, Bouma BJ, Vriend JW, Vliegen HW, Groenink M, Mulder BJ. Usefulness of exercise‐induced hypertension as predictor of chronic hypertension in adults after operative therapy for aortic isthmic coarctation in childhood. Am J Cardiol. 2011;108:435–439. doi: 10.1016/j.amjcard.2011.03.063 [DOI] [PubMed] [Google Scholar]
- 29. Baumgartner H, De Backer J, Babu‐Narayan SV, Budts W, Chessa M, Diller GP, lung B, Kluin J, Lang IM, Meijboom F, et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554 [DOI] [PubMed] [Google Scholar]
- 30. Meijs TA, Warmerdam EG, Slieker MG, Krings GJ, Molenschot MMC, Meijboom FJ, Sieswerda GT, Doevendans PA, Bouma BJ, de Winter RJ, et al. Medium‐term systemic blood pressure after stenting of aortic coarctation: a systematic review and meta‐analysis. Heart. 2019;105:1464–1470. doi: 10.1136/heartjnl-2019-314965 [DOI] [PubMed] [Google Scholar]
- 31. Egbe AC, Miranda WR, Bonnichsen CR, Warnes CA, Connolly HM. Potential benefits of ambulatory blood pressure monitoring in coarctation of aorta. J Am Coll Cardiol. 2020;75:2089–2090. doi: 10.1016/j.jacc.2020.02.053 [DOI] [PubMed] [Google Scholar]
- 32. Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273 [DOI] [PubMed] [Google Scholar]
- 33. de Divitiis M, Pilla C, Kattenhorn M, Zadinello M, Donald A, Leeson P, Wallace S, Redington A, Deanfield JE. Vascular dysfunction after repair of coarctation of the aorta: impact of early surgery. Circulation. 2001;104:I165–I170. doi: 10.1161/hc37t1.094900 [DOI] [PubMed] [Google Scholar]
- 34. Heger M, Willfort A, Neunteufl T, Rosenhek R, Gabriel H, Wollenek G, Wimmer M, Maurer G, Baumgartner H. Vascular dysfunction after coarctation repair is related to the age at surgery. Int J Cardiol. 2005;99:295–299. doi: 10.1016/j.ijcard.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 35. Vriend JW, de Groot E, Mulder BJ. Limited effect of early repair on carotid arterial wall stiffness in adult post‐coarctectomy patients. Int J Cardiol. 2005;100:335–336. doi: 10.1016/j.ijcard.2004.11.033 [DOI] [PubMed] [Google Scholar]
- 36. Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874–879. doi: 10.1001/jama.298.8.874 [DOI] [PubMed] [Google Scholar]
- 37. Vesey JM, Otto CM. Complications of prosthetic heart valves. Curr Cardiol Rep. 2004;6:106–111. doi: 10.1007/s11886-004-0007-x [DOI] [PubMed] [Google Scholar]
- 38. Johnson S, Stroud MR, Kratz JM, Bradley SM, Crawford FA Jr, Ikonomidis JS. Thirty‐year experience with a bileaflet mechanical valve prosthesis. J Thorac Cardiovasc Surg. 2019;157:213–222. doi: 10.1016/j.jtcvs.2018.09.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4
Figure S1


