Abstract
Background
Diabetes is a major risk factor for atrial fibrillation (AF). However, it remains unclear whether individual AF phenotype and related comorbidities differ between patients who have AF with and without diabetes. This study investigated the association of diabetes with AF phenotype and cardiac and neurological comorbidities in patients with documented AF.
Methods and Results
Participants in the multicenter Swiss‐AF (Swiss Atrial Fibrillation) study with data on diabetes and AF phenotype were eligible. Primary outcomes were parameters of AF phenotype, including AF type, AF symptoms, and quality of life (assessed by the European Quality of Life‐5 Dimensions Questionnaire [EQ‐5D]). Secondary outcomes were cardiac (ie, history of hypertension, myocardial infarction, and heart failure) and neurological (ie, history of stroke and cognitive impairment) comorbidities. The cross‐sectional association of diabetes with these outcomes was assessed using logistic and linear regression, adjusted for age, sex, and cardiovascular risk factors. We included 2411 patients with AF (27.4% women; median age, 73.6 years). Diabetes was not associated with nonparoxysmal AF (odds ratio [OR], 1.01; 95% CI, 0.81–1.27). Patients with diabetes less often perceived AF symptoms (OR, 0.74; 95% CI, 0.59–0.92) but had worse quality of life (β=−4.54; 95% CI, −6.40 to −2.68) than those without diabetes. Patients with diabetes were more likely to have cardiac (hypertension [OR, 3.04; 95% CI, 2.19–4.22], myocardial infarction [OR, 1.55; 95% CI, 1.18–2.03], heart failure [OR, 1.99; 95% CI, 1.57–2.51]) and neurological (stroke [OR, 1.39, 95% CI, 1.03–1.87], cognitive impairment [OR, 1.75, 95% CI, 1.39–2.21]) comorbidities.
Conclusions
Patients who have AF with diabetes less often perceive AF symptoms but have worse quality of life and more cardiac and neurological comorbidities than those without diabetes. This raises the question of whether patients with diabetes should be systematically screened for silent AF.
Registration
URL: https://www.clinicaltrials.gov; Unique Identifier: NCT02105844.
Keywords: atrial fibrillation, cardiovascular disease, cognitive impairment, diabetes, quality of life
Subject Categories: Atrial Fibrillation; Epidemiology; Diabetes, Type 2
Nonstandard Abbreviations and Acronyms
- EQ‐5D
European Quality of Life‐5 Dimensions Questionnaire
- MoCA
Montreal Cognitive Assessment
- ORBIT‐AF
Outcomes Registry for Better Informed Treatment of Atrial Fibrillation
- Swiss‐AF
Swiss Atrial Fibrillation
Clinical Perspective
What Is New?
In this multicenter cohort study of 2411 patients with documented atrial fibrillation, we found that patients with diabetes less often perceive atrial fibrillation symptoms, although they have worse quality of life and more cardiac and neurological comorbidities than patients without diabetes.
What Are the Clinical Implications?
Our findings further raise the question of whether patients with diabetes should be systematically screened for silent atrial fibrillation.
Patients with concomitant AF and diabetes deserve more attentive care.
Diabetes is a major risk factor for atrial fibrillation (AF). 1 The underlying mechanisms linking diabetes to AF involve electrical, structural, and autonomic remodeling in the atria. 2 In addition, metabolic alterations such as insulin resistance, inflammation, and abnormal hemostasis can lead to endothelial dysfunction and atherogenesis, which further predispose to AF development. 3
Hypothetically, diabetes may also affect the individual manifestation of AF episodes. However, data are limited and previous studies have provided inconsistent results. 4 , 5 , 6 One study reported that patients with diabetes have more sustained AF episodes and more pronounced AF symptoms. 4 On the contrary, another study reported that patients with diabetes perceive AF symptoms less often than those without diabetes, 5 whereas few other studies found no association of diabetes with AF type or symptoms. 6 , 7 , 8 , 9 Moreover, the individual perception of quality of life in patients who have AF with and without diabetes is currently unknown.
Diabetes can promote the development of hypertension, myocardial infarction, and heart failure, thus creating a favorable substrate for the initiation and maintenance of AF. 10 , 11 , 12 Moreover, diabetes can predispose to neurological complications 13 , 14 and has also been incorporated within risk scores for stroke prediction (eg, CHA2DS2‐VASc or CHADS2 score) in patients with AF. 15 , 16 , 17 However, in light of the important advances in the management of diabetes over the past years, it remains unclear whether diabetes and antidiabetic medications are independently associated with cardiac and neurological comorbidities in patients with AF.
In this study, we hypothesized that AF phenotype and cardiac and neurological comorbidities differ between patients who have AF with and without diabetes. Our primary aim was to investigate the association of diabetes with parameters of AF phenotype, including AF type, AF symptoms, and quality of life. Our secondary aims were to investigate the association of diabetes with: (1) cardiac comorbidities, including history of hypertension, myocardial infarction, heart failure; and (2) neurological comorbidities, including history of stroke and cognitive impairment.
Methods
Availability of Data
Patient informed consent forms, as approved by the responsible ethics committee (Ethikkommission Nordwest‐ und Zentralschweiz), do not allow the data to be made publicly available. The participants signed a consent form, which states that their data, containing personal and medical information, are exclusively available for research institutions in an anonymized form. Researchers interested in obtaining the data for research purposes can contact the Swiss‐AF (Swiss Atrial Fibrillation) study scientific lead. Contact information is provided on the Swiss‐AF website (http://www.swissaf.ch/contact.htm). Authorization of the responsible ethics committee is mandatory before the requested data can be transferred to external research institutions.
Study Population
The Swiss‐AF study is an ongoing prospective cohort study including 2415 patients with a history of documented AF. The study protocol and objectives have been previously described in detail. 18 Study participants were enrolled between 2014 and 2017 among 14 centers in Switzerland. Patients were recruited by comprehensive screening in participating hospitals and/or by contacting general practitioners in the area. Patients were excluded if they had secondary forms of AF, had acute illness within the past 4 weeks, or were unable to provide informed consent. All data were collected in a standardized manner by trained study personnel. At enrollment, participants underwent a clinical examination, blood sampling, cognitive assessment, quality‐of‐life assessment, and 5‐minute resting 16‐lead ECG. Detailed information on personal characteristics, risk factors, and comorbidities was obtained through standardized case report forms, which have been previously validated. 18 , 19 The Swiss AF study was approved by local research ethics committees in all participating sites. Each study participant provided written informed consent. For the current analysis, participants of the Swiss‐AF study with complete data available on diabetes and AF phenotype were considered eligible (Figures S1 and S2).
Assessment of Diabetes and Antidiabetic Medication
Diabetes was defined on the basis of documented medical history and use of antidiabetic treatment. Participants were classified according to the use of antidiabetic medications into: patients without diabetes, patients with noninsulin‐requiring diabetes, and patients with insulin‐requiring diabetes. Participants were classified as having noninsulin‐requiring diabetes if they had a diagnosis of diabetes and were on a diet and/or taking antidiabetic medications other than insulin/insulin analogues. Participants were classified as having insulin‐requiring diabetes if they had a diagnosis of diabetes and were taking insulin/insulin analogues.
Assessment of AF Phenotype
AF type was determined by the local study investigator during the baseline visit on the basis of all available clinical patient data over the years before enrollment, documented by medical records, ECG, and/or rhythm monitoring device. AF type was classified into paroxysmal, persistent, and permanent AF according to the guidelines of the European Society of Cardiology. 20 Paroxysmal AF was defined as self‐terminating AF lasting <7 days and that did not require cardioversion and that was documented at least twice within the past 5 years. 20 Persistent AF was defined as AF sustained for at least 7 days and/or AF requiring cardioversion, documented within the past 5 years by ECG or rhythm monitoring devices. 20 Permanent AF was defined as AF in which cardioversion therapy failed or was not attempted. 20 To assess the association of diabetes with self‐terminating versus nonself‐terminating AF, the study participants were categorized as having paroxysmal and nonparoxysmal AF.
Participants provided detailed information on AF‐related symptoms, including palpitations, dizziness, chest pain, exercise intolerance, dyspnea, tiredness, syncope, or other symptoms.
Quality of life was measured based on the European Quality of Life‐5 Dimensions Questionnaire (EQ‐5D), which is a widely used, nondisease‐specific instrument. 18 , 21 EQ‐5D was composed of 5 domains, including mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. For each of the 5 domains, participants selected 1 of the following responses: “no problems,” “some problems,” or “extreme problems.” Moreover, the instrument includes a visual analogue scale, on which participants score their health state between 0 (worst imaginable health state) and 100 (best imaginable health state). 22 Hence, the score ranges from 0 to 100, with higher values indicating better quality of life.
Assessment of Cardiac and Neurological Comorbidities
Information on the history of hypertension, myocardial infarction, and heart failure was collected from patients’ reports and medical records. History of heart failure was clinically defined as a syndrome in which patients had typical symptoms (eg, breathlessness, ankle swelling, and fatigue) and typical signs (eg, elevated jugular venous pressure, pulmonary crackles, and displaced apex beat) related to abnormalities of cardiac structure or function.
Information on the history of stroke was collected from the patients’ reports and verified by medical records. Neurocognitive function was assessed by calculating the Montreal Cognitive Assessment (MoCA) score. MoCA score evaluated various domains of cognitive and executive functions, including visuospatial ability, memory, orientation, abstraction, language, mental flexibility, and attention. 18 , 23 Each patient could obtain a minimum of 0 points and a maximum of 30 points, with higher scores indicating a better neurocognitive function. For those who achieved <30 points and had ≤12 years of education, 1 point was added to the MoCA total score. 18 Cognitive impairment was defined as a MoCA score of ≤25. 23
Additional Measurements
Smoking status was classified as current, former, and never smoking. Body weight was obtained from each participant in light clothing and without shoes using a calibrated device. Body height was measured without shoes using a calibrated device. Body mass index was calculated as weight in kilograms divided by height in meters squared. Extensive information on medication intake, such as use of anticoagulation medications (including vitamin K antagonists and nonvitamin K antagonists), antiarrhythmic medications (including amiodarone, dronedarone, sotalol, flecainide, and propafenone), and β‐blockers, was obtained from the patients` reports and verified by medical records. Additional information on prior procedures, including history of pulmonary vein isolation and electrical cardioversion, was obtained. Rhythm control intervention was defined as either a pulmonary vein isolation and/or electrical cardioversion and/or use of antiarrhythmic medications, which altogether represent the most effective rhythm control intervention currently available. We also calculated CHA2DS2‐VASc score, which includes congestive heart failure; hypertension; age ≥75 years; diabetes; prior stroke, transient ischemic attack, or thromboembolism; vascular disease; age 65 to 74 years; and sex (female). In a subsample of our population, left ventricular ejection fraction (LVEF) and left atrial size (diameter in parasternal long axis) were measured by transthoracic echocardiography, which was performed within the last 3 months before the patients’ enrollment.
Statistical Analysis
Baseline characteristics were stratified by absence or presence of diabetes. Categorical variables were presented as numbers (percentages), and between‐group differences were compared using Pearson chi‐square tests. Continuous variables were presented as mean (±SD) or median (interquartile range), and between‐group differences were compared using Wilcoxon rank sum tests.
We used logistic regression to cross‐sectionally investigate the association of diabetes (no versus yes) and antidiabetic medication (no diabetes versus noninsulin‐requiring diabetes versus insulin‐requiring diabetes) with AF symptoms (no versus yes), AF type (paroxysmal versus nonparoxysmal), history of hypertension (no versus yes), history of myocardial infarction (no versus yes), history of heart failure (no versus yes), history of stroke (no versus yes), and cognitive impairment (no versus yes). Odds ratios (ORs) and 95% CIs were reported. We used ordinary least‐squares linear regression to investigate the association of diabetes and antidiabetic medication with quality‐of‐life score. β Coefficients and CIs were reported.
For the analyses, 2 models were created. The first model (model 1) was adjusted for age (continuous) and sex (men versus women). The second model (model 2) was adjusted for potential confounders that were selected based on biological plausibility and previous literature. For the analyses on AF symptoms, model 2 was adjusted for age (continuous), sex (men versus women), use of β‐blockers (no versus yes), and use of antiarrhythmic medications (no versus yes). For the other analyses, model 2 was adjusted for age (continuous), sex (men versus women), smoking status (never versus former versus current), body mass index (continuous), and (in case hypertension was not the outcome) history of hypertension (no versus yes).
Assumptions for logistic regression were checked and met. Multicollinearity was examined to ensure that continuous independent variables were not closely correlated. Assumptions for linear regression were checked, and no violations were observed. The assumption of homoscedasticity was checked by examining plots of the residuals versus the fitted values. The possibility of multicollinearity was assessed by the variance inflation factor. The influential points were inspected with Cook`s distance. The assumption of independence of residuals was checked by Durbin Watson test. The assumption of normal distribution of error terms was checked by examining plots of the residuals. We checked for potential effect modification by separately adding product interaction terms of the exposure with each of the covariates of the most adjusted model, but none of the interaction terms were significant. Multiple imputations were performed in case of missing covariates. Statistical analyses were performed using SPSS version 21 (IBM) and R statistical software (rms package, R Project for Statistical Computing, Institute for Statistics and Mathematics, R Core Team, version 4.0.1).
Sensitivity Analyses
To test the robustness of our findings, we performed the following sensitivity analyses:
To evaluate the potential influence of study centers on our results, we additionally adjusted our analyses for study centers.
To account for a potential influence of AF duration on our results, we additionally adjusted our analyses for AF duration (ie, time since first documented AF episode); and we stratified our analyses by median AF duration (ie, 3.6 years).
To account for a potential influence of rhythm control strategy on our results, we additionally adjusted our analyses for rhythm control intervention.
To account for a potential influence of anticoagulation medications on our results, we additionally adjusted our analyses for use of anticoagulation medications.
We separately explored the association of diabetes with the 5 domains of the quality‐of‐life score, namely mobility (no impairment versus moderate or extreme impairment), self‐care (no impairment versus moderate or extreme impairment), usual activities (no impairment versus moderate or extreme impairment), pain/discomfort (no pain/discomfort versus moderate or extreme pain/discomfort), and anxiety/depression (no anxiety/depression versus moderate or extreme anxiety/depression).
To evaluate the impact of diabetes on cardiac structure and function, we investigated the association of diabetes and antidiabetic medication with left atrial size and LVEF, using ordinary squares linear regression.
We evaluated potential sex differences by performing the analyses in men and women separately.
Results
Of a total of 2415 patients enrolled in the Swiss‐AF cohort study, complete data on history of diabetes and AF phenotype parameters were available in 2411 (99.8%) patients (Figure S1). Of these, 420 (17.4%) had known diabetes (Table 1). The baseline characteristics of the study population are shown in Table 1. The mean age was 73.2 years; the median age was 73.6 years (interquartile range, 68.2–78.0 years); and 27.4% were women (Table 1). Compared with those without diabetes, patients with diabetes were more frequently older, men, smokers, and had a higher body mass index (Table 1). Patients with diabetes had a higher stroke risk compared with those without diabetes as estimated by median CHA2DS2‐VASc score (5 versus 3, P<0.001).
Table 1.
Baseline Characteristics of Included Participants*
| All (n=2411) | No diabetes (n=1991) | Diabetes (n=420) | P value † | |
|---|---|---|---|---|
| Age, y | 73.2 (8.4) | 73.0 (8.5) | 74.2 (7.7) | 0.02 |
| Women, n (%) | 661 (27.4) | 583 (29.3) | 78 (18.6) | <0.001 |
| Smoking, n (%) | <0.001 | |||
| Current | 175 (7.3) | 127 (6.4) | 48 (11.4) | |
| Former | 1180 (48.9) | 933 (46.9) | 247 (58.8) | |
| Never | 1056 (43.8) | 931 (46.8) | 125 (29.8) | |
| Body mass index, kg/m2 | 27.7 (4.7) | 27.2 (4.5) | 29.8 (5.2) | <0.001 |
| Systolic blood pressure, mm Hg | 133.8 (18.7) | 133.7 (18.8) | 134.6 (18.2) | 0.3 |
| Diastolic blood pressure, mm Hg | 77.3 (11.8) | 77.7 (11.7) | 75.7 (12.3) | 0.001 |
| Diabetes treatment, n (%) | ||||
| Diet alone, no antidiabetic treatment | 80 (19) | |||
| Intake of antidiabetic medication other than insulin or insulin analogue | 212 (50.5) | |||
| Intake of insulin or insulin analogue | 128 (30.5) | |||
| AF duration, y | 3.6 (0.9–8.5) | 3.5 (0.9–8.1) | 3.8 (0.9–9.9) | 0.08 |
| CHA2DS2‐VASc score | 3 (2–5) | 3 (2–4) | 5 (4–6) | <0.001 |
| CHA2DS2‐VASc score, n (%) | ||||
| 0 | 83 (3.4) | 83 (4.2) | 0 (0) | |
| 1 | 218 (9) | 218 (10.9) | 0 (0) | |
| 2 | 411 (17) | 397 (19.9) | 14 (3.3) | |
| 3 | 527 (21.9) | 469 (23.6) | 58 (13.8) | |
| 4 | 502 (20.8) | 407 (20.4) | 95 (22.6) | |
| 5 | 369 (15.3) | 256 (12.9) | 113 (26.9) | |
| 6 | 201 (8.3) | 112 (5.6) | 89 (21.2) | |
| 7 | 78 (3.2) | 42 (2.1) | 36 (8.6) | |
| 8 | 19 (0.8) | 4 (0.2) | 15 (3.6) | |
| Use of oral anticoagulation, n (%) | 2179 (90.3) | 1789 (89.9) | 389 (92.6) | 0.08 |
| Use of vitamin K antagonist | 950 (39.4) | 746 (37.5) | 204 (48.6) | <0.001 |
| Use of nonvitamin K antagonist | 1227 (50.9) | 1043 (52.4) | 184 (43.8) | 0.001 |
| Use of antiarrhythmic medications, n (%) | 516 (21.4) | 439 (22) | 77 (18.3) | 0.09 |
| Use of β‐blockers, n (%) | 1695 (70.3) | 1357 (68.2) | 338 (80.5) | <0.001 |
| Prior procedures, n (%) | ||||
| History of pulmonary vein isolation | 489 (20.3) | 443 (22.3) | 46 (11) | <0.001 |
| History of electrical cardioversion | 861 (35.7) | 712 (35.8) | 149 (35.5) | 0.9 |
| Rhythm control intervention, n (%) | 1311 (54.4) | 1111 (55.8) | 200 (47.6) | 0.002 |
| Any AF symptoms, n (%) | 1493 (61.9) | 1266 (63.6) | 227 (54) | <0.001 |
| Palpitations | 871 (36.1) | 761 (38.2) | 110 (26.2) | <0.001 |
| Dizziness | 341 (14.1) | 280 (14.1) | 61 (14.5) | 0.8 |
| Chest pain | 237 (9.8) | 190 (9.5) | 47 (11.2) | 0.3 |
| Exercise intolerance | 539 (22.4) | 464 (23.3) | 75 (17.9) | 0.01 |
| Dyspnea | 589 (24.4) | 486 (24.4) | 103 (24.5) | 0.9 |
| Tiredness | 386 (16) | 323 (16.2) | 63 (15) | 0.5 |
| Syncope | 78 (3.2) | 65 (3.3) | 13 (3.1) | 0.8 |
| Other symptoms | 336 (13.9) | 294 (14.8) | 42 (10) | 0.01 |
| AF type, n (%) | 0.07 | |||
| Paroxysmal AF | 1078 (44.7) | 907 (45.6) | 171 (40.7) | |
| Nonparoxysmal AF | 1333 (55.3) | 1084 (54.5) | 249 (59.3) | |
| Nonparoxysmal AF | ||||
| Persistent AF | 737 (30.6) | 619 (31.1) | 118 (28.1) | |
| Permanent AF | 596 (24.7) | 465 (23.4) | 131 (31.2) | |
| Quality of life | 72.1 (17.4) | 73.3 (17.0) | 66.9 (18.5) | <0.001 |
| History of hypertension, n (%) | 1684 (69.8) | 1311 (65.8) | 373 (88.8) | <0.001 |
| History of myocardial infarction, n (%) | 390 (16.2) | 284 (14.3) | 106 (25.2) | <0.001 |
| History of heart failure, n (%) | 625 (25.9) | 454 (22.8) | 171 (40.7) | <0.001 |
| Left atrial size, mm | 44.6 (7.8) | 44.3 (7.9) | 46.3 (7.2) | 0.04 |
| LVEF (%) | 54.3 (11.8) | 54.7 (11.4) | 52.2 (11.9) | 0.02 |
| LVEF <50%, n (%) | 188 (7.8) | 150 (7.5) | 38 (9) | 0.08 |
| History of stroke, n (%) | 318 (13.2) | 245 (12.3) | 73 (17.4) | 0.005 |
| Neurocognitive function (MoCA score) | 25.3 (3.1) | 25.6 (3.1) | 24.1 (3.4) | <0.001 |
Rhythm control intervention was defined as either a history of pulmonary vein isolation and/or electrical cardioversion and/or use of antiarrhythmic medications, which altogether represent the most effective rhythm control interventions currently available.
AF indicates atrial fibrillation; CHA2DS2‐VASc score includes congestive heart failure; hypertension; age ≥75 years; diabetes; prior stroke, transient ischemic attack, or thromboembolism; vascular disease; age 65 to 74 years; and sex (female); LVEF indicates left ventricular ejection fraction.
Data are presented as mean (SD) or median (interquartile range), unless otherwise specified. Data on history of heart failure and data on history of stroke were available in 2409 and 2410 participants, respectively. Data on neurocognitive function were available in 2398 participants. Echocardiographic data on indicates left atrial size and ejection fraction were available in a subsample of 476 and 711 participants, respectively.
For categorical variables, differences between groups were compared using the Pearson chi‐square test. For continuous variables, differences between groups were compared using the Wilcoxon rank sum test. The quality‐of‐life score ranges from 0 to 100, with higher values indicating better quality of life. The Montreal Cognitive Assessment (MoCA) score ranges from 0 to 30, with higher values indicating better neurocognitive function.
Diabetes and AF Phenotype
Diabetes and antidiabetic medications were not associated with AF type (Tables 2 and 3, Figure, Figure S3). Compared with patients without diabetes, patients with diabetes less often perceived AF symptoms (OR, 0.75; 95% CI, 0.61–0.94 in patients with diabetes; OR, 0.64; 95% CI, 0.44–0.92 in patients with insulin‐requiring diabetes) (Tables 2 and 3, Figure, Table S1, Figure S3).
Table 2.
Association of Diabetes With AF Phenotype*
| Model 1 | Model 2 | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Nonparoxysmal AF | 1.13 (0.91 to 1.41) | 1.01 (0.81 to 1.27) |
| Any AF symptoms | 0.75 (0.61 to 0.94) † | 0.74 (0.59 to 0.92) † |
| Palpitations | 0.65 (0.51 to 0.83) † | 0.64 (0.50 to 0.81) † |
| Dizziness | 1.12 (0.83 to 1.52) | 1.12 (0.83 to 1.52) |
| Chest pain | 1.33 (0.95 to 1.88) | 1.29 (0.91 to 1.83) |
| Exercise intolerance | 0.76 (0.58 to 0.99) † | 0.76 (0.58 to 1.01) |
| Dyspnea | 1.07 (0.84 to 1.37) | 1.04 (0.81 to 1.33) |
| Tiredness | 1.01 (0.75 to 1.36) | 1.02 (0.76 to 1.38) |
| Syncope | 1.02 (0.55 to 1.88) | 0.99 (0.54 to 1.83) |
| Other symptoms | 0.70 (0.50 to 1.00) | 0.70 (0.49 to 0.99) † |
Model 1: adjusted for age and sex.
Model 2 for outcomes “AF type” and “quality of life”: adjusted for age, sex, smoking status, body mass index, and prevalent hypertension. Model 2 for outcome “AF symptoms”: adjusted for age, sex, use of β‐blockers, and use of antiarrhythmic medications.
AF indicates atrial fibrillation; and OR, odds ratio.
The beta regression coefficients (β) indicate predicted differences in mean quality‐of‐life score between patients with diabetes and those without diabetes (reference). The quality‐of‐life score ranges from 0 to 100, with higher values indicating better quality of life.
P‐value lower than 0.05.
Table 3.
Association of Antidiabetic Medication With AF Phenotype and Cardiac and Neurological Comorbidities†
| Model 1 | Model 2 | |
|---|---|---|
| Antidiabetic medication and AF phenotype | ||
| Nonparoxysmal AF | OR (95% CI) | OR (95% CI) |
| No diabetes | 1 (Reference) | 1 (Reference) |
| Noninsulin‐requiring diabetes | 1.27 (0.99 to 1.64) | 1.15 (0.88 to 1.50) |
| Insulin‐requiring diabetes | 0.88 (0.61 to 1.26) | 0.76 (0.53 to 1.10) |
| Any AF symptoms * | OR (95% CI) | OR (95% CI) |
| No diabetes | 1 (Reference) | 1 (Reference) |
| Noninsulin‐requiring diabetes | 0.81 (0.63 to 1.05) | 0.80 (0.62 to 1.04) |
| Insulin‐requiring diabetes | 0.64 (0.44 to 0.92) ‡ | 0.61 (0.42 to 0.89) ‡ |
| Quality of life * | β (95% CI) | β (95% CI) |
| No diabetes | 0 (Reference) | 0 (Reference) |
| Noninsulin‐requiring diabetes | −5.36 (−7.46 to −3.26) ‡ | −3.62 (−5.77 to −1.48) ‡ |
| Insulin‐requiring diabetes | −8.59 (−11.6 to −5.54) ‡ | −6.65 (−9.73 to −3.57) ‡ |
| Antidiabetic medication and cardiac comorbidities | ||
| Hypertension | OR (95% CI) | OR (95% CI) |
| No diabetes | 1 (Reference) | 1 (Reference) |
| Noninsulin‐requiring diabetes | 4.82 (3.22 to 7.21) ‡ | 3.79 (2.51 to 5.71) ‡ |
| Insulin‐requiring diabetes | 2.84 (1.73 to 4.69) ‡ | 1.96 (1.17 to 3.27) ‡ |
| Myocardial infarction * | OR (95% CI) | OR (95% CI) |
| No diabetes | 1 (Reference) | 1 (Reference) |
| Noninsulin‐requiring diabetes | 1.45 (1.06 to 1.98) ‡ | 1.24 (0.90 to 1.71) |
| Insulin‐requiring diabetes | 2.71 (1.82 to 4.04) ‡ | 2.40 (1.60 to 3.62) ‡ |
| Heart failure * | OR (95% CI) | OR (95% CI) |
| No diabetes | 1 (Reference) | 1 (Reference) |
| Noninsulin‐requiring diabetes | 2.03 (1.57 to 2.64) ‡ | 1.82 (1.39 to 2.38) ‡ |
| Insulin‐requiring diabetes | 2.66 (1.84 to 3.84) | 2.44 (1.67 to 3.55) ‡ |
| Antidiabetic medication and AF‐related complications | ||
| Stroke | OR (95% CI) | OR (95% CI) |
| No diabetes | 1 (Reference) | 1 (Reference) |
| Noninsulin‐requiring diabetes | 1.54 (1.11 to 2.15) ‡ | 1.45 (1.04 to 2.04) ‡ |
| Insulin‐requiring diabetes | 1.28 (0.78 to 2.11) | 1.23 (0.74 to 2.05) |
| Cognitive impairment * | OR (95% CI) | OR (95% CI) |
| No diabetes | 1 (Reference) | 1 (Reference) |
| Noninsulin‐requiring diabetes | 1.74 (1.35 to 2.25) ‡ | 1.57 (1.21 to 2.04) ‡ |
| Insulin‐requiring diabetes | 2.57 (1.74 to 3.80) ‡ | 2.30 (1.55 to 3.41) ‡ |
Model 1: adjusted for age and sex.
Model 2 for outcome AF symptoms: adjusted for age, sex, use of β‐blockers, and use of antiarrhythmic medications; model 2 for other outcomes: adjusted for age, sex, smoking status, body mass index, and (in case hypertension is not the outcome) prevalent hypertension. AF indicates atrial fibrillation; and OR, odds ratio.
For this analysis, participants were classified into: patients without diabetes (reference), patients with noninsulin‐requiring diabetes, and patients with insulin‐requiring diabetes. Data on history of hypertension and history of myocardial infarction were available in 2411 participants. Data on history of heart failure and history of stroke were available in 2409 and 2410 participants, respectively. Data on cognitive impairment were available in 2398 participants. The beta regression coefficients (β) indicate predicted differences in mean values between patients without diabetes (reference), patients with noninsulin‐requiring diabetes, and patients with insulin‐requiring diabetes. The quality‐of‐life score ranges from 0 to 100, with higher values indicating better quality of life.
Results suggest a dose‐response association.
P‐value lower than 0.05.
Figure 1. Association of diabetes with atrial fibrillation (AF) phenotype and cardiac and neurological comorbidities.
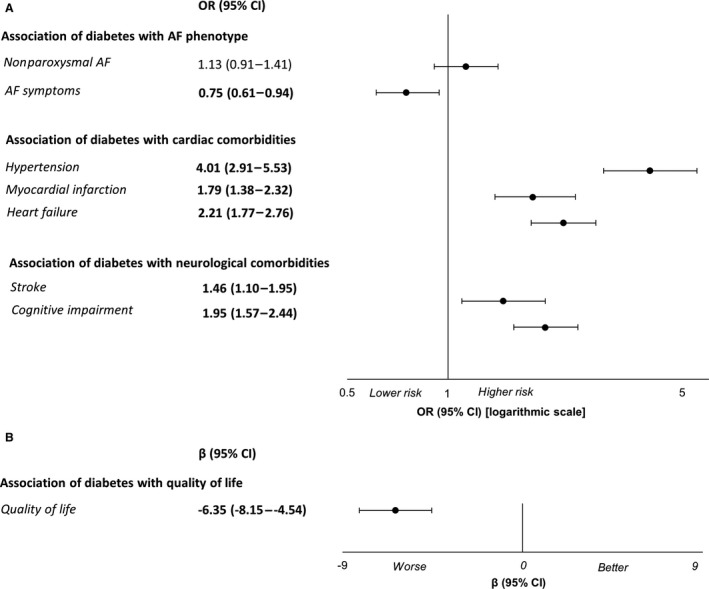
A, Age‐ and sex‐ adjusted odds ratios (ORs) and 95% CIs are derived based on logistic regression. The vertical line represents an OR of 1. B, Age‐ and sex‐adjusted beta regression coefficient (β) and 95% CIs are derived based on linear regression. The vertical line represents a β of 0. The quality‐of‐life score ranges from 0 to 100, with higher values indicating better quality of life.
Patients with diabetes had worse quality of life (predicted mean difference in EQ‐5D score: β, −6.35; 95% CI, −8.15 to −4.54 in patients with diabetes; β, −8.59; 95% CI, −11.6 to −5.54 in patients with insulin‐requiring diabetes) compared with patients without diabetes (Tables 2 and 3, Figure, Figure S3). When we focused our analyses on the specific domains of the quality‐of‐life score, we observed that diabetes was associated with impaired mobility, self‐care, and usual activities but was not associated with anxiety/depression (Table S2). Overall, the results did not substantially change after primary and additional adjustments for potential confounders; therefore, we further report the most adjusted model (model 2).
Diabetes and Cardiac Comorbidities
Patients with diabetes were more likely to have a history of hypertension (OR, 3.04; 95% CI, 2.19–4.22), myocardial infarction (OR, 1.55; 95% CI, 1.18–2.03), and heart failure (OR, 1.99; 95% CI, 1.57–2.51) compared with patients without diabetes (Table 4, Figure). In particular, patients with insulin‐requiring diabetes were more likely to have a history of myocardial infarction (OR, 2.40; 95% CI, 1.60–3.62) and heart failure (OR, 2.44; 95% CI, 1.67–3.55) (Table 3, Figure S3).
Table 4.
Association of Diabetes With Cardiac and Neurological Comorbidities†
| Model 1 | Model 2 | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Association of diabetes with cardiac comorbidities | ||
| Hypertension | 4.01 (2.91–5.53) | 3.04 (2.19–4.22) |
| Myocardial infarction | 1.79 (1.38–2.32) | 1.55 (1.18–2.03) |
| Heart failure | 2.21 (1.77–2.76) | 1.99 (1.57–2.51) |
| Association of diabetes with neurological comorbidities | ||
| Stroke | 1.46 (1.10–1.95) | 1.39 (1.03–1.87) |
| Cognitive impairment | 1.95 (1.57–2.44) | 1.75 (1.39–2.21) |
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, smoking status, body mass index, and (in case hypertension is not the outcome) prevalent hypertension.
OR indicates odds ratio.
Data on history of hypertension and history of myocardial infarction were available in 2411 participants. Data on history of heart failure and data on history of stroke were available in 2409 and 2410 participants, respectively. Data on cognitive impairment were available in 2398 participants.
For a subset of patients, echocardiographic data on LVEF (n=711) and left atrial size (n=476) were available. We observed similar baseline characteristics: (1) when comparing participants with and without data available on left atrial size; and (2) when comparing participants with and without data available on LVEF (Table S3). Patients with diabetes had a higher mean left atrial size and lower mean LVEF than patients without diabetes (Table 1). However, the association of diabetes with echocardiographic parameters did not reach statistical significance in the most adjusted model (Table S4).
Diabetes and Neurological Comorbidities
Compared with patients without diabetes, patients with diabetes were more likely to have a history of stroke (OR, 1.39; 95% CI, 1.03–1.87) and cognitive impairment (OR, 1.75; 95% CI, 1.39–2.21) (Table 4, Figure). In particular, patients with insulin‐requiring diabetes were more likely to have cognitive impairment (OR, 2.30; 95% CI, 1.55–3.41) (Table 3, Figure S3).
Additional Analyses
Results were similar in men and women. The sensitivity analyses adjusting for study centers and AF duration and stratifying by median AF duration provided consistent results. The sensitivity analyses adjusting for rhythm control intervention and use of anticoagulation medications provided consistent results (Table S5).
Discussion
In a large cohort of 2411 patients with documented AF, we investigated whether patients with and without diabetes differ in terms of AF phenotype and comorbidities. We showed that patients with diabetes less often perceive AF symptoms, but have worse quality of life and more cardiac and neurological comorbidities than patients without diabetes.
Previous evidence on the association of diabetes with AF symptoms has been scarce and controversial. 4 , 8 One study did not find an association between diabetes and AF symptoms in a cohort of patients with new‐onset AF. 8 Nevertheless, this study may have been underpowered, as it included a smaller sample size compared with ours. Another study, which was performed in an American AF cohort (ORBIT‐AF [Outcomes Registry for Better Informed Treatment of Atrial Fibrillation]), evaluated the prevalence of specific AF symptoms in patients with and without diabetes. 4 The study reported that patients with diabetes experience fewer episodes of palpitations or syncope and more episodes of dyspnea, exercise intolerance, and tiredness compared with patients without diabetes. These results, however, need to be interpreted with caution, given that the study did not account for potential confounding factors such as age, sex, β‐blockers, antiarrhythmic medications, or ethnicity. 4 Conversely, our study comprehensively assessed the overall burden of any AF symptoms in patients with and without diabetes, also accounting for confounders.
Various factors can explain the reduced manifestation of AF symptoms in patients with diabetes. First, a potential mechanism through which diabetes can affect AF symptoms is cardiac autonomic neuropathy. This complication of diabetes is typically known for masking cardiac symptoms secondary to myocardial infarction. 24 , 25 , 26 In a similar manner, diabetes‐induced cardiac autonomic neuropathy can decrease the sensitivity of cardiac nerves and eventually attenuate the perception of AF symptoms. 27 Second, patients who had AF with diabetes in our cohort had a slightly longer AF duration and a more frequent use of β‐blockers than patients without diabetes. The reduced manifestation of AF symptoms might thus be attributable to the relatively long AF duration and use of β‐blockers, rather than because of diabetes itself. However, our results did not change after adjusting for or stratifying by AF duration or β‐blockers, indicating that the link between diabetes and AF symptoms is independent of these factors.
One can also hypothesize that the hyperglycemic state favors AF progression and occurrence of nonparoxysmal AF episodes by altering cardiac morphology and electrophysiologic properties. 4 , 28 However, the available evidence on the association of diabetes with AF progression is inconclusive. Some studies not accounting for cardiovascular risk factors have reported that patients with diabetes have an increased likelihood of nonparoxysmal AF. 29 , 30 On the other hand, studies accounting for cardiovascular risk factors have usually not detected an association between diabetes and AF progression. 9 , 31 Our study did not find an association of diabetes with AF progression. This result needs to be interpreted with caution and we cannot rule out that our study might have been underpowered to detect an association. In the raw model, as well as in the age‐ and sex‐adjusted model, we observed a borderline significant association, with an OR of 1.13. The effect estimate was largely attenuated (ie, OR, 1.01) after additionally adjusting for cardiovascular risk factors such as obesity and hypertension, which may also be involved in the pathways that link diabetes to AF progression. 12 , 32 Future adequately powered studies with long‐term follow‐up are needed to explore the interplay between glycemic fluctuations, diabetes, and other cardiovascular risk factors in relation to AF progression over time. Additional mechanisms that can explain the link between diabetes and AF progression remain to be further elucidated.
Over the past decades, several studies have consistently reported a worse quality of life in patients who have AF with diabetes compared with those without diabetes. 4 , 8 , 29 , 33 Despite advances in the management of diabetes, our study confirmed that these differences in quality of life still exist. Most likely, the lower quality of life in patients who have AF with diabetes can be ascribed to their increased prevalence of comorbidities. This is supported by our data, showing that patients who have AF with diabetes have more cardiac (hypertension, myocardial infarction, and heart failure) and neurological (stroke and cognitive impairment) comorbidities than patients without diabetes. Notably, our study provides novel insights by revealing significant differences in the neurocognitive function of patients who have AF with and without diabetes. Two previous studies, which were characterized by relatively small sample sizes of patients with AF (n=260 and n=218) and lack of adjustment for potential confounding, were not able to detect an association of diabetes with neurocognitive function. 14 , 34
We further evaluated the clinical characteristics of patients with insulin‐requiring diabetes, those with noninsulin‐requiring diabetes, and those without diabetes, respectively. Of these, patients with diabetes on insulin therapy generally manifested less AF symptoms and more comorbidities. A potential explanation for this result is that elevated concentrations of insulin in the setting of insulin resistance can promote atherosclerosis and AF‐related complications. 35 , 36 On the other hand, it is also plausible that insulin intake may reflect long‐standing diabetes, which is characterized by prolonged exposure to hyperglycemia. In turn, chronic exposure to hyperglycemia and/or poor glycemic control promote the accumulation of glycation end products, which can favor the development of cardiac autonomic neuropathy, reduced perception of AF symptoms, and increased likelihood of comorbidities. Based on these mechanisms, one can assume that among patients with diabetes, especially those with insulin‐requiring diabetes, those with prolonged diabetes duration and/or those with poor glycemic control can have a reduced perception of AF symptoms. This assumption needs to be addressed by further studies with data available on diabetes duration and glycemic control. Future observational and experimental studies are also needed to prospectively investigate the potential impact of insulin and novel antidiabetic treatments (especially glucagon‐like peptide‐1 receptor agonists and sodium‐glucose transport protein 2 inhibitors) on the overall health of patients with AF.
Strengths and Limitations
Our study has several strengths. Using a comprehensive approach, we provide information on AF phenotype and cardiac and neurological comorbidities in patients who have AF with and without diabetes. The study was embedded within a large and well‐characterized cohort of patients with AF. In our study population, we had detailed and extensive information on cardiovascular risk factors, AF type, AF symptoms, and cardiac and neurological comorbidities. The definitions of paroxysmal, persistent, and permanent AF were based on AF guidelines. Another strength is the utilization of a standardized methodology for data collection. Our analyses were adjusted for relevant potential confounders, and multiple sensitivity analyses provided consistent findings.
Several potential limitations should also be acknowledged. The diagnosis of diabetes was based on the medical history of participants rather than laboratory criteria. Therefore, the prevalence of diabetes in our population might have been underreported. Also, we did not have data available on the duration of diabetes or the degree of glycemic control. Nevertheless, we had extensive information on insulin and noninsulin‐based treatments. Furthermore, the cross‐sectional design does not allow us to draw conclusions on causality. The possibility of residual confounding cannot be excluded in an observational study. Last, the generalizability of our findings to other populations needs to be further investigated.
Conclusions
In the Swiss‐AF cohort population, we showed that patients with diabetes less often perceive AF symptoms than those without diabetes. This finding suggests that we may need to increase vigilance for AF detection in patients with diabetes, further raising the question of whether patients with diabetes should be systematically screened for silent AF. The reduced perception of AF symptoms in patients with diabetes might result in a delayed AF diagnosis and consequently more adverse events, especially cardioembolic stroke. Cardiac autonomic neuropathy is the most plausible mechanism through which diabetes can reduce the perception of AF symptoms, and further mediation analyses are needed to verify this hypothesis. In addition, our study shows that patients with diabetes have an increased likelihood of comorbidities and decreased quality of life, which indicates that patients who have AF with diabetes may deserve more attentive care compared with those without diabetes.
Sources of Funding
The Swiss‐AF study (clinical trial number NCT02105844) is supported by grants of the Swiss National Science Foundation (grant numbers 33CS30_148474, 33CS30_177520, and 32473B_176178), the Swiss Heart Foundation, the Foundation for Cardiovascular Research Basel, and the University of Basel.
Disclosures
David Conen holds a McMaster University Department of Medicine Mid‐Career Research Award, and has received speaker fees from Servier Canada, outside of the submitted work. Nicolas Rodondi received a grant from the Swiss Heart Foundation. Jürg H. Beer reports grants from the Swiss National Foundation of Science and The Swiss Heart Foundation; grants from Bayer; and lecture fees from Sanofi Aventis and Amgen, to the institution outside the submitted work. Giorgio Moschovitis has received consultant fees for taking part in advisory boards from Novartis, Astra Zeneca, Bayer, and Böhringer Ingelheim, outside of the submitted work. Giulio Conte reports a research grant from the Swiss National Science Foundation, and research grants from Boston Scientific Inc. Christian Sticherling has received speaker honoraria from Biosense Webster, Boston Scientific, and Medtronic; and research grants from Biosense Webster, Daiichi‐Sankyo, and Medtronic. He is a proctor for Medtronic (Cryoballoon). Christine S. Zuern reports a research grant from Medtronic and speaker fees from Vifor Pharma and Novartis. Michael Kühne reports personal fees from Bayer, Böhringer Ingelheim, Pfizer BMS, Daiichi Sankyo, Medtronic, Biotronik, Boston Scientific, and Johnson & Johnson; and grants from Bayer, Pfizer BMS, and Boston Scientific. Laurent Roten receives speaker honoraria from Abbott/SJM and consulting honoraria from Medtronic. Richard Kobza received institutional grants from Abbott, Biosense‐Webster, Biotronik, Boston‐Scientific, Medtronic, and SIS‐Medica. Tobias Reichlin has received research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union [Eurostars 9799 – ALVALE), and the Cardiovascular Research Foundation Basel, all for work outside the submitted study. He has received speaker/consulting honoraria or travel support from Abbott/SJM, Astra Zeneca, Brahms, Bayer, Biosense‐Webster, Biotronik, Boston‐Scientific, Daiichi Sankyo, Medtronic, Pfizer‐BMS, and Roche, all for work outside the submitted study. He has received support for his institution’s fellowship program from Abbott/SJM, Biosense‐Webster, Biotronik, Boston‐Scientific, and Medtronic, for work outside the submitted study. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
Tables S1–S5
Figures S1–S3
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021800
For Sources of Funding and Disclosures, see page 11.
References
- 1. Huxley RR, Filion KB, Konety S, Alonso A. Meta‐analysis of cohort and case‐control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The association between diabetes mellitus and atrial fibrillation: clinical and mechanistic insights. Front Physiol. 2019;10:135. doi: 10.3389/fphys.2019.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tadic M, Cuspidi C. Type 2 diabetes mellitus and atrial fibrillation: from mechanisms to clinical practice. Arch Cardiovasc Dis. 2015;108:269–276. doi: 10.1016/j.acvd.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 4. Echouffo‐Tcheugui JB, Shrader P, Thomas L, Gersh BJ, Kowey PR, Mahaffey KW, Singer DE, Hylek EM, Go AS, Peterson ED, et al. Care patterns and outcomes in atrial fibrillation patients with and without diabetes: ORBIT‐AF registry. J Am Coll Cardiol. 2017;70:1325–1335. doi: 10.1016/j.jacc.2017.07.755 [DOI] [PubMed] [Google Scholar]
- 5. Sugishita K, Shiono E, Sugiyama T, Ashida T. Diabetes influences the cardiac symptoms related to atrial fibrillation. Circ J. 2003;67:835–838. doi: 10.1253/circj.67.835 [DOI] [PubMed] [Google Scholar]
- 6. Bakhai A, Darius H, De Caterina R, Smart A, Le Heuzey JY, Schilling RJ, Zamorano JL, Shah M, Bramlage P, Kirchhof P. Characteristics and outcomes of atrial fibrillation patients with or without specific symptoms: results from the PREFER in AF registry. Eur Heart J – Qual Care Clin Outcomes. 2016;2(4):299–305. doi: 10.1093/ehjqcco/qcw031 [DOI] [PubMed] [Google Scholar]
- 7. Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, et al. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT‐AF). Circ Cardiovasc Qual Outcomes. 2015;8:393–402. doi: 10.1161/CIRCOUTCOMES.114.001303 [DOI] [PubMed] [Google Scholar]
- 8. Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new‐onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152:1097–1103. doi: 10.1016/j.ahj.2006.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruperti Repilado FJ, Doerig L, Blum S, Aeschbacher S, Krisai P, Ammann P, Erne P, Moschovitis G, di Valentino M, Shah D, et al. Prevalence and predictors of atrial fibrillation type among individuals with recent onset of atrial fibrillation. Swiss Med Wkly. 2018;148:w14652. doi: 10.4414/smw.2018.14652 [DOI] [PubMed] [Google Scholar]
- 10. Kadappu KK, Boyd A, Eshoo S, Haluska B, Yeo AE, Marwick TH, Thomas L. Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction? Eur Heart J Cardiovasc Imaging. 2012;13:1016–1023. doi: 10.1093/ehjci/jes084 [DOI] [PubMed] [Google Scholar]
- 11. van Oort S, Beulens JW, van Ballegooijen AJ, Grobbee DE, Larsson SC. Association of cardiovascular risk factors and lifestyle behaviors with hypertension: a mendelian randomization study. Hypertension. 1979;2020(76):1971–1979. [DOI] [PubMed] [Google Scholar]
- 12. Sun D, Zhou T, Heianza Y, Li X, Fan M, Fonseca VA, Qi L. Type 2 diabetes and hypertension. Circ Res. 2019;124:930–937. doi: 10.1161/CIRCRESAHA.118.314487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Stroke Risk in Atrial Fibrillation Working Group . Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69:546–554. doi: 10.1212/01.wnl.0000267275.68538.8d [DOI] [PubMed] [Google Scholar]
- 14. Ball J, Carrington MJ, Stewart S. Mild cognitive impairment in high‐risk patients with chronic atrial fibrillation: a forgotten component of clinical management? Heart. 2013;99:542–547. doi: 10.1136/heartjnl-2012-303182 [DOI] [PubMed] [Google Scholar]
- 15. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864 [DOI] [PubMed] [Google Scholar]
- 16. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 17. Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D'Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new‐onset atrial fibrillation in the community: the Framingham Heart study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049 [DOI] [PubMed] [Google Scholar]
- 18. Conen D, Rodondi N, Mueller A, Beer J, Auricchio A, Ammann P, Hayoz D, Kobza R, Moschovitis G, Shah D, et al. Design of the Swiss atrial fibrillation cohort study (SWISS‐AF): structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med Wkly. 2017;147:w14467. [DOI] [PubMed] [Google Scholar]
- 19. Conen D, Rodondi N, Müller A, Beer JH, Ammann P, Moschovitis G, Auricchio A, Hayoz D, Kobza R, Shah D, et al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol. 2019;73:989–999. doi: 10.1016/j.jacc.2018.12.039 [DOI] [PubMed] [Google Scholar]
- 20. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 21. Räsänen P, Roine E, Sintonen H, Semberg‐Konttinen V, Ryynänen OP, Roine R. Use of quality‐adjusted life years for the estimation of effectiveness of health care: a systematic literature review. Int J Technol Assess Health Care. 2006;22:235–241. doi: 10.1017/S0266462306051051 [DOI] [PubMed] [Google Scholar]
- 22. Witassek F, Springer A, Adam L, Aeschbacher S, Beer JH, Blum S, Bonati LH, Conen D, Kobza R, Kühne M, et al. Health‐related quality of life in patients with atrial fibrillation: the role of symptoms, comorbidities, and the type of atrial fibrillation. PLoS One. 2019;14:e0226730. doi: 10.1371/journal.pone.0226730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 24. Gersh B, Braunwald E, Bonow RO. Silent Myocardial Ischemia. Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. Philadelphia: Saunders; 2001:1330–1332. [Google Scholar]
- 25. O’Rourke RA, Schlant RC. Asymptomatic (silent) ischemia in stable cad. In: Fuster V, Alexander RW, O’rourke RA, eds. Hurst’s the Heart. 10th ed. New York: Mcgraw‐Hill; 2001:1219–1220. [Google Scholar]
- 26. Langer A, Freeman MR, Josse RG, Armstrong PW. Metaiodobenzylguanidine imaging in diabetes mellitus: assessment of cardiac sympathetic denervation and its relation to autonomic dysfunction and silent myocardial ischemia. J Am Coll Cardiol. 1995;25:610–618. doi: 10.1016/0735-1097(94)00459-4 [DOI] [PubMed] [Google Scholar]
- 27. Rizzo MR, Sasso FC, Marfella R, Siniscalchi M, Paolisso P, Carbonara O, Capoluongo MC, Lascar N, Pace C, Sardu C, et al. Autonomic dysfunction is associated with brief episodes of atrial fibrillation in type 2 diabetes. J Diabetes Complicat. 2015;29:88–92. doi: 10.1016/j.jdiacomp.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 28. Wang A, Green JB, Halperin JL, Piccini JP Sr. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1107–1115. doi: 10.1016/j.jacc.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 29. Fumagalli S, Said SA, Laroche C, Gabbai D, Boni S, Marchionni N, Boriani G, Maggioni AP, Musialik‐Lydka A, Sokal A, et al. Management and prognosis of atrial fibrillation in diabetic patients: an EORP‐AF general pilot registry report. Eur Heart J Cardiovasc Pharmacother. 2018;4:172–179. doi: 10.1093/ehjcvp/pvx037 [DOI] [PubMed] [Google Scholar]
- 30. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, Dan GA, Kalarus Z, Tavazzi L, Maggioni AP, et al. 'Real‐world' management and outcomes of patients with paroxysmal vs. Non‐paroxysmal atrial fibrillation in Europe: the EURObservational Research Programme–Atrial Fibrillation (EORP‐AF) General Pilot Registry. Europace. 2016;18:648.1–657. doi: 10.1093/europace/euv390 [DOI] [PubMed] [Google Scholar]
- 31. Sandhu RK, Conen D, Tedrow UB, Fitzgerald KC, Pradhan AD, Ridker PM, Glynn RJ, Albert CM. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014;3:e000916. doi: 10.1161/JAHA.114.000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang T, Ma X, Tang T, Jin L, Peng D, Zhang R, Chen M, Yan J, Wang S, Yan D, et al. Overall and central obesity with insulin sensitivity and secretion in a Han Chinese population: a Mendelian randomization analysis. Int J Obes. 2005;2016:1736–1741. doi: 10.1038/ijo.2016.155 [DOI] [PubMed] [Google Scholar]
- 33. Berg J, Lindgren P, Nieuwlaat R, Bouin O, Crijns H. Factors determining utility measured with the EQ‐5D in patients with atrial fibrillation. Qual Life Res. 2010;19:381–390. doi: 10.1007/s11136-010-9591-y [DOI] [PubMed] [Google Scholar]
- 34. Bostrom JA, Saczynski JS, Hajduk A, Donahue K, Rosenthal LS, Browning C, Ennis C, Floyd KC, Richardson H, Esa N, et al. Burden of psychosocial and cognitive impairment in patients with atrial fibrillation. Crit Pathw Cardiol. 2017;16:71–75. doi: 10.1097/HPC.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes–part I: pathways of vascular disease in diabetes. Vascul Pharmacol. 2011;54:68–74. doi: 10.1016/j.vph.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 36. Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes–part II: cellular mechanisms and therapeutic targets. Vascul Pharmacol. 2011;54:75–79. doi: 10.1016/j.vph.2011.03.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S5
Figures S1–S3
Data Availability Statement
Patient informed consent forms, as approved by the responsible ethics committee (Ethikkommission Nordwest‐ und Zentralschweiz), do not allow the data to be made publicly available. The participants signed a consent form, which states that their data, containing personal and medical information, are exclusively available for research institutions in an anonymized form. Researchers interested in obtaining the data for research purposes can contact the Swiss‐AF (Swiss Atrial Fibrillation) study scientific lead. Contact information is provided on the Swiss‐AF website (http://www.swissaf.ch/contact.htm). Authorization of the responsible ethics committee is mandatory before the requested data can be transferred to external research institutions.


