Abstract
Background
To clarify differences in clinical significance of intracardiac thrombi in nonvalvular atrial fibrillation‐associated stroke as identified by transesophageal echocardiography (TEE) and transthoracic echocardiography (TTE).
Methods and Results
Using patient data on nonvalvular atrial fibrillation‐associated ischemic stroke between 2011 and 2014 from 15 South Korean stroke centers (n=4841) and 18 Japanese centers (n=1192), implementation rates of TEE/TTE, and detection rates of intracardiac thrombi at each center were correlated. The primary outcome was recurrent ischemic stroke at 1 year after the onset. A total of 5648 patients (median age, 75 years; 2650 women) were analyzed. Intracardiac thrombi were detected in 75 patients (1.3%) overall. Thrombi were detected in 7.8% of patients with TEE (either TEE alone or TEE+TTE: n=679) and in 0.6% of those with TTE alone (n=3572). Thrombus detection rates varied between 0% and 14.3% among centers. As TEE implementation rates at each center increased from 0% to 56.7%, thrombus detection rates increased linearly (detection rate [%]=0.11×TEE rate [%]+1.09 [linear regression], P<0.01). TTE implementation rates (32.3%–100%) were not associated with thrombus detection rates (P=0.53). Intracardiac thrombi were associated with risk of recurrent ischemic stroke overall (adjusted hazard ratio [aHR] 2.35, 95% CI, 1.07–5.16). Thrombus‐associated ischemic stroke risk was high in patients with TEE (aHR, 3.13; 95% CI, 1.17–8.35), but not in those with TTE alone (aHR, 0.89; 95% CI, 0.12–6.51).
Conclusions
Our data suggest clinical relevance of TEE for accurate detection and risk stratification of intracardiac thrombi in nonvalvular atrial fibrillation‐associated stroke.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01581502.
Keywords: atrial fibrillation, echocardiography, stroke
Subject Categories: Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- CRCS‐K
Clinical Research Collaboration for Stroke in Korea
- NIHSS
National Institutes of Health Stroke Scale
- NVAF
nonvalvular atrial fibrillation
- SAMURAI
Stroke Acute Management with Urgent Risk‐Factor Assessment and Improvement
- TEE
transesophageal echocardiography
- TTE
transthoracic echocardiography
Clinical Perspective
What Is New?
The clinical significance of intracardiac thrombi in patients with nonvalvular atrial fibrillation‐associated stroke has not been confirmed; as a result, the role of transesophageal echocardiography compared with transthoracic echocardiography in the clinical practice of stroke care for patients with nonvalvular atrial fibrillation has also not been clearly defined.
In this multinational registry (n=5648) of pooled individual patient data from 2 prospective registries in South Korea and Japan, intracardiac thrombi were detected in 7.8% of patients with transesophageal echocardiography and in 0.6% of those with transthoracic echocardiography alone.
Intracardiac thrombi were associated with risk of recurrent ischemic stroke overall (adjusted hazard ratio [aHR], 2.35; 95% CI, 1.07–5.16).
What Are the Clinical Implications?
Intracardiac thrombus‐associated ischemic stroke risk was high in patients with transesophageal echocardiography (aHR, 3.13; 95% CI, 1.17–8.35), but not in those with transthoracic echocardiography alone (aHR, 0.89; 95% CI, 0.12–6.51), suggesting clinical relevance of transesophageal echocardiography for accurate detection and risk stratification of intracardiac thrombi in nonvalvular atrial fibrillation‐associated stroke.
Patients with ischemic stroke with nonvalvular atrial fibrillation (NVAF) can be assumed to have a higher prevalence of intracardiac thrombi compared with patients with non‐stroke with NVAF. 1 , 2 , 3 , 4 Since the thrombus usually forms in the left atrial appendage in patients with NVAF, transesophageal echocardiography (TEE) can be more effective for identifying patients with a high risk of recurrent ischemic stroke compared with other tools. 1 , 2 , 3 , 4 , 5 , 6 However, the clinical significance of intracardiac thrombi in patients with NVAF‐associated stroke has not been confirmed. As a result, the role of TEE in the clinical practice of stroke care for patients with NVAF has also not been clearly defined. 7
A rational assumption would be that the implementation of TEE, rather than transthoracic echocardiography (TTE), is better suited to accurate diagnosis and risk stratification of intracardiac thrombi among patients with NVAF‐associated ischemic stroke. 1 , 2 , 3 , 4 , 5 , 6 However, sufficient data to substantiate this hypothesis have not been reported for such a patient population. The objective of the present study was to clarify differences in the clinical significance of intracardiac thrombi in NVAF‐associated stroke identified by TEE and TTE, using the EAST‐AF (East‐Asian Ischemic Stroke Patients With Atrial Fibrillation) registry, pooled individual patient data from 2 prospective registries in South Korea and Japan. 8
METHODS
Anonymized data supporting the findings of this study are available from the corresponding author, upon reasonable request and after permission from the ethics committees involved.
Study Population
The CRCS‐K (Clinical Research Collaboration for Stroke in Korea) registry is a prospective, ongoing, nationwide, multicenter acute stroke registry in South Korea (15 stroke centers) that has recruited patients with acute stroke or transient ischemic attack (TIA) admitted within 7 days from onset. 9 , 10 The SAMURAI‐NVAF (Stroke Acute Management with Urgent Risk‐Factor Assessment and Improvement‐NVAF) registry was a prospective, multicenter, observational study in Japan (18 stroke centers) that registered patients hospitalized within 7 days after the onset of ischemic stroke/TIA and diagnosed with NVAF between September 2011 and March 2014. 11 , 12 , 13 , 14 The study was registered with ClinicalTrials.gov (NCT01581502) and the Japanese University Hospital Medical Information Network Clinical Trials Registry (UMIN000006930). For the SAMURAI‐NVAF registry, secondary use of the data set for this study was approved by the ethics committee of the National Cerebral and Cardiovascular Center. For the CRCS‐K registry, data collection and analysis for this study were approved by the ethics committees of participating centers. Written informed consent was obtained from the patients or their families if the patient was incapable of providing consent.
At Korea University, patient information from the CRCS‐K registry from January 2011 to December 2014 was pooled with that from the SAMURAI‐NVAF registry. From the CRCS‐K registry, we included patients aged ≥18 years who had: (1) ischemic stroke or TIA; and (2) a diagnosis of NVAF. NVAF was diagnosed from 12‐lead electrocardiography or monitoring for ≥24 hours during acute hospitalization, or from previous medical documents. We excluded patients with: (1) history of prosthetic valve replacement or hemodynamically relevant mitral valve stenosis; or (2) follow‐up data unavailable. Consequently, we made a full cohort of the EAST‐AF registry (n=6033) using data of all patients registered to the SAMURAI‐NVAF registry (n=1192) and of 4841 eligible patients from the CRCS‐K registry. In this study, we excluded 385 cases of in‐hospital death (Figure 1). 8
Figure 1. Study flowchart.
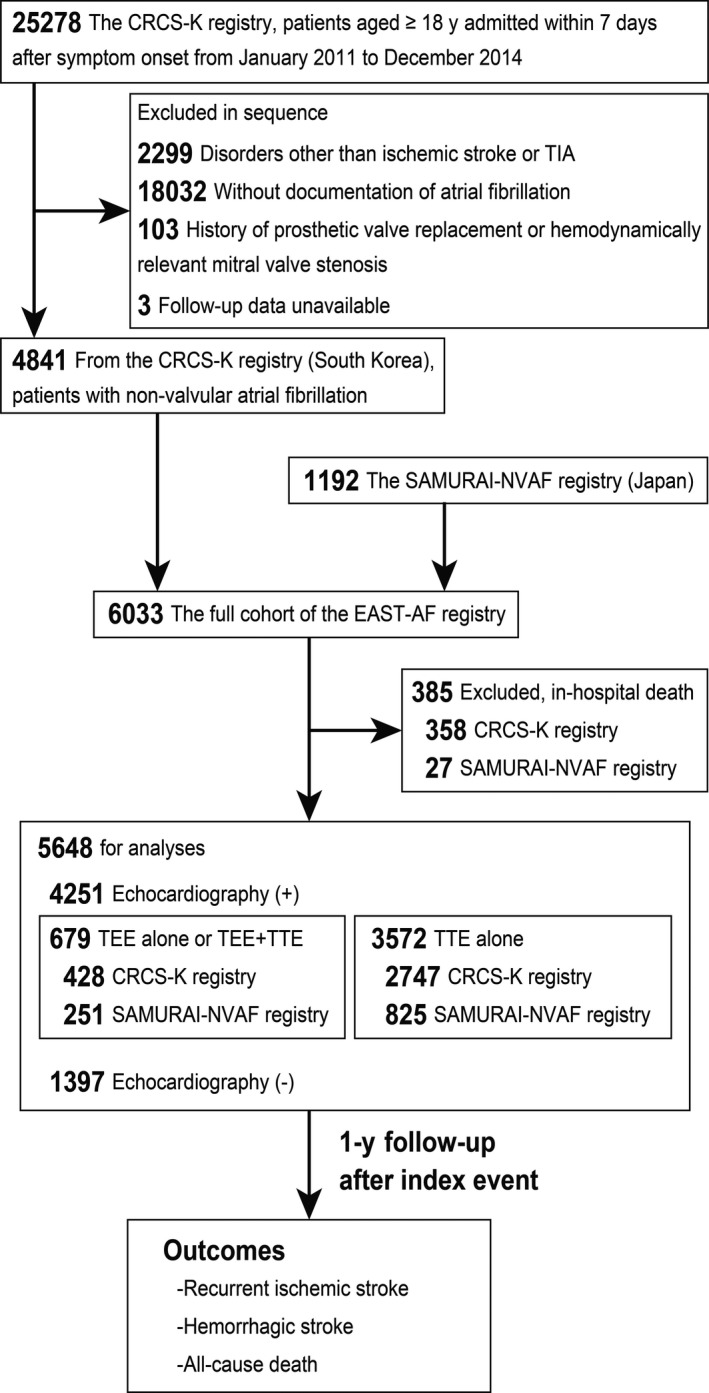
CRCS‐K indicates Clinical Research Collaboration for Stroke in Korea; EAST‐AF, East‐Asian Ischemic Stroke Patients With Atrial Fibrillation; SAMURAI‐NVAF, Stroke Acute Management with Urgent Risk‐Factor Assessment and Improvement‐Nonvalvular Atrial Fibrillation; TEE, transesophageal echocardiography; TIA, transient ischemic attack; and TTE, transthoracic echocardiography.
Clinical Data Collection
The following clinical data were collected: registry (CRCS‐K [South Korea] or SAMURAI‐NVAF [Japan]), participating center, age, sex, body weight, smoking status, timing of NVAF detection relative to the index event (known prior or detected after), congestive heart failure (CRCS‐K: clinical diagnosis by the treating physician; SAMURAI‐NVAF: presence of symptoms or left ventricular ejection fraction ≤40%), vascular risk factors (hypertension, diabetes mellitus, and hyperlipidemia), past medical history (stroke and coronary heart disease), prestroke oral anticoagulants, prestroke antiplatelets, type of the index event (ischemic stroke or TIA), baseline National Institutes of Health Stroke Scale (NIHSS) score, 15 laboratory data (white blood cell count, hemoglobin, platelet count, glucose, and prothrombin time‐international normalized ratio), renal dysfunction (creatinine clearance <30 mL/min), implementation of TTE and/or TEE during hospitalization, medications at discharge (antiplatelets, warfarin, direct oral anticoagulants, and statins), duration of hospital stay, and modified Rankin Scale score at discharge. 16 Indications for TTE and TEE were determined by the physician at the registering centers. Creatinine clearance was calculated using the Cockcroft‒Gault equation. 17 Antiplatelets included aspirin, clopidogrel, dipyridamole, ticlopidine, and cilostazol. Direct oral anticoagulants included dabigatran, apixaban, and rivaroxaban; edoxaban had not yet been approved in Japan or South Korea during the study period. 8
Intracardiac Thrombus Detection
Intracardiac thrombi were considered present if a mass detected in the cardiac cavities appeared to be distinct from the underlying endocardium, was not caused by pectinate muscles or trabeculae carneae, and was detected in >1 imaging plane. The presence of intracardiac thrombus was adjudicated locally at the registering center.
Outcome Events and Follow‐Up Period
The primary outcome was recurrent ischemic stroke. Secondary outcomes were hemorrhagic stroke and death. In the SAMURAI‐NVAF registry, follow‐up to assess outcome events was performed at 3 months, 1 year, and 2 years after the index event, personally in the clinic or by telephone interview of the patient or caregivers by the study physicians or trained study coordinators at participating hospitals. In the CRCS‐K registry, outcome events were collected at 3 months and 1 year after the index event by identification of the events in usual clinical practice or in structured telephone interviews by trained study coordinators at the participating hospitals. In this study, outcome events were captured up to 1 year after the index event.
Statistical Analysis
Demographic and clinical characteristics are summarized as median (interquartile range [IQR]) for continuous variables and as frequencies and percentages for categorical variables. Statistical differences between groups were assessed using the Mann‐Whitney U test or Fisher exact test, as appropriate. The association between rate of echocardiography (TTE or TEE) and the detection rate of intracardiac thrombus at each participating center was estimated using univariate linear regression models. The regression model for TEE was adjusted using the patient number registered by each center.
Two multivariable logistic regression models were created to elucidate factors associated with detection of intracardiac thrombi. For Model 1, TEE and TTE plus the following prespecified variables were included: age, sex, congestive heart failure, 4 hypertension, diabetes mellitus, stroke before index event, coronary heart disease, and prestroke oral anticoagulants. 8 For Model 2, TEE and TTE plus those variables showing values of P<0.05 on univariate logistic regression analyses were included. The same multivariable logistic regression models were applied to a subgroup of patients who underwent TEE. C statistics and Hosmer‒Lemeshow χ2 statistics were used as goodness‐of‐fit indices for each multivariable logistic model. Odds ratios (ORs) with 95% CIs were calculated.
Cumulative incidences of the primary and secondary outcomes at 1 year were estimated. Univariate Kaplan‒Meier survival probabilities were estimated for patients with and without intracardiac thrombi, and we used the log‐rank testing to compare groups. We constructed multivariable Cox proportional hazard models with adjustments for registry (CRCS‐K and SAMURAI‐NVAF), age, sex, congestive heart failure, hypertension, diabetes mellitus, history of stroke, coronary heart disease, baseline NIHSS score, warfarin at discharge, and direct oral anticoagulants at discharge. To account for the heterogeneity caused by unmeasured variables among centers, shared gamma distributed frailty clustered by participating center was included in the multivariable Cox models. Hazard ratios (HRs) with 95% CIs were calculated. Because hemorrhagic stroke and death could compete with recurrent ischemic stroke events, we constructed Fine and Gray competing‐risks models, where the above‐mentioned prespecified adjusting covariates were included. Sensitivity analysis for the recurrent ischemic stroke was also performed using patients with echocardiography (TTE or TEE, n=4251, Figure 1).
Exploratory subgroup analyses for the association between intracardiac thrombi and recurrent ischemic stroke risk were performed on the basis of implemented echocardiography (patients with TEE or patients with TTE alone), institutional TEE implementation rates (dichotomized at the median rate), congestive heart failure (yes or no), and oral anticoagulants at discharge (warfarin or direct oral anticoagulants).
All reported P values were 2‐tailed and P<0.05 was considered statistically significant. Pairwise deletion was used for missing data handling. All statistical analyses were performed using the Stata/IC statistical package (version 15.1; Stata Corp LP, College Station, TX).
RESULTS
In this study, data from 5648 patients were analyzed (Figure 1). Number of patient registration at each center ranged from 7 to 1007 (median, 90; IQR, 40–215). Median age was 75 years (IQR, 69–81 years), and 2650 patients (46.9%) were women. TTE alone was performed for 3572 patients (63.2%), TEE alone was performed for 84 patients (1.5%), and 595 patients underwent both TTE and TEE (10.5%). No echocardiography was performed in 1397 patients (24.7%).
The implementation rate of TTE and TEE was heterogeneous among participating centers. Institutional TTE implementation rates varied between 32.3% and 100.0% (median, 88.9%; IQR, 78.9%–95.6%), and rates for TEE implementation were between 0.0% and 56.7% (median, 4.1%; IQR, 1.3%–12.1%) among centers. Compared with patients examined using TTE alone (n=3572), patients who underwent TEE (either TEE alone or TEE+TTE: n=679) were less frequently from the CRCS‐K registry (P<0.01), were younger (P<0.01), and were less frequently women (P<0.01, Table S1). Prestroke use of oral anticoagulants was more frequent (P=0.03) and baseline NIHSS score was lower (P<0.01) in patients with TEE than in those with TTE alone.
Intracardiac thrombi were detected in 75 patients (1.3%) overall. Thrombi were detected in 7.8% of the 679 patients examined with TEE and in 0.6% of the 3572 patients examined with TTE alone. Detection rates of intracardiac thrombi at each center were 0.0% to 14.3%. As the institutional rate of TEE implementation increased, the detection rate of intracardiac thrombi increased in a linear manner, and the association was significant (thrombus detection rate [%]=0.11×TEE implementation rate [%]+1.09 [linear regression], P<0.01, Figure 2). This association remained significant after adjustment with the patient number registered by each center (thrombus detection rate [%]=0.11×TEE implementation rate [%]+1.85, P<0.01). No significant association between institutional TTE rate and intracardiac thrombus detection rate was identified (P=0.53).
Figure 2. Institutional rates of echocardiography and detection rates of intracardiac thrombus.
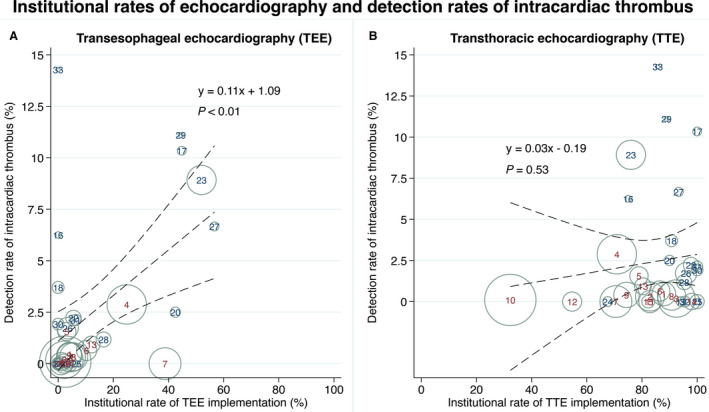
Scatter plots of TEE (A) and TTE rate (B) and the detection rate of intracardiac thrombus in each center. Markers are sized according to the patient number registered by each center. Numbers at each marker indicate center identifiers (maroon: CRCS‐K registry; navy: SAMURAI‐NVAF registry). Dotted lines represent fitted regression lines with 95% CIs. CRCS‐K indicates Clinical Research Collaboration for Stroke in Korea; SAMURAI‐NVAF, Stroke Acute Management with Urgent Risk‐Factor Assessment and Improvement‐Nonvalvular Atrial Fibrillation; TEE, transesophageal echocardiography; and TTE, transthoracic echocardiography.
Compared with patients without intracardiac thrombi (n=5573), patients in whom thrombi were detected (n=75) were less frequently from the CRCS‐K registry (P<0.01), and NVAF had more frequently been documented before the index event (P<0.01, Table 1). The rate of intracardiac thrombus detection in patients with NVAF detected after index event was 0.9% (21/2425). The rate of intracardiac thrombus detection in patients with NVAF known before index event (without detected AF) was 1.7% (54/3223). In patients with thrombi, congestive heart failure and oral anticoagulant use before the index event were more frequent (P<0.01 each). Baseline NIHSS score was marginally lower (P=0.06), and white blood cell count and platelet count were lower (P=0.03 each) in patients with thrombi than in patients without. Median hospital stay was longer in overall patients with intracardiac thrombi (18 days) than in patients without (11 days; P<0.01). Similar findings were seen for patients who underwent TEE with and without intracardiac thrombi (18 days versus 11.5 days, respectively; P<0.01) and patients who underwent TTE alone with and without intracardiac thrombi (20.5 days versus 13 days, respectively; P=0.10). Thirty‐seven patients were diagnosed as developing small‐vessel occlusion and 71 as developing large‐artery atherosclerosis although they had NVAF; all of them did not have intracardiac thrombi.
Table 1.
Data in Patients With and Without Intracardiac Thrombus Detection (n=5648)
| Intracardiac thrombus (n=75) | No thrombus detection (n=5573) | Missing data, n (%) | |
|---|---|---|---|
| CRCS‐K (South Korea), n (%) | 27 (36.0) | 4456 (79.9) | 0 (0.00) |
| Age, median (IQR), y | 73 (70–81) | 75 (69–81) | 0 (0.00) |
| Women, n (%) | 35 (46.7) | 2615 (46.9) | 0 (0.00) |
| Body weight, median (IQR), kg | 57.8 (50–65) | 60 (51.3–68) | 55 (0.97) |
| Smoking, n (%) | 28 (37.3) | 1712 (30.8) | 7 (0.12) |
| NVAF known before index event, n (%) | 54 (72.0) | 3169 (56.9) | 0 (0.00) |
| Congestive heart failure, n (%) | 19 (25.3) | 446 (8.0) | 0 (0.00) |
| Vascular risk factor, n (%) | |||
| Hypertension | 54 (72.0) | 4075 (73.2) | 4 (0.07) |
| Diabetes mellitus | 20 (26.7) | 1488 (26.7) | 5 (0.09) |
| Hyperlipidemia | 30 (40.0) | 1625 (29.2) | 7 (0.12) |
| Clinical history, n (%) | |||
| Stroke before index event | 22 (29.3) | 1357 (24.4) | 5 (0.09) |
| Coronary heart disease | 10 (13.3) | 678 (12.2) | 0 (0.00) |
| Prestroke oral anticoagulants, n (%) | 30 (40.0) | 1099 (19.7) | 3 (0.05) |
| Prestroke antiplatelets, n (%) | 26 (34.7) | 2030 (36.5) | 3 (0.05) |
| Ischemic stroke as index event, n (%) | 69 (92.0) | 5412 (97.2) | 5 (0.09) |
| Baseline NIHSS score, median (IQR) | 5 (2–12) | 8 (3–16) | 0 (0.00) |
| Laboratory data, median (IQR) | |||
| White blood cell count, /µL | 6900 (5500–8830) | 7500 (6050–9500) | 7 (0.12) |
| Hemoglobin, g/dL | 13.9 (12.2–14.8) | 13.5 (12.2–14.7) | 8 (0.14) |
| Platelet count, ×103/µL | 178 (150–226) | 195 (160–236) | 10 (0.18) |
| Glucose, mg/dL | 128 (111–153) | 124 (105–153) | 188 (3.33) |
| PT‐INR | 1.06 (1.01–1.3) | 1.06 (1–1.15) | 61 (1.08) |
| Renal dysfunction, n (%)* | 3 (4.1) | 612 (11.1) | 66 (1.17) |
| TTE, n (%) | 71 (94.7) | 4096 (73.5) | 0 (0.00) |
| TEE, n (%) | 53 (70.7) | 626 (11.2) | 0 (0.00) |
| Medication at discharge, n (%) | |||
| Antiplatelets | 16 (21.3) | 1793 (32.2) | 0 (0.00) |
| Warfarin | 56 (74.7) | 3512 (63.0) | 0 (0.00) |
| Direct oral anticoagulants | 16 (21.3) | 565 (10.1) | 0 (0.00) |
| Dabigatran | 6 (8.0) | 259 (4.7) | |
| Apixaban | 0 (0.0) | 31 (0.6) | |
| Rivaroxaban | 10 (13.3) | 275 (4.9) | |
| Statins | 35 (46.7) | 3707 (66.5) | 0 (0.00) |
| Hospital stay, median (IQR), d | 18 (10–29) | 11 (7–20) | 0 (0.00) |
| mRS score at discharge, median (IQR) | 2 (1–3) | 3 (1–4) | 0 (0.00) |
CRCS‐K indicates Clinical Research Collaboration for Stroke in Korea; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; NVAF, nonvalvular atrial fibrillation; PT‐INR, prothrombin time/international normalized ratio; TEE, transesophageal echocardiography; and TTE, transthoracic echocardiography.
Renal dysfunction was defined as creatinine clearance <30 mL/min.
Factors Associated With Detection of Intracardiac Thrombus
In the multivariable logistic models (Table 2), TEE implementation (adjusted OR, 16.09; 95% CI, 9.65–26.87), TTE implementation (adjusted OR, 4.15; 95% CI, 1.49–11.56), congestive heart failure (adjusted OR, 2.85; 95% CI, 1.60–5.07), and prestroke oral anticoagulants (adjusted OR, 1.80; 95% CI, 1.04–3.14) consistently showed significantly positive associations with detection of intracardiac thrombus. In the analysis of patients who underwent TEE, congestive heart failure was also significantly associated with intracardiac thrombus detection (adjusted OR, 2.18; 95% CI, 1.06–4.49) (Table S2).
Table 2.
Logistic Regression Models for Intracardiac Thrombus Detection (n=5648)
| Univariate | Multivariable (model 1)* | Multivariable (model 2) † | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| TEE | 19.04 (11.50–31.51) | <0.01 | 17.03 (10.17–28.53) | <0.01 | 16.09 (9.65–26.87) | <0.01 |
| TTE | 6.40 (2.33–17.56) | <0.01 | 4.16 (1.49–11.61) | <0.01 | 4.15 (1.49–11.56) | <0.01 |
| Age (per 10‐y increase) | 0.97 (0.78–1.21) | 0.80 | 1.05 (0.81–1.35) | 0.72 | … | … |
| Women | 0.99 (0.63–1.56) | 0.96 | 1.22 (0.74–2.00) | 0.44 | … | … |
| NVAF known before index event | 1.95 (1.18–3.24) | 0.01 | … | … | 1.54 (0.86–2.78) | 0.15 |
| Congestive heart failure | 3.90 (2.29–6.62) | <0.01 | 2.97 (1.66–5.31) | <0.01 | 2.85 (1.60–5.07) | <0.01 |
| Hypertension | 0.94 (0.57–1.57) | 0.82 | 0.82 (0.47–1.40) | 0.46 | … | … |
| Diabetes mellitus | 0.99 (0.59–1.67) | 0.99 | 0.92 (0.53–1.59) | 0.77 | … | … |
| Stroke before index event | 1.29 (0.78–2.13) | 0.32 | 1.21 (0.69–2.13) | 0.50 | … | … |
| Coronary heart disease | 1.11 (0.57–2.17) | 0.75 | 0.99 (0.49–2.01) | 0.98 | … | … |
| Prestroke oral anticoagulants | 2.71 (1.70–4.32) | <0.01 | 2.14 (1.26–3.65) | <0.01 | 1.80 (1.04–3.14) | 0.03 |
| Ischemic stroke as index event | 0.33 (0.14–0.77) | 0.01 | … | … | 0.67 (0.27–1.67) | 0.39 |
NVAF indicates nonvalvular atrial fibrillation; OR, odds ratio; TEE, transesophageal echocardiography; and TTE, transthoracic echocardiography.
Model 1: adjusted for TEE and TTE plus prespecified variables of age, sex, congestive heart failure, hypertension, diabetes mellitus, stroke before index event, coronary heart disease, and prestroke oral anticoagulants. The model showed a c‐statistic of 0.87 and a Hosmer‒Lemeshow Chi‐squared statistic of 7.14 (P=0.52). Number of observations=5641; log likelihood ratio Chi‐squared test statistic=180.10 (P<0.01); and McFadden R 2=0.23.
Model 2: adjusted for TEE and TTE plus those variables showing P<0.05 on univariate models. The model showed a c‐statistic of 0.88 and a Hosmer‒Lemeshow Chi‐squared statistic of 2.28 (P=0.80). Number of observations=5640; log likelihood ratio Chi‐squared test statistic=181.34 (P<0.01); and McFadden R 2=0.23.
Outcome Events Between Patients With and Without Intracardiac Thrombus
The 5648 patients provided 4619 patient‐years of follow‐up data (median follow‐up 365 days, IQR, 335–365 days): 69 patient‐years in patients with intracardiac thrombi and 4550 patient‐years in patients without thrombi. The number of patients who were lost to follow‐up was 323 (5.7%) at 3 months and 662 (11.7%) at 1 year. In this population, 190 recurrent ischemic stroke events and 42 hemorrhagic stroke events were encountered. During follow‐up, 905 patients died. The cumulative incidence of recurrent ischemic stroke was 10.7% in patients with intracardiac thrombi and 3.3% in patients without thrombi (Table 3). On log‐rank testing, recurrent ischemic stroke was significantly more frequent in patients with thrombi than in patients without thrombi (Figure 3). Risk for recurrent ischemic stroke was significantly higher in patients with intracardiac thrombi than in those without (adjusted HR, 2.35; 95% CI, 1.07–5.16; multivariable Cox shared‐frailty model). Likewise, competing‐risks models showed significantly higher risk for ischemic stroke in patients with thrombi than in those without (hemorrhagic stroke as competing event: adjusted subhazard ratio, 2.53; 95% CI, 1.13–5.67; P=0.02; death as competing event: adjusted subhazard ratio, 2.35; 95% CI, 1.05–5.24; P=0.03). The sensitivity analysis cohort consisting of patients who received echocardiography (TTE or TEE) also demonstrated a higher risk for ischemic stroke in patients with thrombi than in those without thrombus detection (adjusted HR, 2.28; 95% CI, 1.02–5.04; multivariable Cox shared‐frailty model).
Table 3.
Outcomes Between Patients With and Without Intracardiac Thrombus Detection (n=5648)
| Cumulative incidence (total number) | Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI)* | Adjusted hazard ratio with shared frailty (95% CI) † | |
|---|---|---|---|---|
| Recurrent ischemic stroke | ||||
| Intracardiac thrombus (n=79) | 10.7% (8) | 2.76 (1.29–5.86); P<0.01 | 2.33 (1.08–5.06); P=0.03 | 2.35 (1.07–5.16); P=0.03 |
| No thrombus detection (n=5569) | 3.3% (182) | 1 (reference) | 1 (reference) | 1 (reference) |
| Hemorrhagic stroke | ||||
| Intracardiac thrombus (n=79) | 0.0% (0) | … | … | … |
| No thrombus detection (n=5569) | 0.8% (42) | 1 (reference) | 1 (reference) | 1 (reference) |
| All‐cause death ‡ | ||||
| Intracardiac thrombus (n=79) | 6.7% (5) | 0.37 (0.15–0.89); P=0.02 | 0.69 (0.28–1.66); P=0.40 | 0.73 (0.30–1.78); P=0.49 |
| No thrombus detection (n=5569) | 16.2% (900) | 1 (reference) | 1 (reference) | 1 (reference) |
CRCS‐K indicates Clinical Research Collaboration for Stroke in Korea; and SAMURAI‐NVAF, Stroke Acute Management with Urgent Risk‐Factor Assessment and Improvement‐Nonvalvular Atrial Fibrillation.
Adjusted for registry (CRCS‐K and SAMURAI‐NVAF), age, sex, congestive heart failure, hypertension, diabetes mellitus, history of stroke, coronary heart disease, baseline National Institutes of Health score, warfarin at discharge, and direct oral anticoagulants at discharge.
Adjusted for age, sex, congestive heart failure, hypertension, diabetes mellitus, history of stroke, coronary heart disease, baseline National Institutes of Health score, warfarin at discharge, and direct oral anticoagulants at discharge. Shared gamma distributed frailty clustered by participating centers is included into the model.
Causes of the 905 deaths were stroke (6.1%), cardiovascular events (3.1%), infection (19.6%), and unclear (71.2%).
Figure 3. Unadjusted Kaplan‒Meier curves for recurrent ischemic stroke (A), hemorrhagic stroke (B), and death (C).
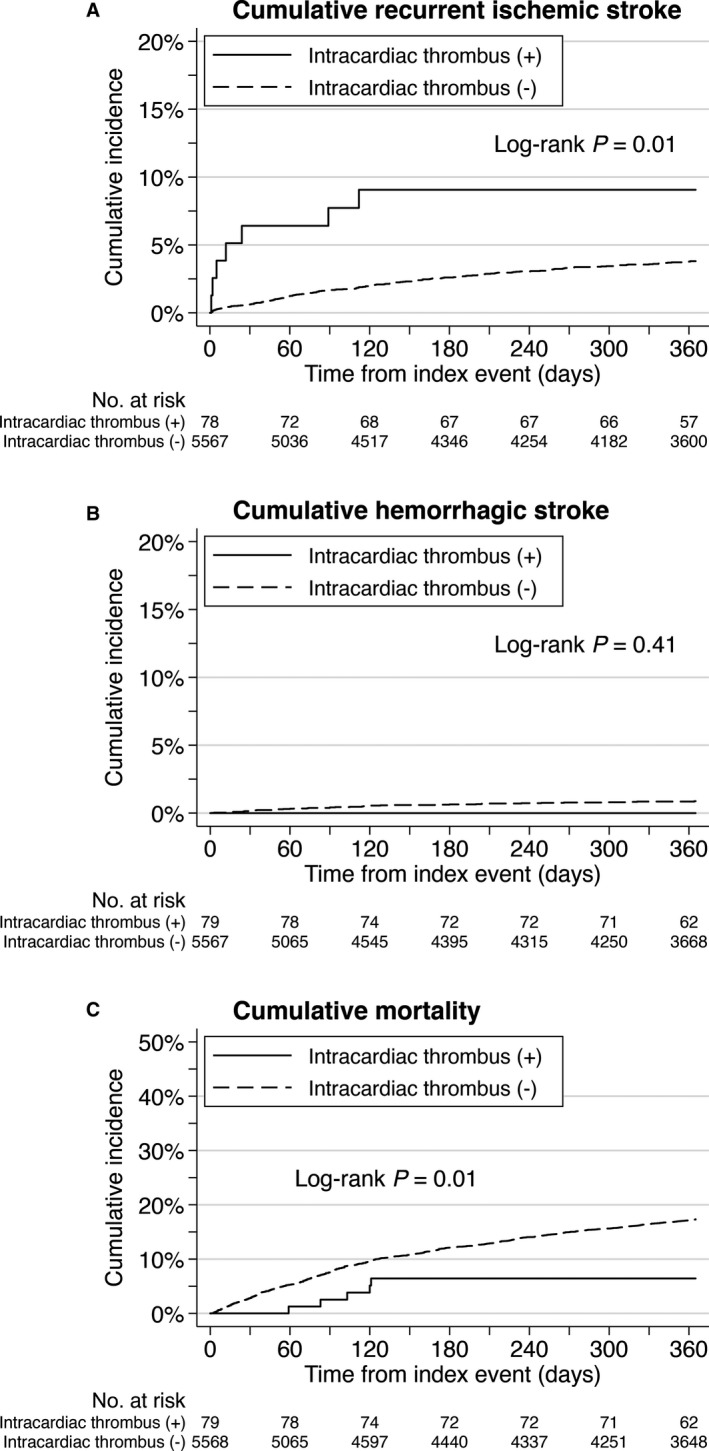
Recurrent ischemic strokes are more frequent in patients with intracardiac thrombi than in those without thrombi.
Cumulative incidence of hemorrhagic stroke was 0.0% in patients with intracardiac thrombi and 0.8% in patients without thrombi. Cumulative mortality rates were 6.3% and 16.2%, respectively. No significant differences in the risks for hemorrhagic stroke and death were evident between groups (Figure 3, Table 3).
Subgroup Analysis of Risk for Recurrent Ischemic Stroke With Intracardiac Thrombus
No significant interactions were identified in each subgroup analysis. Intracardiac thrombi were associated with a high risk of recurrent ischemic stroke within patients who underwent TEE (adjusted HR, 3.13; 95% CI, 1.17–8.35); this association was not seen within patients with TTE alone (adjusted HR, 0.89; 95% CI, 0.12–6.51; P for interaction=0.265; Figure 4).
Figure 4. Subgroup analyses of risk for recurrent ischemic stroke with intracardiac thrombus.
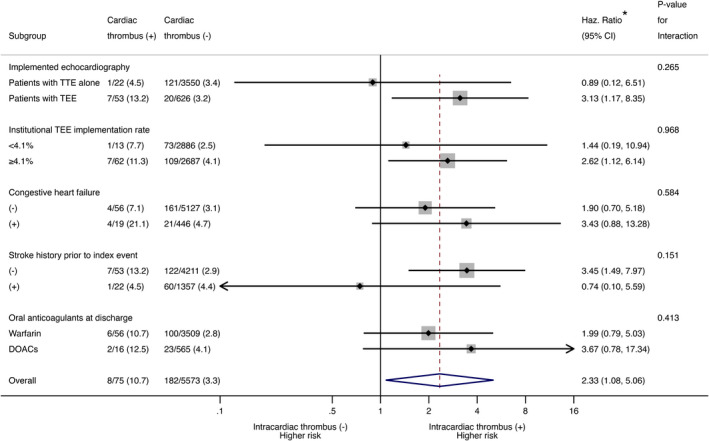
CRCS‐K indicates Clinical Research Collaboration for Stroke in Korea; DOACs, direct oral anticoagulants; Haz., hazard; NIHSS, National Institutes of Health Stroke Scale; SAMURAI‐NVAF, Stroke Acute Management with Urgent Risk‐Factor Assessment and Improvement‐Nonvalvular Atrial Fibrillation; TEE, transesophageal echocardiography; and TTE, transthoracic echocardiography. *Adjusted for registry (CRCS‐K and SAMURAI‐NVAF), age, sex, congestive heart failure, hypertension, diabetes mellitus, history of stroke, coronary heart disease, baseline National Institutes of Health Stroke Scale score, warfarin at discharge, and DOACs at discharge.
DISCUSSION
This multinational East Asian cohort that comprised pooled individual patient data from 2 prospective, observational multicenter registries showed a 1.3% (75/5648) detection rate for intracardiac thrombi in patients with NVAF‐associated ischemic stroke/TIA, in which TEE was implemented in 12.0% of patients (679/5648). Although the thrombus detection rate was markedly lower than rates reported for patients with atrial fibrillation, the subgroup of patients who underwent TEE showed a 7.8% detection rate for intracardiac thrombi, within the 2.8% to 21.1% detection rates reported from TEE studies on patients with atrial fibrillation. 1 , 2 , 3 , 18 , 19 , 20 Moreover, the institutional TEE rate showed a significantly positive association with the detection rate of intracardiac thrombi at each center, whereas no association was seen between the TTE rate and thrombus detection rate (Figure 2). Although no data have been reported for TEE implementation rates in NVAF‐associated stroke, with the insufficient TEE implementation as seen in this study, many thrombi may be missed under current stroke care, which would result in underestimation of the clinical significance of intracardiac thrombi and delayed development of specific interventions for intracardiac thrombi in stroke patients.
No specific data have been reported on the risk of recurrent embolic events associated with intracardiac thrombi in patients after stroke. Considering that patients with stroke/TIA in this study had NVAF and that most intracardiac thrombi were detected in patients who underwent TEE (53/75, 70.7%), the principal site of thrombi in the present data would be the left atrial appendage. 21 In addition, the 1‐year incidence of recurrent ischemic stroke was 10.7% in the present patients with intracardiac thrombi, broadly consistent with reported risks for embolic events of left atrial thrombi. 3 , 5 Although the risk of recurrent ischemic stroke doubled in patients with intracardiac thrombi compared with those without thrombus documentation (Table 3), in our data, thrombi detected with TTE alone notably showed a much lower risk of recurrent ischemic stroke compared with thrombi detected with TEE (Figure 4). As an explanation for this difference in risk of intracardiac thrombi between patients with TEE and those with TTE alone, the influence of a selection bias on TEE implementation should be considered first. Unmeasured confounders associated with ischemic stroke risk in NVAF (eg, sustained or paroxysmal AF, duration of AF, left atrial enlargement, untreated obstructive sleep apnea, natriuretic peptides, or troponins) may have affected the decision to perform TEE. 22 However, considering that TTE often does not provide a suitable window for observing the left atrial appendage, the thrombi identified in patients with TTE alone might have contained false‐positive results. 6 , 21 Our data may indicate the clinical relevance of TEE, rather than TTE, for accurate detection and risk stratification of intracardiac thrombi in NVAF‐associated stroke. The greater frequency of death in patients without intracardiac thrombi than in patients with thrombi (Figure 3) might be attributable to the bias that patients with TEE were younger and showed lower NIHSS scores than patients with TTE alone (Table S1).
Among the patient characteristics, congestive heart failure consistently showed positive associations with thrombus detection (Table 2). Systolic left ventricular dysfunction was reported to correlate with thrombus detection in a TEE study of patients with ischemic stroke. 4 The positive association between prestroke anticoagulants and thrombus detection seems contradictory. As a possible cause of this paradoxical finding, patients with prestroke anticoagulation and positive thrombus detection might have poor time in therapeutic range of warfarin anticoagulation since the range <40% was reported to show a higher risk of stroke than no warfarin medication. 23 Prestroke anticoagulant use might be associated with prior history of cardioembolism and patients with recurrent cardioembolic stroke might have higher possibility to have intracardiac thrombi, although prior history of any stroke was not independently related to thrombus detection. Stroke occurrence despite anticoagulation also suggests the presence of higher risk profiles for NVAF including left atrial appendage dysfunction or the presence of a prothrombotic state such as in antiphospholipid syndrome or malignancy. 8 , 24 Although performing TEE in all cases with NVAF‐associated ischemic stroke is not realistic, the presence of congestive heart failure and the high risk profile of NVAF may be key findings in proper patient selection for TEE. The present study analyzed patients with NVAF‐associated stroke, including both NVAF known before and NVAF detected after the index stroke, but optimization of antithrombotic therapy is often required for patients of embolic ischemic stroke with no detection of atrial fibrillation in genuine clinical settings. Evaluation of left atrial volume and function by 3‐dimensional echocardiography is reportedly useful particularly in patients without known atrial fibrillation for understanding the mechanism of ischemic stroke, 25 , 26 although such data were not collected in our registries.
Although patients with ischemic stroke/TIA with atrial fibrillation are qualified for anticoagulant therapy regardless of the detection of intracardiac thrombi, optimal anticoagulant therapy for NVAF‐associated stroke with intracardiac thrombi may differ from general stroke patients with NVAF since the risk for developing thromboembolism would be higher with intracardiac thrombi than without thrombi. Large retrospective studies of cardiac surgery in patients with atrial fibrillation demonstrated a positive effect of simultaneous surgical occlusion of the left atrial appendage during cardiac surgery on stroke prevention, 27 , 28 but the efficacy of left atrial appendage closure alone is unknown, as is its efficacy in patients with NVAF‐associated stroke with intracardiac thrombi. As seen in the present study, estimation of the impact of intracardiac thrombi on the risk for recurrent embolic events can be biased because TEE is basically performed only for patients who could expect benefit over risk of TEE. Further studies are warranted to unbiasedly estimate the impact of intracardiac thrombi on the embolic risk after NVAF‐associated stroke based on accurate determination of the presence or absence of thrombi. Use of cardiac computed tomography, which is less invasive than TEE and possibly as sensitive as TEE, may be used in combination with TEE when conducting such studies. 29
Some limitations need to be considered when interpreting the present results. First, the timing of TEE and TTE relative to the index event was unknown. Considering the duration of hospital stay, both would have been performed within ≈10 to 20 days after onset in most cases. Second, indications of TEE for stroke would vary among the participating centers as well as between countries, which might have been reflected in the outliers seen in Figure 2A and in the different rates of thrombus detection between South Korea and Japan (Table 1). In addition, between South Korea and Japan, there are differences in drug indications, as well as stroke care process and healthcare environments. 30 Third, the information on the modality by which intracardiac thrombi were detected was unavailable from the CRCS‐K and SAMURAI‐NVAF registries. The location of thrombi was also unknown. Fourth, the present data could not show the clinical relevance of TEE when compared with computed tomography or magnetic resonance imaging. 31 , 32 Fifth, the presence of unmeasured confounders, including the clinical types of NVAF (eg, paroxysmal, persistent, or permanent), should be considered. 12 Last, the applicability of our findings to ethnicities other than East‐Asian is unknown.
In this multinational cohort, the clinical significance of TEE for accurate diagnosis and risk stratification of intracardiac thrombi in patients with stroke with NVAF was noted. Clarification of the role of TEE would lead to more accurate risk stratification and further optimization of preventive strategies after NVAF‐associated stroke.
Sources of Funding
This study was supported by a Grant‐in‐Aid (H23‐JunkankiIppan‐010; 19K17023) from the Ministry of Health, Labour and Welfare, Japan; the Japan Agency for Medical Research and Development (AMED) (19lk0201094h0001); Bristol‐Myers Squibb Korea; and the Korea Centers for Disease Control and Prevention (no. 2017ER620101). The funding sources did not participate in any part of the study, from study conception to article preparation.
Disclosures
Dr Koga reports personal fees from Bayer Yakuhin, BMS/Pfizer, Daiichi‐Sankyo, Nippon Boehringer Ingelheim, and Ono; and grants from Takeda, Daiichi‐Sankyo, Nippon Boehringer Ingelheim, Astellas, and Shionogi, outside this work. Dr Cha received lecture honoraria from Sanofi, Otsuka, and Pfizer and a site investigator of multicenter clinical trial sponsored by BMS Korea, Shinpoong Pharm. Co., Boehringer Ingelheim, Daiichi‐Sankyo, Esai, AstraZeneca Korea, Servier Korea, Jeil Pham., outside this work. B.‐C. Lee reports grants from Boehringer Ingelheim, Bayer, Daiichi‐Sankyo, Eisai, and Astra Zeneca, outside this work. Dr Bae is a principal investigator, a member of the steering committee, and/or a site investigator of multicenter clinical trials or clinical studies that are sponsored by BMS Korea, Shinpoong Pharm. Co. Ltd., Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, Esai, AstraZeneca Korea, Servier Korea, Yuhan Corporation, Jeil Pharmaceutical Co., Shire Korea, JLK inspection, Chong Gun Dang Pharmaceutical Corp., and Dong‐A Pharmaceutical and received lecture honoraria from Esai, Shire Korea, Amgen, and Otsuka Korea, outside this work. Dr Toyoda received lecture honoraria from Nippon Boehringer Ingelheim, Bayer Yakuhin, Daiichi‐Sankyo, and Bristol‐Myers Squibb, outside this work. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
Tables S1–S2
Acknowledgments
Author contributions: Drs Tanaka, Koga, Kim, K.‐J. Lee, and Bae had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Koga, Tanaka, Kim, K.‐J. Lee, Yoshimura, Bae, Toyoda. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Tanaka, Mizoguchi, Koge, Koga, Toyoda. Critical revision of the manuscript for important intellectual content: Koga, Kim, Cha, B.‐C. Lee, Koge, Toyoda, Bae. Statistical analysis: Tanaka, Mizoguchi, Park, J. Lee. Study supervision: Bae, Toyoda, Koga.
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–459. doi: 10.1016/0735-1097(94)00396-8 [DOI] [PubMed] [Google Scholar]
- 2. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X [DOI] [PubMed] [Google Scholar]
- 3. Leung DY, Davidson PM, Cranney GB, Walsh WF. Thromboembolic risks of left atrial thrombus detected by transesophageal echocardiogram. Am J Cardiol. 1997;79:626–629. doi: 10.1016/S0002-9149(96)00828-4 [DOI] [PubMed] [Google Scholar]
- 4. Sen S, Laowatana S, Lima J, Oppenheimer SM. Risk factors for intracardiac thrombus in patients with recent ischaemic cerebrovascular events. J Neurol Neurosurg Psychiatry. 2004;75:1421–1425. doi: 10.1136/jnnp.2004.038687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. SPAF III Investigators . Transesophageal echocardiographic correlates of thromboembolism in high‐risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann Intern Med. 1998;128:639–647. [DOI] [PubMed] [Google Scholar]
- 6. Omran H, Jung W, Rabahieh R, Wirtz P, Becher H, Illien S, Schimpf R, Lüderitz B. Imaging of thrombi and assessment of left atrial appendage function: a prospective study comparing transthoracic and transoesophageal echocardiography. Heart. 1999;81:192–198. doi: 10.1136/hrt.81.2.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 8. Tanaka K, Koga M, Lee K‐J, Kim BJ, Park EL, Lee J, Mizoguchi T, Yoshimura S, Cha J‐K, Lee B‐C, et al. Atrial fibrillation‐associated ischemic stroke patients with prior anticoagulation have higher risk for recurrent stroke. Stroke. 2020;51:1150–1157. doi: 10.1161/STROKEAHA.119.027275 [DOI] [PubMed] [Google Scholar]
- 9. Kim BJ, Park J‐M, Kang K, Lee SJ, Ko Y, Kim JG, Cha J‐K, Kim D‐H, Nah H‐W, Han M‐K, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke‐fifth division registry in South Korea. J Stroke. 2015;17:38–53. doi: 10.5853/jos.2015.17.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim BJ, Cho Y‐J, Hong K‐S, Lee J, Kim J‐T, Choi KH, Park TH, Park S‐S, Park J‐M, Kang K, et al. Trajectory groups of 24‐hour systolic blood pressure after acute ischemic stroke and recurrent vascular events. Stroke. 2018;49:1836–1842. doi: 10.1161/STROKEAHA.118.021117 [DOI] [PubMed] [Google Scholar]
- 11. Toyoda K, Arihiro S, Todo K, Yamagami H, Kimura K, Furui E, Terasaki T, Shiokawa Y, Kamiyama K, Takizawa S, et al. Trends in oral anticoagulant choice for acute stroke patients with nonvalvular atrial fibrillation in Japan: the SAMURAI‐NVAF study. Int J Stroke. 2015;10:836–842. doi: 10.1111/ijs.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koga M, Yoshimura S, Hasegawa Y, Shibuya S, Ito Y, Matsuoka H, Takamatsu K, Nishiyama K, Todo K, Kimura K, et al. Higher risk of ischemic events in secondary prevention for patients with persistent than those with paroxysmal atrial fibrillation. Stroke. 2016;47:2582–2588. doi: 10.1161/STROKEAHA.116.013746 [DOI] [PubMed] [Google Scholar]
- 13. Yoshimura S, Koga M, Sato S, Todo K, Yamagami H, Kumamoto M, Itabashi R, Terasaki T, Kimura K, Yagita Y, et al. Two‐year outcomes of anticoagulation for acute ischemic stroke with nonvalvular atrial fibrillation‐SAMURAI‐NVAF Study. Circ J. 2018;82:1935–1942. [DOI] [PubMed] [Google Scholar]
- 14. Mizoguchi T, Tanaka K, Toyoda K, Yoshimura S, Itabashi R, Takagi M, Todo K, Shiozawa M, Yagita Y, Yoshimoto T, et al. Early initiation of direct oral anticoagulants after onset of stroke and short‐ and long‐term outcomes of patients with nonvalvular atrial fibrillation. Stroke. 2020;51:883–891. doi: 10.1161/STROKEAHA.119.028118 [DOI] [PubMed] [Google Scholar]
- 15. Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, Haley EC, Grotta J, Marler J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25:2220–2226. doi: 10.1161/01.STR.25.11.2220 [DOI] [PubMed] [Google Scholar]
- 16. Shinohara Y, Minematsu K, Amano T, Ohashi Y. Modified Rankin scale with expanded guidance scheme and interview questionnaire: interrater agreement and reproducibility of assessment. Cerebrovasc Dis. 2006;21:271–278. doi: 10.1159/000091226 [DOI] [PubMed] [Google Scholar]
- 17. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 18. Frenkel D, D'Amato SA, Al‐Kazaz M, Markowitz SM, Liu CF, Thomas G, Ip JE, Sharma SK, Yang H, Singh P, et al. Prevalence of left atrial thrombus detection by transesophageal echocardiography: a comparison of continuous non‐vitamin K antagonist oral anticoagulant versus warfarin therapy in patients undergoing catheter ablation for atrial fibrillation. JACC Clin Electrophysiol. 2016;2:295–303. [DOI] [PubMed] [Google Scholar]
- 19. Wu M, Gabriels J, Khan M, Shaban N, D'Amato S, Liu CF, Markowitz SM, Ip JE, Thomas G, Singh P, et al. Left atrial thrombus and dense spontaneous echocardiographic contrast in patients on continuous direct oral anticoagulant therapy undergoing catheter ablation of atrial fibrillation: comparison of dabigatran, rivaroxaban, and apixaban. Heart Rhythm. 2018;15:496–502. doi: 10.1016/j.hrthm.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 20. Hwang J, Park H‐S, Jun S‐W, Choi S‐W, Lee CH, Kim I‐C, Cho Y‐K, Yoon H‐J, Kim H, Nam C‐W, et al. The incidence of left atrial appendage thrombi on transesophageal echocardiography after pretreatment with apixaban for cardioversion in the real‐world practice. PLoS One. 2018;13:e0208734. doi: 10.1371/journal.pone.0208734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Troughton RW, Asher CR, Klein AL. The role of echocardiography in atrial fibrillation and cardioversion. Heart. 2003;89:1447–1454. doi: 10.1136/heart.89.12.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calenda BW, Fuster V, Halperin JL, Granger CB. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol. 2016;13:549–559. doi: 10.1038/nrcardio.2016.106 [DOI] [PubMed] [Google Scholar]
- 23. Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res. 2009;124:37–41. doi: 10.1016/j.thromres.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 24. Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, Macha K, Tsivgoulis G, Ambler G, Arihiro S, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87:677–687. doi: 10.1002/ana.25700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sade LE, Keskin S, Can U, Çolak A, Yüce D, Çiftçi O, Özin B, Müderrisoğlu H. Left atrial mechanics for secondary prevention from embolic stroke of undetermined source. Eur Heart J Cardiovasc Imaging. 2020:jeaa311. doi: 10.1093/ehjci/jeaa311 [DOI] [PubMed] [Google Scholar]
- 26. Tanaka K, Koga M, Sato K, Suzuki R, Minematsu K, Toyoda K. Three‐dimensional analysis of the left atrial appendage for detecting paroxysmal strial fibrillation in acute ischemic stroke. Int J Stroke. 2014;9:1045–1051. [DOI] [PubMed] [Google Scholar]
- 27. Friedman DJ, Piccini JP, Wang T, Zheng J, Malaisrie SC, Holmes DR, Suri RM, Mack MJ, Badhwar V, Jacobs JP, et al. Association between left atrial appendage occlusion and readmission for thromboembolism among patients with atrial fibrillation undergoing concomitant cardiac surgery. JAMA. 2018;319:365–374. doi: 10.1001/jama.2017.20125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yao X, Gersh BJ, Holmes DR Jr, Melduni RM, Johnsrud DO, Sangaralingham LR, Shah ND, Noseworthy PA. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing cardiac surgery. JAMA. 2018;319:2116–2126. doi: 10.1001/jama.2018.6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aimo A, Kollia E, Ntritsos G, Barison A, Masci PG, Figliozzi S, Klettas D, Stamatelopoulos K, Delialis D, Emdin M, et al. Echocardiography versus computed tomography and cardiac magnetic resonance for the detection of left heart thrombosis: a systemic review and meta‐analysis. Clin Res Cardiol. 2020. doi: 10.1007/s00392-020-01741-7 [DOI] [PubMed] [Google Scholar]
- 30. Song D, Tanaka E, Lee K, Sato S, Koga M, Kim YD, Nagatsuka K, Toyoda K, Heo JH. Factors associated with early hospital arrival in patients with acute ischemic stroke. J Stroke. 2015;17:159–167. doi: 10.5853/jos.2015.17.2.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pathan F, Hecht H, Narula J, Marwick TH. Roles of transesophageal echocardiography and cardiac computed tomography for evaluation of left atrial thrombus and associated pathology: a review and critical analysis. JACC Cardiovasc Imaging. 2018;11:616–627. [DOI] [PubMed] [Google Scholar]
- 32. Kitkungvan D, Nabi F, Ghosn MG, Dave AS, Quinones M, Zoghbi WA, Valderrabano M, Shah DJ. Detection of LA and LAA thrombus by CMR in patients referred for pulmonary vein isolation. JACC Cardiovasc Imaging. 2016;9:809–818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S2


