Abstract
Background
Food insecurity (FI) has been associated with an increased atherosclerotic cardiovascular disease (ASCVD) risk; however, the pathways by which FI leads to worse cardiovascular health are unknown. We tested the hypothesis that FI is associated with ASCVD risk through nutritional/anthropometric (eg, worse diet quality and increased weight), psychological/mental health (eg, increased depressive symptoms and risk of substance abuse), and access to care pathways.
Methods and Results
We conducted a cross‐sectional study of adults (aged 40–79 years) using the 2007 to 2016 National Health and Nutrition Examination Survey. Our primary exposure was household FI, and our outcome was 10‐year ASCVD risk categorized as low (<5%), borderline (≥5% –<7.5%), intermediate (≥7.5%–<20%), and high risk (≥20%). We used structural equation modeling to evaluate the pathways and multiple mediation analysis to determine direct and indirect effects. Of the 12 429 participants, 2231 (18.0%) reported living in a food‐insecure household; 5326 (42.9%) had a low ASCVD risk score, 1402 (11.3%) borderline, 3606 (29.0%) intermediate, and 2095 (16.9%) had a high‐risk score. In structural models, we found significant path coefficients between FI and the nutrition/anthropometric (β, 0.130; SE, 0.027; P<0.001), psychological/mental health (β, 0.612; SE, 0.043; P<0.001), and access to care (β, 0.110; SE, 0.036; P=0.002) pathways. We did not find a significant direct effect of FI on ASCVD risk, and the nutrition, psychological, and access to care pathways accounted for 31.6%, 43.9%, and 15.8% of the association, respectively.
Conclusions
We found that the association between FI and ASCVD risk category was mediated through the nutrition/anthropometric, psychological/mental health, and access to care pathways. Interventions that address all 3 pathways may be needed to mitigate the negative impact of FI on cardiovascular disease.
Keywords: atherosclerotic cardiovascular disease, food insecurity, social determinants of health
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Epidemiology, Mental Health, Risk Factors
Nonstandard Abbreviations and Acronyms
- FI
food insecurity
- HEI‐15
Healthy Eating Index‐2015
- NHANES
National Health and Nutrition Examination Survey
- PCE
pooled cohort equation
- PHQ‐9
Patient Health Questionnaire‐9
- SEM
structural equation modeling
- USDA
United States Department of Agriculture
Clinical Perspective
What Is New?
We found that food insecurity was associated with higher odds of participants being in an elevated atherosclerotic cardiovascular disease risk category.
Over 90% of the association between food insecurity and atherosclerotic cardiovascular disease risk was mediated by the nutritional/anthropometric, psychological/mental health, and access to care pathways, with the psychological pathway accounting for almost half of the effect.
What Are the Clinical Implications?
Food insecurity likely leads to adverse health outcomes through multiple pathways.
Interventions that address food insecurity and all 3 pathways may be necessary to reduce the impact of food insecurity on cardiovascular health.
The social determinants of health, or the conditions in which people are born, grow, live, and age, have a profound impact on morbidity and mortality. 1 , 2 , 3 One way social determinants of health can affect health is through structuring the distribution of unmet health‐related social needs for individual patients. Unmet health‐related social needs are associated with worse health outcomes and disparities in care. 1 One unmet social need that is prevalent in the United States is food insecurity (FI), or the lack of consistent access to enough food for an active and healthy life. 4 Before the COVID‐19 pandemic, 10.5% of US households, or over 35 million people, were food insecure. 4 An additional 17 million people in the United States are estimated to have become food insecure due to the social and economic impacts of the pandemic. 5
FI is associated with numerous negative health outcomes including cardiometabolic disease and increased atherosclerotic cardiovascular disease (ASCVD) risk. 6 , 7 , 8 , 9 , 10 , 11 The mechanisms or pathways by which FI affects cardiovascular health are still unclear, but 3 have been hypothesized: (1) nutritional/anthropometric, (2) psychological or mental health, and (3) compensatory or behavioral (Figure 1). 12 , 13 , 14 In the nutritional/anthropometric pathway, individuals living in food‐insecure households resort to consuming cheap, calorie‐dense foods because of cost, which can affect cardiovascular disease (CVD) risk both through diet quality and by leading to weight gain and obesity. 11 , 15 In the psychological pathway, FI leads to increased stress and feelings of depression because of concerns about having enough food. 16 , 17 Depression has been independently associated with progression of atherosclerosis and an elevated CVD risk. 18 , 19 In the compensatory pathway, the competing priorities of FI (ie, having to choose between spending money on food versus medical care) lead to individuals having to compensate for their inadequate food budget by reducing consumption of other goods and services necessary for health. 20 , 21 , 22 These can include delays in seeking medical care (because of the cost of accessing health care) and taking less medication than recommended because of cost. Although each pathway is plausible alone, few studies have comprehensively tested these pathways together or quantified their relative contributions. 12 Understanding the complex relationship between FI and CVD and the relative importance of these pathways could inform both interventions and policies designed to reduce FI and improve cardiovascular health.
Figure 1. Conceptual framework for examining the association between food insecurity and increased atherosclerotic cardiovascular disease (ASCVD) risk.

Compensatory/behavioral includes 2 aspects, access to care and medication adherence.
To fill this gap in the literature, we conducted a cross‐sectional analysis of national data to evaluate the pathways through which FI is associated with increased ASCVD risk. Specifically, we evaluated the association between household FI and 10‐year risk of developing a first ASCVD event based on the 2013 American College of Cardiology and American Heart Association Pooled Cohort risk equations (PCEs). 23 Elucidating these pathways could inform targets for interventions designed to mitigate the negative effect of FI on cardiovascular health and identify mediators of the association between FI and ASCVD risk.
Methods
Study Design and Data Sources
Based on a prior published conceptual framework, 12 , 13 , 14 we evaluated the nutrition/ anthropometric, psychological/mental health, and one aspect of the compensatory/behavioral (access to care) pathways through which FI may be associated with ASCVD risk. We conducted a cross‐sectional analysis and used structural equation modeling (SEM) to test these pathways using data from the National Health and Nutrition Examination Survey (NHANES). SEM is a statistical approach that uses a combination of techniques, including path analysis and factor analysis, to evaluate multivariate pathways between categorical variables, measured or indicator variables, and latent constructs. 24 SEM provides a framework for evaluating complex relationships between multiple variables and tests the validity of theoretical models (for this study evaluating the pathways through which FI may be associated with ASCVD risk). As opposed to regression analysis, SEM allows variables in the model to correlate, simultaneously testing the relationships among multiple observed and latent variables, and accounts for measurement error in both latent and observed variables. 24 , 25 , 26 NHANES is collected by the National Center for Health Statistics and is a series of large, cross‐sectional surveys conducted in 2‐year cycles. 27 We combined data from the 2007 to 2008, 2009 to 2010, 2011 to 2012, 2013 to 2014, and 2015 to 2016 waves of the NHANES, the most recent years available that include data on FI. The data obtained in the NHANES include responses to an in‐home interview (in either English or Spanish) on a variety of demographic and health characteristics, findings from a physical examination and 24‐hour dietary recall conducted at a mobile examination center, and laboratory measurements. Trained interviewers using a computer‐assisted personal interviewing system collected all questionnaire data. Further details of the NHANES study design and recruitment procedures have been previously published. 27 , 28 The data that support the findings of this study are available from the corresponding author upon reasonable request. Informed consent was obtained as part of the National Health and Nutrition Examination nation survey. The data we included in this study are from their survey but our study team did not obtain informed consent.
Study Sample
We limited inclusion to participants who were aged 40 to 79 years (N=17 389), because the ASCVD PCE risk score has been validated in this age group. 23 We then excluded participants who were pregnant (N=14) or missing FI data (N=243). We also excluded participants with a self‐reported history of CVD (coronary heart disease), myocardial infarction, angina, or stroke (N=2083), and participants who were missing data necessary to calculate the PCE score (cholesterol results, systolic blood pressure, or medication history [N=2620]), yielding a final sample size of 12 429 (71.5%).
Exposure
Our exposure of interest was household FI. FI was measured in the NHANES using the 18‐item US Department of Agriculture (USDA) Household Food Security Survey Module. 29 This validated questionnaire was developed by the USDA to measure household FI over the prior 12 months. NHANES administers the survey in accordance with recommendations from the USDA and uses the established scoring system for responses to categorize household food security: high food security (0 affirmative responses), marginal food security (1–2 affirmative responses), low food security (3–7 affirmative responses), and very low food security (8–18 affirmative responses). Consistent with USDA definitions, we defined FI as households that reported low or very low food security. In this study, we analyzed the data using both the categorical food security variable (high food security, marginal food security, low food security, and very low food security) and FI as a binary variable by categorizing participants as food secure (0–2 affirmative responses) or food insecure (≥3 affirmative responses). 29
Outcome
Our primary outcome was 10‐year ASCVD risk based on the 2013 American College of Cardiology/American Heart Association sex and race‐specific PCE. 23 The American College of Cardiology/American Heart Association PCE is a validated tool to evaluate individuals’ 10‐year risk of developing a first hard ASCVD event, including coronary heart disease death, nonfatal myocardial infarction, and fatal or nonfatal stroke. The risk estimates were derived from a combination of established cardiovascular risk factors examined prospectively in 5 cohorts. The variables include: age, sex, race and ethnicity (non‐Hispanic White, non‐Hispanic Black, other [including Hispanic, Asian, other race or multiracial]), smoking status, hypertension treatment status, systolic blood pressure, total and high‐density lipoprotein cholesterol, and diabetes. The PCE was validated for non‐Hispanic White and non‐Hispanic Black men and women. As recommended, 23 we used the equations for non‐Hispanic White participants for other racial and ethnic groups. We calculated the risk score for participants using the stepwise approach provided by the American College of Cardiology and American Heart Association. 23 For this study, age, sex, race and ethnicity, smoking status (current or not), and if a participant was taking a medication to manage blood pressure were based on self‐report. Systolic blood pressure was based on the average of 3 readings as measured at the NHANES examination. Total cholesterol and high‐density lipoprotein cholesterol were based on direct laboratory measures at the time of the NHANES examination. Diabetes was defined as having any of the following: self‐report of diabetes, glycated hemoglobin >6.5%, fasting glucose >126, or taking a diabetes medication. 10 , 30 As in prior studies, 31 we categorized participants based on their estimated 10‐year ASCVD risk into the following groups: low (<5%), borderline (≥5%–<7.5%), intermediate (≥7.5%–<20%), and high (≥20%).
Measures
We constructed variables based on the questionnaire and physical examination data collected in the NHANES for 3 latent constructs: nutrition/anthropometric, psychological/mental health, and access to care.
Nutrition/Anthropometric Latent Construct
The nutrition/anthropometric latent construct was based on 3 indicator variables: Healthy Eating Index (HEI)‐2015 score, body mass index (BMI), and waist circumference (centimeters). Researchers from the USDA’s Center for Nutrition Policy and Promotion and the National Cancer Institute developed the HEI‐2015 score to assess individuals’ conformance to the 2015 to 2020 Dietary Guidelines for Americans. 32 , 33 , 34 The HEI‐2015 consists of 13 components: total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium, added sugars, and saturated fats. The HEI‐2015 score assesses whether individuals met the recommended amounts of the initial 9 components and did not exceed the recommended amounts from the last 4 dietary components. We calculated the HEI‐2015 total score using the standard scoring algorithm established by the USDA and National Cancer Institute based on one 24‐hour dietary recall. The score ranges from 0 to 100, with a higher HEI‐2015 score reflecting closer adherence to the 2015 to 2020 dietary guidelines or a healthier diet. BMI (weight in kilograms divided by height in meters squared) based on weight and height, and waist circumference (in centimeters) were measured at the time of the NHANES examination.
Psychological/Mental Health Latent Construct
The psychological latent construct was based on 4 indicator variables: depressive symptoms, illicit drug use, alcohol abuse, and contact with a mental health provider. Depressive symptoms were measured by the validated Patient Health Questionnaire‐9 (PHQ‐9). 35 , 36 The PHQ‐9 consists of 9 items that assess the frequency of symptoms over the prior 2 weeks. Responses include not at all, several days, more than half the days, and nearly every day, and are given a point range from 0 to 3. We evaluated depressive symptoms as a continuous variable based on the total sum of the item responses (0–27). Illicit drug use was based on if participants self‐reported ever using cocaine, heroin, or methamphetamines (yes or no). Alcohol abuse was based on one question that asked if participants drank 4 (women)/5 (men) or more alcoholic beverages of any kind almost every day (yes or no). Contact with a mental health provider was based on self‐report (yes or no) to one question asked to all participants, “During the past 12 months, have you seen or talked to a mental health professional such as a psychologist, psychiatrist, psychiatric nurse, or clinical social worker about your health?”
Access to Preventative Care Latent Construct
As described above, 2 key elements of the compensatory pathway include forgoing care and having suboptimal medication adherence, because of the resource constraints of FI. Unfortunately, the NHANES does not contain data needed to assess medication adherence. Therefore, we examined only the aspect of the compensatory pathway related to access to care. This latent construct was based on 3 indicator variables, with a higher score representing worse access to preventative care: routine place to go for health care, usual source of care, and frequency of health care use in the past year. Routine place to go for health care was based on one question asking participants if there is a place they go if they are sick or need advice about health (yes or no). Usual source of care was based on one question asking participants what kind of place they go for health care (clinic/doctor’s office, or other). Frequency of health care use was based on one question asking participants how many times they had seen a doctor or other health care professional in the past 12 months, not including if they had been seen in the emergency room or been hospitalized (none or ≥1 times).
Covariates
Covariates included demographic characteristics that have been associated with FI and ASCVD risk. 4 These included age, household income‐to‐poverty ratio, highest education level achieved (lower than high school, high school graduate, or higher than high school), and current marital status (married/living with a partner, divorced/widowed/separated, or never married). Household income‐to‐poverty ratio is calculated by dividing the total household income by the poverty guidelines, based on family size, year, and state. Because sex and race and ethnicity (non‐Hispanic White, non‐Hispanic Black, other [including Hispanic, Asian, other race or multiracial]) are used to determine the PCE used to calculate ASCVD risk, we did not include them as covariates in the multivariable model or structural model, but did use sex and race and ethnicity to characterize the study population and evaluate for differences in bivariate analysis. We did not control for smoking status, blood pressure, cholesterol, or diabetes because they were used to calculate ASCVD risk score.
Statistical Analysis
We performed univariate analysis and bivariate analysis, using χ2 test or t test, to evaluate the association between FI and each individual measure and covariate. We used multivariate normal multiple imputation with 10 imputed data sets to quantify uncertainty related to missing values for HEI score (5.3% missing), BMI (1.0% missing), waist circumference (3.1% missing), PHQ‐9 (7.2% missing), and income‐to‐poverty ratio (8.1% missing). 24 We performed bivariate analyses, using χ2 tests, t tests, or analysis of variance to compare characteristics between food‐insecure and food‐secure participants, and we used multivariable ordinal logistic regression to evaluate the association between FI and ASCVD risk categories. Afterward, SEM was conducted to assess the relationship between FI and ASCVD risk. We followed a 2‐step approach to evaluate the model; first, the measurement models were tested and refined, and second, the structural model was tested using weighted least squares. Controlling for age, income, education level, and marital status, we initially tested the model with FI as a binary variable and then tested the model using FI as a categorical variable. All continuous variables (HEI‐2015, BMI, waist circumference, PHQ‐9, age, income‐to‐poverty ratio) included in the SEM analysis were standardized. For the SEM analyses, we reverse coded the HEI‐2015 score (100–total score), so higher scores represented worse dietary quality, and the directionality would be similar to other variables included in the nutrition latent construct (eg, worse diet quality and higher BMI would be more likely to lead to increased ASCVD risk). Model fit was determined using accepted relative fit indices: the comparative fit index, Tucker‐Lewis index, the root mean square error of approximation , and the standardized root mean square residual, with values >0.95 comparative fit index, >0.95 Tucker‐Lewis index, <0.06 root mean square error of approximation, and <0.08 standardized root mean square residual recognized as good fit. 37 We used modification indices to fine tune the model for improvement. Modification indices represent a commonly used, data‐driven improvement in the fit of the hypothesized model if a previously omitted parameter was added and freely estimated. The largest modification indices may be associated with parameters that are unsupported by theory and represent idiosyncratic characteristics of the data; therefore, we only used modification indices that were theoretically plausible (eg, the correlation between PHQ‐9 and BMI). 38 We used bias‐corrected bootstrapping, with 1000 iterations, to determine direct and indirect effects with 95% CIs of the relationship between FI and ASCVD risk. Because our goal was to investigate the relationship between FI and ASCVD risk score, and not to generate nationally representative estimates, we did not apply the sample weights included in the NHANES for this study. We used a 2‐sided hypothesis test and considered a P value of <0.05 statistically significant. All analyses were conducted using Stata 15.1 (StataCorp, College Station, TX) and Mplus V8 software (Muthén & Muthén). The Wake Forest School of Medicine Institutional Review Board deemed this study of publicly available, deidentified data exempt from human subject research.
Results
Study Population Characteristics
Of the 12 429 participants, the plurality were women (51.7%), non‐Hispanic White (41.3%), and had higher than a high school education level (51.0%) (Table 1). Of the participants, 2231 (18.0%) reported living in a food‐insecure household, of whom 1373 (11.1%) reported low food security, and 858 (6.9%) reported very low food security. In the study population, 5326 (42.9%) had a low ASCVD risk score, 1402 (11.3%) had a borderline risk score, 3606 (29.0%) had an intermediate score, and 2095 (16.9%) were identified as having a high ASCVD risk score.
Table 1.
Study Population Characteristics (N=12,429)
| Characteristic | Value | |
|---|---|---|
| Age, y | Mean (SD) | 56.5 (10.7) |
| Sex | Women | 6423 (51.7) |
| Race and ethnicity | Non‐Hispanic White | 5131 (41.3) |
| Non‐Hispanic Black | 2610 (21.0) | |
| Hispanic | 3417 (27.5) | |
| Other | 1271 (10.2) | |
| Marital status | Married/living with a partner | 8076 (65.0) |
| Divorced/widowed/separated | 3221 (25.9) | |
| Never married | 1125 (9.1) | |
| Adult education level | <High school | 3335 (26.8) |
| High school graduate | 2755 (22.2) | |
| >High school | 6339 (51.0) | |
| Income‐to‐poverty ratio | Mean (SD) | 2.67 (1.6) |
| HEI‐2015 score | Mean (SD) | 52.4 (13.5) |
| Body mass index | Mean (SD) | 29.4 (6.5) |
| Waist circumference | Mean (SD) | 101.0 (15.3) |
| PHQ‐9 total | Mean (SD) | 3.1 (4.2) |
| Drug use | Yes | 1685 (13.6) |
| Alcohol abuse | Yes | 990 (8.0) |
| Mental health provider | Yes | 908 (7.3) |
| Place for care | None | 1525 (12.3) |
| Usual source of care | Not a clinic or doctor’s office | 2230 (17.9) |
| Health care use | None | 1841 (14.8) |
| Household food security | Food secure | 8912 (71.7) |
| Marginal food security | 1286 (10.4) | |
| Low food security | 1373 (11.1) | |
| Very low food security | 858 (6.9) | |
| Food insecurity | Yes | 2231 (18.0) |
| ASCVD category | Low, <5% | 5326 (42.9) |
| Borderline, ≥5–<7.5% | 1402 (11.3) | |
| Intermediate, ≥7.5–<20% | 3606 (29.0) | |
| High, ≥20% | 2095 (16.9) |
Data are presented as number (percent) unless otherwise specified. ASCVD indicates atherosclerotic cardiovascular disease; HEI‐2015, Healthy Eating Index‐2015; and PHQ‐9, Patient Health Questionnaire‐9.
In bivariate analysis, FI was associated with younger age, being a woman, identifying as being non‐Hispanic Black or Hispanic, lower educational attainment, and lower household income‐to‐poverty ratio (Table 2; all P<0.001). Participants who were food insecure, compared with participants who were food secure, were more likely to have a lower mean HEI‐2015 score (49.4 versus 53.1; P<0.001), higher mean BMI (30.3 versus 29.2 kg/m2; P<0.001), and higher waist circumference (102.4 versus 100.7 cm, P<0.001). Food‐insecure participants were also more likely to report a higher mean PHQ‐9 total (5.5 versus 2.7; P<0.001), illicit drug use (17.6% versus 12.7%; P<0.001), alcohol abuse (11.2% versus 7.3%; P<0.001), and seeing a mental health provider in the past 12 months (12.5% versus 6.2%; P<0.001). Food‐insecure participants were significantly more likely to report not having a routine place to go for health care (19.2% versus 10.8%; P<0.001), usual source of care was not a clinic or doctor’s office (28.6% versus 15.6%; P<0.001), and not seeing a doctor or other health care professional in the prior 12 months (20.3% versus 13.6%). In multivariable models controlling for age, income, education, and marital status, FI was significantly associated with participants being in a higher risk ASCVD category (β, 0.17; 95% CI, 0.07–0.28; P=0.001) (Table 3). This association was primarily seen among participants who lived in very low food‐secure households (β, 0.38; 95% CI, 0.22–0.54; P<0.001).
Table 2.
Characteristics of Food‐Secure Compared With Food‐Insecure Participants
| Characteristic | Food secure, N=9698 | Food insecure, N=2176 | P value | |
|---|---|---|---|---|
| Age, y | Mean (SD) | 57.1 (10.7) | 54.1 (10.0) | <0.001 |
| Sex | ||||
| Men | 4991 (48.9) | 1015 (45.5) | 0.003 | |
| Women | 5207 (51.1) | 1216 (54.5) | ||
| Race and ethnicity | ||||
| Non‐Hispanic White | 4494 (44.1) | 637 (28.6) | <0.001 | |
| Non‐Hispanic Black | 2077 (20.4) | 533 (23.9) | ||
| Hispanic | 2509 (24.6) | 908 (40.7) | ||
| Other | 1118 (11.0) | 153 (6.9) | ||
| Marital status | ||||
| Married/living with a partner | 6885 (67.6) | 1191 (53.4) | <0.001 | |
| Divorced/widowed/separated | 2466 (24.2) | 755 (33.9) | ||
| Never married | 841 (8.3) | 284 (12.7) | ||
| Education level | ||||
| <High school | 2343 (23.0) | 992 (44.5) | <0.001 | |
| High school graduate | 2226 (21.8) | 529 (23.7) | ||
| >High school | 5629 (55.2) | 710 (31.8) | ||
| Income‐to‐poverty ratio | Mean (SD) | 2.9 (1.6) | 1.4 (1.0) | <0.001 |
| HEI‐2015 score | Mean (SD) | 53.1 (13.5) | 49.4 (13.2) | <0.001 |
| Body mass index | Mean (SD) | 29.2 (6.4) | 30.3 (7.0) | <0.001 |
| Waist circumference | Mean (SD) | 100.7 (15.2) | 102.4 (15.8) | <0.001 |
| PHQ‐9 total | Mean (SD) | 2.7 (3.7) | 5.1 (5.5) | <0.001 |
| Drug use | Yes | 1292 (12.7) | 393 (17.6) | <0.001 |
| Alcohol abuse | Yes | 741 (7.3) | 249 (11.2) | <0.001 |
| Mental health provider | Yes | 630 (6.2) | 278 (12.5) | <0.001 |
| Place for care | None | 1096 (10.8) | 429 (19.2) | <0.001 |
| Usual source of care | Not a clinic or doctor’s office | 1592 (15.6) | 638 (28.6) | <0.001 |
| Health care use | None | 1388 (13.6) | 453 (20.3) | <0.001 |
Data are presented as number (percent) unless otherwise specified. HEI‐2015 indicates Healthy Eating Index‐2015; and PHQ‐9, Patient Health Questionnaire‐9.
Table 3.
Multivariable Analysis Evaluating Association Between Food Security and Atherosclerotic Cardiovascular Disease Risk Category
| Binary FI model | Categorical FI model | ||||
|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | ||
| Food secure | Ref | Ref | |||
| Food insecure | 0.17 (0.07–0.28) | 0.001 | … | ||
| Marginal food security | … | 0.15 (0.02–0.28) | 0.02 | ||
| Low food security | … | 0.10 (−0.03 to 0.23) | 0.14 | ||
| Very low food security | … | 0.38 (0.22–0.54) | <0.001 | ||
| Age | 0.20 (0.20–0.21) | <0.001 | 0.20 (0.20–0.21) | <0.001 | |
| Marital status | Married/living with a partner | Ref | Ref | ||
| Divorced/widowed/separated | −0.27 (−0.36 to −0.18) | <0.001 | −0.27 (−0.36 to −0.18) | <0.001 | |
| Never married | 0.11 (−0.02 to 0.25) | 0.11 | 0.11 (−0.03 to 0.25) | 0.11 | |
| Education level | <High school | 0.26 (0.16–0.36) | <0.001 | 0.26 (0.16–0.36) | <0.001 |
| High school graduate | 0.32 (0.22–0.42) | <0.001 | 0.32 (0.23–0.42) | <0.001 | |
| >High school | Ref | Ref | |||
| Income‐to‐poverty ratio | −0.15 (−0.18 to −0.13) | <0.001 | −0.15 (−0.17 to −0.12) | <0.001 | |
FI indicates food insecurity; and Ref, reference.
Pathways Through Which FI Was Associated With ASCVD Risk Category
Figure 2 depicts the structural model for the association between FI and ASCVD risk category controlling for all covariates. The initial model did not achieve acceptable goodness of fit across all metrics. Therefore, we used modification indices to add 5 correlations to indicator variables in the model (Table 4). After modifications, the model with FI as a binary variable (food secure versus food insecure) showed good model fit (Figure 2a). We found a significant path coefficient between FI and the nutrition/anthropometric (β, 0.130; SE, 0.027; P<0.001), psychological (β, 0.612; SE, 0.043; P<0.001), and access to care (β, 0.110; SE, 0.036; P=0.002) latent constructs. There was a significant association between the nutrition/anthropometric (β, 0.137; SE, 0.007; P<0.001), psychological/mental health (β, 0.040; SE, 0.017; P=0.02), and access to care (β, 0.085; SE, 0.012; P<0.001) latent constructs and an elevated ASCVD risk category. In mediation analysis, we did not find a significant direct effect between FI and ASCVD risk category after adjusting for the indirect effects, and the indirect effect of the pathways accounted for 91.2% of the total effect (Table 5). The nutritional/anthropometric, psychological/mental health, and access to care paths accounted for 31.6%, 43.9%, and 15.8%, respectively, of the association of FI with ASCVD risk category.
Figure 2. Path diagram showing the relationship between food insecurity and atherosclerotic cardiovascular disease (ASCVD) risk category.
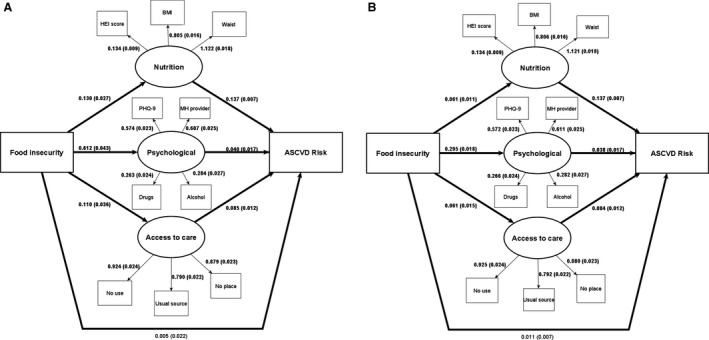
A, Binary food insecurity (food insecure vs food secure). B, Categorical food security (high, marginal, low, and very low food security). All associations adjusted for age, income‐to‐poverty ratio, highest education level achieved, and marital status. Includes β coefficient (SEs), and bold indicates significance of P<0.05. BMI indicates body mass index; Drugs, self‐reported use of cocaine, heroin, or methamphetamines; HEI, Healthy Eating Index‐2015; MH, mental health (provider seen in the past 12 months); No place, place participant goes if sick or needs advice about health (yes or no); No use, how many times participant saw a doctor or other health care professional in the past 12 months (none or ≥1 time); PHQ‐9, Patient Health Questionnaire‐9; Usual source, place participant goes for health care (clinic/doctor’s office or other); and Waist, waist circumference.
Table 4.
Goodness‐of‐Fit Indices for Each Model Modification
| RMSEA (90% CI) | CFI | TLI | SRMR | |
|---|---|---|---|---|
| Binary FI model | ||||
| Initial model | 0.050 (0.048–0.052) | 0.94 | 0.90 | 0.059 |
| Adding correlation between PHQ‐9 and BMI | 0.048 (0.046–0.050) | 0.95 | 0.91 | 0.059 |
| Adding correlation between PHQ‐9 and waist circumference | 0.047 (0.045–0.049) | 0.95 | 0.92 | 0.058 |
| Adding correlation between PHQ‐9 and no health care use | 0.045 (0.043–0.047) | 0.96 | 0.92 | 0.057 |
| Adding correlation between no health care use and seeing a mental health provider | 0.041 (0.039–0.043) | 0.96 | 0.94 | 0.046 |
| Adding correlation between no routine place to go for health care and seeing a mental health provider (final model) | 0.040 (0.038–0.042) | 0.97 | 0.94 | 0.041 |
| Categorical FI model | ||||
| Initial model | 0.050 (0.048–0.052) | 0.94 | 0.91 | 0.060 |
| Adding correlation between PHQ‐9 and BMI | 0.048 (0.046–0.050) | 0.95 | 0.91 | 0.060 |
| Adding correlation between PHQ‐9 and waist circumference | 0.047 (0.045–0.049) | 0.95 | 0.92 | 0.059 |
| Adding correlation between PHQ‐9 and no health care use | 0.045 (0.043–0.047) | 0.96 | 0.92 | 0.058 |
| Adding correlation between no health care use and seeing a mental health provider | 0.042 (0.040–0.044) | 0.96 | 0.94 | 0.048 |
| Adding correlation between no routine place to go for health care and seeing a mental health provider (final model) | 0.040 (0.038–0.042) | 0.97 | 0.94 | 0.042 |
BMI indicates body mass index; CFI, comparative fit index; FI, food insecurity; PHQ‐9, Patient Health Questionnaire‐9; RMSEA, root mean square error of approximation; SRMR, the standardized root mean square residual; and TLI, Tucker‐Lewis index.
Table 5.
Mediation Path of the Relationship Between Food Insecurity and Atherosclerotic Cardiovascular Disease Risk Category
| Effect | Bootstrap 95% CI | P value | % Total effect | |
|---|---|---|---|---|
| Binary food insecurity | ||||
| Total effect | 0.057 | 0.019–0.095 | 0.005 | |
| Total indirect effects | 0.052 | 0.03–0.076 | <0.001 | 91.2% |
| Direct effect of food insecurity | 0.005 | −0.04 to 0.047 | 0.83 | … |
| Indirect paths | ||||
| Nutrition/anthropometric | 0.018 | 0.011–0.26 | <0.001 | 31.6% |
| Psychological/mental health | 0.025 | 0.005–0.046 | 0.02 | 43.9% |
| Access to care | 0.009 | 0.003–0.017 | 0.005 | 15.8% |
| Categorical food insecurity | ||||
| Total effect | 0.034 | 0.017–0.049 | <0.001 | |
| Total indirect effects | 0.025 | 0.014–0.036 | <0.001 | 73.5% |
| Direct effect of food insecurity | 0.009 | −0.009 to 0.027 | 0.35 | … |
| Indirect paths | ||||
| Nutrition/anthropometric | 0.008 | 0.005–0.012 | <0.001 | 23.5% |
| Psychological/mental health | 0.011 | 0.001–0.021 | 0.03 | 44.0% |
| Access to care | 0.005 | 0.003–0.008 | <0.001 | 14.7% |
The model with FI as a categorical variable (high, marginal, low, and very low food security) also showed good model fit after modifications (Table 4, Figure 2B). Similarly, we found a significant path coefficient between FI and the nutrition/anthropometric (β, 0.061; SE, 0.011; P<0.001), psychological/mental health (β, 0.295; SE, 0.018; P<0.001), and access to care (β, 0.061; SE, 0.015; P<0.001) latent constructs. There was a significant association between the nutrition/anthropometric (β, 0.137; SE, 0.007; P<0.001), psychological/mental health (β, 0.038; SE, 0.011; P=0.03), and access to care (β, 0.084; SE, 0.012; P<0.001) latent constructs and ASCVD risk category. We did not find a significant direct effect of FI after adjusting for the indirect effects, and the indirect effect accounted for 73.5% of the total effect (Table 5). The nutritional/anthropometric, psychological/mental health, and access to care paths accounted for 23.5%, 44.0%, and 14.7%, respectively, of the association of FI with ASCVD risk category.
Discussion
There is a growing recognition of the impact of unmet health‐related social needs on the development of CVD and CVD risk factors and the need to address the social and economic factors that lead to CVD heath disparities. 2 , 39 In this study, we found that FI, primarily among individuals who lived in a household with very low food security, was associated with greater odds of participants having a higher predicted ASCVD risk score category. The association of FI with ASCVD risk category was mediated by the nutrition/anthropometric, psychological/mental health, and access to care pathways, and these 3 pathways accounted for over 90% of the total effect of the association between FI and ASCVD risk category.
Our results are consistent with the growing body of literature showing FI to be associated with an increased risk of ASCVD risk and CVD. 6 , 7 , 8 , 9 , 10 , 11 , 30 This study adds to this prior research by evaluating the complex relationship between FI and ASCVD risk and testing the theory that the association between FI and ASCVD is mediated by the nutrition/anthropometric, psychological/mental health, and access to care pathways. We found that the association of FI and an elevated ASCVD risk category was mediated through all 3 of these potentially modifiable pathways. Because of limited food budgets, studies have found that individuals in food‐insecure households are incentivized to consume cheap, calorie‐dense foods that could directly lead to cardiometabolic risk factors (eg, diabetes or hyperlipidemia) or indirectly lead to increased ASCVD risk through increased weight gain and obesity. 15 , 40 , 41 Although studies often focus on the impact of FI on nutrition, prior studies, as well as our results, provide support that the association between FI and ASCVD risk is also mediated through the psychological/mental health and access to care pathways. 17 , 42 , 43 FI can lead to increased anxiety and depression, which have been shown to be associated with increased CVD risk. Studies have also shown that FI is associated with increased risk of substance abuse, which has been associated with increased CVD risk. 44 , 45 FI can also lead to delays in individuals seeking medical care, which could lead to the development or worsening of underlying health conditions and increased ASCVD risk. 22 , 46 , 47 The results of this study advance our understanding of the potential reasons FI could be associated with elevated CVD risk, and these results can inform future interventions designed to mitigate the negative effect of FI on ASCVD risk (ie, including proximal dietary and mental health outcomes in studies designed to address FI).
The association between FI and ASCVD risk category was primarily mediated through the nutrition/anthropometric and psychological/mental health pathways, with 31.6% of the total effect through the nutrition/anthropometric pathway and 43.9% of the total effect through the psychological/mental health pathway. One prior study that evaluated this same conceptual framework in individuals with HIV found that FI primarily affected HIV outcomes (eg, viral suppression and CD4 count) through the psychological and compensatory pathways. 12 It could be that FI negatively affects populations and chronic health conditions differently. It could also be that because we were only able to evaluate one aspect of the compensatory pathway, access to care, this resulted in lower estimates of the association explained by this pathway. Several studies have shown that FI can lead to delays or missed doses of prescribed medications because individuals have to choose between spending money on food versus medications, and we may have seen a larger indirect effect of the compensatory pathway if we had been able to include data on medication adherence. 21 , 48 Future studies can help confirm this conceptual framework in other populations and other health conditions.
This study has both important public health and clinical implications. FI is a major public health problem that affects over 35 million people in the United States and disproportionately impacts low‐income and minority households. 4 FI has increased because of the economic and social impacts of the COVID‐19 pandemic, and developing further interventions to mitigate the negative impacts of FI on health is important given the likely lasting economic effects of the pandemic. 5 There has been growing interest among national health care organizations in addressing FI and other unmet social needs in clinical settings. 49 , 50 , 51 These interventions have primarily focused on providing food‐insecure patients nutrition support (eg, referrals to local food pantries). 52 , 53 Interventions that address only the nutritional pathway may be less effective than interventions that address multiple pathways or FI directly. Multipronged interventions, such as pairing mental health services with the delivery of healthy food, may more effectively address the potential effect of FI on CVD risk. Addressing FI directly could also affect all 3 pathways by which FI may be associated with cardiometabolic health. The Supplemental Nutrition Assistance Program may serve as an example of such an intervention. By providing a near‐cash transfer to lower income households, Supplemental Nutrition Assistance Program participation could improve food access, improve mental health, and free up resources that can be devoted to health care and self‐management activities. Because the Supplemental Nutrition Assistance Program is the largest program in the United States to combat FI, examining associations between Supplemental Nutrition Assistance Program participation and cardiometabolic health is of great importance. 54 , 55 , 56 Given confounding related to the decision of eligible individuals to participate, however, we were not able to examine these associations in this study. We think this is an important step for future work. Additionally, broader public health and policy changes that address the underlying causes of FI, such as poverty, racism, and access to healthy foods, could be necessary to significantly address the negative health effects of FI.
There are several limitations to this study that should be acknowledged. First, the analysis used cross‐sectional data, so causation cannot be determined. Whether FI leads to an elevated ASCVD risk category or an elevated ASCVD risk category leads to FI is unclear. This was somewhat mitigated by excluding participants with a previous history of CVD, but future longitudinal studies should be conducted to test the pathways by which FI could lead to CVD risk. Second, the ASCVD PCE score has only been validated in non‐Hispanic White and non‐Hispanic Black populations. The score may overestimate the 10‐year risk in other racial and ethnic groups. Also, we evaluated the association between FI and ASCVD risk category. FI may influence the association between ASCVD risk and actual ASCVD, which could be a future area of research. Third, the HEI‐2015 score was based on one 24‐hour dietary intake, which may not represent a participants’ usual intake. Fourth, we were unable to include data on stress or anxiety in the psychological/mental health pathway, because the NHANES did not consistently measure these data through the years included in this study. Additionally, the NHANES does not include data on other health‐related social needs, such as lack of transportation, that often cluster with FI. It is unclear if FI is a marker for these other unmet social needs or on the causal pathway in the development of the ASCVD risk.
Conclusions
In this study, we found that FI was associated with higher odds of participants being in an elevated ASCVD risk category. Over 90% of the association between FI and ASCVD risk was mediated by the nutritional/anthropometric, psychological/mental health, and access to care pathways, with the psychological pathway accounting for almost half of the effect. Although further research is needed to confirm our associations, interventions that directly address FI and these 3 pathways may be needed to mitigate the negative impact of FI on CVD.
Sources of Funding
Dr Palakshappa is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL146902. Dr Berkowitz’s work on this project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Institute under award number K23DK109200. Dr Rosenthal reports grants from the National Institutes of Health during the conduct of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication. Dr Berkowitz reported receiving personal fees from the Aspen Institute outside of the submitted work.
Disclosures
None.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Solar O, Irwin A. A conceptual framework for action on the social determinants of health. Social Determinants of Health Discussion Paper 2 (Policy and Practice). http://www.who.int/sdhconference/resources/ConceptualframeworkforactiononSDH_eng.pdf. Accessed February 22, 2021.
- 2. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American heart association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 3. Hayman LL, Worel JN. Reducing disparities in cardiovascular health: social determinants matter. Cardiovasc Nurse. 2016;31:288–290. doi: 10.1097/JCN.0000000000000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coleman‐Jensen A, Rabbit MP, Gregory CA, Singh A. Household Food Insecurity in the United States in 2019, ERR‐275. U.S. Department of Agriculture Economic Research Service.
- 5. Gundersen C, Hake M, Dewey A, Engelhard E. Food insecurity during COVID‐19. Appl Econ Perspect Policy. 2021;43:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vercammen KA, Moran AJ, McClain AC, Thorndike AN, Fulay AP, Rimm EB. Food security and 10‐year cardiovascular disease risk among U.S. Adults. Am J Prev Med. 2019;56:689–697. doi: 10.1016/j.amepre.2018.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun Y, Liu B, Rong S, Du Y, Xu G, Snetselaar LG, Wallace RB, Bao W. Food insecurity is associated with cardiovascular and all‐cause mortality among adults in the United States. J Am Heart Assoc. 2020;9:e014629. doi: 10.1161/JAHA.119.014629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford ES. Food security and cardiovascular disease risk among adults in the United States: findings from the National Health and Nutrition Examination Survey, 2003–2008. Prev Chronic Dis. 2013;1:E202. doi: 10.5888/pcd10.130244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. J Gen Intern Med. 2007;22:1018–1023. doi: 10.1007/s11606-007-0192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005–2012. PLoS One. 2017;12:e0179172. doi: 10.1371/journal.pone.0179172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung C, Tester J, Laraia B. Household food insecurity and ideal cardiovascular health factors in US adults. JAMA Intern Med. 2017;177:730–732. doi: 10.1001/jamainternmed.2017.0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiser SD, Sheira LA, Palar K, Kushel M, Wilson TE, Adedimeji A, Merenstein D, Cohen M, Turan JM, Metsch L, et al. Mechanisms from food insecurity to worse HIV treatment outcomes in US women living with HIV. AIDS Patient Care STDS. 2020;34:425–435. doi: 10.1089/apc.2020.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiser SD, Palar K, Hatcher A, Young S, Frongillo E, Laraia B. Food Insecurity and health: A conceptual framework. In: Ivers L, ed. Food Insecurity and Public Health. CRC Press; 2015:23–50. [Google Scholar]
- 14. Seligman HK, Berkowitz SA. Aligning programs and policies to support food security and public health goals in the United States. Annu Rev Public Health. 2019;40:319–337. doi: 10.1146/annurev-publhealth-040218-044132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanson KL, Connor LM. Food insecurity and dietary quality in US adults and children: a systematic review. Am J Clin Nutr. 2014;100:684–692. doi: 10.3945/ajcn.114.084525 [DOI] [PubMed] [Google Scholar]
- 16. Melchior M, Chastang JF, Falissard B, Galera C, Tremblay RE, Cote SM, Boivin M. Food insecurity and children's mental health: a prospective birth cohort study. PLoS One. 2012;7:e52615. doi: 10.1371/journal.pone.0052615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pourmotabbed A, Moradi S, Babaei A, Ghavami A, Mohammadi H, Jalili C, Symonds ME, Miraghajani M. Food insecurity and mental health: a systematic review and meta‐analysis. Public Health Nutr. 2020;23:1778–1790. doi: 10.1017/S136898001900435X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55:511–523. doi: 10.1016/j.pcad.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 19. Seldenrijk A, Vogelzangs N, Batelaan NM, Wieman I, van Schaik DJ, Penninx BJ. Depression, anxiety and 6‐year risk of cardiovascular disease. J Psyosom Res. 2015;78:123–129. doi: 10.1016/j.jpsychores.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 20. Berkowitz SA, Meigs JB, DeWalt D, Seligman HK, Barnard LS, Bright OJM, Schow M, Atals SJ, Wexler DJ. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the measuring economic insecurity in diabetes study. JAMA Intern Med. 2015;175:257–265. DOI: 10.1001/jamainternmed.2014.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost‐related medication underuse, and unmet needs. Am J Med. 2014;127(303–310):e303. doi: 10.1016/j.amjmed.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 22. Charkhchi P, Fazeli Dehkordy S, Carlos RC. Housing and food insecurity, care access, and health status among the chronically Ill: an analysis of the behavioral risk factor surveillance system. J Gen Intern Med. 2018;33(5):644–650. doi: 10.1007/s11606-017-4255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;63:2935–2959. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartwell ML, Khojasteh J, Wetherill MS, Croff JM, Wheeler D. Using structural equation modeling to examine the influence of social, behavioral, and nutritional variables on health outcomes based on NHANES data: Addressing complex design, Nonnormally distributed variables, and missing information. Curr Dev Nutr. 2019;3(nzz010). doi: 10.1093/cdn/nzz010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beran TN, Violato C. Structural equation modeling in medical research: a primer. BMC Res Notes. 2010;3:267. PMID: 20969789. doi: 10.1186/1756-0500-3-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paek MS, Ip EH, Levine B, Avis NE. Longitudinal reciprocal relationships between quality of life and coping strategies among women with breast cancer. Ann Behav Med. 2016;50:775–783. PMID: 27272631. doi: 10.1007/s12160-016-9803-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Center for Health Statistics . About the National Health and Nutrition Examination Survey. National Center of Health Statistics. http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed February 22, 2021.
- 28. Measuring Guides for the Dietary Recall Interview . National Center of Health Statistics 2015. http://www.cdc.gov/nchs/nhanes/measuring_guides_dri/measuringguides.htm. Accessed February 21, 2021.
- 29. Food Security in the U.S.: Survey Tools . US Department of Agriculture; http://www.ers.usda.gov/topics/food‐nutrition‐assistance/food‐security‐in‐the‐us/definitions‐of‐food‐security/.
- 30. Palakshappa D, Speiser JL, Rosenthal GE, Vitolins MZ. Food insecurity is associated with an increased risk of comorbid medical conditions in obese adults. J Gen Intern Med. 2019;34:1486–1493. doi: 10.1007/s11606-019-05081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khera R, Pandey A, Ayers CR, Carnethon MR, Greenland P, Ndumele CE, Nambi V, Seliger SL, Chaves PHM, Safford MM, et al. Performance of the pooled cohort equations to estimate atherosclerotic cardiovascular disease risk by body mass index. JAMA Netw Open. 2020;3:e2023242. DOI: 10.1001/jamanetworkopen.2020.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reedy J, Lerman JL, Krebs‐Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, Subar AF, Kahle LL, Tooze JA, et al. Evaluation of the healthy eating index‐2015. J Acad Nutr Diet. 2018;118:1622–1633. doi: 10.1016/j.jand.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirkpatrick SI, Reedy J, Krebs‐Smith SM, Pannucci TE, Subar AF, Wilson MM, Lerman JL, Tooze JA. Applications of the healthy eating index for surveillance, epidemiology, and intervention research: considerations and caveats. J Acad Nutr Diet. 2018;118:1603–1621. doi: 10.1016/j.jand.2018.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krebs‐Smith SM, Pannucci TE, Subar AF, Kirkpatric SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the healthy eating index: HEI‐2015. J Acad Nutr Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Medi. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patel JS, Oh Y, Rand KL, Wu W, Cyders MA, Kroenke K, Stewart JC. Measurement invariance of the patient health questionnaire‐9 (PHQ‐9) depression screener in U.S. adults across sex, race/ethnicity, and education level: NHANES 2005‐2016. Depress Anxiety. 2019; 36:813–823. doi: 10.1002/da.22940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hooper D, Coughlan J, Mullen MR. Structural equation modeling: guidelins for determining model fit. The Electronic Journal of Business Research Methods. 2008;6:53–60. [Google Scholar]
- 38. Saris WE, Satorra A, van der Veld WM. Testing structural equation models or detection of misspecifications? Struct Eq Model Multi J. 2009;16:561–582. doi: 10.1080/10705510903203433 [DOI] [Google Scholar]
- 39. Goff DC, Buxton DB, Pearson GD, Wei GS, Gosselin TE, Addou EA, Stoney CM, Desvigne‐Nickens P, Srinivas PR, Galis ZS, et al. Implementing the national heart, lung, and blood institute's strategic vision in the division of cardiovascular sciences. Circ Res. 2019;124:491–497. doi: 10.1161/CIRCRESAHA.118.314338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ranjit N, Macias S, Hoelscher D. Factors related to poor diet quality in food insecure populations. Transl Behav Med. 2020;10:1297–1305. doi: 10.1093/tbm/ibaa028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leung CW, Epel ES, Ritchie LD, Crawford PB, Laraia BA. Food insecurity is inversely associated with diet quality of lower‐income adults. J Acad Nutr Diet. 2014;114(1943–1953):e1942. doi: 10.1016/j.jand.2014.06.353 [DOI] [PubMed] [Google Scholar]
- 42. Loh IH, Oddo VM, Otten J, Otten J. Food insecurity is associated with depression among a vulnerable workforce: early care and education workers. International Journal of Environmental Research and Public Health. 2021;18(1):170– 10.3390/ijerph18010170. DOI: 10.3390/ijerph18010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Myers CA. Food insecurity and psychological distress: a review of the recent literature. Curr Nutr Rep. 2020;9:107–118. doi: 10.1007/s13668-020-00309-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Day E, Rudd JHF. Alcohol use disorders and the heart. Addiction. 2019;114:1670–1678. doi: 10.1111/add.14703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Talarico GP, Crosta ML, Giannico MB, Summaria F, Calò L, Patrizi R. Cocaine and coronary artery diseases: a systematic review of the literature. J Cardiovasc Med. 2017;18:291–294. doi: 10.2459/JCM.0000000000000511 [DOI] [PubMed] [Google Scholar]
- 46. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low‐income Americans. J Gen Intern Med. 2006;21:71–77. doi: 10.1111/j.1525-1497.2005.00278.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Janio EA, Sorkin DH. Food insecurity and healthcare access, utilization, and quality among middle and later life adults in California. J Aging Health. 2020;33(3–4):171–186. doi: 10.1177/0898264320967563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McDougall JA, Anderson J, Adler Jaffe S, Guest DD, Sussman AL, Meisner ALW, Wiggins CL, Jimenez EY, Pankratz VS. Pankratz vs food insecurity and forgone medical care among cancer survivors. JCO Oncol Pract. 2020;16(9):e922–e932. doi: 10.1200/JOP.19.00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Byhoff E, Kangovi S, Berkowitz SA, DeCamp M, Dzeng E, Earnest M, Gonzalez CM, Hartigan S, Karani R, Memari M, et al. A society of general internal medicine position statement on the internists' role in social determinants of health. J Gen Intern Med. 2020;35(9):2721–2727. doi: 10.1007/s11606-020-05934-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Daniel H, Bornstein SS, Kane GC. Addressing social determinants to improve patient care and promote health equity: an American College of Physicians Position Paper. Ann Intern Med. 2018;168:577–578. doi: 10.7326/M17-2441 [DOI] [PubMed] [Google Scholar]
- 51. Dzau VJ, McClellan MB, McGinnis JM, Burke SP, Coye MJ, Diaz A, Daschle TA, Frist WH, Gaines M, Hamburg MA, et al. Vital directions for health and health care: priorities from a national academy of medicine initiative. JAMA. 2017;317:1461–1470. doi: 10.1001/jama.2017.1964 [DOI] [PubMed] [Google Scholar]
- 52. De Marchis EH, Torres JM, Benesch T, Fichtenber C, Allen IE, Whitaker EM, Gottlieb LM. Interventions addressing food insecurity in health care settings: A systematic review. Ann Fam Med. 2019;17:436–447. doi: 10.1370/afm.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Palakshappa D, Vasan A, Khan S, Seifu L, Feudtner C, Fiks AG. Clinicians' perceptions of screening for food insecurity in suburban pediatric practice. Pediatrics. 2017;140(1):e20170319. DOI: 10.1542/peds.2017-0319 [DOI] [PubMed] [Google Scholar]
- 54. Berkowitz SA, Seligman HK, Rigdon J, Meigs JB, Basu S. Supplemental nutrition assistance program (SNAP) participation and health care expenditures among low‐income adults. JAMA Intern Med. 2017;177:1642–1649. doi: 10.1001/jamainternmed.2017.4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanjeevi N, Freeland‐Graves JH, Sachdev PK. Association of loss of Supplemental Nutrition Assistance Program benefits with food insecurity and dietary intake of adults and children. The Am J Clin Nutr. 2021;114:683–689. doi: 10.1093/ajcn/nqab082 [DOI] [PubMed] [Google Scholar]
- 56. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34:1830–1839. doi: 10.1377/hlthaff.2015.0645 [DOI] [PubMed] [Google Scholar]


