Abstract
Background
Cardiovascular health (CVH) status is associated with cardiovascular diseases (CVD). However, evidence for association of CVH change with risk of CVD is scarce.
Methods and Results
Seven metrics (smoking status, body mass index, physical activity, diet, total cholesterol, blood pressure, and fasting blood glucose) were used to evaluate the CVH status. Having 0 to 2, 3 to 4, and 5 to 7 ideal cardiovascular metrics were categorized as low, moderate, and high CVH status, respectively. Change in CVH status was assessed from 2006/2007 to 2010/2011. We calculated lifetime risk of CVD using a modified Kaplan–Meier method, and life expectancy was evaluated via the multistate lifetable method. There were 82 349 participants included in our analysis. At 35 years index age, the age‐adjusted incident rate and lifetime risk of CVD were increased with decreasing number of ideal CVH metrics. The direction of change in status of CVH was consistently associated with age‐adjusted incident rate and lifetime risk of CVD. At 35 years index age, improvement from low to moderate (37.6% [95% CI, 32.8%–42.4%]) or to high status (24.4% [95% CI, 12.7%–36.0%]) had lower lifetime risk of CVD compared with consistently low status (44.6% [95% CI, 40.8%–48.5%]). The improvement in CVH could prolong the years of life free from CVD. The pattern of incident rate and lifetime risk across change in CVH status was similar at 45 and 55 years index age.
Conclusions
Higher number of CVH metrics was associated with lower lifetime risk of CVD. The improvement of CVH status could reduce the lifetime risk of CVD and prolonged the year of life free from CVD.
Keywords: cardiovascular disease, cardiovascular health status, life expectancy, lifetime risk
Subject Categories: Cardiovascular Disease, Lifestyle, Risk Factors
Nonstandard Abbreviation and Acronym
- CVH
cardiovascular health
Clinical Perspective
What Is New?
Ideal cardiovascular health metrics was associated with lifetime risk of cardiovascular disease.
The improvement in cardiovascular health status could lower the lifetime risk of cardiovascular disease and prolonged the years of life free from cardiovascular diseases.
What Are the Clinical Implications?
The promotion of cardiovascular health might have important implications for prevention of cardiovascular diseases.
Although cardiovascular disease (CVD) outcomes have been substantially improved in past decades, CVD remains the largest single contributor to global morbidity and mortality. 1 , 2 China had the highest number of CVD deaths, which was far higher than any other countries. 2 In 2010, the American Heart Association made recommendations for cardiovascular health (CVH) promotion and disease reduction by monitoring and preventing the development of risk factors before they emerge. 3 There were 7 metrics determined to monitor the CVH, which consisted of 4 health behaviors (smoking status, body mass index [BMI], physical activity, and diet) and 3 health factors (blood pressure, fasting blood glucose, and total cholesterol). 3
Numerous studies have investigated the benefit of ideal CVH metrics on CVD worldwide. Results showed that people with higher number of ideal CVH metrics have lower risk of CVD and CVD‐related mortality. 4 , 5 , 6 Considering the dynamic nature of CVH status, a handful of studies have assessed the change in CVH status and its association with risk of CVD, but the results were not consistent. 7 , 8 , 9 , 10 Results from the Whitehall Ⅱ study did not find a consistent relationship of direction of change in CVH with incident of CVD and mortality, 7 while Gaye et al observed significant reduction on risk of CVD resulting from improvement in CVH. 9 Two other American and British studies also demonstrated that the change of CVH status could alter the risk of subsequent CVD events. 8 , 10 However, prior studies were conducted based on relatively small samples, and evidence about the effect of change in CVH status on CVD risk in the Chinese population is still limited. In addition, previous studies evaluated the association between change in CVH status and risk of CVD using metrics of hazards ratio or incidence rate. 8 , 9 , 10 , 11 Lifetime risk has proved to be a useful quantification of the absolute risk over a lifetime. It is defined as the cumulative probability of developing a disease for a given time period after accounting for competing risk of death, and has been widely used in previous studies. 12 , 13 , 14 , 15 The lifetime risk, which served as an indispensable supplement to the use of short‐term relative risk in clinical practice, is relatively easy to interpret and can readily be used by relevant stakeholders compared with traditional metrics of risk of disease. The lifetime risk of CVD and its relationship with some risk factors have been assessed before, such as BMI, blood pressure, and diabetes. 14 , 15 , 16 However, to our knowledge, no study has evaluated the association of lifetime risk for CVD with change in CVH status over time.
In the present study, we used data from a prospective cohort study to quantify the lifetime risk of CVD according to number of ideal CVH metrics and change in status of CVH. We also evaluated the effect of change in CVH status on total life expectancy, and life expectancy free from CVD and with CVD.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
This study was based on a prospective cohort study performed in the community of Kailuan in Tangshan, China. A total of 101 510 employees (including the retired) of Kailuan Group company were enrolled in the Kailuan cohort study in 2006 and were followed up every 2 years to obtain related information. The protocol of the Kailuan study was approved by the Ethnic Committees of Kailuan General Hospital. Written informed consent was obtained from all participants. More details about the Kailuan study are available in previous publications. 17 In the present study, participants included for analysis of associations between lifetime risk of CVD and number of ideal CVH metrics met the following criteria: aged equal or older than index age at baseline; without history of CVD before 2006; and without missing values of CVH metrics in 2006. To examine whether changes in status of CVH (from baseline in 2006–2007 to a follow‐up visit in 2010–2011) altered the lifetime risk of CVD, participants included were: aged equal or older than index age at visit in 2010 to 2011; without history of CVD before 2010; and without missing values of CVH metrics in 2006 or in 2010. Figure S1 presents the flowchart of participants included for analysis at the index age of 35 years.
CVH Metrics and Status
The information on health behaviors, including smoking status, physical activity, and dietary intake, was obtained via standard questionnaires. Body mass index (BMI) was calculated as weight (kg) divided by square of height (m). Blood pressure was measured using a mercury sphygmomanometer with a cuff of appropriate size by trained nurse and physicians after at least 5 minutes of rest in the sitting position. All procedures followed the standard recommended procedures from the Joint National Committee (JNC) 7 report. 18 Systolic blood pressure is the point at which the first of ≥2 Korotkoff sounds is heard, and the disappearance of Korotkoff sound is used to define diastolic blood pressure. At least 2 readings of blood pressure were taken at a 5‐minute interval. The average value of the multiple blood pressure measures was used for further analysis. A blood sample was drawn after an overnight fast to detect the levels of fasting blood glucose and total cholesterol. The information about dietary salt intake was obtained by asking participants to rate their habitual daily salt intake as low, moderate, or high, which was defined as <6 g/d (<2400 mg/d sodium intake), 6 to 10 g/d (2400–4000 mg/d sodium intake), and >10 g/d (>4000 mg/d sodium intake), respectively. Based on the definition proposed by the American Heart Association, 7 ideal metrics in our study were defined as never smoked, BMI <25 kg/m2, daily salt intake <6 g/d, physical activity >80 minutes per week, total cholesterol <200 mg/dL without medication treatment, fasting blood glucose <100 mg/dL without medication treatment, and blood pressure <120/80 mm Hg without medication treatment. We defined low, moderate, and high CVH status for 0 to 2 ideal metrics, 3 to 4 ideal metrics, and 5 to 7 ideal metrics, respectively. We examined change in status of CVH between 2006/2007 and 2010/2011. We further categorized the participants by change in status of CVH into 9 groups: consistently low, low to moderate, low to high, moderate to low, consistently moderate, moderate to high, high to low, high to moderate, and consistently high. The method of classification was adopted from previous studies. 7
Outcomes
We defined CVD events as myocardial infarction (MI), stroke (including hemorrhagic stroke and ischemic stroke), and heart failure (HF). Cases of MI (I21), stroke (I60, I61, and I63), and HF (I50) were identified according to International Classification of Diseases, Tenth Revision (ICD‐10) codes. The occurrence of cardiovascular events was ascertained through the Municipal Social Insurance Institution and the Hospital Discharge Register. To avoid omissions of suspected CVD, we reviewed the discharge records of 11 Kailuan Hospitals and inquired about the history of CVD at each visit. Data on death were obtained from provincial vital statistics offices and were reviewed by clinicians. More details on identification of cardiovascular events and deaths have been published previously. 11 , 17 , 19 All participants included were followed until death, age 95 years, or end of follow‐up (December 31, 2017).
Statistical Analysis
We used mean (SD) and proportion (%) to describe the characteristics of participants included at index age of 35, 45, and 55 years. In the present study, the Practical Incidence Estimators macro, a modified Kaplan–Meier method, was applied to calculate the probability of developing CVD (lifetime risk of CVD) with age as the time scale. 20 The Practical Incidence Estimators Macro can produce age‐specific incidence rates using direct standardization to the combined group, and estimate the remaining lifetime risk conditional on survival event‐free age (index age of 35, 45, and 55 years) to 95 years old. This method can account for the competing risk of death, which may lead to underestimation of absolute risk for CVD. The age‐adjusted incident rate and lifetime risk of total CVD, MI, stroke, and HF were estimated in subgroups according to number of ideal CVH metrics at baseline. Because of the small sample size of participants with all 7 ideal CVH metrics, we combined them with participants having 6 ideal CVH into 1 group. The cumulative lifetime risk of CVD according to CVH status in 2006/2007 was also assessed. To further explore the association between change in CVH status and CVD risk, we also evaluated the age‐adjusted incident rate and remaining lifetime risk in 9 subgroups according to change in status of CVH.
We also computed the total life expectancy, life expectancy free from CVD, and life expectancy with CVD in participants with different transitions in status of CVH. The life expectancy was evaluated using the multistate lifetable method from the SPACE program (Stochastic Population Analysis for Complex Events). 21 There were 3 different health states: free of CVD, living with CVD, and death, and only 3 transitions between these states were allowed in our analysis: from free of CVD to CVD (incident CVD), CVD to death (mortality among participants with CVD), and free of CVD to death (non‐CVD mortality among participants without CVD). More details have been described by Cai et al. 21
All analyses were performed in SAS (version 9.4; SAS Institute Inc.). P<0.05 was considered statistically significant in this analysis.
RESULTS
There were 82 349, 64 279, and 33 887 participants included for estimation of lifetime risk of CVD at index age of 35, 45, and 55 years, respectively. As index age increased from 35 to 55 years, an increase of blood pressure was observed (systolic blood pressure from 131.97 to 137.92 mm Hg, and diastolic blood pressure from 84.10 to 84.80 mm Hg). There was no obvious difference in the presence of ideal CVH metrics among samples at 35, 45, and 55 years index age. Characteristics of participants for different index age are presented in Table 1.
Table 1.
Characteristics of Participants at Baseline in 3 Study Samples
| Characteristics | Index age | ||
|---|---|---|---|
| 35 y (n=82 349) | 45 y (n=64 279) | 55 y (n=33 887) | |
| Age at baseline, y | 53.31 (10.22) | 56.91 (8.52) | 63.06 (7.19) |
| Sex | |||
| Female | 16 563 (20.11) | 11 968 (18.62) | 5357 (15.81) |
| Male | 65 786 (79.89) | 52 311 (81.38) | 28 530 (84.19) |
| Body mass index, kg/m2 | 25.10 (3.43) | 25.11 (3.41) | 25.08 (3.48) |
| Systolic blood pressure, mm Hg | 131.97 (20.94) | 134.17 (21.17) | 137.92 (21.62) |
| Diastolic blood pressure, mm Hg | 84.10 (11.77) | 84.60 (11.67) | 84.80 (11.50) |
| Fasting blood glucose, mmol/L | 5.52 (1.72) | 5.58 (1.80) | 5.60 (1.89) |
| Total cholesterol, mmol/L | 4.99 (1.16) | 5.02 (1.17) | 5.01 (1.20) |
| Smoking status | |||
| Nonideal | 32 556 (39.53) | 25 321 (39.39) | 12 675 (37.40) |
| Ideal | 49 793 (60.47) | 38 958 (60.61) | 21 212 (62.60) |
| Dietary salt intake | |||
| Nonideal | 74 876 (90.93) | 58 423 (90.89) | 30 645 (90.43) |
| Ideal | 7473 (9.07) | 5856 (9.11) | 3242 (9.57) |
| Physical activities | |||
| Nonideal | 69 284 (84.13) | 52 311 (81.38) | 24 996 (73.76) |
| Ideal | 13 065 (15.87) | 11 968 (18.62) | 8891 (26.24) |
| Body mass index | |||
| Nonideal | 40 316 (48.96) | 31 690 (49.30) | 16 629 (49.07) |
| Ideal | 42 033 (51.04) | 32 589 (50.70) | 17 258 (50.93) |
| Total cholesterol | |||
| Nonideal | 33 408 (40.57) | 27 122 (42.19) | 14 288 (42.16) |
| Ideal | 48 941 (59.43) | 37 157 (57.81) | 19 599 (57.84) |
| Blood pressure | |||
| Nonideal | 67 298 (81.72) | 54 243 (84.39) | 29 729 (87.73) |
| Ideal | 15 051 (18.28) | 10 036 (15.61) | 4158 (12.27) |
| Fasting blood glucose | |||
| Nonideal | 25 428 (30.88) | 20 863 (32.46) | 11 058 (32.63) |
| Ideal | 56 921 (69.12) | 43 416 (67.54) | 22 829 (67.37) |
Data are presented as mean (SD) or number (proportion %).
Ideal CVH and Incident of CVD
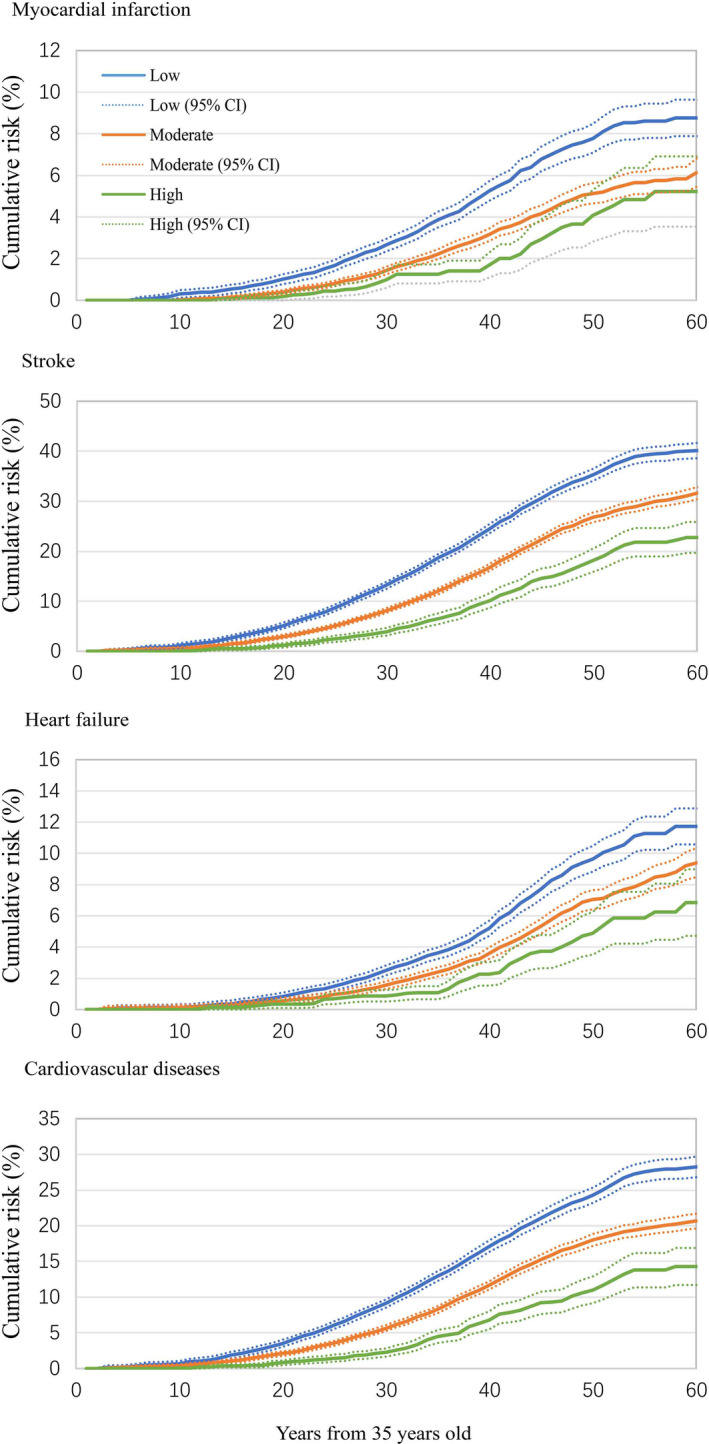
A gradual increase in age‐adjusted incident rate of total CVD and specific‐CVD of MI, stroke, and HF was observed as the number of ideal CVH metrics declined (Table S1). Among participants without any ideal CVH metrics, the age‐adjusted incident rate of CVD from index age of 35 was 12.92 per 1000 person‐years, and that of MI, stroke, and HF were 2.67, 8.09, and 2.60 per 1000 person‐years, respectively. Among participants with ≥6 ideal CVH metrics, the age‐adjusted incident rate from 35 years old of CVD was 3.02 per 1000 person‐years, and that of MI, stroke, and HF was 0.41, 2.00, and 0.84 per 1000 person‐years, respectively. Table 2 reports the lifetime risk of CVD related to the number of ideal CVH metrics. The lifetime risk of developing CVD was substantially decreased from low to high CVH status. The highest lifetime risk of CVD was 44.3% (38.0%–50.5%) for index age 35 years, 42.1% (35.9%–48.4%) for index age 45 years, and 39.4% (32.7%–46.2%) for index age 55 years in participants with no ideal CVH metrics. Participants with >3 ideal CVH metrics had significantly lower lifetime risk of CVD compared with participants with no ideal CVH metrics. Figure 1 presents the curve of cumulative risk of CVD from 35 years of age in subgroups of participants according to status of CVH at baseline.
Table 2.
Lifetime Risk (%) of Cardiovascular Diseases by Number of Ideal Cardiovascular Health Metrics at Index Age of 35, 45, and 55 Years
| No. of ideal metrics | Index age | |||||
|---|---|---|---|---|---|---|
| 35 y | 45 y | 55 y | ||||
| Lifetime risk (95% CI) | P value | Lifetime risk (95% CI) | P value | Lifetime risk (95% CI) | P value | |
| Myocardial infarction | ||||||
| 0 | 12.43 (7.61–17.25) | Reference | 11.07 (6.32–15.83) | Reference | 10.64 (5.72–15.56) | Reference |
| 1 | 8.73 (7.40–10.06) | 0.147 | 8.67 (7.33–10.00) | 0.339 | 7.90 (6.56–9.25) | 0.292 |
| 2 | 8.41 (7.30–9.52) | 0.111 | 8.28 (7.17–9.39) | 0.262 | 7.91 (6.78–9.05) | 0.289 |
| 3 | 6.68 (5.76–7.60) | 0.022 | 6.69 (5.76–7.62) | 0.076 | 6.29 (5.35–7.23) | 0.088 |
| 4 | 5.32 (4.32–6.32) | 0.005 | 5.33 (4.32–6.34) | 0.021 | 5.17 (4.15–6.19) | 0.033 |
| 5 | 5.40 (3.55–7.24) | 0.008 | 5.40 (3.56–7.25) | 0.029 | 5.23 (3.36–7.10) | 0.044 |
| ≥6 | 4.00 (0.06–7.93) | 0.008 | 4.00 (0.06–7.93) | 0.025 | 4.04 (0.06–8.02) | 0.041 |
| Stroke | ||||||
| 0 | 28.25 (22.46–34.05) | Reference | 26.78 (21.31–32.26) | Reference | 24.03 (18.23–29.83) | Reference |
| 1 | 30.85 (27.77–33.93) | 0.437 | 30.69 (27.59–33.80) | 0.223 | 28.77 (25.49–32.05) | 0.163 |
| 2 | 26.97 (25.29–28.65) | 0.676 | 26.67 (24.99–28.36) | 0.971 | 25.38 (23.63–27.13) | 0.663 |
| 3 | 21.73 (20.38–23.08) | 0.032 | 21.61 (20.25–22.96) | 0.072 | 20.32 (18.93–21.70) | 0.222 |
| 4 | 18.92 (17.29–20.56) | 0.002 | 18.50 (16.88–20.11) | 0.004 | 17.72 (16.07–19.37) | 0.040 |
| 5 | 15.11 (12.24–17.99) | <0.001 | 15.08 (12.20–17.96) | <0.001 | 14.52 (11.59–17.46) | 0.004 |
| ≥6 | 9.86 (4.55–15.18) | <0.001 | 9.86 (4.55–15.18) | <0.001 | 9.40 (4.05–14.75) | <0.001 |
| Heart failure | ||||||
| 0 | 11.69 (7.30–16.07) | Reference | 11.42 (7.04–15.81) | Reference | 10.93 (6.40–15.45) | Reference |
| 1 | 12.62 (10.46–14.77) | 0.709 | 12.46 (10.34–14.58) | 0.677 | 11.71 (9.53–13.90) | 0.759 |
| 2 | 11.28 (9.86–12.69) | 0.862 | 11.38 (9.95–12.81) | 0.985 | 11.16 (9.69–12.63) | 0.924 |
| 3 | 10.19 (9.00–11.39) | 0.520 | 10.22 (9.02–11.42) | 0.603 | 9.95 (8.72–11.18) | 0.684 |
| 4 | 8.57 (6.98–10.15) | 0.190 | 8.41 (6.85–9.98) | 0.205 | 8.26 (6.67–9.85) | 0.276 |
| 5 | 7.01 (4.67–9.35) | 0.065 | 7.02 (4.67–9.36) | 0.082 | 6.83 (4.44–9.21) | 0.117 |
| ≥6 | 5.38 (1.43–9.32) | 0.036 | 5.38 (1.43–9.32) | 0.044 | 5.07 (1.14–9.00) | 0.056 |
| Total cardiovascular diseases | ||||||
| 0 | 44.25 (37.96–50.54) | Reference | 42.11 (35.86–48.36) | Reference | 39.43 (32.67–46.19) | Reference |
| 1 | 42.52 (39.53–45.51) | 0.626 | 42.24 (39.23–45.25) | 0.971 | 39.84 (36.61–43.08) | 0.914 |
| 2 | 38.62 (36.79–40.45) | 0.092 | 38.36 (36.53–40.20) | 0.260 | 36.98 (35.05–38.92) | 0.495 |
| 3 | 33.15 (31.56–34.75) | 0.001 | 33.10 (31.50–34.70) | 0.006 | 31.58 (29.91–33.24) | 0.027 |
| 4 | 29.22 (27.21–31.22) | <0.001 | 28.74 (26.75–30.73) | <0.001 | 27.84 (25.80–29.89) | 0.001 |
| 5 | 23.91 (20.50–27.31) | <0.001 | 23.89 (20.47–27.30) | <0.001 | 23.16 (19.66–26.66) | <0.001 |
| ≥6 | 16.02 (9.55–22.48) | <0.001 | 16.02 (9.55–22.48) | <0.001 | 15.30 (8.79–21.80) | <0.001 |
Figure 1. Cumulative risk (percent) of cardiovascular diseases from index age of 35 years by cardiovascular health status.
Change in Status of CVH and Incidence of CVD
Change in CVH status was examined among 52 627 individuals. From 2006/2007 to 2010/2011, 20.5% (n=10 797) of participants improved their CVH status mostly from low to moderate (13.7%) and from moderate to high (5.8%), while only 1.0% improved from low to high. On the contrary, among 11 005 (20.9%) participants having worse CVH status, 14.9% changed from moderate to low and 0.9% and 5.1% changed from high to low and moderate, respectively. More than half of the participants (58.6%) stayed at the same status of CVH (22.9% in low status, 32.7% in moderate status, and 3.0% in high status).
Table S2 shows the age‐adjusted incident rate of total CVD, MI, stroke, and HF by change in status of CVH. Participants with consistently low CVH status had the highest age‐adjusted incident rate of CVD (11.40, 12.51, and 16.04 per 1000 person‐years at index age of 35, 45, and 55 years old, respectively), and the lowest (2.70, 3.01, and 4.04 per 1000 person‐years at index age of 35, 45, and 55 years old, respectively) was observed in participants with consistently high CVH status. The improvement in CVH status lowered the age‐adjusted incident rate of CVD compared with corresponding stable status of CVH. For instance, the age‐adjusted incident rates of CVD at 35 index age for participants with CVH status changed from low to moderate and from low to high were 7.81 and 5.02 per 1000 person‐years, which were lower than that for participants with consistently low status (11.40 per 1000 person‐years).
At index age of 35 years, the lifetime risk of CVD for the consistently low group (44.6% [95% CI, 40.8%–48.5%]) was significantly higher than that of the low to moderate group (37.6% [95% CI, 32.8%–42.4%]) and low to high group (24.4% [95% CI, 12.7%–36.0%]). Compared with the consistently moderate group (29.7% [95% CI, 26.7%–32.6%]), the lifetime risk of CVD at 35 index age in the moderate to low group (40.4% [95% CI, 33.1%–47.7%]) was higher, while that in the moderate to high group was much lower (19.7% [95% CI, 14.7%–24.7%]). Participants with consistently high status showed lowest lifetime risk from 35 to 95 years old (14.7% [95% CI, 6.9%–22.6%]), and the change in status from high to moderate or low significantly increased the lifetime risk of CVD (22.0% [95% CI, 15.6%–28.4%] for the high to moderate group; 28.6% [95% CI, 16.7%–40.5%] for the high to low group). The associations between change in status of CVH and lifetime risk of CVD at index age of 45 or 55 years were similar to those at 35 index age (Table 3).
Table 3.
Lifetime Risk (%) of Cardiovascular Diseases by Change in Status of Cardiovascular Health From 2006/2007 to 2010/2011
| Change in CVH status | Index age | |||||
|---|---|---|---|---|---|---|
| 35 y | 45 y | 55 y | ||||
| Lifetime risk (95% CI) | P value | Lifetime risk (95% CI) | P value | Lifetime risk (95% CI) | P value | |
| Myocardial infarction | ||||||
| Consistently low | 6.58 (5.14– 8.03) | Reference | 6.45 (5.01–7.89) | Reference | 5.63 (4.18–7.09) | Reference |
| Low to moderate | 7.35 (5.23–9.47) | 0.558 | 7.21 (5.09–9.33) | 0.563 | 7.10 (4.92–9.28) | 0.272 |
| Low to high | 1.99 (0.00–4.90) | 0.005 | 2.02 (0.00–4.96) | 0.008 | 1.43 (0.00–4.22) | 0.009 |
| Moderate to low | 7.69 (5.26–10.11) | 0.443 | 7.69 (5.24–10.14) | 0.392 | 7.32 (4.82–9.81) | 0.252 |
| Consistently moderate | 4.60 (3.59–5.61) | 0.027 | 4.59 (3.58–5.60) | 0.038 | 4.32 (3.30–5.34) | 0.148 |
| Moderate to high | 4.00 (1.76–6.25) | 0.058 | 4.02 (1.76–6.27) | 0.074 | 4.08 (1.79–6.36) | 0.261 |
| High to low | 12.03 (1.47–22.60) | 0.316 | 12.03 (1.47–22.60) | 0.305 | 11.27 (0.53–22.01) | 0.308 |
| High to moderate | 3.45 (0.67–6.23) | 0.050 | 3.45 (0.67–6.23) | 0.060 | 3.53 (0.69–6.37) | 0.197 |
| Consistently high | 2.77 (0.00–5.76) | 0.024 | 2.77 (0.00–5.76) | 0.030 | 2.51 (0.00–5.47) | 0.063 |
| Stroke | ||||||
| Consistently low | 33.10 (29.04–37.16) | Reference | 33.17 (29.04–37.31) | Reference | 31.51 (27.15–35.87) | Reference |
| Low to moderate | 27.77 (22.86–32.68) | 0.101 | 27.46 (22.48–32.43) | 0.083 | 26.40 (21.19–31.61) | 0.141 |
| Low to high | 18.36 (8.24–28.47) | 0.008 | 18.60 (8.36–28.84) | 0.010 | 16.29 (5.82–26.77) | 0.009 |
| Moderate to low | 27.31 (19.54–35.08) | 0.196 | 27.03 (19.11–34.95) | 0.177 | 25.44 (17.18–33.71) | 0.203 |
| Consistently moderate | 20.86 (18.22–23.5) | <0.001 | 20.82 (18.16–23.48) | <0.001 | 19.84 (17.11–22.56) | <0.001 |
| Moderate to high | 13.78 (9.14–18.42) | <0.001 | 13.67 (9.01–18.33) | <0.001 | 13.01 (8.28–17.74) | <0.001 |
| High to low | 16.62 (7.47–25.76) | 0.002 | 16.62 (7.47–25.76) | 0.002 | 15.25 (6.05–24.45) | 0.003 |
| High to moderate | 16.08 (9.95–22.21) | <0.001 | 16.08 (9.95–22.21) | <0.001 | 16.19 (9.91–22.47) | <0.001 |
| Consistently high | 11.72 (4.08–19.36) | <0.001 | 11.72 (4.07–19.36) | <0.001 | 11.18 (3.48–18.88) | <0.001 |
| Heart failure | ||||||
| Consistently low | 11.72 (8.93–14.51) | Reference | 11.75 (8.92–14.57) | Reference | 11.11 (8.20–14.02) | Reference |
| Low to moderate | 9.90 (6.38–13.43) | 0.428 | 9.81 (6.26–13.35) | 0.401 | 9.64 (5.98–13.30) | 0.537 |
| Low to high | 6.37 (0.00–14.18) | 0.206 | 6.45 (0.00–14.37) | 0.217 | 6.72 (0.00–14.97) | 0.325 |
| Moderate to low | 11.69 (7.33–16.04) | 0.990 | 11.75 (7.34–16.16) | 0.999 | 11.50 (6.98–16.01) | 0.889 |
| Consistently moderate | 7.79 (5.75–9.82) | 0.025 | 7.71 (5.67–9.75) | 0.023 | 7.42 (5.35–9.49) | 0.043 |
| Moderate to high | 4.04 (1.85–6.24) | <0.001 | 4.06 (1.86–6.26) | <0.001 | 3.66 (1.46–5.86) | <0.001 |
| High to low | 12.60 (2.18–23.02) | 0.873 | 12.60 (2.18–23.02) | 0.877 | 10.93 (0.40–21.45) | 0.974 |
| High to moderate | 4.61 (1.61–7.61) | 0.001 | 4.61 (1.61–7.61) | 0.001 | 4.41 (1.36–7.45) | 0.002 |
| Consistently high | 0.89 (0.00–1.92) | <0.001 | 0.89 (0.00–1.92) | <0.001 | 0.69 (0.00–1.65) | <0.001 |
| Total cardiovascular diseases | ||||||
| Consistently low | 44.61 (40.75–48.47) | Reference | 44.70 (40.77–48.63) | Reference | 42.62 (38.39–46.84) | Reference |
| Low to moderate | 37.56 (32.75–42.37) | 0.025 | 37.11 (32.23–41.99) | 0.018 | 36.07 (30.92–41.21) | 0.054 |
| Low to high | 24.38 (12.72–36.04) | 0.001 | 24.71 (12.91–36.51) | 0.002 | 22.24 (9.98–34.50) | 0.002 |
| Moderate to low | 40.39 (33.13–47.65) | 0.315 | 40.34 (32.93–47.74) | 0.308 | 38.73 (30.92–46.53) | 0.391 |
| Consistently moderate | 29.65 (26.74–32.55) | <0.001 | 29.56 (26.63–32.49) | <0.001 | 28.30 (25.28–31.33) | <0.001 |
| Moderate to high | 19.68 (14.68–24.69) | <0.001 | 19.61 (14.59–24.63) | <0.001 | 18.70 (13.58–23.81) | <0.001 |
| High to low | 28.64 (16.74–40.54) | 0.018 | 28.64 (16.74–40.54) | 0.018 | 26.16 (13.85–38.47) | 0.020 |
| High to moderate | 21.99 (15.61–28.37) | <0.001 | 21.99 (15.61–28.37) | <0.001 | 21.96 (15.43–28.50) | <0.001 |
| Consistently high | 14.74 (6.87–22.61) | <0.001 | 14.74 (6.87–22.61) | <0.001 | 13.80 (5.85– 21.76) | <0.001 |
CVH indicates cardiovascular health.
Figure 2 shows estimates of life expectancy by change in CVH status. At index age of 35 years, years of life expectancy free from CVD were 38.36, 45.00, and 50.34 in the group with consistently low, moderate, and high status of CVH, respectively. The improvement in CVH status prolonged the life expectancy free from CVD (41.70 years for low to moderate status, 43.40 years for low to high status, and 47.26 years for moderate to high status), and the worsening of CVH shortened the life expectancy free from CVD in the group with moderate to low status (43.00 years), high to moderate status (48.31 years), and high to low status (43.44 years). The patterns of life expectancy at the index age of 45 and 55 years were similar to that at 35 years old (Table S3).
Figure 2. Total life expectancy, life expectancy free from cardiovascular disease, and life expectancy with cardiovascular diseases (years) by change in cardiovascular health status from 2006/2007 to 2010/2011.
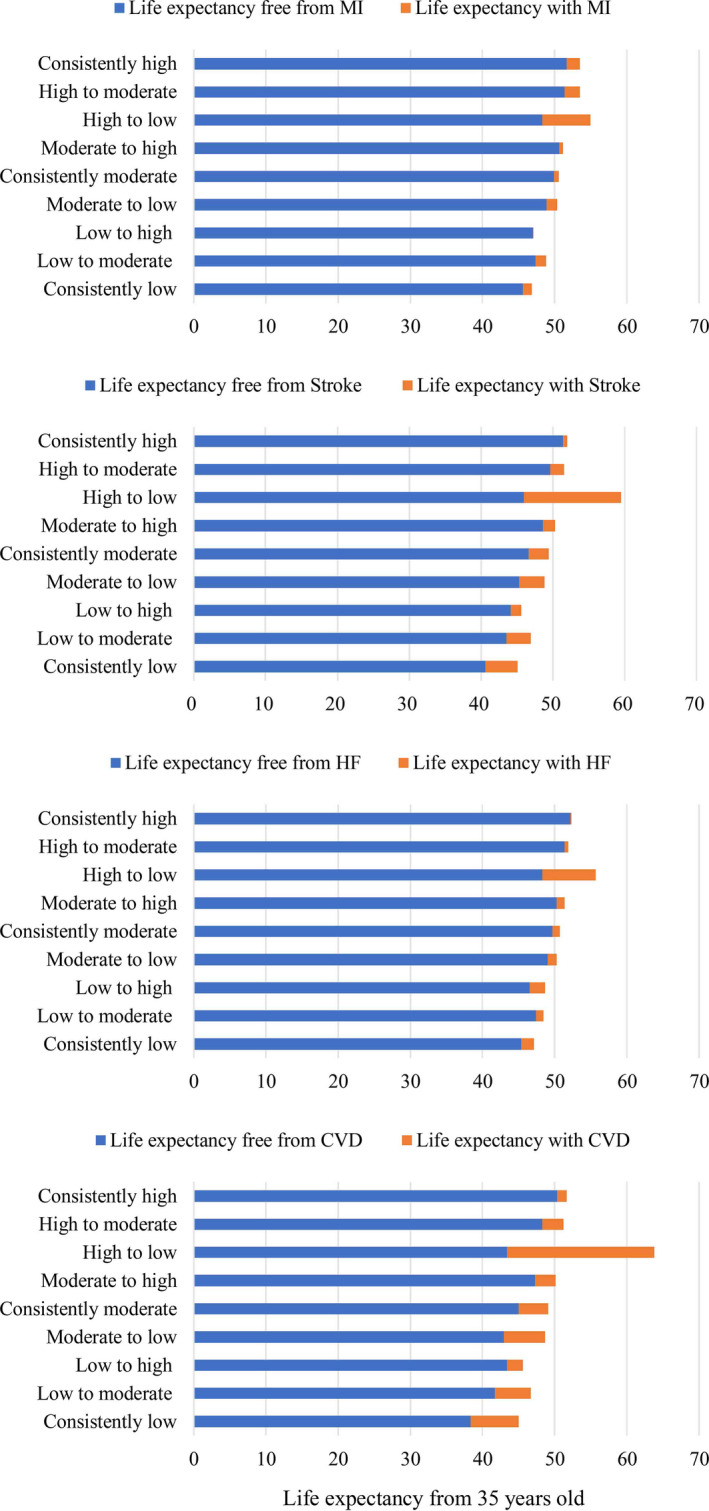
CVD indicates cardiovascular diseases; HF, heart failure; and MI, myocardial infarction.
DISCUSSION
In the present study, participants with a higher number of CVH metrics at baseline had a lower lifetime risk of CVD. The improvement of CVH status could significantly reduce the lifetime risk of CVD and prolonged the life expectancy free from CVD. Instead, the worsening of CVH increased the lifetime risk of CVD and shortened the years of life free from CVD.
The associations between ideal CVH metrics and cardiovascular outcomes have been widely assessed before. Prior studies have consistently reported that a higher number of ideal CVH metrics was associated with a lower risk of cardiovascular events, such as MI, stroke, and CVD mortality. 4 , 22 , 23 However, the association between CVH and risk of CVD was mostly estimated using the metric of hazards ratio 4 , 23 , 24 or incident rate. 25 Several studies have estimated the lifetime risk of cardiovascular events by 4 cardiovascular‐related risk factors (level of total cholesterol, blood pressure, diabetic status, and smoking status). 14 , 26 For example, Berry et al found that the lifetime risk of MI and stroke at 45 years of age increased from 1.7% to 42.0% and from 6.7% to 10.3%, respectively, with the increasing number of elevated risk factors. 26 Similar results were also observed in the Chinese Multi‐Provincial Cohort study. Among 21 953 participants, the lifetime risk of CVD at the index age of 35 years was associated with levels of blood pressure, non‐HDL (non–high‐density lipoprotein), HDL, BMI, diabetic status, and smoking status individually and in combination. 27 However, to date, the evidence for association between lifetime risk of CVD and ideal CVH metrics is scarce. Results of our study consistently showed a negative association of ideal CVH metrics at baseline with lifetime risk of CVD.
CVH status is determined by 7 health metrics, which can be altered by change in lifestyles and behaviors over time. The ARIC (Atherosclerosis Risk in Communities) study reported that 38.2% of participants changed their CVH status between 1997 to 1998 and 1993 to 1995. 9 In the Whitehall II study, about half (42.1%) of participants’ CVH status changed during 10.4 years of follow‐up. 7 In the present study, only 58.6% participants stayed at the same status of CVH over ≈4 years of follow‐up. The above evidence supports the notion that the CVH status is under dynamic change.
The effect of change in CVH status on the cardiovascular system has been assessed before. Aatola et al reported that each 1‐point increase in ideal CVH metrics was associated with 0.09 m/s decrease in pulse wave velocity, an indicator of arterial stiffness. 28 Hwang et al demonstrated that each unit decrease in ideal CVH metrics was related to a 15% higher risk of coronary artery calcium, although the association was not statistically significant. 29
However, to date, only 4 longitudinal studies have assessed the prevalence of change in CVH status and its association with CVD. In the Framingham offspring study and British Regional Heart Study, the score of 2, 1, 0 was assigned to ideal, intermediate, and poor status for each metric, respectively, and status of CVH was further defined as high (≥8 score) and low (≤7 score). 8 , 10 Results from both studies showed that the risk of CVD increased in an order from low to low group, low to high group, high to low group, to high to high group. 8 , 10 To more precisely evaluate the change in CVH status, the Whitehall II study and ARIC study adopted the method of classification by categorizing participants into 9 subgroups according to change in status of CVH, 7 , 9 which was also applied in our analysis. The ARIC study demonstrated that improvement in CVH status was associated with a lower risk of incident CVD and all‐cause mortality. 9 However, the Whitehall II study did not find any consistent relationships between the direction of change in status of CVH and risk of CVD, 7 which were observed in the present study. Except for the much larger sample size of our study, the discrepancy in results may also be attributable to the diversity in participants. The target population for the Whitehall II study was British civil servant, and was aged 35 to 55 years and mostly White race, while the subjects in the present study were all from the Kailuan company in Tangshan, China, and were much older and in a wider age range of 35 to 95 years old. Since the proportion of participants having low CVH status in the Whitehall II study at baseline was 23.5%, and that of our study was 39.3%, it seemed that subjects in the Whitehall II study might have been relatively highly health conscious and were living under healthier conditions. In addition, assessment of change in CVH status in the Whitehall II study was conducted over an 11‐year period, approximately twice as long as that of our study. The change in CVH status over a longer period was more vulnerable to natural aging so that deteriorating physical condition was more likely to be observed, which might also explain the difference in results between the Whitehall II study and the present study. Moreover, we also evaluated the life expectancy by change in CVH. Our findings showed that the improvement in CVH could prolong the years of life free from CVD, which matched with the primary conclusions in our study.
Our study was derived from the Kailuan cohort study and was conducted with a large sample size. To our knowledge, this is the first study to evaluate the effect of ideal CVH metrics and change in CVH status on CVD using metrics of lifetime risk and life expectancy. However, there are some limitations that should be taken into consideration. First, our study's follow‐up is relatively short. However, the significant association between change in CVH status and lifetime risk of CVD was still observed in our study, suggesting that change in CVH status even over a few years had considerable effect on CVD risk. Second, with more categorical groups, the sample size in some categories was small, which may influence the stability of our results. Third, the diagnosis of CVD was identified by ICD‐10 code, which might cause misclassification bias and typically reduce the precision of the estimates. However, this error generally biases the risk estimates downward. In addition, to avoid omissions of suspected CVD, we reviewed the discharge records of 11 Kailuan Hospitals and inquired about the history of CVD at each visit, which would significantly reduce the potential bias caused by misclassification. Fourth, HF can be categorized as HF with reduced ejection fraction, HF with moderately reduced ejection fraction, and HF with preserved ejection fraction, which represents different entities, but the data were not available in our study. Further studies should pay attention to the subtypes of HF.
CONCLUSIONS
In conclusion, our study suggested that ideal CVH metrics were associated with lifetime risk of CVD. In addition, the direction of change in CVH status was associated with lifetime risk of CVD and life expectancy free from CVD. The improvement in CVH could lower the lifetime risk of CVD and prolonged the years of life without CVD.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Shouling Wu, Email: drwusl@163.com.
Yaohua Tian, Email: yaohua_tian@hust.edu.cn.
REFERENCES
- 1. Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005–2013. MMWR Suppl. 2014;63:3–27. [PubMed] [Google Scholar]
- 2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 4. Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li NA, Bian L, Wu J, Jia Q, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–2456. doi: 10.1161/STROKEAHA.113.678839 [DOI] [PubMed] [Google Scholar]
- 5. Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta‐analysis of prospective studies. Clin Cardiol. 2017;40:1339–1346. doi: 10.1002/clc.22836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaye B, Canonico M, Perier MC, Samieri C, Berr C, Dartigues JF, Tzourio C, Elbaz A, Empana JP. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the Three‐City Study. J Am Coll Cardiol. 2017;69:3015–3026. doi: 10.1016/j.jacc.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 7. van Sloten TT, Tafflet M, Périer MC, Dugravot A, Climie RED, Singh‐Manoux A, Empana JP. Association of change in cardiovascular risk factors with incident cardiovascular events. JAMA. 2018;320:1793–1804. doi: 10.1001/jama.2018.16975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Enserro DM, Vasan RS, Xanthakis V. Twenty‐year trends in the American Heart Association Cardiovascular health score and impact on subclinical and clinical cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc. 2018;7:e008741. doi: 10.1161/JAHA.118.008741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaye B, Tajeu GS, Vasan RS, Lassale C, Allen NB, Singh‐Manoux A, Jouven X. Association of changes in cardiovascular health metrics and risk of subsequent cardiovascular disease and mortality. J Am Heart Assoc. 2020;9:e017458. doi: 10.1161/JAHA.120.017458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed A, Pinto Pereira SM, Lennon L, Papacosta O, Whincup P, Wannamethee G. Cardiovascular health and stroke in older british men: prospective findings from the British Regional Heart Study. Stroke. 2020;51:3286–3294. doi: 10.1161/STROKEAHA.120.030546 [DOI] [PubMed] [Google Scholar]
- 11. Wu Z, Jin C, Vaidya A, Jin W, Huang Z, Wu S, Gao X. Longitudinal patterns of blood pressure, incident cardiovascular events, and all‐cause mortality in normotensive diabetic people. Hypertension. 2016;68:71–77. doi: 10.1161/HYPERTENSIONAHA.116.07381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;4:321–329. doi: 10.1056/NEJMoa1012848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, McManus DD, Ko D, Weng L‐C, Lunetta KL, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018;361:k1453. doi: 10.1136/bmj.k1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd‐Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801. doi: 10.1001/jama.2012.14312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, Lloyd‐Jones DM. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–287. doi: 10.1001/jamacardio.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd‐Jones DM, Wilkins JT. Hypertension, obesity, diabetes, and heart failure‐free survival: the cardiovascular disease lifetime risk pooling project. JACC Heart Fail. 2016;4:911–919. doi: 10.1016/j.jchf.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang C, Yuan YU, Zheng M, Pan AN, Wang M, Zhao M, Li Y, Yao S, Chen S, Wu S, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75:2921–2930. doi: 10.1016/j.jacc.2020.04.038 [DOI] [PubMed] [Google Scholar]
- 18. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 19. Wu S, Song Y, Chen S, Zheng M, Ma Y, Cui L, Jones JB. Blood pressure classification of 2017 associated with cardiovascular disease and mortality in young Chinese adults. Hypertension. 2020;76:251–258. doi: 10.1161/HYPERTENSIONAHA.119.14239 [DOI] [PubMed] [Google Scholar]
- 20. Beiser A, D'Agostino RB Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer's disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–1522. doi: [DOI] [PubMed] [Google Scholar]
- 21. Cai L, Hayward MD, Saito Y, Lubitz J, Hagedorn A, Crimmins E. Estimation of multi‐state life table functions and their variability from complex survey data using the SPACE Program. Demogr Res. 2010;22:129–158. doi: 10.4054/DemRes.2010.22.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Younus A, Aneni EC, Spatz ES, Osondu CU, Roberson L, Ogunmoroti O, Malik R, Ali SS, Aziz M, Feldman T, et al. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non‐US populations. Mayo Clin Proc. 2016;91:649–670. doi: 10.1016/j.mayocp.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 23. Isiozor NM, Kunutsor SK, Voutilainen A, Kurl S, Kauhanen J, Laukkanen JA. Ideal cardiovascular health and risk of acute myocardial infarction among Finnish men. Atherosclerosis. 2019;289:126–131. doi: 10.1016/j.atherosclerosis.2019.08.024 [DOI] [PubMed] [Google Scholar]
- 24. Han C, Liu F, Yang X, Chen J, Li J, Cao J, Li Y, Shen C, Yu L, Liu Z, et al. Ideal cardiovascular health and incidence of atherosclerotic cardiovascular disease among Chinese adults: the China‐PAR project. Sci China Life Sci. 2018;61:504–514. doi: 10.1007/s11427-018-9281-6 [DOI] [PubMed] [Google Scholar]
- 25. Ommerborn MJ, Blackshear CT, Hickson DA, Griswold ME, Kwatra J, Djoussé L, Clark CR. Ideal cardiovascular health and incident cardiovascular events: the Jackson Heart Study. Am J Prev Med. 2016;51:502–506. doi: 10.1016/j.amepre.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Liu J, Wang W, Wang M, Qi Y, Xie W, Li Y, Sun J, Liu J, Zhao D. Lifetime risk for cardiovascular disease in a Chinese population: the Chinese Multi‐Provincial Cohort Study. Eur J Prev Cardiol. 2015;22:380–388. doi: 10.1177/2047487313516563 [DOI] [PubMed] [Google Scholar]
- 28. Aatola H, Hutri‐Kähönen N, Juonala M, Laitinen TT, Pahkala K, Mikkilä V, Telama R, Koivistoinen T, Lehtimäki T, Viikari JSA, et al. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. J Am Heart Assoc. 2014;3:e000532. doi: 10.1161/JAHA.113.000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hwang SJ, Onuma O, Massaro JM, Zhang X, Fu YP, Hoffmann U, Fox CS, O'Donnell CJ. Maintenance of ideal cardiovascular health and coronary artery calcium progression in low‐risk men and women in the Framingham Heart Study. Circ Cardiovasc Imaging. 2018;11:e006209. doi: 10.1161/CIRCIMAGING.117.006209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


